Abstract
Deregulation of protein synthesis is a hallmark of cancer cell proliferation, survival, and metastatic progression. eIF5A1, and its highly related isoform eIF5A2, are translation initiation factors that have been implicated in a range of human malignancies, but how they control cancer development and disease progression is still poorly understood. Here, we investigated how eIF5A proteins regulate pancreatic ductal adenocarcinoma (PDAC) pathogenesis. eIF5A proteins are the only known proteins regulated by a distinct posttranslational modification termed hypusination, which is catalyzed by two enzymes, deoxyhypusine synthase (DHPS) and deoxyhypusine hydroxylase (DOHH). The highly selective nature of the hypusine modification and its amenability to pharmacological inhibition make eIF5A proteins attractive therapeutic targets. We found that the expression and hypusination of eIF5A proteins are upregulated in human PDAC tissues and in premalignant pancreatic intraepithelial neoplasia (PanIN) tissues isolated from Pdx-1-Cre: LSL-KRASG12D mice. Knockdown of eIF5A proteins in PDAC cells inhibited their growth in vitro and orthotopic tumor growth in vivo, whereas amplification of eIF5A proteins increased PDAC cell growth and tumor formation in mice. Small molecule inhibitors of DHPS and DOHH both suppressed eIF5A hypusination, preventing PDAC cell growth. Interestingly, we found that eIF5A proteins regulate PDAC cell growth by modulating the expression of PEAK1, a non-receptor tyrosine kinase essential for PDAC cell growth and therapy resistance. Our findings suggest that eIF5A proteins utilize PEAK1 as a downstream effector to drive PDAC pathogenesis, and that pharmacological inhibition of the eIF5A-hypusine-PEAK1 axis may provide a novel therapeutic strategy to combat this deadly disease.
Keywords: pancreatic ductal adenocarcinoma, eukaryotic translation initiation factor 5A, pseudopodium-enriched atypical kinase one, chemoresistance, ciclopirox olamine
Introduction
A widespread feature of cancer pathogenesis is deregulation of protein synthesis, which is characterized by hyperactive ribosome biogenesis and reprogramming of mRNA translation in a manner that favors proliferation, survival and metastasis [1-3]. Recent work has suggested that targeting regulatory components of oncogenic protein synthesis represents an effective anti-neoplastic strategy [1-4]. One of the candidate targets is eIF5A (eukaryotic translation initiation factor 5A), an 18-kDa protein that is highly conserved from archae to humans. eIF5A is indispensible for normal mammalian development, is involved in translation elongation and mRNA transport, and is important for cell cycle progression and proliferation [5-10]. Vertebrates carry two genes that encode two highly homologous eIF5A isoforms, eIF5A1 and eIF5A2, which in humans is 84% identical. eIF5A1 is ubiquitously expressed in all tissues, whereas eIF5A2 expression is primarily restricted to brain and testis [11].
Important recent work has shown that EF-P (translation elongation factor P), the bacterial homolog of eIF5A, alleviates ribosome stalling during the synthesis of polyproline motif-containing proteins [12, 13]. Interestingly, the pyrrolidine ring of proline imposes structural constraints on the positioning of the amino acid in the peptidyl transfer center, which reduces peptide bond formation leading to ribosome stalling. EF-P is proposed to alleviate this by stabilizing peptidyl-tRNA on the ribosome and by optimizing the positioning of substrates. eIF5A has also been reported to perform a similar function in yeast [14]. These findings suggest that a major function of EF-P/eIF5A is to enhance and fine-tune the production of a set of polyproline tract-containing proteins. This unique modulatory function may be crucial for hyper-proliferating cancer cells, which have substantial demands for oncogenic and metabolic proteins containing polyproline domains. Indeed, many cell cycle, apoptosis, and signal transduction proteins contain polyproline regions [15-17]. Increased demands for such proteins may explain why eIF5A expression is increased in several cancers including glioblastoma, leukemia, liver, colon, lung, cervical, and ovarian cancer [18-25].
It is notable that the mRNA translational activity of both eIF5A proteins is uniquely regulated by the formation of an unusual amino acid, hypusine ([N-(4-amino-2-hydroxybutyl) lysine]) [11, 26]. Hypusine is formed by the transfer of the butylamine portion of the polyamine, spermidine, to the ε-amino group of a specific lysine substrate of eIF5A, which is catalyzed by deoxyhypusine synthase (DHPS). Carbon 2 of the transferred 4-aminobutyl moiety is then hydroxylated by deoxyhypusine hydroxylase (DOHH). Each step in the enzymatic reaction are amenable to specific pharmacological inhibition by N(1)-guanyl-1,7,-diamineoheptane (GC7), an inhibitor of DHPS, and ciclopirox olamine (CPX), a bidentate iron chelator that inhibits DOHH [8, 26-28]. The fact that eIF5A proteins are upregulated in proliferating cancer cells, regulate translation of polyproline-containing proteins, and are the only proteins known to be hypusine-modified make them attractive therapeutic targets to treat cancer. Such new therapeutic targets and strategies are sorely needed to treat pancreatic ductal adenocarcinoma (PDAC), which has a dismal 5-year survival rate of 3-5% [29]. However, the role of eIF5A and hypusine regulation have not been previously investigated in PDAC. Therefore, the goal of the current study is to determine if eIF5A is critically involved in PDAC. Our findings demonstrate that eIF5A proteins are overexpressed and hypusinated in murine pancreatic intraepithelial neoplasia (PanIN) and human PDAC tissues. We also demonstrate that pharmacological inhibition of eIF5A hypusination, or genetic knockdown of eIF5A proteins, inhibits PDAC cell growth in vitro and orthotopic tumor formation in vivo. Finally, we show that eIF5A proteins control the expression of the novel tyrosine kinase PEAK1 (pseudopodium-enriched atypical kinase 1; Sgk269), which we have previously shown is overexpressed in human PDAC tissues, and is critical for PDAC tumor growth, metastasis and gemcitabine resistance [30-33].
Materials and Methods
Cell lines, siRNAs and DNA constructs
Three PDAC cell lines (FG, PANC1, 779E) and HPNE (Human Pancreatic Nestin Expressing) cells were used in this study and maintained as described [30, 34]. 779E cells were established from a patient-derived tumor (moderately-to-poorly differentiated PDAC) by A. M. Lowy. FG cells were kindly gifted from Dr. David Cheresh (UCSD), while PANC1 cells and HPNE cells were obtained from American Type Culture Collection. The cell lines were regularly authenticated based on morphological and growth characteristics as well as analysis of KRas mutation, which was most recently conducted in March 2014. Details of siRNAs and DNA constructs are in Supplementary Methods.
Antibodies, chemicals and tissue microarrays
Antibodies directed at eIF5A1 (rabbit monoclonal (EP526Y): ab32443) and eIF5A2 (mouse monoclonal (1E7): H00056648-M01) were purchased from AbCam and Abnova, respectively. Anti-Src and phospho-Src (Tyr416) antibodies were from Cell Signaling Technology. Anti-GAPDH antibody was from Abcam, anti-survivin antibody was from Biolegend, anti-tubulin, DHPS and DOHH antibodies were from Sigma, anti-KRas antibody was from Santa Cruz Biotechnology (sc-30) and anti-PEAK1 antibody was from Millipore. The antibody specifically recognizing hypusinated eIF5A1 (NIH353) was a kind gift from Dr. Myung-Hee Park (NIH), which has been validated for use in western blotting [35] and immunohistochemistry [5, 36] in human as well as mouse tissues and cell lines. GC7 (N1-guanyl-1, 7-diaminoheptane) was purchased from Calbiochem, CPX (ciclopirox olamine) was from Santa Cruz Biotechnology. Cisplatin was purchased from Enzo Life Sciences, and gemcitabine was from Eli Lilly and used as described previously [30]. Human pancreatic cancer tissue arrays were purchased from US Biomax (PA2081a except for DOHH staining, where PA803 was used).
Immunohistochemistry
Human pancreatic cancer tissue array was purchased from US Biomax as described above. PDX-1-Cre:LSL-KRASG12D mouse pancreas sections were provided by A. M. Lowy. Samples from patients BK-17 and BK-19 were collected and processed as described previously [30], in accordance with UCSD (La Jolla, CA) Institutional Review Board (IRB) #071136X. Determination of eIF5A1, eIF5A2 and hypusinated eIF5A1 protein expression was performed as described previously using specific antibodies (ab32443 (Abcam) for eIF5A1, H00056648-M01 (Abnova) for eIF5A2 and NIH353 for hypusinated eIF5A1) [30, 35, 36]. Images were collected with a Leica DM2500 microscope using a 40x objective lens.
Western blotting and quantitative PCR (qPCR)
Western blotting and qPCR were performed as described previously [30]. Briefly, cells were lysed in RIPA (radioimmunoprecipitation assay) buffer (20 mM Tris-HCl pH7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with Complete protease inhibitor cocktail (Roche), and equal amounts of cell extracts were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with appropriate primary and HRP-conjugated secondary antibodies (Jackson ImmunoResearch) for western blotting. For experiments in Fig. 2B and C, cells were serum-starved overnight prior to lysis to assess the effect of KRas activation without stimulation from growth factors present in the serum. For qPCR, mRNA was isolated using Trizol (Invitrogen) and RNeasy kit (Qiagen), and cDNA was synthesized using iScript cDNA synthesis kit (BioRad). All PCR reactions were carried out using GoTaq Flexi DNA Polymerase kit (Promega).
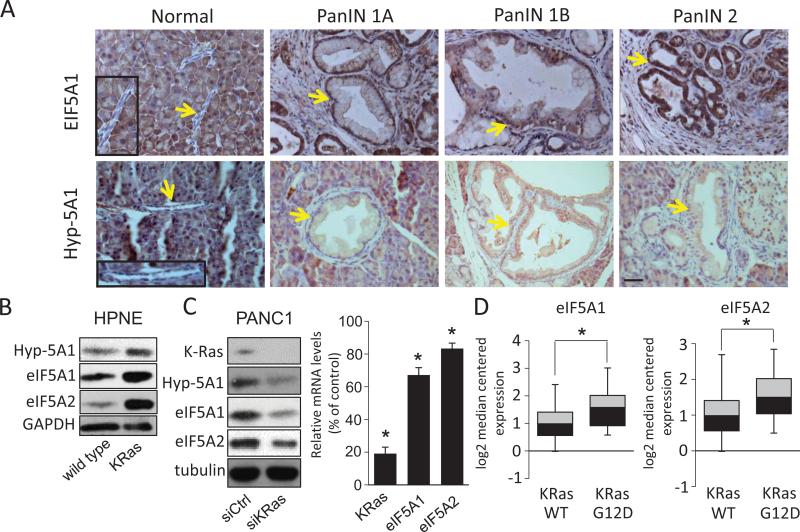
Figure 2.
eIF5A protein levels and hypusination are upregulated in PanINs and PDAC cell lines in response to KRas activation. A, Immunohistochemical staining of eIF5A1 and hypusinated eIF5A1 (Hyp-5A1) in PanINs of Pdx-Cre:LSL-KRASG12D mouse tissues. Arrows indicate normal pancreatic ducts or PanIN tissues and boxed regions show normal ducts with corresponding high magnification images. Bar= 100 μm. B, Normal human pancreatic cells (HPNE) with or without exogenous KRasG12D expression were western blotted for eIF5A1, eIF5A2, and Hyp-5A. C, PANC1 cells that harbor the KRasG12D mutation, were depleted of KRas by siRNA-mediated knockdown (siKRas) and western blotted for the indicated proteins. Right panel, mRNA levels of KRas, eIF5A1, and eIF5A2 in KRas-knockdown cells relative to control siRNA-transfected cells (siCtrl). D, Oncomine analyses of eIF5A1 and eIF5A2 mRNA levels in lung adenocarcinoma patient samples harboring wild-type KRas (WT) or the KRasG12D mutation (G12D), as derived from Okayama et al [39]. * represents P values of < 0.05 as determined by Student's t-test.
Cell proliferation assay, soft agar growth assay and clonogenic survival assay
Cell proliferation assay was performed using the CyQuant direct cell proliferation assay kit (Invitrogen) as described in Supplementary Methods. Soft agar growth assay was conducted as detailed in Supplementary Methods, and the number and size of colonies were analyzed using ImageJ software (NIH). Clonogenic survival assay was performed by plating cells without or with drug treatment into 6-well plates (50 cells/well) and allowing them to form colonies, as described in Supplementary Methods. Subsequently, cells were fixed and stained with crystal violet (Sigma-Aldrich), and the number of colonies was manually counted.
Orthotopic implantation experiments
Orthotopic implantation experiments were performed as described previously [30] and detailed in Supplementary Methods. Briefly, 1× 106 cells were orthotopically injected into the tail of the pancreas of 4-6 weeks old female athymic mice (Jackson Laboratory), which were subsequently sacrificed at the indicated time points to assess the weight of primary tumors.
Oncomine analyses
Normalized eIF5A1, eIF5A2, DHPS, DOHH and PEAK1 expression data were downloaded from Oncomine (Compendia Bioscience). Datasets with statistical significance (p-values< 0.05) were used for the analysis. Heatmaps were generated by Microsoft Excel.
Statistical analyses
All quantified data were plotted and analyzed in GraphPad Prism 6.0 with ANOVA, Student's t-test, or nonlinear regression analysis. Data are representative of at least 3 independent experiments and are reported as mean +/- SD, and * represents P-values < 0.05.
Results
eIF5A1, eIF5A2 and hypusinated eIF5A are increased in human and mouse PDAC tissues in response to activated KRas
We first sought to determine the relative expression levels of hypusinated and total eIF5A proteins in normal and PDAC tissues. Tissue microarrays (TMAs) representing normal pancreatic and PDAC tissues were immunohistochemically stained using antibodies specific to eIF5A1, eIF5A2, and the active, hypusinated form of eIF5A1. Importantly, antibody specificity to each eIF5A isoform was validated by western blotting as shown in Fig. S1A. Normal pancreatic ducts showed only weak expression of eIF5A proteins or hypusinated eIF5A1, whereas robust eIF5A protein expression and hypusination was observed in the majority of PDAC tissues regardless of the differentiation status (Fig. 1A-C). These findings were confirmed in matched PDAC and adjacent uninvolved pancreatic tissues from the same patients (Fig. 1D-F). In contrast, we found that the expression of DHPS and DOHH are high in both normal pancreatic ducts and PDAC tissues (Fig. S1B, C). In fact, there was no significant difference in DHPS levels between normal ducts and PDAC tissues, while DOHH levels were slightly decreased in PDAC tissues (Fig. S1C). These results indicate that eIF5A proteins and hypusination levels are amplified in human PDAC compared to normal pancreatic duct tissues, without a concomitant increase in the expression of DHPS or DOHH.
Figure 1.
Immunohistochemical staining of eIF5A1, eIF5A2, and hypusinated eIF5A1 (Hyp-eIF5A1) in human PDAC or normal pancreatic tissue sections. A-C, Immunohistochemical staining of eIF5A1 (A), eIF5A2 (B) and Hyp-eIF5A1 (C) in normal human pancreatic tissues and PDAC tissues of varying tumor grades. A-C, Graphs show quantitative analyses of blind scoring on a 0-3 scale. Immunohistochemical staining of eIF5A1 (D), eIF5A2 (E), and Hyp-eIF5A1 (F) in matched normal and tumor tissues from PDAC patients BK-17 and BK-19. A-F, arrows indicate normal pancreatic ducts or tumor tissues and boxed regions show normal ducts with corresponding high magnification images. Bars = 100 μm. * represents P values of < 0.05 as determined by Student's t-test.
Activating point mutations in the KRas proto-oncogene are the primary oncogenic drivers in human PDAC [29, 37]. The Pdx-1-Cre;LSL-KRASG12D transgenic mouse model faithfully recapitulates early stages of pancreatic intraepithelial neoplasia (PanIN) development, which are induced by KRas activation [38]. We isolated pancreatic tissues at various stages of PanIN development from these animals and stained them with eIF5A1 and hypusinated eIF5A1 antibodies. We were unable to determine the levels of eIF5A2, due to the lack of a suitable antibody to mouse eIF5A2. Interestingly, both eIF5A1 and hypusinated eIF5A1 are increased in PanIN tissues compared to normal pancreatic duct tissues (Fig. 2A). These findings suggest that activated eIF5A1 is upregulated during early stages of PDAC progression in response to KRas activation. In support of these findings, introduction of KRasG12D into human pancreatic nestin-positive (HPNE) cells strongly increased eIF5A1/2 protein levels and hypusination (Fig. 2B). In contrast, knockdown of activated KRas in PANC1 cells significantly reduced eIF5A1/2 protein levels and hypusination (Fig. 2B). Quantitative PCR (qPCR) analysis showed a modest, but statistically significant, decrease of eIF5A1/2 mRNA levels in KRas-depleted PANC1 cells (Fig. 2C), indicating that eIF5A upregulation by KRas can occur transcriptionally. In support of these findings, Oncomine analyses revealed that eIF5A1/2 mRNA expression levels are increased in patient-derived lung adenocarcinoma tissues with activated KRasG12D compared to those harboring wild-type KRas (Fig. 2D) [39]. Taken together, these results indicate that KRas activation upregulates eIF5A1/2 expression as well as hypusination during the early stages of PDAC progression.
Inhibition of eIF5A expression or hypusination suppresses PDAC cell growth, whereas overexpression of eIF5A enhances PDAC cell growth in vitro
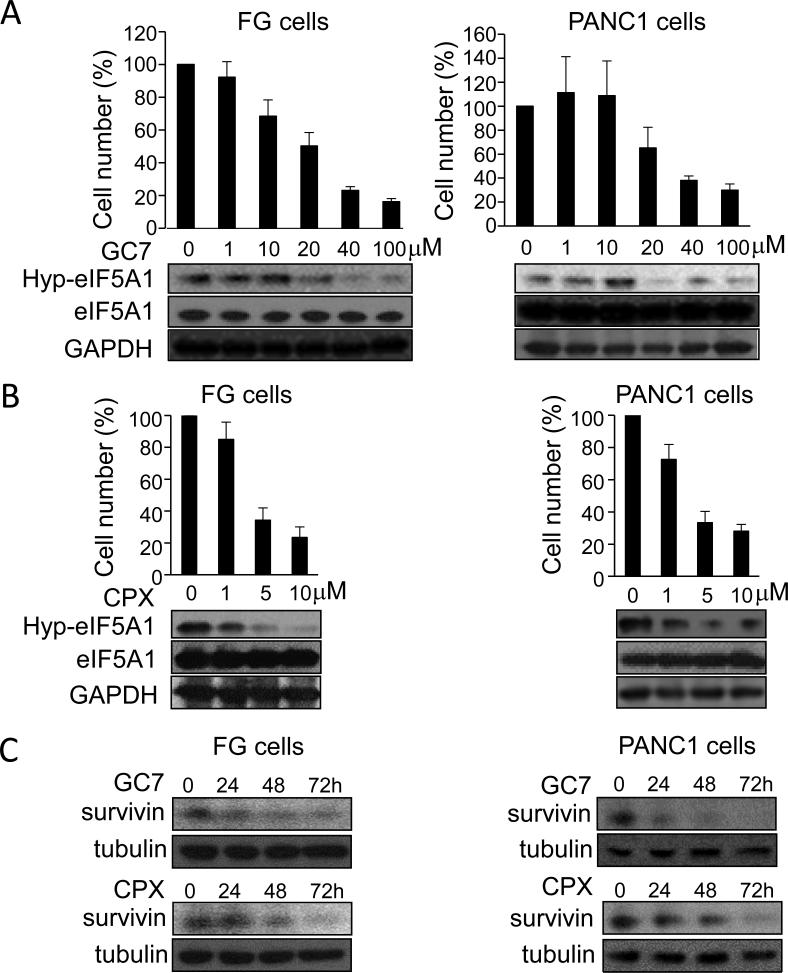
Hypusination of eIF5A proteins is required for its translational activity in eukaryotic cells [11, 26]. GC7 and CPX are established inhibitors of eIF5A hypusination that target DHPS and DOHH, respectively [8, 18, 19, 26-28]. Previous work has shown that low micromolar doses of CPX induce growth arrest and apoptosis in various cancer cells, as evidenced by decreased survivin expression [40]. Treatment of FG and PANC1 cells with GC7 or CPX potently suppressed cell proliferation, eIF5A1 hypusination, and survivin expression in a dose-dependent manner. Together these findings indicate that eIF5A hypusination is necessary for PDAC cell growth in vitro (Fig. 3A-C).
Figure 3.
Pharmacological inhibition of eIF5A hypusination suppresses PDAC cell proliferation in vitro. A and B, bar graphs represent viable cell number of FG or PANC1 cells treated for 72 hours with GC7 (A) or CPX (B) at the indicated concentrations relative to vehicle-treated cells. Lower panels, western blots showing dose-dependent inhibition of eIF5A1 hypusination (Hyp-eIF5A1) by GC7 or CPX relative to total eIF5A1 and GAPDH protein levels. C, Western blot analyses of survivin and tubulin (loading control) in FG and PANC1 cells treated with 40 μM GC7 or 5 μM CPX for the indicated times.
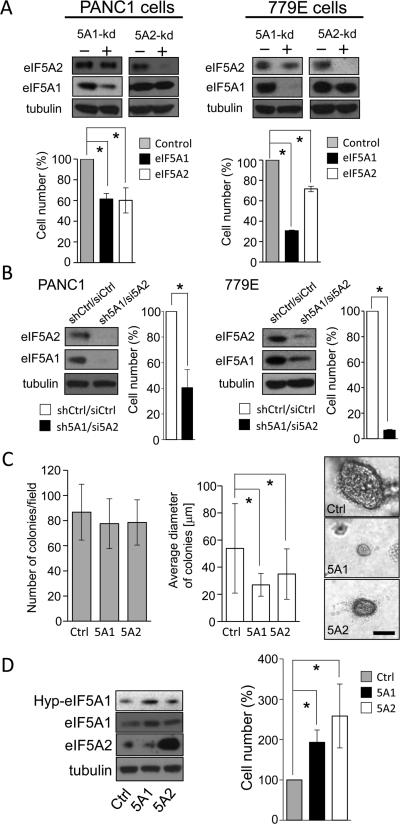
To directly determine whether eIF5A protein expression is necessary for PDAC cell growth in vitro, we depleted eIF5A1 or eIF5A2 in PANC1 and 779E cells using specific shRNAs. Using this approach we were able to selectively deplete eIF5A1 by greater than 80% and eIF5A2 by greater than 90% in both cell lines (Fig. 4A). Importantly, eIF5A1 knockdown significantly suppressed cell growth by approximately 40% and 70% in PANC1 and 779E cells, respectively, whereas eIF5A2 depletion reduced cell proliferation by 40% in both cell lines. These results were confirmed using an independent set of shRNAs in PANC1 cells (Fig. S3A). In addition, combined depletion of eIF5A1 by shRNA and eIF5A2 by siRNA inhibited growth of PANC1 and 779E cells by approximately 60% and 90%, respectively (Fig. 4B). Furthermore, depletion of either eIF5A1 or eIF5A2 in PANC1 inhibited anchorage-independent growth in soft-agar as evidenced by the significantly reduced size of individual colonies (Fig. 4C). Finally, overexpression of eIF5A1 or eIF5A2 dramatically enhanced growth of PANC1 cells (Fig. 4D) and 779E cells (data not shown). Time course analyses confirmed that eIF5A1/2 knockdown or overexpression mainly affects cell proliferation, not cell death (Fig. S2). These results suggest that eIF5A proteins and hypusination are essential for anchorage-dependent and -independent growth in vitro.
Figure 4.
eIF5A proteins are necessary and sufficient for PDAC cell proliferation in vitro. A, Bar graphs represent viable cell number of PANC1 or 779E cells containing eIF5A1 or eIF5A2 shRNAs (5A1-kd and 5A2-kd, respectively) relative to control shRNA-containing cells after 7 days of growth. Upper panels show corresponding western blots for tubulin (loading control), eIF5A1, and eIF5A2. B, The number of viable PANC1 and 779E cells containing both eIF5A1 shRNA and eIF5A2 siRNA (sh5A1/si5A2) was determined as in (A), and is shown relative to control cells containing shRNA and siRNA (shCtrl/siCtrl). Tubulin (loading control), eIF5A1, and eIF5A2 protein levels were determined as in (A). C, PANC1 cells containing control (Ctrl), eIF5A1 (5A1), or eIF5A2 (5A2) shRNAs were grown in soft agar for 25 days, and the average number of colonies and their average diameter were determined as indicated in Materials and Methods. Right panels, representative phase-contrast photomicrographs of Ctrl, 5A1, and 5A2 cell colonies in soft agar. Bar=200 μm. D, Bar graph represents viable cell number of PANC1 cells overexpressing eIF5A1 (5A1) or eIF5A2 (5A2) relative to mock-transfected (Ctrl) cells after 7 days of growth. Western blots show the expression levels of eIF5A1, eIF5A2, and hypusinated eIF5A1 (HypeIF5A1). * represents P values of < 0.05 as determined by Student's t-test.
eIF5A is essential for PDAC tumor growth in vivo
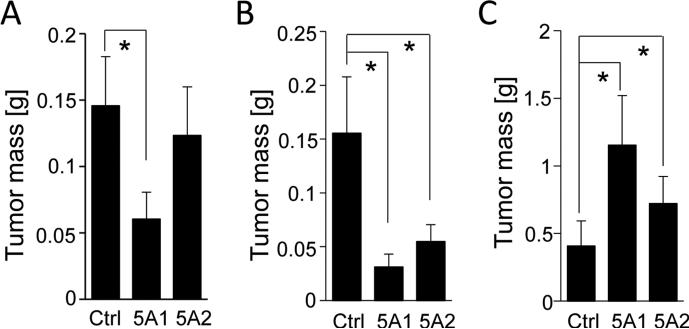
To determine whether eIF5A proteins are essential for PDAC tumor growth in vivo, PANC1 or 779E cells expressing control, eIF5A1 or eIF5A2 shRNAs together with GFP, were orthotopically implanted into the pancreas of nude mice. Changes in tumor development were assessed using fluorescence whole-body imaging and by measuring primary weight of resected tumors. eIF5A1 depletion in PANC1 and 779E cells reduced tumor weight by greater than 50% compared to control tumors (Fig. 5A, Fig. S3B). Similarly, eIF5A2 depletion in the 779E cells reduced tumor formation compared to control tumors (Fig. 5B, Fig. S3B). Also, eIF5A2 depletion in PANC1 cells reduced tumor formation, but did not reach statistical significance (Fig. 5A). The presence of eIF5A1 or eIF5A2 knockdown in the orthotopic tumor tissues was validated by qPCR (Fig. S3C and D). These findings suggest that eIF5A1 is essential for PDAC tumor growth, whereas eIF5A2 expression contributes to PDAC growth in vivo in a context-dependent manner.
Figure 5.
eIF5A proteins are sufficient and necessary for orthotopic PDAC tumor growth. PANC1 (A) or 779E (B) cells expressing control (Ctrl), eIF5A1 (5A1), or eIF5A2 (5A2) shRNAs were orthotopically implanted into athymic mice and allowed to form tumors for 23 days and 16 days, respectively. Bar graphs represent the average tumor weights for each group. C, PANC1 cells overexpressing eIF5A1 (5A1), eIF5A2 (5A2), or mock-transfected (Ctrl) PANC1 cells were orthotopically implanted and allowed to form tumors for 55 days. The average tumor weight from each group was measured as above. * represents P values of < 0.05 as determined by Student's t-test.
To determine the effect of overexpression of eIF5A proteins for PDAC tumor growth in vivo, we assessed orthotopic tumor formation of PANC1 cells overexpressing eIF5A1 or eIF5A2. As shown in Fig. 5C, the average tumor mass of eIF5A1-overexpressing PANC1 cells was significantly increased compared to control tumors. eIF5A2 overexpression also enhanced tumor growth albeit to a lesser degree (Fig. 5C). The presence of eIF5A1 or eIF5A2 overexpression in the tumor tissues was determined as above (Fig. S3E). Taken together these findings underscore the importance of eIF5A1 and eIF5A2 in driving PDAC tumor growth in vitro and in vivo.
eIF5A regulates the expression of PEAK1, a novel non-receptor tyrosine kinase
PEAK1 is a newly identified non-receptor tyrosine kinase that modulates Src kinase activity and plays an essential role in driving PDAC malignancy [30-33]. Like eIF5A, PEAK1 is amplified in PanINs and PDAC patient tissues in response to KRas activation [30]. Interestingly, PEAK1 possesses a polyproline motif suggesting that it could be translationally regulated by eIF5A [12-14]. This prompted us to determine if eIF5A is an upstream regulator of PEAK1 expression. Depletion eIF5A1 or eIF5A2 significantly reduced PEAK1 protein levels and Src activity in PANC1 and 779E cells (Fig. 6A, Fig. S4A, B). These results were confirmed by eIF5A1 or eIF5A2 knockdown using an independent set of shRNAs (Fig. S3A). In addition, simultaneous depletion of eIF5A1 by shRNA and eIF5A2 by siRNA, as well as treatment with GC7 or CPX, also robustly inhibited PEAK1 expression and Src activity compared to depletion of individual isoforms (Fig. 6B, C). On the other hand, overexpression of eIF5A1 or eIF5A2 significantly increased PEAK1 protein levels and Src activity (Fig. 6D and data not shown). Collectively, these results demonstrate that eIF5A is sufficient and necessary for PEAK1 protein expression in PDAC cells.
Figure 6.
eIF5A proteins regulate PEAK1 protein levels to drive PDAC cell proliferation in vitro. A, PANC1 cells expressing eIF5A1 (5A1-kd) or eIF5A2 (5A2-kd) shRNAs were western blotted for PEAK1, total and kinase activated/phosphorylated (Y416) Src, and GAPDH. B, PANC1 cells containing both eIF5A1 shRNA and eIF5A2 siRNA (sh5A1/si5A2) or expressing control shRNA and siRNA (shCtrl/siCtrl) were western blotted as in (A). C, PANC1 cells treated with GC7 (40 μM) or CPX (5 μM) for 72 hrs were western blotted as above. D, PANC1 cells containing control (Ctrl), eIF5A1- (5A1) or eIF5A2-expressing vectors (5A2) were western blotted for the indicated proteins. E, Bar graphs represent viable cell number in control, eIF5A1- or eIF5A2-overexpressing PANC1 cells also transfected with PEAK1 siRNA (siPEAK1) after 7 days of growth. Results are shown relative to control siRNA-transfected cells. F, Cell growth was measured as in (E) for PANC1 cells expressing eIF5A1 or eIF5A2 shRNAs also transfected with either control or PEAK1-expressing vectors. Corresponding western blots from lysates of cells after 7 days of growth show the expression levels of PEAK1 and tubulin. * represents P values of < 0.05 as determined by Student's t-test.
eIF5A-PEAK1 axis is essential to drive PDAC cell proliferation in vitro
To determine whether PEAK1 is the downstream effector of eIF5A in driving PDAC cell proliferation, we employed two independent approaches. First, we investigated whether PEAK1 knockdown can offset the enhanced PDAC cell proliferation induced by eIF5A overexpression. As shown in Fig. 6E, PEAK1 knockdown reduced the proliferation of eIF5A1- or eIF5A2-overexpressing cells by 50%. Secondly, we investigated whether overexpression of PEAK1 could rescue the anti-proliferative effect of eIF5A1 or eIF5A2 depletion. PANC1 cells expressing shRNAs were transiently transfected with plasmids encoding GFP or GFP-tagged PEAK1, and subjected to western blotting and cell proliferation analyses (Fig. 6F). PEAK1 was successfully overexpressed in PANC1 cells containing eIF5A1 or eIF5A2 shRNAs, which rescued the anti-proliferative effect of eIF5A1 or eIF5A2 depletion. Thus, exogenous introduction of PEAK1 was sufficient to overcome downregulation of PEAK1 in these cells to drive cell proliferation. PEAK1 overexpression did not affect cell proliferation in control PANC1 cells in a statistically significant manner, most likely because PEAK1 is expressed at high levels in these cells and forced expression of PEAK1 above the elevated endogenous levels may not be sufficient to promote PANC1 cell growth. However, it significantly enhanced cell proliferation in PANC1 cells with eIF5A1 or eIF5A2 knockdown (Fig. 6F). Altogether, these results indicate that PEAK1 is a necessary downstream effector of eIF5A proteins in enhancing PDAC cell proliferation.
Targeting eIF5A hypusination increases gemcitabine sensitivity in PDAC cells in vitro
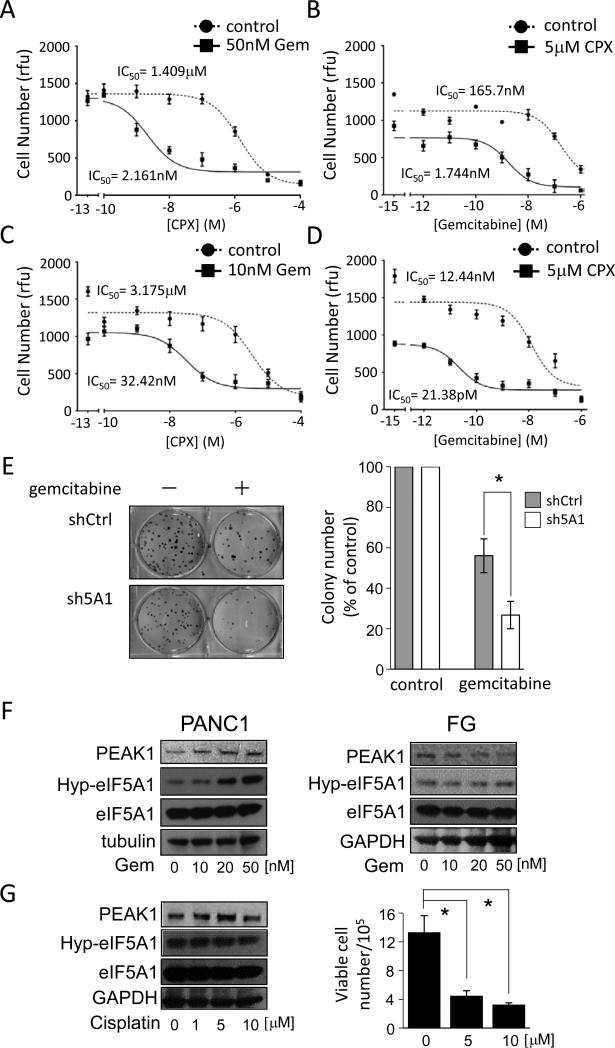
In our previous work, we demonstrated that knockdown of PEAK1 renders PDAC cells sensitive to gemcitabine, the current first-line chemotherapy for PDAC treatment [30]. Having determined the role of eIF5A in regulating PEAK1 levels, we hypothesized that targeting eIF5A would increase sensitivity to gemcitabine in both drug-resistant (PANC1) and -sensitive (FG) PDAC cell lines [41]. As shown in Fig. 7A-D and Fig. S4C, inhibiting eIF5A hypusination with CPX dramatically enhanced gemcitabine sensitivity in both lines. In fact, IC50 value of gemcitabine decreased from 165 nM to 1.74 nM and 12.4 nM to 21.3 pM upon co-treatment with CPX in PANC1 cells and FG cells, respectively. Moreover, we found that knockdown of eIF5A1, eIF5A2 or PEAK1 renders PANC1 or FG cells significantly more susceptible to gemcitabine (Fig. 7E and Fig. S4D-F). Interestingly, we found that gemcitabine treatment of resistant PANC1 cells robustly increased eIF5A1 hypusination and PEAK1 protein expression in a dose-dependent manner (Fig. 7F). This response was not observed in gemcitabine-sensitive FG cells (Fig. 7F) and it was specific to gemcitabine, as treatment with the DNA damaging and cytotoxic agent, cisplatin, did not significantly alter eIF5A1 hypusination or PEAK1 expression (Fig. 7G). These findings support a mechanism by which eIF5A-mediated PEAK1 expression contributes to gemcitabine sensitivity in PDAC cells, and suggests that combination therapies involving gemcitabine and CPX could benefit PDAC patients.
Figure 7.
Inhibiting eIF5A function in PDAC cells increases gemcitabine sensitivity. A, Analyses of viable cell number after 7 days of growth for PANC1 cells treated with increasing doses of CPX in the presence of 50 nM gemcitabine. B, Analyses of viable cell number after 7 days of growth for PANC1 cells treated with increasing doses of gemcitabine in the presence of 5 μM CPX. C, Analyses of viable cell number after 7 days of growth for FG cells treated with increasing doses of CPX in the presence of 10 nM gemcitabine. D, Analyses of viable cell number for FG cells performed as in B. IC50 values represent the concentration of a drug that is required for 50% inhibition of cell proliferation as compared to vehicle-treated cells. Rfu=relative fluorescence unit. E, Clonogenic growth of PANC1 cells containing control (shCtrl) or eIF5A1 (sh5A1) shRNAs was determined after gemcitabine treatment (100 nM, 24 hrs). Left panel, representative bright-field photomicrographs of colonies. Right panel, bar graph represents the number of colonies in gemcitabine-treated cells relative to vehicle-treated control cells. F, Western blot analyses of PEAK1, hypusinated eIF5A1 (Hyp-5A1) and total eIF5A1 levels in PANC1 and FG cells treated with the indicated concentrations of gemcitabine for 72 hrs. G, Left panel, PANC1 cells treated with the indicated concentrations of cisplatin for 72 hrs were western blotted as in (F). Right panel, the bar graph represents the number of viable cells after 72 hrs of growth in the presence or absence cisplatin at the indicated concentrations. * represents P values of < 0.05 as determined by Student's t-test.
Discussion
We report here that eIF5A protein amplification is an early component of PanIN development and a critical contributor to PDAC growth in vitro and in vivo. Immunohistochemical analyses demonstrated the upregulation of hypusinated and total eIF5A1 and eIF5A2 in patient-derived PDAC tissues (Fig. 1), suggesting an involvement of eIF5A proteins and their activity in PDAC oncogenesis. eIF5A proteins function downstream of activating KRas mutations to drive PEAK1 protein expression, a non-receptor tyrosine kinase essential for PDAC progression [30]. Pharmacological intervention of eIF5A activities inhibited PEAK1 expression as well as proliferation of multiple PDAC cell lines. Together these findings implicate KRas/eIF5A/PEAK1 as a new signaling module and therapeutic target functionally important for human PDAC, which supports the emerging notion that eIF5A hypusination can be therapeutically targeted to inhibit a wide range of cancers, including those driven by oncogenic KRas [18-21, 23, 24].
Our findings demonstrate that KRas activation is both sufficient and necessary for eIF5A protein amplification in PDAC cell lines (Fig. 2). Oncomine analyses also revealed that eIF5A mRNA is increased in lung cancer patient tissues that harbor activating KRas mutations (Fig. 2D), which accounts for up to 30% of lung cancer cases [42]. Together these findings suggest that oncogenic mutations in KRas promote eIF5A1/2 mRNA and protein expression in PDAC and possibly other cancers where KRas is the oncogenic driver. In future work it will be important to determine how KRas signaling controls eIF5A1/2 transcription and protein expression in cancer cells. It will also be important to determine the precise role that eIF5A proteins play downstream of KRas activation. In this regard, important recent work showed that in bacteria and in yeast, eIF5A and its counterpart EF-P, specifically facilitate the translation of polyproline motif-containing proteins [12-14]. This raises the intriguing possibility that eIF5A proteins control gene expression in a specialized manner by modulating translation of a subset of proteins with defined signaling and protein-protein interaction motifs. In fact, eIF5A depletion only reduces global protein synthesis by approximately 5% in mammalian cells [43]. Thus, hyper-proliferating cancer cells likely upregulate specific eIF5A proteins due to increased demands for polyproline domain-containing proteins such as Abl and PIK3R2 [15]. Polyproline-containing proteins are known to control oncogenic signaling by integrating protein-protein interactions mediated by SH3 (Src homology-3), WW, and EVH1 (ENA/VASP Homology 1) domains [15, 16]. It is also interesting that the ability of eIF5A to selectively regulate protein translation may extend beyond polyproline domain containing proteins, as EF-P was recently shown to regulate the synthesis of proteins with other motifs including APP, YIRYIR or GSCGPG [44]. Altogether these findings suggest that regulation of eIF5A activity provides an additional level of translational control for a select set of proteins that drive PDAC cell growth and oncogenesis in response to KRas activation. Our finding that the expression of PEAK1, a polyproline domain-containing protein, is modulated by eIF5A in PDAC cells supports this notion (Figs. 6A-D).
We have previously shown that, like eIF5A, PEAK1 protein expression is increased in PanINs, PDAC patient tissues, and PDAC cell lines in response to KRas activation [30]. PEAK1 is a cytoskeleton-associated tyrosine kinase and scaffold protein that transmits growth factor and integrin adhesion signals from the cell's exterior, which is important for cell proliferation and migration [30-33, 45]. Our findings reported here demonstrate that PEAK1 is an important downstream mediator of eIF5A function in PDAC cell proliferation. Knockdown or pharmacological inhibition of eIF5A proteins resulted in reduced PEAK1 protein expression (Fig. 5), whereas overexpression of eIF5A proteins upregulated PEAK1 levels (Fig. 6A) and enhanced proliferation of PANC1 cells (Fig. 4D). Importantly, enhanced cell proliferation by eIF5A overexpression was substantially, although not completely, offset by PEAK1 knockdown (Fig. 6E). In addition, PEAK1 overexpression restored cell proliferation in eIF5A-depleted cells (Fig. 6F). These results indicate that eIF5A proteins accelerate PDAC cell proliferation, at least partially, through upregulation of PEAK1. Considering the recent report showing the involvement of PEAK1 in basal breast cancer oncogenesis [46], it will be of interest to determine whether the eIF5A-PEAK1 signaling axis is also important for the pathogenesis of breast cancer as well as other cancers. Indeed, Oncomine analysis revealed that increased expression of eIF5A isoforms together with PEAK1 is observed across a broad spectrum of cancer types including breast cancer (Fig. S5), indicating that the eIF5A-PEAK1 axis may be a widely conserved mechanism that drives tumorigenesis. In future work, it will be important to determine whether eIF5A proteins promote cancer pathogenesis by modulating PEAK1's kinase activity and/or through its reported scaffolding functions [31, 32, 45].
Our finding that genetic or pharmacological inhibition of eIF5A increases gemcitabine sensitivity has important clinical implications (Fig. 7A, B). While gemcitabine is the first-line chemotherapy for PDAC, many patients display innate resistance or acquire resistance during the course of treatment. We found that in gemcitabine-resistant PANC1 cells, gemcitabine induced eIF5A hypusination and increased PEAK1 expression in a dose-dependent manner. These observations indicate that gemcitabine-resistant cells may possess the ability to activate the eIF5A-PEAK1 pathway upon exposure to gemcitabine to promote survival. Importantly, inhibition of eIF5A function by shRNA or CPX (Fig. 7B), in these gemcitabine-resistant cells, restored sensitivity to gemcitabine. Thus, eIF5A-PEAK1 signaling contributes to gemcitabine resistance, and eIF5A-PEAK1 expression may have utility as a biomarker to identify gemcitabine-sensitive and -refractory patients. However, they do not rule out the possibility that other pathways exist to regulate gemcitabine resistance/sensitivity in PDAC cells.
While we demonstrated that CPX inhibits PDAC cell proliferation by targeting eIF5A, the underlying mechanisms may be multifaceted. For example, the anti-cancer activities of CPX are dependent, in part, on its iron-chelating activity - but not as a general chelator of extracellular and/or intracellular iron [47]. Rather, CPX is a selective chelator of iron associated with non-heme enzymes, including DOHH, ribonucleotide reductase (RNR) and prolyl hydroxylase(s) [28, 48]. Indeed, CPX was reported to inhibit RNR, which is essential for DNA synthesis, by iron chelation [40]. The fact that RNR is irreversibly inhibited by gemcitabine [49] likely explains the potent synergistic effects of CPX and gemcitabine in killing, not only gemcitabine-resistant, but also gemcitabine-sensitive PDAC cells (Fig. 7A). These findings suggest that hypusination inhibitors may have clinical applications in treating PDAC patients. Indeed, CPX is already approved by the FDA (US Food and Drug Administration) for use as an anti-fungal agent and was recently shown to be relatively safe for use in cancer patients [50]. In fact, no serious health risks were observed in a recent open-label, ascending dose Phase I study, which evaluated the efficacy of CPX in treating patients with relapsed or refractory hematological malignancies [50]. In light of these findings, additional studies are warranted to determine if CPX can be safely repurposed for use in PDAC patients, alone or in combination with gemcitabine.
In summary, there is an urgent need to understand the detailed mechanisms that initiate and drive PDAC progression so that specific diagnostic markers and new therapeutic strategies can be designed. Our findings provide new mechanistic insights into PDAC development and drug resistance by implicating KRas/eIF5A/PEAK1 as a new signaling module and functional biomarker that can be therapeutically targeted by small molecule compounds like CPX. The efficiency at which the FDA could repurpose CPX and its reported safety in human cancer patients could rapidly benefit PDAC patients that suffer from this deadly disease.
Supplementary Material
Acknowledgments
The authors thank members of the Klemke, Bouvet and Lowy laboratories, Dr. David Cheresh (UCSD) for kindly providing the FG cell line and Drs. Hartmut Hanauske-Abel (Rutgers University) and Myung-Hee Park (National Institute of Dental and Craniofacial Research) for kindly providing the NIH353 antibody, constructs encoding human eIF5A1 and eIF5A2 and helpful comments.
Financial Support
This work was supported by the NIH-F32 Postdoctoral Fellowship F32CA180374 (K. Fujimura) and NIH grants CA097022 (R.L. Klemke), CA132971 (M. Bouvet) and CA155620 (A.M. Lowy).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Authors' Contributions
Conception and design: K. Fujimura, J.A. Kelber, R.L. Klemke
Development of methodology: K. Fujimura, T. Wright, J. Strnadel, J.A. Kelber, S. Kaushal, C. Metildi, A.M. Lowy, R.L. Klemke
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): K. Fujimura, T. Wright, J.A. Kelber, J. Strnadel, S. Kaushal, C. Metildi, A.M. Lowy, M. Bouvet, R.L. Klemke
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): K. Fujimura, T. Wright, J. Strnadel, J.A. Kelber, R.L. Klemke
Writing, review, and/or revision of the manuscript: K. Fujimura, J.A. Kelber, R.L. Klemke
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K. Fujimura, T. Wright, J.A. Kelber, R.L. Klemke
Study supervision: R.L. Klemke
References
- 1.Pandolfi PP. Aberrant mRNA translation in cancer pathogenesis: an old concept revisited comes finally of age. Oncogene. 2004;23:3134–7. doi: 10.1038/sj.onc.1207618. [DOI] [PubMed] [Google Scholar]
- 2.Ruggero D. Translational control in cancer etiology. Cold Spring Harb Perspect Biol. 2013;5:a012336. doi: 10.1101/cshperspect.a012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol. 2009;11:903–8. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 4.Grzmil M, Hemmings BA. Translation regulation as a therapeutic target in cancer. Cancer Res. 2012;72:3891–900. doi: 10.1158/0008-5472.CAN-12-0026. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura K, Lee SB, Park JH, Park MH. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids. 2012;42:703–10. doi: 10.1007/s00726-011-0986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–21. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landau G, Bercovich Z, Park MH, Kahana C. The role of polyamines in supporting growth of mammalian cells is mediated through their requirement for translation initiation and elongation. J Biol Chem. 2010;285:12474–81. doi: 10.1074/jbc.M110.106419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, et al. The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest. 2010;20:2156–70. doi: 10.1172/JCI38924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3105–14. doi: 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi XP, Yin KC, Ahern J, Davis LJ, Stern AM, Waxman L. Effects of N1-guanyl-1,7-diaminoheptane, an inhibitor of deoxyhypusine synthase, on the growth of tumorigenic cell lines in culture. Biochim Biophys Acta. 1996;1310:119–26. doi: 10.1016/0167-4889(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 11.Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J Biochem. 2006;139:161–9. doi: 10.1093/jb/mvj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ude S, Lassak J, Starosta AL, Kraxenberger T, Wilson DN, Jung K. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science. 2013;339:82–5. doi: 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- 13.Doerfel LK, Wohlgemuth I, Kothe C, Peske F, Urlaub H, Rodnina MV. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013;339:85–8. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez E, Shin BS, Woolstenhulme CJ, Kim JR, Saini P, Buskirk AR, et al. eIF5A promotes translation of polyproline motifs. Mol. Cell. 2013;51:35–45. doi: 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–41. [PubMed] [Google Scholar]
- 16.Xi G, Shen X, Clemmons DR. p66shc inhibits insulin-like growth factor-I signaling via direct binding to Src through its polyproline and Src homology 2 domains, resulting in impairment of Src kinase activation. J. Biol. Chem. 2010;285:6937–51. doi: 10.1074/jbc.M109.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamuro D, Sabbatini P, White E, Prendergast GC. The polyproline region of p53 is required to activate apoptosis but not growth arrest. Oncogene. 1997;15:887–98. doi: 10.1038/sj.onc.1201263. [DOI] [PubMed] [Google Scholar]
- 18.Preukschas M, Hagel C, Schulte A, Weber K, Lamszus K, Sievert H, et al. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: implications for new targeted therapies. PLOS One. 2012;7:e43468. doi: 10.1371/journal.pone.0043468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balabanov S, Gontarewicz A, Ziegler P, Hartmann U, Kammer W, Copland M, et al. Hypusination of eukaryotic initiation factor 5A (eIF5A): a novel therapeutic target in BCR-ABL-positive leukemias identified by a proteomics approach. Blood. 2007;109:1701–11. doi: 10.1182/blood-2005-03-037648. [DOI] [PubMed] [Google Scholar]
- 20.Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, et al. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51:1255–63. doi: 10.1002/hep.23451. [DOI] [PubMed] [Google Scholar]
- 21.Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, et al. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852–64. doi: 10.1016/j.cell.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He LR, Zhao HY, Li BK, Liu YH, Liu MZ, Guan XY, et al. Overexpression of eIF5A-2 is an adverse prognostic marker of survival in stage I non-small cell lung cancer patients. Int J Cancer. 2011;129:143–50. doi: 10.1002/ijc.25669. [DOI] [PubMed] [Google Scholar]
- 23.Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ, Liao YJ, et al. Overexpression of EIF5A2 promotes colorectal carcinoma cell aggressiveness by upregulating MTA1 through C-myc to induce epithelialmesenchymaltransition. Gut. 2012;61:562–75. doi: 10.1136/gutjnl-2011-300207. [DOI] [PubMed] [Google Scholar]
- 24.Mémin E, Hoque M, Jain MR, Heller DS, Li H, Cracchiolo B, et al. Blocking eIF5A modification in cervical cancer cells alters the expression of cancer-related genes and suppresses cell proliferation. Cancer Res. 2014;74:552–62. doi: 10.1158/0008-5472.CAN-13-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, Yang JM. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res. 2001;61:3806–9. [PubMed] [Google Scholar]
- 26.Maier B, Tersey SA, Mirmira RG. Hypusine: a new target for therapeutic intervention in diabetic inflammation. Discov Med. 2010;10:18–23. [PubMed] [Google Scholar]
- 27.Hoque M, Hanauske-Abel HM, Palumbo P, Saxena D, D'Alliessi Gandolfi D, Park MH, et al. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology. 2009;6:90. doi: 10.1186/1742-4690-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clement PM, Hanauske-Abel HM, Wolff EC, Kleinman HK, Park MH. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int J Cancer. 2002;100:491–8. doi: 10.1002/ijc.10515. [DOI] [PubMed] [Google Scholar]
- 29.Kern SE, Shi C, Hruban RH. The complexity of pancreatic ductal cancers and multidimensional strategies for therapeutic targeting. J Pathol. 2011;223:295–306. doi: 10.1002/path.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelber JA, Reno T, Kaushal S, Metildi C, Wright T, Stoletov K, et al. Kras induces a Src/PEAK1/ErbB2 kinase amplification loop that drives metastatic growth and therapy resistance in pancreatic cancer. Cancer Res. 2012;72:2554–2564. doi: 10.1158/0008-5472.CAN-11-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Kelber JA, Tran Cao HS, Cantin GT, Lin R, Wang W, et al. Pseudopodium-enriched atypical kinase 1 regulates the cytoskeleton and cancer progression. PNAS. 2010;107:10920–5. doi: 10.1073/pnas.0914776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelber JA, Klemke RL. PEAK1, a novel kinase target in the fight against cancer. Oncotarget. 2010;1:219–23. doi: 10.18632/oncotarget.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bristow JM, Reno TA, Jo M, Gonias SL, Klemke RL. Dynamic phosphorylation of tyrosine 665 in pseudopodium-enriched atypical kinase 1 (PEAK1) is essential for the regulation of cell migration and focal adhesion turnover. J Biol Chem. 2013;288:123–31. doi: 10.1074/jbc.M112.410910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KM, Nguyen C, Ulrich AB, Pour PM, Ouellette MM. Immortalization with telomerase of the Nestin-positive cells of the human pancreas. Biochem. Biophys. Res. Commun. 2003;301:1038–44. doi: 10.1016/s0006-291x(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 35.Clement PM, Johansson HE, Wolff EC, Park MH. Differential expression of eIF5A-1 and eIF5A-2 in human cancer cells. FEBS J. 2006;273:1102–14. doi: 10.1111/j.1742-4658.2006.05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cracchiolo BM, Heller DS, Clement PM, Wolff EC, Park MH, Hanauske-Abel HM. Eukaryotic initiation factor 5A-1 (eIF5A-1) as a diagnostic marker for aberrant proliferation in intraepithelial neoplasia of the vulva. Gynecol Oncol. 2004;94:217–22. doi: 10.1016/j.ygyno.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leach SD. Mouse models of pancreatic cancer: the fur is finally flying! Cancer Cell. 2004;5:7–11. doi: 10.1016/s1535-6108(03)00337-4. [DOI] [PubMed] [Google Scholar]
- 39.Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012;72:100–11. doi: 10.1158/0008-5472.CAN-11-1403. [DOI] [PubMed] [Google Scholar]
- 40.Eberhard Y, McDermott SP, Wang X, Gronda M, Venugopal A, Wood TE, et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood. 2009;114:3064–73. doi: 10.1182/blood-2009-03-209965. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, VandenBoom TG, 2nd, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–12. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dogan S, Shen R, Ang DC, Johnson ML, D'Angelo SP, Paik PK, et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3,026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS-Mutant Cancers. Clin. Cancer Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li CH, Ohn T, Ivanov P, Tisdale S, Anderson P. eIF5A Promotes Translation Elongation, Polysome Disassembly and Stress Granule Assembly. PLoS One. 2010;5:e9942. doi: 10.1371/journal.pone.0009942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hersch SJ, Wang M, Zou SB, Moon KM, Foster LJ, Ibba M, et al. Divergent Protein Motifs Direct Elongation Factor P-Mediated Translational Regulation in Salmonella enterica and Escherichia coli. MBio. 2013;4:00180–13. doi: 10.1128/mBio.00180-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Y, Zhang C, Croucher DR, Soliman MA, St-Denis N, Pasculescu A, et al. Temporal regulation of EGF signalling networks by the scaffold protein Shc1. Nature. 2013;499:166–71. doi: 10.1038/nature12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Croucher DR, Hochgräfe F, Zhang L, Liu L, Lyons RJ, Rickwood D, et al. Involvement of Lyn and the atypical kinase SgK269/PEAK1 in a basal breast cancer signaling pathway. Cancer Res. 2013;73:1969–80. doi: 10.1158/0008-5472.CAN-12-1472. [DOI] [PubMed] [Google Scholar]
- 47.Hanauske-Abel HM, Saxena D, Palumbo PE, Hanauske AR, Luchessi AD, Cambiaghi TD, et al. Drug-induced reactivation of apoptosis abrogates HIV-1 infection. Plos One. 2013;8:e74414. doi: 10.1371/journal.pone.0074414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YS, Kang KR, Wolff EC, Bell JK, McPhie P, Park MH. Deoxyhypusine hydroxylase is a Fe(II)-dependent, HEAT-repeat enzyme. Identification of amino acid residues critical for Fe(II) binding and catalysis. J. Biol. Chem. 2006;281:13217–25. doi: 10.1074/jbc.M601081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, et al. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2′,2′-difluorodeoxycytidine. Mol. Pharmacol. 1990;38:567–72. [PubMed] [Google Scholar]
- 50.Minden MD, Hogge DE, Weir SJ, Kasper J, Webster DA, Patton L, et al. Oral ciclopirox olamine displays biological activity in a phase I study in patients with advanced hematologic malignancies. Am J Hematol. 2013 doi: 10.1002/ajh.23640. doi: 10.1002/ajh.23640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.