Abstract
Objective
To determine whether biomarkers of myocardial stress and fibrosis improve prediction of mode of death in patients with chronic heart failure.
Background
The two most common modes of death in patients with chronic heart failure are pump failure and sudden cardiac death. Prediction of mode of death may facilitate treatment decisions. The relationship between NT-proBNP, galectin-3, and ST2, biomarkers that reflect different pathogenic pathways in heart failure (myocardial stress and fibrosis), and mode of death is unknown.
Methods
HF-ACTION was a randomized controlled trial of exercise training vs. usual care in patients with chronic heart failure due to left ventricular systolic dysfunction (LVEF<35%). An independent clinical events committee prospectively adjudicated mode of death. NT-proBNP, galectin-3, and ST2 levels were assessed at baseline in 813 subjects. Associations between biomarkers and mode of death were assessed using cause-specific Cox-proportional hazards modeling, and interaction testing was used to measure differential association between biomarkers and pump failure versus sudden cardiac death. Discrimination and risk reclassification metrics were used to assess the added value of galectin-3 and ST2 in predicting mode of death risk beyond a clinical model that included NT-proBNP.
Results
After a median follow up of 2.5 years, there were 155 deaths: 49 from pump failure 42 from sudden cardiac death, and 64 from other causes. Elevations in all biomarkers were associated with increased risk of both pump failure and sudden cardiac death in both adjusted and unadjusted analyses. In each case, increases in the biomarker had a stronger association with pump failure than sudden cardiac death but this relationship was attenuated after adjustment for clinical risk factors. Clinical variables along with NT-proBNP levels were stronger predictors of pump failure (C statistic: 0.87) than sudden cardiac death (C statistic: 0.73). Addition of ST2 and galectin-3 led to improved net risk classification of 11% for sudden cardiac death, but not pump failure.
Conclusions
Clinical predictors along with NT-proBNP levels were strong predictors of pump failure risk, with insignificant incremental contributions of ST2 and galectin-3. Predictability of sudden cardiac death risk was less robust and enhanced by information provided by novel biomarkers.
Keywords: heart failure, biomarker, prognosis, mode of death
Introduction
Heart failure is among the leading causes of mortality and morbidity worldwide (1,2). The predominant modes of death in heart failure patients are pump failure and sudden cardiac death (3). Decisions regarding costly and complex interventions such as implantable cardioverter defibrillators (ICD) and left ventricular assist devices (LVAD) require careful weighing of the risks and benefits that may be significantly informed by understand the risk of specific modes of death (4). Current risk stratification models focus on predicting total mortality among patients with heart failure, but lack sensitivity and specificity when applied to prediction of cause-specific death (5). Plasma biomarkers that reflect the degree of myocardial stress and fibrosis, pathologic processes associated with the risk for pump failure and sudden cardiac death, may enhance risk stratification (6). On the basis of several large studies, three biomarkers have been cleared by the Food and Drug Administration (FDA) as aids for assessing prognosis of patients with chronic heart failure: natriuretic peptides (NT-proBNP and BNP), galectin-3, and soluble ST2 (ST2)(7).
Several studies have reported on the overall prognostic implications of these biomarkers in patients with heart failure. However, whether their use alongside commonly available clinical measures can improve risk prediction of mode of death— on the basis of which complex clinical decisions such as ICD implantation in heart failure are regularly made—is unknown (8). For this reason, we sought to evaluate the relationships between elevated levels of NT-proBNP, galectin-3, ST2, and mode of death in a cohort of 813 chronic heart failure patients on optimal medical therapy.
Methods
Study Population
Details of the design, rationale, and primary results of the Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION) study have been published elsewhere (9,10). Briefly, HF-ACTION (clinicaltrials.gov, NCT00047437) was a randomized clinical trial evaluating the effect of exercise training vs. usual care on long-term morbidity and mortality in patients with chronic heart failure due to left ventricular systolic dysfunction (NYHA class II–IV, left ventricular ejection fraction (LVEF) < 35%). The primary endpoint of HF-ACTION was a composite of all-cause mortality and all-cause hospitalization over a median follow up of 2.5 years. HF-ACTION was approved by local Institutional Review Boards, and all enrolled patients provided written informed consent.
Adjudication of Mode of Death
An independent clinical events committee that comprised of 11 cardiologists blinded to treatment allocation reviewed and adjudicated all suspected events by consensus. Definitions were as follows:
Pump Failure
Death from worsening/intractable heart failure which generally occurred during hospitalization but could occur at home during hospice care. Terminal arrhythmias associated with pump failure deaths were classified as a pump failure death. Pump failure secondary to recent myocardial infarctions were not included.
Sudden Cardiac Death
Unexpected and otherwise unexplained death in a previously stable patient. This included patients who were comatose then died after attempted resuscitation. Patients in this category should have had recent human contact before the event. Patients who died who had been out of contact for prolonged or unknown periods of time were classified as unknown.
Other causes of death were myocardial infarction, cerebrovascular accident, cardiovascular procedure related, non-cardiovascular, and unknown. Follow-up events were prospectively ascertained every 6 months via direct patient contact and verified through death certificates, medical records, and contact with patients’ family members by dedicated research personnel.
Biomarker Measurements
A sub-set of patients enrolled in the HF-ACTION study who agreed to participate in the biomarker substudy underwent plasma collection of at baseline. Baseline blood samples were obtained on the same day as baseline exercise testing but were obtained prior to exercise. Samples were collected via peripheral vein into EDTA containing tubes, and then centrifuged immediately and stored at −70°C prior to analysis at core laboratories. Plasma NT-proBNP was measured by a standard electrochemiluminesence immunoassay (Elecsys proBNP, Roche Diagnostics, Indianapolis, IN). The inter-assay and intra-assay coefficients of variation were 2.9 and 6.1%, respectively. Galectin-3 was assessed using enzyme linked immunosorbent assays (BGM Galectin-3, BG Medicine, Waltham, MA). The inter-assay and intra-assay variances were 5.6 and 8.6%, respectively. ST2 was measured from banked plasma samples via a highly sensitive sandwich monoclonal immunoassay (Presage ST2 assay, Critical Diagnostics, New York, NY). The inter-assay and intra-assay coefficients of variation were less than 2.5% and 4.0%, respectively. The core laboratories were blinded to all clinical data.
Statistical Analysis
Baseline patient characteristics were described using medians and intra-quartile ranges or proportions, first in the entire cohort and then stratified by median level of each biomarker. For continuous variables, differences were compared using the ANOVA and Kruskal-Wallis tests. Categorical variables were compared using the chi-square and exact tests. The primary outcome variables of interests were death from pump failure and sudden cardiac death; other modes of death were treated as competing risks in the analysis. ST2 and NT-proBNP were log-base2 transformed to approximate normality. After assessment of various methods for handling galectin-3, including log transformation, the best model fit was provided by handling galectin-3 as a continuous variable with truncation at >20 ng/mL to avoid violation of the linearity assumption. To describe the timing and frequency of pump failure and sudden cardiac death and their relationship to the 3 biomarkers, cumulative incidence curves stratified by median level of each biomarker were plotted for each outcome. Using Gray’s K-sample test for comparing the cumulative incidence, P-values were derived that tested the null hypothesis of equality between the median-stratified sub-distributions (11).
To determine whether each biomarker was associated with pump failure or sudden cardiac death, and whether the association differed (in magnitude or direction) between the two outcomes, a series of cause-specific Cox proportional hazard models was fit. Cause-specific Kaplan-Meier curves were created in order to select the appropriate cause-specific modeling strategy; because if the curves crossed, an indicator for mode of death was included as a stratification variable in the cause-specific Cox regression model using the data augmentation method described by Lunn and McNeil (12). The association between each biomarker and outcome of interest was characterized by a hazard ratio (HR) and the corresponding 95% confidence interval (CI). To determine if each biomarker had a differential effect on mode of death, the interaction between biomarker and mode of death was tested in the cause-specific Cox regression model described above and the differential association P-value was reported. Models included adjustment first for a comprehensive set of predictors (BMI, sex, dosage of loop diuretic, LVEF, Canadian Cardiovascular Society angina classification, creatinine, and resting ECG ventricular conduction abnormalities) that had been identified as independently contributing to risk prediction in the final adjusted model in the overall HF-ACTION cohort for all-cause mortality, and then additionally for the other two biomarkers (13).
The prognostic utility of each biomarker was examined using two metrics. First, Therneau’s survival concordance index was provided to help assess each model’s discriminative ability. This metric is useful in determining a model’s ability to assign higher risk to individuals that experienced an event. Second, the categorical net reclassification improvement (NRI) was derived to help assess the added predictive ability of these novel biomarkers. The “benchmark” model used to construct the NRI tables was the HF-ACTION adjustment model covariates and NT-proBNP. Patients were categorized as low/high risk based on the Kaplan-Meier failure rate at 4 years. ST2 and galectin-3 levels were added to the benchmark model individually and simultaneously in order to compare the reclassification of risk. Next, we estimated the expected number of cases and controls at 4-years and tabulated the NRI among cases and controls separately. All analyses were performed with SAS 9.3 (SAS Institute Incorporated, Cary, NC) and R 3.0.0 (R Development Core Team). A P-value ≤ 0.05 was considered statistically significant for all analyses. The authors had full access to and take full responsibility for the integrity of the data.
Results
Baseline characteristics
Baseline plasma samples on NT-proBNP, galectin-3, and ST2 were available for 813 patients, who were broadly similar to the HF-ACTION cohort as a whole (N=2331, data not shown). Baseline characteristics for the overall cohort and stratified by median biomarker levels are shown in Table 1. Median age of the study cohort was 59 years; 64% were white, and 71% were male. Median biomarker levels were 852 pg/mL (NT-proBNP), 13.9 ng/mL (galectin-3), and 23.8 pg/dL (ST2). Patients with elevated biomarker levels were older, had a higher prevalence of co-morbidities and NYHA function class, and impaired functional capacity on exercise testing.
Table 1.
Baseline Patient Characteristics by Median Levels of NT-proBNP, Galectin-3, and ST2
| Baseline Characteristic | Total (N=813) | NT-proBNP (pg/mL) | Galectin-3 (ng/mL) | ST2 (pg/dL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| <852 (N=406) | ≥852 (N=407) | P value | <13.9 (N=406) | ≥13.9 (N=407) | P value | <23.8 (N=407) | ≥23.8 (N=406) | P value | ||
| Age, years | 59 (51,68) | 54 (46,63) | 63 (55,73) | <.001 | 55 (46,63) | 63 (54,73) | <.001 | 55 (48,66) | 62 (54,71) | <.001 |
|
| ||||||||||
| Female, % | 29.0 | 30.3 | 27.8 | 0.427 | 35.7 | 22.4 | <.001 | 40.3 | 17.7 | <.001 |
|
| ||||||||||
| Caucasian, % | 62.7 | 57.1 | 68.2 | 0.002 | 56.9 | 68.4 | <.001 | 57.5 | 67.7 | 0.011 |
|
| ||||||||||
| Diabetes, % | 32.7 | 26.8 | 38.6 | <.001 | 31.3 | 34.2 | 0.383 | 26.3 | 39.2 | <.001 |
|
| ||||||||||
| MI, % | 41.5 | 32.8 | 50.1 | <.001 | 33.5 | 49.4 | <.001 | 33.2 | 49.8 | <.001 |
|
| ||||||||||
| Hypertension, % | 65.0 | 62.1 | 67.9 | 0.085 | 66.1 | 64.0 | 0.524 | 63.0 | 67.1 | 0.220 |
|
| ||||||||||
| Abnormal EKG,% | 55.7 | 48.2 | 63.2 | <.001 | 46.4 | 65.1 | <.001 | 48.8 | 63.5 | <.001 |
|
| ||||||||||
| NYHA class | ||||||||||
| II | 64.2 | 70.9 | 57.5 | 70.9 | 57.5 | 70.8 | 57.6 | |||
| III | 34.3 | 28.6 | 40.0 | <.001 | 28.6 | 40.0 | <.001 | 28.7 | 39.9 | <.001 |
| IV | 1.5 | 0.5 | 2.5 | 0.5 | 2.5 | 0.5 | 2.5 | |||
|
| ||||||||||
| LVEF, % | 24 (19,30) | 25 (20,30) | 24 (19,30) | 0.198 | 26 (21,32) | 22 (18,28) | <.001 | 25 (20,30) | 24 (19,30) | 0.020 |
|
| ||||||||||
| Cr, mg/dL | 1.2 (1.0,1.5) | 1.1 (0.9,1.3) | 1.4 (1.1,1.8) | <.001 | 1.1 (0.9,1.3) | 1.3 (1.1,1.7) | <.001 | 1.1 (0.9,1.4) | 1.3 (1.1,1.6) | <.001 |
|
| ||||||||||
| BUN, mg/dL | 20.0 (15.0,28.0) | 17.0 (13.0,22.0) | 25.0 (18.0,35.0) | <.001 | 17.0 (13.0,23.0) | 24.0 (17.0,35.0) | <.001 | 18.0 (14.0,24.0) | 23.0 (16.0,32.0) | <.001 |
|
| ||||||||||
| Hgb, g/dL | 13.3 (12.1,14.5) | 13.6 (12.5,14.9) | 12.9 (11.7,14.2) | <.001 | 13.3 (12.3,14.8) | 13.3 (12.0,14.3) | 0.015 | 13.2 (12.2,14.5) | 13.4 (12.1,14.5) | 0.729 |
|
| ||||||||||
| Exercise duration, min | 9.5 (7.0,12.0) | 10.4 (8.4,13.1) | 8.5 (6.0,10.7) | <.001 | 10.3 (8.3,13.0) | 8.5 (6.3,11.0) | <.001 | 10.3 (8.1,12.9) | 8.8 (6.3,11.0) | <.001 |
|
| ||||||||||
| Peak V02, mL/kg/min | 14.5 (11.7,17.4) | 15.5 (13.1,18.7) | 13.2 (10.9,16.1) | <.001 | 15.5 (12.9,18.8) | 13.3 (10.8,16.3) | <.001 | 15.5 (12.4,18.7) | 13.6 (10.9,16.3) | <.001 |
|
| ||||||||||
| 6-Minute walk, meters | 367 (300,436) | 388 (322,457) | 350 (278,411) | <.001 | 378 (316,442) | 353.6 (278,427) | 0.001 | 384.8 (321,454) | 348 (276,419) | <.001 |
|
| ||||||||||
| Beta-Blocker,% | 94.3 | 95.1 | 93.6 | 0.367 | 95.3 | 93.4 | 0.228 | 94.6 | 94.1 | 0.755 |
|
| ||||||||||
| ACE-I/ARB, % | 95.1 | 97.8 | 92.4 | <.001 | 97.0 | 93.1 | 0.010 | 95.6 | 94.6 | 0.511 |
|
| ||||||||||
| Aldosterone antagonist, % | 44.5 | 46.1 | 43.0 | 0.380 | 46.8 | 42.3 | 0.193 | 45.0 | 44.1 | 0.802 |
|
| ||||||||||
| ICD, % | 46.4 | 37.9 | 54.8 | <.001 | 36.2 | 56.5 | <.001 | 34.9 | 57.9 | <.001 |
N Values are median (interquartile range), or N (%). NT-proBNP indicates aminoterminal pro B-type natriuretic peptide; MI, myocardial infarction; EKG, electrocardiogram; LVEF, left ventricular ejection fraction; New York Heart Association; and ICD, implantable cardioverter defibrillator.
Biomarkers and Risk of Mode of Death
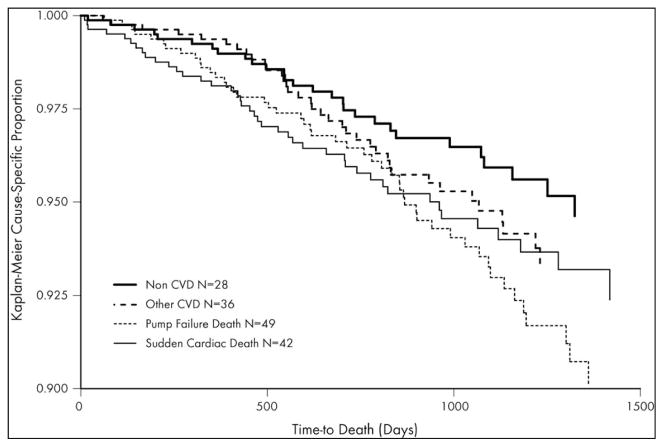
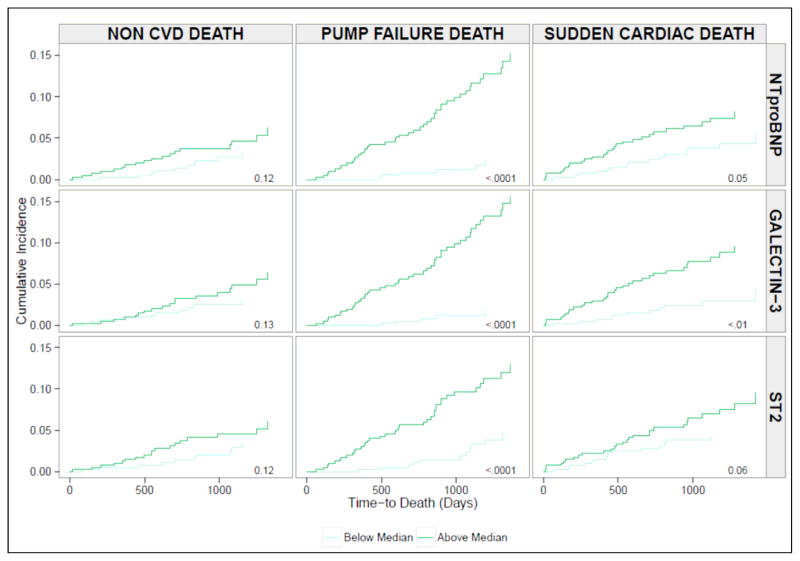
During a median follow-up time of 32 months, there were 155 deaths. 49 were classified as due to pump failure, 42 as due to sudden cardiac death, 36 from other cardiac causes (CVA, cardiac procedure-related, fatal myocardial infarction, or unknown), and 28 from non-cardiac causes. Figures 1 shows the cumulative probability of events according to mode of death. Figure 2 shows the cumulative incidences of non-cardiovascular, pump failure, and sudden cardiac deaths, stratified by median biomarker levels. As shown, the incidence of non-cardiovascular death was similar regardless of biomarker levels, whereas it differed significantly in case of cardiovascular causes of death. Patients with higher than median levels of NT-proBNP, galectin-3, and ST2 were more likely to die of pump failure (P<0.001, all). Sudden cardiac death occurred more commonly in patients with higher than median levels of NT-proBNP (P=0.05) and galectin-3 (P<0.01), while the association in the case of ST2 was of borderline significance (P=0.06).
Figure 1. Kaplan-Meier Cause-Specific Survival Curves.
The cumulative probability of events according to mode of death. Pump failure death was most common (N=49), followed by sudden cardiac death (N=42). 36 patients died from other cardiac causes (CVA, cardiac procedure-related, fatal myocardial infarction, or unknown), and 28 from non-cardiac causes.
Figure 2. Cumulative Incidence of Sudden Cardiac Death, Pump Failure, and Non-Cardiovascular Death According to Levels of Biomarkers.
The cumulative incidence functions of adverse outcomes according to above versus below median biomarker level groups. Incidence of non-cardiovascular death was equivalent between patient groups. Patients with more than the medial level of all three biomarkers had a significantly greater incidence of pump failure (P<0.001, all). Incidence of sudden cardiac death was significantly greater in those with more than median levels of galectin-3 (P<0.01) and NT-proBNP (P=0.05), and of borderline significance in case of ST2 (P=0.06).
To explore the differential association between each biomarker and mode of death, we first looked at the unadjusted effect, then adjusted for known clinical predictors of adverse outcomes, and lastly for the two remaining biomarkers (Table 2). After adjustment for clinical predictors, all three biomarkers remained significantly associated with both pump failure and sudden cardiac death. After additional adjustment for other biomarkers (ST+galectin-3), NT-proBNP remained predictive of both pump failure and sudden cardiac death. In case of galectin-3, the associations with either mode of death were attenuated, but remained significant, after adjustment for clinical predictors and NT-proBNP, whereas ST2 only retained a significant association with risk of pump failure.
Table 2.
Biomarkers and Risk According to of Mode of Death
| Prediction Models | Pump Failure HR (95%CI) |
Sudden Cardiac Death HR (95%CI) |
Differential P value |
|---|---|---|---|
| Log2 NT-proBNP univariate | 3.71 (2.65–5.18) | 1.95 (1.39–2.73) | <0.01 |
| Adjusted for Clinical Model* | 2.85 (1.92–4.25) | 1.97 (1.30–2.99) | 0.21 |
| Adjusted for Clinical Model* + Galectin-3 + ST2 | 1.91 (1.24–2.95) | 1.65 (1.06–2.58) | 0.65 |
|
| |||
| Galectin-3 Truncated at 20ng/dL univariate | 2.77 (1.93–3.99) | 1.68 (1.21–1.32) | 0.04 |
| Adjusted for Clinical Model* | 1.92 (1.27–2.93) | 1.70 (1.12–2.58) | 0.68 |
| Adjusted for Clinical Model*+ NT-proBNP | 1.60 (1.05–2.42) | 1.51 (0.99–2.30) | 0.85 |
| Adjusted for Clinical Model*+ NT-proBNP + ST2 | 1.38 (0.90–2.11) | 1.47 (0.96–2.25) | 0.83 |
|
| |||
| Log2 ST2 univariate | 2.37 (1.94–2.89) | 1.66 (1.28–2.15) | 0.03 |
| Adjusted for Clinical Model* | 2.18 (1.69–2.81) | 1.49 (1.10–2.01) | 0.06 |
| Adjusted for Clinical Model*+ NT-proBNP | 1.75 (1.31–2.35) | 1.27 (0.90–1.78) | 0.16 |
| Adjusted for Clinical Model*+ NT-proBNP + Galectin-3 | 1.67 (1.24–2.26) | 1.22 (0.87–1.72) | 0.17 |
HF-ACTION Clinical Adjustment Model: Serum creatinine level, Body Mass Index, Sex, Dosage of loop diuretic, Left Ventricular ejection fraction, CCS angina classification, and resting ECG Ventricular Conduction abnormality. NT-proBNP indicates amino-terminal proB-type natriuretic peptide; HR, hazard ratio; CI, confidence interval.
There was a differential association between pump failure and sudden cardiac death for all three biomarkers in the unadjusted models (Table 2). In all cases, increases in the biomarker had a stronger association with pump failure than with sudden cardiac death (P≤0.04). After adjustment for clinical risk factors, there was no further evidence of a significant differential association for NT-proBNP and galectin-3, whereas ST2 still had borderline evidence for a stronger association with pump failure (P=0.06).
Due to potential confounding that may results from patients with ICD therapies, we performed an additional sensitivity analysis by using ICD shocks as an SCD equivalent (Supplementary Table 1), and noted trends similar to those reported in Table 2. We also noted statistical evidence of a differentially stronger association of all three biomarkers with risk of pump failure.
Incremental Changes in Prediction of Mode of Death Using Biomarkers
To explore the clinical implications of our findings, we calculated changes in C statistics for prediction of pump failure and sudden cardiac death with incremental addition of biomarkers to the clinical model (Table 3). For pump failure, the C statistic for the clinical model was 0.82 (95% CI: 0.73–0.91), and similar to that for univariate biomarkers: 0.76 (95% CI: 0.67–0.84) for galectin-3, 0.79 (95% CI: 0.70–0.88) for ST2, and 0.83 (95% CI: 0.74–0.91) for NT-proBNP. Addition of NT-proBNP led to an increase in the C statistic (0.82→0.87); similarly subsequent additions of ST2 and galectin-3 increased the C statistic (0.87→0.89). The ability to discriminate among individuals that experienced a pump failure death was better than that for sudden cardiac death using the clinical model: 0.68 (95% CI: 0.58–0.78)(Table 3). Addition of NT-proBNP to the clinical model improved the C statistic (0.68→0.73), and subsequent additions of ST2 and galectin-3 increased it to 0.75.
Table 3.
Increases in C-Statistic for Prediction of Pump Failure and Sudden Cardiac Death with Incremental Addition of Biomarkers
| Prediction Models | Pump Failure C-Statistic |
Sudden Cardiac Death C-Statistic |
|---|---|---|
| Clinical Model* | 0.82 (0.73–0.91) | 0.68 (0.58–0.78) |
|
| ||
| NT-proBNP univariate | 0.83 (0.74–0.91) | 0.67 (0.58–0.76) |
| Galectin-3 univariate | 0.76 (0.67–0.84) | 0.66 (0.57–0.75) |
| ST2 univariate | 0.79 (0.70–0.88) | 0.64 (0.55–0.73) |
|
| ||
| Clinical Model * + NT-proBNP | 0.87 (0.78–0.96) | 0.73 (0.64–0.83) |
| Clinical Model * + Galectin-3 | 0.83 (0.74–0.92) | 0.71 (0.62–0.81) |
| Clinical Model* + ST2 | 0.86 (0.77–0.95) | 0.72 (0.62–0.82) |
|
| ||
| Clinical Model* + NT-proBNP + Galectin-3 | 0.88 (0.79–0.97) | 0.74 (0.64–0.84) |
| Clinical Model* + NT-proBNP + ST2 | 0.88 (0.79–0.96) | 0.75 (0.65–0.85) |
| Clinical Model* + NT-proBNP + ST2 + Galectin-3 | 0.89 (0.80–0.98) | 0.75 (0.65–0.85) |
HF-ACTION Clinical Adjustment Model: Serum creatinine level, Body Mass Index, Sex, Dosage of loop diuretic, Left Ventricular ejection fraction, CCS angina classification, and resting ECG Ventricular Conduction abnormality. NT-proBNP indicates amino-terminal proB-type natriuretic peptide.
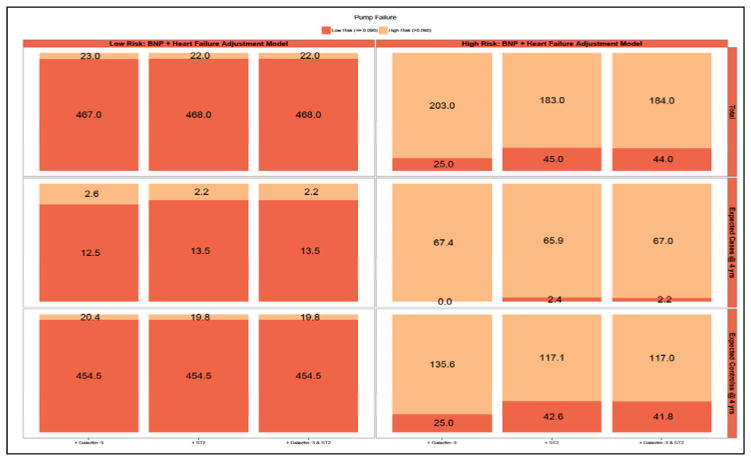
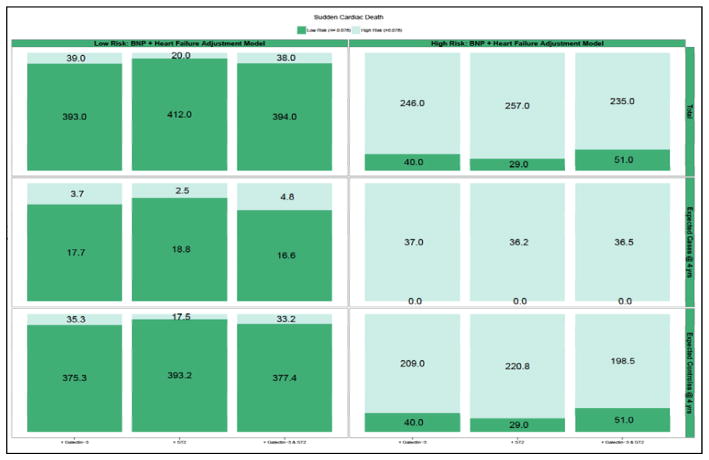
Due to potential insensitivities in detection of clinically important risk differences using AUC methods, we quantified the NRI for pump failure and sudden cardiac death. We calculated whether addition to the biomarkers to a risk model with clinic predictors and NT-proBNP would lead to more appropriate classification of risk for each mode of death. Patients were classified into risk categories (low/high) of pump failure and sudden cardiac death based on a dichotomy of risk from a cause-specific model that included clinical covariates and NT-proBNP levels. The Kaplan-Meier failure rate at 4 years was chosen as the cut-off for high/low risk. For pump failure, this value was 10%, for sudden cardiac death this value was 8%. For the endpoint of pump failure (Figure 3; Supplementary Table 2), addition of ST2, galectin-3, or ST2 + galectin-3 to the clinical model + NT-proBNP did not result in a significant amount of net reclassification. In the case of sudden cardiac death (Figure 4; Supplementary Table 3), the addition of ST2 + galectin-3 led to a significant degree of appropriate reclassification [NRI: 0.11 (95% CI: 0.02 – 0.20]. These findings suggest that, after consideration of clinical risk factors and NT-proBNP, addition of novel biomarkers galectin-3 and ST2 may lead to improvements in risk classification of sudden cardiac death.
Figure 3. Reclassification of 4 Year Predictive Probabilities of Pump Failure with Addition of Novel Biomarkers.
Graphic representation of reclassification across low and high risk of pump failure and change in predicted probabilities with addition of galectin-3 and ST2 to Clinical model + NT-proBNP in entire cohort (top panel), cases (middle panel), and controls (bottom panel). Overlapping shading in risk categories represents the number of patients reclassified with addition of galectin-3 (left column), ST2 (middle column), and ST2+galectin-3 (right column). For example, among all patients (top panel), the addition of galectin-3 (left most column) moved 23 low risk patients into the high risk category, and 25 high risk patients to the low risk category.
Figure 4. Reclassification of 4 Year Predictive Probabilities of Sudden Cardiac Death with Addition of Novel Biomarkers.
Graphic representation of reclassification across low and high risk of sudden cardiac death and change in predicted probabilities with addition of galectin-3 and ST2 to Clinical model + NT-proBNP in entire cohort (top panel), cases (middle panel), and controls (bottom panel). Overlapping shading in risk categories represents the number of patients reclassified with addition of galectin-3 (left column), ST2 (middle column), and ST2+galectin-3 (right column). For example, among all patients (top panel), the addition of galectin-3+ST2 (right most column) moved 38 low risk patients into the high risk category, and 51 high risk patients to the low risk category.
Discussion
In this analysis of 813 ambulatory systolic heart failure patients, we found that biomarkers of myocardial stress and fibrosis were strong independent predictors of death from pump failure and sudden cardiac death. When considering individual patient risk, prediction models comprising of clinical factors and NT-proBNP levels were stronger predictors of pump failure than sudden cardiac death. Inclusion of novel biomarkers improved risk discrimination for sudden cardiac death, but not pump failure. These findings suggest that readily available clinical measurements along with NT-proBNP are excellent predictors of risk of pump failure, and inclusion of novel biomarkers might play a role in improving prediction of sudden cardiac death.
We believe these data are of interest for several reasons. This is the first study to simultaneously report on the association between galectin-3, ST2, and mode specific death in patients with chronic heart failure (14,15). In a recent study of 876 ambulatory heart failure patients, Beyes-Genis and colleagues found that addition of ST2 to clinical risk factors and NT-proBNP improved prediction of all-cause and cardiovascular mortality, whereas the incremental predictive contribution of galectin-3 was trivial (16). Based on these results, they recommended incorporation of ST2, but not galectin-3, into clinical practice. Our study differed by focusing on the therapeutic implications of measuring additional biomarkers by studying the added value of novel biomarkers for prediction of cause-specific death, rather than all cause or cardiovascular mortality. This was because key clinical decisions in heart failure patients are generally based on forecasting risk of mode of death. Our findings also brought us to a different conclusion: knowledge of clinical variables and NT-proBNP levels already provide extremely accurate assessments of pump failure risk, whereas further inclusion of both ST2 and galectin-3 may improve prediction of sudden cardiac death. This underscores the need for validation of results in separate patient populations, as well as considering the therapeutic implications of reporting on purely prognostic performances of biomarkers, as these may be ambiguous when examined against a consolidated group of distinct clinical outcomes.
Second, we demonstrated a clear difference in our ability to predict pump failure versus sudden cardiac death using currently available data and novel heart failure biomarkers. Previously, only one study has examined this question: a case-control study of 36 patients reported on correlations between ST2 levels and risk of sudden cardiac death in heart failure, but was limited by its small size, reliance on observational data, and a lack of information about the competing risk of pump failure (17). Despite the theoretic possibility that galectin-3, a marker of myocardial fibrosis, might associate more strongly with sudden cardiac death, and ST2, a marker for biomechanical stretch, with pump failure, we did not find this to be the case (18,19). In fact, both biomarkers showed stronger associations with pump failure, and significant improvements in risk reclassification of sudden cardiac death occurred only with consideration of both biomarkers. Furthermore, clinical predictors and NT-proBNP yielded a C statistic of 0.87 for prediction of pump failure, while inclusion of all clinical factors and biomarkers led to a C statistic of 0.75 for prediction of sudden cardiac death. This suggests that pump failure may be a more clearly defined and predictable outcome that results from concomitant dysfunction in several overlapping biological pathways, and the incremental prognostic value of novel biomarkers after inclusion of natriuretic peptides might be minimal. On the other hand, there is a need for improved prediction of sudden cardiac death risk and these results suggest that clinical use of ST2 and galectin-3 might be useful for this purpose.
Third, our study raises key questions about the added clinical utility of novel biomarkers in heart failure. While ST2 and galectin-3 have been FDA approved for use in heart failure based on their prognostic significance, their use at the bedside remains uncertain. Particularly promising has been the notion that adding these biomarkers to clinical data could assist clinicians with challenging decisions in instances where current prediction models lack sufficient accuracy, and interventions focus on use of expensive technologies (e.g. ICDs and LVADs), limited resources (e.g. cardiac transplantation), and end of life care. Since such interventions are aimed at preventing specific causes of cardiac death, examining the association of novel biomarkers with mode of death is of central significance prior to their widespread clinical use. Our finding that addition of ST2 and galectin-3 to clinically available data and NT-proBNP may enhance the ability to reclassify risk of sudden cardiac death implies that these biomarkers may play a future role in assisting physicians with recommendations surrounding use of ICDs. Moreover, our finding that currently available clinical predictors, along with consideration of NT-proBNP, are robust predictors of pump failure implies that use of novel biomarkers for this purpose may not improve clinical decision making to the degree that the added cost and complexity is justified. An additional point to consider is that clinical use of biomarkers can provide important objective, biologically meaningful, and reproducible measures of heart failure prognosis that may be of more value than currently used subjective, imprecise, and irreproducible measures. For example, qualification for use of ICD for prevention of sudden death in heart failure is based solely on NYHA class and LVEF, both highly variable and mediocre measures of disease state (20). Further studies are warranted to examine whether biomarker-based risk assessments might streamline and improve care of heart failure patients in a cost-effective manner. Lastly, undergoing studies are examining whether available treatments for heart failure, or novel inhibitors of ST2 or galectin-3 might modulate the disease process, and attenuate risk of specific outcomes, quantifiable with serum biomarker measurements (21–23). Based on these studies, serial measures of these biomarkers may play a role in prediction of therapeutic response (24).
Several potential limitations of our study deserve consideration. First, our analysis was limited to the subset of HF-ACTION patients in whom plasma levels of NT-proBNP, galectin-3, and ST2 were available. However, this is the largest study of biomarkers and mode of death in heart failure to date, and the magnitudes of reported hazard ratios are consistent with previously reported risks of adverse clinical outcomes (14,15). Additionally, baseline characteristics of patients with and without available NT-proBNP levels were statistically similar. We did not have detailed information on appropriate versus inappropriate shocks in patients with ICDs; since appropriate shocks may represent a sudden cardiac death event, this may have led us to underestimate the occurrence of this mode of death; however, any misclassification of this kind would be expected to bias the results towards the null (25). We also provide a sensitivity analysis using ICD shocks as a SCD equivalent, with results largely consistent with our main findings. For purposes of reclassification, we categorized patients as high/low risk, despite the absence of well-defined risk categories in heart failure. This likely led to a conservative estimation of the NRI, as increases in risk categories would be expected to increase degrees of reclassification. The study population for this analysis was derived from the HF-ACTION trial, and as such is susceptible to the limitations inherent in clinical trial populations such as limited follow-up time. Our study population consisted only of ambulatory patients with impaired ejection fraction (LVEF < 35%), so our results cannot be extrapolated to the population of patients with heart failure and preserved ejection fraction.
Conclusions
In summary, we found that novel biomarkers of myocardial stress and fibrosis were strongly associated with increased risk of death from pump failure and sudden cardiac death. Available clinical predictors along with NT-proBNP levels were strong predictors of pump failure risk, with insignificant incremental contributions with consideration of ST2 and galectin-3. Predictability of sudden cardiac death risk was less robust and enhanced by information provided by novel biomarkers. These findings suggest that despite the independent prognostic value of ST2 and galectin-3 elevations in heart failure, their clinical use might focus on improved prediction of sudden cardiac death risk.
Supplementary Material
Acknowledgments
Funding Sources
Dr. Ahmad received support from the Daland Fellowship in Clinical Investigation and a training grant from the NHLBI (T32HL069749). The HF-ACTION study was funded by grants from the NHLBI. The biomarkers assays were funded by grants from Roche Diagnostics, BG Medicine, and Critical Diagnostics.
We wish to thank all the participants and investigators of the HF-ACTION clinical trial. We also wish to thank Kevin Anstrom, PhD, and Karen Pieper, MS, for their substantial input in reviewing the statistical analysis plan and results.
Abbreviation List
- HF
heart failure
- PF
pump failure
- SCD
sudden cardiac death
- ICD
implantable cardioverter defibrillators
- LVAD
Left ventricular assist device
- NT-proBNP
amino terminal proB-type natriuretic peptide
- Soluble ST2
ST2
- HF-ACTION
Heart failure and a controlled trial investigating outcomes of exercise training
- NRI
Net Reclassification Index
- HR
hazard ratio
- CI
confidence interval
Footnotes
Disclosures
Drs. Felker, Fiuzat, and O’Connor have received research funding from BG Medicine, Critical Diagnostics, and Roche Diagnostics. Drs. Felker has served as a consultant for BG Medicine, Singulex, and Roche Diagnostics. Dr. Zannad has received research funding from BG Medicine and Roche Diagnostics, and served as a consultant for BG Medicine. Dr. Kitzman serves as a consultant for Relypsa, Inc. Dr. Piña is a consultant for General Electric and Novartis. None of the other authors report any conflicts.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, et al. Trends in Heart Failure Incidence and Survival in a Community-Based Population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Korngold EC, Januzzi JL, Jr, Gantzer ML, Moorthy MV, Cook NR, Albert CM. Amino-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein as predictors of sudden cardiac death among women. Circulation. 2009;119:2868–76. doi: 10.1161/CIRCULATIONAHA.108.832576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HN, Januzzi JL., Jr Biomarkers in the management of heart failure. Current treatment options in cardiovascular medicine. 2010;12:519–31. doi: 10.1007/s11936-010-0096-3. [DOI] [PubMed] [Google Scholar]
- 5.Tapanainen JM, Lindgren KS, Makikallio TH, Vuolteenaho O, Leppaluoto J, Huikuri HV. Natriuretic peptides as predictors of non-sudden and sudden cardiac death after acute myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2004;43:757–63. doi: 10.1016/j.jacc.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Havmoller R, Chugh SS. Plasma biomarkers for prediction of sudden cardiac death: another piece of the risk stratification puzzle? Circ Arrhythm Electrophysiol. 2012;5:237–43. doi: 10.1161/CIRCEP.111.968057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Kimmenade RR, Januzzi JL., Jr Emerging biomarkers in heart failure. Clinical chemistry. 2012;58:127–38. doi: 10.1373/clinchem.2011.165720. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E. Heart Failure. JACC: Heart Failure. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA: the journal of the American Medical Association. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whellan DJ, O’Connor CM, Lee KL, et al. Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION): Design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Daniels LB, Maisel AS. Natriuretic peptides. Journal of the American College of Cardiology. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European heart journal. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor CM, Whellan DJ, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circulation Heart failure. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smart NA, Steele M. Systematic review of the effect of aerobic and resistance exercise training on systemic brain natriuretic peptide (BNP) and N-terminal BNP expression in heart failure patients. International journal of cardiology. 2010;140:260–5. doi: 10.1016/j.ijcard.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Januzzi JL, Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. European heart journal. 2012;33:2265–71. doi: 10.1093/eurheartj/ehs191. [DOI] [PubMed] [Google Scholar]
- 16.Bayes-Genis A, de Antonio M, Vila J, et al. Head-to-head comparison of two myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 vs. Galectin-3. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.07.087. [DOI] [PubMed] [Google Scholar]
- 17.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, et al. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. Journal of the American College of Cardiology. 2009;54:2174–9. doi: 10.1016/j.jacc.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 18.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–8. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 19.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–49. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Ruifrok WP, Meissner M, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circulation Heart failure. 2013;6:107–17. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 22.Calvier L, Miana M, Reboul P, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad T, O’Connor CM. Therapeutic implications of biomarkers in chronic heart failure. Clinical pharmacology and therapeutics. 2013;94:468–79. doi: 10.1038/clpt.2013.139. [DOI] [PubMed] [Google Scholar]
- 25.Yu B, Barbalic M, Brautbar A, et al. Association of Genome-Wide Variation with Highly Sensitive Cardiac Troponin-T (hs-cTnT) Levels in European- and African-Americans: A Meta-Analysis from the Atherosclerosis Risk in Communities and the Cardiovascular Health Studies. Circulation Cardiovascular genetics. 2012 doi: 10.1161/CIRCGENETICS.112.963058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.