Abstract
Pericytes of the central nervous system (CNS) are uniquely positioned within a multicellular structure termed the neurovascular unit (NVU) to provide crucial support to blood brain barrier (BBB) formation, maintenance, and stability. Numerous CNS diseases are associated with some aspect of BBB dysfunction. A dysfunction can manifest as one or multiple disruptions to any of the following barriers: physical, metabolic, immunological and transport barrier. A breach in the BBB can notably result in BBB hyper-permeability, endothelial activation and enhanced immune-endothelial interaction. How the BBB is regulated within this integrated unit remains largely unknown, especially as it relates to pericyte-endothelial interaction. We summarize the latest findings on pericyte origin, possible marker expression, and availability within different organ systems. We highlight pericyte-endothelial cell interactions, concentrating on extra- and intra- cellular signaling mechanisms linked to platelet derived growth factor-B, transforming growth factor -β, angiopoietins, Notch, and gap junctions. We discuss the role of pericytes in the NVU under inflammatory insult, focusing on how pericytes may indirectly affect leukocyte CNS infiltration, the direct role of pericyte-mediated basement membrane modifications, and immune responses. We review new findings of pericyte actions in CNS pathologies including Alzheimer’s disease, stroke, multiple sclerosis, diabetic retinopathy, and HIV-1 infection. The uncovering of the regulatory role of pericytes on the BBB will provide key insight into how barrier integrity can be re-established during neuroinflammation.
Keywords: blood brain barrier, pericyte, brain endothelial cells, neuroinflammation, neurovascular unit
Introduction
The adult central nervous system (CNS) requires a complex vascular network to maintain proper homeostasis and function. Communication between the CNS and vasculature, otherwise known as the blood brain barrier (BBB), is mediated through what has been termed the neurovascular unit (NVU) which is comprised of endothelial cells (EC), pericytes, vascular smooth muscle cells (vSMC), astrocytes, neurons, and perivascular macrophages. The interplay between cells within the NVU regulates numerous functions of the BBB including its stability, regulation of CNS blood flow, transport of molecules and nutrients into the CNS, and clearance of toxic products(Armulik et al., 2010; Daneman et al., 2010). Altered communication between the cells that make up the NVU is a key factor in many neurologic diseases(Sengillo et al., 2013).
Pericytes were first described by Eberth (1871) and Rouget (1873) as a population of contractile cells that surround the ECs of small blood vessels(Eberth, 1871; Rouget, 1873). The term “pericyte” was later coined by Zimmerman due to their close proximity to endothelial cells(Zimmerman, 1923). Despite this early emergence in the literature, most that is known about pericytes comes from recent studies.
Pericytes are found on pre-capillary arterioles, capillaries, and post-capillary venules. They extend primary processes along the abluminal, longitudinal axis of the blood vessel, and usually span numerous ECs(Krueger and Bechmann, 2010). Pericytes are encased within the endothelial basement membrane (BM) where they are thought to contribute to and regulate BM assembly via interactions with ECs(Winkler et al., 2011b). In areas where there is no BM present, pericytes make direct peg-socket connections with ECs through gap junctions and adherent junctions (AJ)(Li et al., 2011; Winkler et al., 2011b). These data suggest that pericytes are distributed along the vasculature in such a way as to facilitate, integrate, and coordinate vascular endothelial cell-cell signals.
Much of the recent research has focused on pericyte-endothelial cell interactions and their subsequent role in BBB regulation in the healthy CNS. The present review will focus on pericytes in disease states, highlighting the signaling pathways involved, role of pericytes on BBB regulation during inflammatory insult, and altered function in neuropathologic diseases.
Background
Origin
Current literature proposes numerous developmental lineages of pericytes. It was recently determined that pericytes of the forebrain are derived from neural crest cells, specifically neuroectoderm, and pericytes in other organs (skeletal muscle, liver, skin, and lung) originate from the mesoderm. Much of the data come from quail-chick studies in which quail neuroectoderm cells or mesoderm cells were transplanted into chick embryos. Transplanted neuroectoderm initiated pericyte populations in the forebrain whereas mesoderm transplantation initiated pericyte populations in the mid-brain, brainstem, spinal cord, heart, lung, liver, and gut(Armulik et al., 2011a; Zlokovic, 2011).
Pericytes may also originate from mesothelial cells that have undergone epithelial-to-mesenchymal transition. Mesothelial cells migrate to sites of angiogenesis and subsequently give rise to vSMCs, fibroblasts, and pericytes(Armulik et al., 2011b). It has also been postulated that pericytes arise from trans-differentiated endothelial cells(DeRuiter et al., 1997) as well as from bone marrow elements during adult angiogenesis after acute and chronic CNS injury(Piquer-Gil et al., 2009).
It is hypothesized that pericyte precursors are recruited to sites of angiogenesis/development by chemotactic attractants such as platelet derived growth factor-B (PDGF-B) secreting cells(Dore-Duffy, 2008). Precursor cells migrate into the tissue and begin to differentiate into pericytes as they are enclosed in the BM on the newly formed vessels(Armulik et al., 2011b). Yet, it may be that pericytes do not undergo linear differentiation. Depending on pathologic condition or injury, pericytes may emanate from a number of cell types (vSMCs, immature mesenchyme) utilizing a grouping of pre-set genes that the cell can easily upregulate and use to differentiate into pericytes(Armulik et al., 2011b). These data point to a multitude of lineages for production or differentiation into pericytes. It may be that pericyte populations arise due to a complex battery of signaling mechanisms dependent on pathologic condition. Further evidence is needed to determine all possible sources of pericytes.
Markers
The identification and characterization of pericytes has presented a challenge. Not only is the study of pericytes difficult due to their morphology and close relationship with ECs, but also due to the fact that there is a lack of unique, specific markers. This may be explained by the numerous lineages from which pericytes are derived and that pericytes themselves are multipotent cells. Unfortunately, expression of markers found on pericytes can fluctuate depending on the physiologic or pathophysiologic state.
Pericytes present contractile, cytoskeletal, and surface proteins allowing identification. The primary agreed upon markers under normal conditions include PDGF receptor-β (PDGFR-β), α-smooth muscle actin (αSMA), chondroitin sulfate proteoglycan 4 (NG2), pericytic aminopeptidase N (pAPN), alanyl (membrane) aminopeptidase (CD13), desmin, and regulator of G protein signaling 5 (RGS5)(Bandopadhyay et al., 2001; Armulik et al., 2011a). Depending on the type of blood vessel, resident pericytes display variable expression of contractile proteins such as αSMA. Levels of αSMA are elevated in pre-capillary regions as compared to post-capillary regions(Dore-Duffy, 2008; Krueger and Bechmann, 2010). Interestingly, not all populations of pericytes express αSMA, whereas neighboring cells such as vSMCs do express αSMA(Bandopadhyay et al., 2001).
Other markers associated with pericytes include the adhesion molecules intercellular adhesion molecule 1(ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1)(Dalkara et al., 2011). These molecules are often expressed by neighboring endothelial and glial cells in the CNS as well, which makes specific identifications difficult(Engelhardt and Sorokin, 2009; Krueger and Bechmann, 2010). These observations emphasize the importance of future research to definitively identify specific pericyte markers, or a timeline of marker expression based on differentiation, activation, and lineage of pericytes.
Pericyte density across different organ systems
Pericyte abundance is varied within the mammalian vasculature as is the percentage of the EC abluminal surface coverage. It is regarded that the tissues with the highest abundance of pericytes are the CNS and eye (retina) with a ratio of pericytes to endothelial cells of 1:3 and 1:1, respectively(Sa-Pereira et al., 2012). Other tissues determined to contain significant pericyte populations include the lung, skeletal muscle, and kidney. Pericyte coverage has been linked to EC turnover rate, such that the greater the pericyte coverage, there is less EC turnover(Diaz-Flores et al., 2009). Pericytes also form “umbrella-like” structures at EC junctions where they are twice as likely to be found in comparison to random location distribution(Diaz-Flores et al., 2009). Pericytes are primarily found distal to gaseous exchange areas, especially within the retina where oxygen transfer occurs.
CNS/BBB
In the CNS, as in many other tissues, pericytes reside within pre-capillary arterioles, capillaries, and post-capillary venules(Bonkowski et al., 2011). There is conflicting evidence concerning the percentage of pericyte coverage of ECs in the CNS varying between 22–99% of the abluminal surface(Dalkara et al., 2011). Pericytic coverage is not dependent upon age, but the aged pericyte shows a larger presence of lysosomes and inclusions(Peters and Sethares, 2012) assumed to be phagocytic in origin.
Proper BBB formation during embryogenesis is dependent upon pericytes. Pericyte number around ECs has been determined to inversely correlate with BBB permeability and pericytes themselves regulate tight junction (TJ) formation and transendothelial vesicular transport, although the mechanism by which this occurs is unknown(Daneman et al., 2010). Mice deficient in PDGF-Rβ signaling have demonstrated that a functional BBB is present before astrocytic end-foot generation, and these animals show increased vascular permeability and an increased rate of transcytosis compared to control animals(Armulik et al., 2010; Daneman et al., 2010). TJ abnormalities are also present in these animals. The existence of pericytes is not required for EC expression of BBB-specific genes, but loss of pericyte coverage upregulates genes known to increase vascular permeability [angiopoietin 2 (Ang-2), Plvap, ICAM-1, and Lgals3](Daneman et al., 2010). There is also a decrease in Ang-1, a gene known to decrease vascular permeability. Current evidence provides a broad picture of the necessity of pericyte coverage for proper BBB function during embryogenesis, as well as a complex signaling network between the cells of the BBB for homeostatic vascular permeability.
Cardiac tissue
Pericytes are the second most abundant cell in the human heart(Nees et al., 2012). They form the same type of gap junctions with ECs as within the CNS using CX-43 and are associated with a 1:2 or 1:3 pericyte to EC ratio(Nees et al., 2012). Cardiac pericytes display a characteristic star-shaped morphology and are completely enveloped within a dense extracellular matrix (ECM)(Nees et al., 2012). This subset of pericytes has similar physiologic functions to pericytes of the CNS, including EC proliferation, barrier formation and exchange of solutes through nitrogen oxide (NO) mediated vasodilation, initiation of the coagulation process through expression of tissue factor and pro-thrombinase, regulation of vascular BM remodeling, and leukocyte extravasation during acute inflammation(Nees et al., 2012; Proebstl et al., 2012; Wang et al., 2012). Cardiac pericytes express the prototypical pericyte markers (PDGFR-β, αSMA, NG2, desmin) and participate in a wide range of pathophysiologic conditions. Among these are vessel wall thickening in atherosclerosis, an increase in thrombosis via inflammatory responses and expression of pro-coagulants, and fibrosis via secretion of PAI-1 and procollagen-1(McCullough et al., 2011).
Renal pericytes
Renal pericytes have recently come into the spotlight due to their role in kidney pathophysiology. Pericytes within the kidney are found in and around the glomeruli, on the afferent and efferent arterioles, descending vasa recta (DVR), and peritubular capillaries(Stefanska et al., 2013). Pericytes of the kidney are known as mesangial cells in the glomeruli and pericytes of peritubular capillaries(Stefanska et al., 2013). Markers of renal pericytes are the same as those of the CNS and cardiac muscle (PDGF-Rβ, α-SMA, NG2, RGS5, and desmin), yet this population of pericytes also expresses CD248, a marker for stromal pericytes and fibroblasts of the kidney(Smith et al., 2011).
Functions of pericytes vary within the kidney. PDGF-B expressing ECs attract migrating pericytes during angiogenesis, yet pericytes actively inhibit capillary growth(Orlidge and D‧Amore, 1987). Within the DVR, pericytes help regulate medullary blood flow via a contractile function and contribute to nephron glomerular filtration rate(Schlondorff and Banas, 2009). Pericytic contraction is regulated by several stimuli such as Ang-2, endothelin-1, vasopressin, and adenosine while relaxation is induced by acetylcholine and norepinephrine(Schlondorff and Banas, 2009). Activated renal pericytes play important roles in the immune response by being major sources of cytokines after kidney injury and promoting interstitial fibrosis(Schrimpf et al., 2012); blocking PDGF-B signaling attenuates this immune response and prevents fibrosis(Chen et al., 2011). Renal fibrosis stems from the ability of pericytes to secrete collagen I α1 that is induced by activation of pericytes, their infiltration into the interstitium and differentiation into myofibroblasts, the end result being an increase in fibrosis leading to a decline in renal function(Stefanska et al., 2013). Glomerulosclerosis and tubulo-interstitial fibrosis are characteristics of diabetic nephropathy secondary to pericyte matrix accumulation.
Pericyte-Endothelial cell interaction
The molecular communication between pericytes and ECs has been a major focus of research in the past few years. These signaling mechanisms have proven to be necessary for proper pericyte function and maintenance of the BBB. The intricate relationship between pericytes and ECs has inevitably shown that deficits in one cell type will have a drastic affect on the other. This review focuses on a number of signaling mechanisms including PDGF-B, transforming growth factor-β (TGF-β), Ang-1, Ang-2, Notch, and gap junctions.
PDGF-B/PDGF-Rβ
PDGF-B secretion by ECs is critical for recruitment and migration of pericytes to newly formed vessels during angiogenesis(Gaengel et al., 2009). ECs secrete PDGF-B as a homodimer (PDGF-BB), which is retained in the ECM or on the surface of the ECs via retention motifs within the C terminus of the protein. These retention motifs interact with heparin sulfate proteoglycans (HSPG) to promote localization of PDGF-B. Mutations of these retention motifs, or a decrease of N-sulfated heparin leads to abnormal pericyte coverage, pericyte detachment, delayed migration, and impaired PDGF-B signaling(Abramsson et al., 2007). Pericyte proliferation, however, is not affected by disruption of the retention motifs or HSPGs, and PDGF-B overexpression initiates, but is unable to sustain, angiogenesis in vivo(Rodriguez et al., 2013).
PDGF-B binds to PDGF-Rβ expressed on the surface of pericytes and vSMCs. PDGF-Rβ is a receptor tyrosine kinase; activation of PDGF-Rβ by PDGF-B induces dimerization of the receptor, autophosphorylation of tyrosine residues within the intracellular portion of the receptor, and initiation of downstream signal transduction(Tallquist et al., 2003). Signaling cascades for PDGF-Rβ include PI3K, PLCγ, and the MAPK pathways. Mutations in these pathways result in abnormal pericyte proliferation and migration, and alterations in vSMC numbers(Tallquist et al., 2003). Genetic deletion of either PDGF-B or PDGF-Rβ results in nearly identical phenotypes and is lethal in embryonic mice due to vascular dysfunction and hemorrhage(Soriano, 1994). The vascular leakage seen in the PDGF-B−/− and PDGF-Rβ−/− mice arises from a severe lack of pericytes(Lindahl et al., 1997). Knockout of PDGF-B and PDGF-Rβ also has secondary consequences in ECs leading to endothelial hyperplasia and abnormal gap junctions(Hellstrom et al., 2001). Upregulation of vascular endothelial growth factor A is observed in these animals as well, and may induce greater vascular permeability(Hellstrom et al., 2001). Persistent exposure of PDGF-Rβ to PDGF-B causes reduced expression of PDGF-Rβ leading to decreased integrin α1β1 levels and subsequent impairment of pericyte adhesion to ECM components(Hosaka et al., 2013). This contradicts evidence suggesting that sustained PDGF-B signaling through PDGF-Rβ in the adult CNS is required for pericyte survival(Bell et al., 2010). This inconsistency may stem from the fact that most studies of PDGF-B/PDGF-Rβ signaling have focused on embryonic development rather than in postnatal animals.
TGF-β
TGF-β is a cytokine that mediates numerous cellular processes including pericyte and EC proliferation, differentiation, and vascular remodeling. TGF-β is secreted by both pericytes and ECs in the pro-form, which needs activation by thrombospondin, proteases, or integrins(Gaengel et al., 2009). There are two types of TGF-β receptors: type 1 (TGFβR1) which has two forms (activin-like kinase 1 [ALK1] and 5 [ALK5]) and type 2 (TGFβR2), which has one. Pericytes and ECs express both TGF-β receptors. Activated TGF-β binds to TGFβR2, which recruits and induces phosphorylation of either ALK1 or ALK5, which then elicits distinct intracellular signaling cascades with opposing effects on proliferation, differentiation, and migration(Armulik et al., 2011a).
ECs express both ALK5 and ALK1, yet pericytes only express ALK5(Van Geest et al., 2010). In pericytes, ALK5 activation initiates phosphorylation of the Smad 2/3 complex, which subsequently activates a protein complex with Smad 4. The Smad 2–3–4 complex translocates to the nucleus and promotes vessel maturation, inhibits pericyte proliferation, and induces upregulation of contractile protein expression(Van Geest et al., 2010).
Alternatively, ALK1 activation induces phosphorylation of the Smad 1–5–8 complex and, with endoglin, upregulates target genes associated with proliferation and migration of ECs(Goumans et al., 2002). It is hypothesized that the recruitment and proper attachment of pericytes to ECs leads to activation of ALK5 through secreted TGF-β, which then promotes EC differentiation, inhibition of proliferation, and formation/stabilization of the BM and BBB(Van Geest et al., 2010). Knockout of many of these proteins, especially TGFβ1, ALK1, ALK5, TGFβR2, endoglin, and Smad 4, is embryonically lethal due to severe vascular abnormalities including reduced pericyte coverage in the capillaries, defective formation of pericytes and vSMCs, and pericyte detachment(Winkler et al., 2011b)).
TGF-β signaling also enhances proper pericyte attachment to ECs by upregulation of the adhesion molecule, N-cadherin(Winkler et al., 2011b). Knock out of Smad 4 causes several deficits in the brain vasculature concerning EC proliferation and reduced pericyte coverage, which are linked to a reduced expression of N-cadherin(Gerhardt and Betsholtz, 2003). TGF-β-contingent upregulation of N-cadherin is also dependent upon an organized cooperation with Notch signaling through transcriptional elements acting on the N-cadherin promoter(Li et al., 2011). Regulation of vascular N-cadherin involves additional signaling pathways, including sphingosine-1-phosphate 1 (S1P1). S1P1 is a secreted sphingolipid which signals through specific endothelial G protein coupled receptors and initiates cytoskeletal, adhesive, and junctional alterations(Allende and Proia, 2002). Knock out of the S1P1 gene results in faulty pericyte coverage of microvessels(Liu et al., 2000). S1P1 signaling promotes trafficking of N-cadherin to polarized plasma membrane domains on ECs, thus strengthening EC/pericyte contact(Paik et al., 2004).
Ang-1 and -2
Ang-1 is expressed by pericytes and binds to its main receptor, Tie-2, expressed on ECs. The Ang-1/Tie-2 complex signaling as a paracrine loop is necessary for blood vessel stability and maturation(Gaengel et al., 2009). Stability is achieved by inhibiting vascular leakage and up regulating EC-dependent cytokines (PDGF-B and TGF-β)(von Tell et al., 2006) which recruits migrating pericytes to vessels. Ablation of Ang-1 or Tie-2 in mice leads to development of vascular deficits, lack of pericytes, and embryonic lethality(Patan, 1998). Interestingly, animal models using signaling-deficient Tie-2 receptors exhibit no deficits in pericyte recruitment to angiogenic areas, even though there are significant defects in vascular development(Tachibana et al., 2005). Ang-1 KO animals exhibit no evidence for the necessity of the Ang-1 gene in pericyte recruitment(Jeansson et al., 2011). Pericyte secreted Ang-1 also promotes in vivo angiogenesis, EC migration, and actin remodeling(Lamalice et al., 2007).
Unlike Ang-1, Ang-2 is expressed by ECs and acts in an autocrine, antagonistic manner on Tie-2 receptors(Fiedler et al., 2006). In adult animal models, Ang-2 expression is associated mainly with areas of active vascular remodeling and angiogenesis(Maisonpierre et al., 1997). Ang-2 signaling decreases vessel stability and increases endothelial permeability(Armulik et al., 2005). Ang-2 overexpression results in a similar phenotype to Ang-1/Tie-2 deficient animals including a lack of pericyte coverage with defective angiogenesis(Armulik et al., 2005). With knockout of Ang-2, there is an observed normalization of vessel integrity as well as pericyte coverage(Falcon et al., 2009). The opposing effects of Ang-1/Ang-2 signaling on angiogenesis and pericyte recruitment emphasizes the vital role they play in the development and regulation of the vasculature.
Notch
Notch signaling within the vasculature plays distinct roles in angiogenesis, embryonic vascular development, and arterial cell fate(Kume, 2012). In the mammalian vasculature, there are four heterodimeric receptors (Notch 1, 2, 3, and 4) and five ligands (delta-like 1, 3, and 4 and jagged 1 and 2). Direct cell-to-cell contact is important for Notch signaling, as all Notch ligands and receptors are transmembrane proteins. This allows for proper cellular organization into larger structures, as cells are able to regulate protein expression of neighboring cells, known as lateral inhibition, and is a key aspect of Notch signaling. Once there is appropriate ligand-receptor binding, activated Notch receptors go through a series of proteolytic cleavages within the cell membrane to release the Notch intracellular domain which then translocates to the nucleus, binds to a number of transcription factors, and upregulates transcription of Notch-dependent genes (Li et al., 2011; Winkler et al., 2011a).
Notch signaling defects are implicated in several neuropathologies resulting in pericyte dysfunction and faulty attachment to ECs. Notch inhibition is linked to abnormal blood vessel stability and pericyte detachment(Dimova et al., 2013). Notch signaling, in cooperation with TGF-β/Smad4 signaling, regulates pericyte attachment to ECs via N-cadherin(Li et al., 2011). Selective ablation of Notch signaling, like Smad4 ablation, shows deficits in pericyte attachment to ECs leading to perinatal hemorrhage(Li et al., 2011; Winkler et al., 2011a). Notch3 is also implicated as necessary for pericyte recruitment to angiogenic areas, and its transcription is upregulated upon EC/pericyte contact(Liu et al., 2010). Wang et al.(Wang et al., 2014), using Notch3 mutated zebra fish, showed a decrease in pericyte proliferation and recovery using Notch3 treatment. They reported embryonic hemorrhage in the brains of animals with mutated Notch3, but nowhere else in the developing animal. These data indicate an abnormal BBB in Notch3 mutated animals. Notch3 mutant pericytes in mice show an increase in the number of abnormal pericytes in vivo, and morphologically, these cells display severe cellular injury(Gu et al., 2012). Recent evidence demonstrate that newborn Notch3-deficient mice have greatly reduced expression of PDGF-Rβ on pericytes, and PDGF-Rβ expression was amplified with Notch ligand activation(Jin et al., 2008). It was also determined that use of a PDGF-Rβ inhibitor in Notch3-deficient animals increased brain hemorrhage, and that Notch3-driven proliferation of pericytes requires PDGF-Rβ function(Wang et al., 2014). These data suggest an intrinsic, necessary role of Notch signaling in normal pericyte function.
Cell Junctions
The role of gap junctions and TJ is increasingly being recognized in pericyte function and disease pathogenesis. Gap junctions are aggregates of intracellular channels allowing diffusion of molecules, ions, and metabolites to the cytoplasm of the adjoining cell. Gap junction channels that form between two adjacent cells are made up of one or more different forms of connexin (CX) proteins, of which CX-37, CX-40, CX-43 and CX-45 are found predominantly in the vasculature (Figueroa and Duling, 2009). CX-43 and CX-45 have been detected primarily in the microvasculature and in pericytes both during development and postnatal(Cohen-Salmon et al., 2004). During development, CX-45 predominates over CX-43 expression and induces pericyte differentiation(Kruger et al., 2000). Knockout of CX-45 is embryonically lethal due to vascular defects and a leaky barrier, indicative of impaired pericyte coverage(Kruger et al., 2000). Interestingly, vSMC differentiation and development was impaired in arteries of CX-45−/− mice but not in veins, and this impairment cannot be compensated for by introduction of other connexins(Kruger et al., 2000). CX-43 is principally expressed by ECs and mesenchymal cells in the vasculature after birth(Hirschi et al., 2003). CX-43 mediates coupling of mesenchymal cells to ECs and initiates pericyte differentiation via TGF-β signaling(Hirschi et al., 2003). In CX-43−/− mice, pericytes fail to activate TGF-β signaling. These cells can adhere to ECs normally, but are unable to differentiate, thus it was determined that pericyte differentiation is dependent upon EC contact and TGF-β signaling(Hirschi et al., 2003). In CX-43−/− animals, exogenous CX-45 can induce TGF-β signaling, and subsequently initiate pericyte differentiation(Fang et al., 2013).
Although not studied as in-depth as gap junctions, TJ are now known to be regulated by pericytes. Mice with the PDGF-Rβ+/− phenotype display an age-dependent reduction of the TJ proteins, ZO-1, occludin and claudin-5 within the CNS(Bell et al., 2010). There is only speculation as to the cause of the observed reduction in TJ expression, but the primary hypothesis centers on the loss of pericyte coverage and the necessity of pericyte/EC contact for TJ formation.
BBB under inflammatory insult and role of pericytes
NVU and inflammation
Inflammatory cellular responses of the NVU (endothelial cells, pericytes, astrocytes, basal lamina, perivascular macrophages) to pathologic, immune, or ischemic injury involve the production of cytokines, chemokines, reactive oxygen species (ROS) and chemoattractants. These molecules induce alterations in the components of the NVU leading to altered function, leukocyte adhesion, and extravasation into the injured tissue. Under homeostatic conditions, the BBB is largely regulated by brain microvascular endothelial cells (BMVECs) through a continuous expression of TJ, AJ, and junctional adhesion molecules(Persidsky et al., 2006). BMVECs are not fenestrated and utilize specialized transport mechanisms for conveyance of metabolic molecules and nutrients into the CNS(Persidsky et al., 2006). The interaction of BMVECs with astrocytes, and the astrocytic end-foot projections which connect with greater than 99% of the basement membrane of the NVU, is crucial for proper BBB function(Persidsky et al., 2006). The close proximity of astrocytes to neurons and BMVECs allows for proper molecular communication and regulation of metabolites between the CNS and blood flow. There is contradictory evidence concerning BMVEC-astrocyte interactions and regulation of BMVEC TJ expression; such studies reveal that BMVEC-astrocyte co-culture upregulates TJ expression or demonstrate a lack of need for astrocytic end-foot processes contacting capillary beds for TJ formation(Zlokovic, 2008; Bell et al., 2010; Alvarez et al., 2011). Yet, astrocytic secretion of TGF-β and fibroblast growth factor to BMVECs and pericytes in culture greatly increases barrier function compared to ECs cultured alone(Dohgu et al., 2005). Ultimately, pericytes contribute to BBB function through reducing BMVEC vesicular transport and increasing BMVEC TJ expression(Armulik et al., 2010; Daneman et al., 2010).
Numerous neuroinflammatory conditions (neurodegenerative disorders, ischemia, multiple sclerosis [MS]) are characterized by impaired BBB function and will be discussed in depth in a later section. Upon inflammatory insult with cytokines (TNFα, IL-1β) or viral proteins (HIV-1), BMVEC TJ protein expression and function are altered(Persidsky et al., 2006). Changes in TJ function, along with an increased expression of E- and P-selectin, ICAM-1, and VCAM-1, allow for adhesion of rolling leukocytes to the injured area of vasculature and extravasation into the neuropil(Persidsky et al., 2006). Once past the initial barrier, activated leukocytes and macrophages must pass the astrocytic end-foot processes using a chemokine/cytokine gradient as a guide(Persidsky et al., 2006), and subsequently interact with local astrocytes and increase astrocytic chemokine/cytokine production. Activation of resident microglia and macrophages also exacerbate the inflammatory response and BBB dysfunction(Nishioku et al., 2010).
Kynurenine Pathway in the NVU
Especially important within the NVU during inflammation is activation and production of key elements of the kynurenine pathway (KP). Activation of the KP, a key pathway of L-tryptophan catabolism, occurs primarily with interferon-γ (INF-γ) yet numerous other inflammatory mediators such as lipopolysaccharide (LPS), IL-1β, TNFα, and the HIV-1 regulatory proteins tat and nef can induce activation to a much lesser extent(Takikawa et al., 1988; Hu et al., 1995; Takikawa et al., 1999; Smith et al., 2001). Activation of this pathway plays a critical role in immune tolerance at the BBB and is becoming increasingly better understood. The KP has three major metabolites (kynurenine [KYN], kynurenic acid [KA], and quinolinic acid[QUIN]) which are tightly regulated by a series of enzymatic steps. The first and rate-limiting enzyme in the KP cascade is indoleamine 2,3-dioxygenase (IDO). IDO responds to immunological signals (INFγ, LPS, TNFα) and is expressed by most human brain cells(Guillemin et al., 2005; Ball et al., 2007; Owe-Young et al., 2008). Human BBB ECs and pericytes do not constitutively express IDO yet IDO expression is induced in these cells by INFγ treatment(Owe-Young et al., 2008). The same study by Owe-Young et al. (Owe-Young et al., 2008) determined that both human ECs and pericytes of the NVU do not express kynurenine 3-monooxygenase (KMO). Lack of KMO allows for production of KA and KYN but not QUIN. Even though pericytes do not express KMO, there is constitutive expression of picolinic acid (PIC), a downstream KP metabolite, which is significantly increased upon treatment with INFγ+TNFα(Owe-Young et al., 2008). PIC is known to block the neurotoxic effects of QUIN, in which the constitutive and increased expression by pericytes under inflammatory insult will contribute to brain homeostasis(Beninger et al., 1994; Owe-Young et al., 2008). Patients with amyotrophic lateral sclerosis (ALS) show a disturbed KP in which CSF and serum levels of QUIN are increased and PIC levels are decreased(Chen et al., 2010). This shift in the KP results in greater neurotoxicity as QUIN is a potent excitotoxin(Guillemin et al., 2007). Activation of the KP through increased IDO expression may also be a factor in immune modulation through depletion of L-tryptophan in the microenvironment. This depletion has potent antiparasitic, antiviral, antibacterial, and antifungal activities(Kwidzinski and Bechmann, 2007). L-tryptophan depletion by IDO induction also results in decreased T-cell proliferation and increased apoptotic susceptibility thus suppressing the response to antigen presentation(Munn et al., 1999; Fallarino et al., 2002; Lee et al., 2002). Furthermore, inhibition of IDO in a HIV-1 encephalitis (HIVE) mouse model results in significant elimination of HIV-infected macrophages in the brain demonstrating a robust immunosuppressive function of IDO in HIV infection(Potula et al., 2005). These data suggest a need for determining the role of the KP in the NVU and its function in neuroinflammatory disease pathology.
Pericytes as a secondary checkpoint in leukocyte CNS infiltration
Although much is known about how leukocytes traverse the EC layer, little information is available on how migrating leukocytes navigate the pericyte sheath surrounding BMVECs. Pericytes in physiologic conditions wrap around ECs, with TJ and gap junction connections between the two(Bell et al., 2010; Daneman et al., 2010; Zlokovic, 2011). Upon exposure to secreted molecules (cytokines/chemokines, neurotransmitters, and neurohormones) from components of the NVU, pericytes show a detached, migratory phenotype with a decrease in TJ/gap junction expression(Dore-Duffy and Cleary, 2011). This change in morphology may be due to a change in pericyte function and their ability to act as a second barrier to infiltrating leukocytes. Evidence suggesting this second barrier comes from the observation that migrating leukocytes accumulate between the EC and pericyte/BM layers(Yadav et al., 2003). Wang et al.(Wang et al., 2012) established that pericyte engagement with activated neutrophils induces relaxed pericyte morphology similar to the migratory morphology seen by direct inflammatory insult. In vivo studies demonstrate that, in response to inflammatory insult with TNFα, IL-1β, or LPS, migrating neutrophils access the parenchyma through the pericyte layer via gaps between adjacent pericytes(Engelhardt and Sorokin, 2009). Also noted was that leukocytes crawling along pericyte processes is prerequisite to leukocyte migration through the pericyte layer, and breach of this layer by neutrophils is through preferentially selected gaps between pericytes in which numerous leukocytes exit the venular wall at the same time(Proebstl et al., 2012). Additional studies are needed to fully understand the complexities related to leukocyte migration through the pericyte layer and basement membrane.
Pericyte role in leukocyte adhesion/retention in the perivascular space
Pericytes constitutively express low levels of the adhesion molecules ICAM-1 and VCAM-1 during normal conditions. Upon activation with inflammatory mediators, ICAM-1 and VCAM-1 expression is upregulated and active binding to infiltrating leukocytes can be initiated(Guillemin and Brew, 2004; Proebstl et al., 2012). This upregulation is seen simultaneously with a downregulation of TJ and gap junction proteins, emphasizing a change from a stabilizing cell into a cell that guides an immunological response(Nakagawa et al., 2012). Neutrophil transmigration though the pericyte layer has been determined to be dependent on ICAM-1 and its leukocyte integrin ligands(Proebstl et al., 2012). Pericytes alone do not bind neutrophils as robustly as ECs, but when cultured together in vitro, the level of pericyte-leukocyte adherence is upregulated significantly(Ayres-Sander et al., 2013). The same study also noted that inflammatory cytokine-dependent upregulation of ICAM-1 increased migrating neutrophil adhesion to pericytes, but was not enough to increase transmigration through the pericyte layer. The current evidence points to a guidance role of pericytes in leukocyte infiltration into the parenchyma instead of a solely adhesive and retention role. Further investigation is necessary to determine whether or not leukocyte retention between the endothelial layer and parenchyma is pericyte-dependent, BM-dependent, or due to a complex interplay between all components of the NVU.
BM modifications
As pericytes are embedded within the endothelial BM, it is necessary to understand their interaction and possible remodeling of the BM during inflammation. The endothelial BM in the CNS is composed of laminins, type IV collagen, heparin sulfate proteoglycan (perlecan predominates in the endothelial BM), and nidogens which are arranged as tightly interwoven protein sheets(Engelhardt and Sorokin, 2009).
Laminin isoforms are comprised of a heterotrimeric combination of an a chain, a β chain, and a γ chain in which laminin α4 and α5 chains combined with β1 and γ1 chains dominate vessels in the CNS(Rowe and Weiss, 2008). Type IV collagen is made up of a heterotrimeric combination of six different isoforms: α1α1α2, α3α4α5, and α5α5α6 being the only combinations of the isoforms found in vivo(Rowe and Weiss, 2008). This tightly packed assembly may act as a more of a barrier to infiltrating leukocytes than the pericyte layer does, but communication between the two layers may be necessary for proper extravasation into the neuropil. Pericytes secrete laminin, perlecan, and nidogen-1 in vitro and induce ECs to secrete BM components as well(Brachvogel et al., 2007). Infiltrating leukocytes preferentially penetrate the BM where laminins and type IV collagen are sparsely distributed(Wang et al., 2006).These areas are referred to as low expression regions (LERs). Acute inflammatory insult with IL-1β induces enlarged gaps between pericytes and these areas were positively correlated with observed larger LERs(Wang et al., 2012). The same study notes that direct neutrophil contact with pericytes mediates enlargement of pericyte-pericyte gaps as well as LER increase. These findings suggest that pericytes have an active role in BM remodeling during acute inflammation even though the specific molecular mechanism has yet to be elucidated.
Immune responses
Not only do pericytes actively bind and aid in leukocyte transmigration, these multifunction cells actively promote the inflammatory response within the CNS. Insult to pericytes with inflammatory stimuli such as HIV-1, LPS, and human cytomegalovirus stimulate pericytic release of inflammatory mediators including IL-1β, IL-6, TNFα, ROS, NO, LRP-1, and matrix metalloproteinases (MMP-2 and MMP-9)(Nakagawa et al., 2012). All of these secreted inflammatory components contribute to BBB dysfunction either by autocrine signaling on pericytes (disruption of gap junctions) or through direct action on other aspects of the NVU (BMVEC and TJ loss, BM destruction via MMP-2 and MMP-9).
Pericytes can also contribute to the inflammatory response through antigen presentation and expression of the complement receptors C5a and C3a when activated by infection (bacterial meningitis)(Gasque et al., 1998). The upregulation of these receptors, as well as antigen presentation in itself, recruits macrophages to the site of infection and causes cell activation, further intensifying the immune response.
Further confounding the role of pericytes in the immune response is the observation that pericytes migrate into the perivascular space and become indistinguishable from resident macrophages and activated microglia(Guillemin and Brew, 2004). This may be due to pericytes having macrophage-like phagocytic properties and use these to clear toxic cellular byproducts from the microcirculation(Bell et al., 2010; Winkler et al., 2011a). The phagocytic action of pericytes seems to be tightly regulated such that increased phagocytosis of latex beads was only upregulated by treatment with TNFα and INFγ(Pieper et al., 2014). Simultaneously, major histocompatibility complex class II (MHC II) and CD68, markers for antigen presentation and macrophages respectively, mRNA is upregulated significantly but only with INFγ stimulation(Pieper et al., 2014). These data indicate that pericytes supply inflammatory signals for both the innate and adaptive immune responses, and that these signals are dependent on the type of pathogenic stimulus. Even with recent advances in understanding the mechanisms behind BBB dysfunction, the role pericytes play in this immune response is still greatly understudied.
Pericyte dysfunction under pathologic conditions
The role pericytes play under normal conditions within the CNS microvasculature is well documented, but less known is about the function of pericytes during inflammatory insult and their influence on BBB permeability. Much of the literature focuses on pericytic regulation of capillary blood flow through their contractile function during inflammation. Recent studies point out that the contraction of pericytes is regulated by intracellular Ca2+ concentration(Hamilton et al., 2010) affected by ROS, formation of mega channels, and peroxynitrite(Yemisci et al., 2009). Through all of these mediators, distinct changes in pericyte contractility and capillary blood flow have been found, yet little has been investigated concerning pericyte function on BBB tightness and penetration of inflammatory mediators into the CNS. Below we will focus on a few disease states common to the CNS and what is known about pericyte dysfunction in their pathogenesis.
Neurodegeneration and Alzheimer’s disease (AD)
Neurodegenerative disorders, especially AD, are associated with disruptions within the BBB and NVU as well as microvascular dysfunction(Zlokovic, 2011). AD is characterized by extracellular accumulation of amyloid-β (Aβ) and tau pathology in neurons(Zlokovic, 2011). Pericytes have only recently been studied for their pivotal role in BBB function during AD. During neurodegeneration, there is an increase in pro-inflammatory cytokines, especially IL-1β, IL-6, and TNFα(Zlokovic, 2011), and systemic inflammation correlating with impairment of low-density lipoprotein receptor-related protein-1 (LRP-1), which has been characterized as an efflux transporter of Aβ(Jaeger et al., 2009). In AD, Aβ accumulates around pericytes, especially in capillary beds causing pericyte death and detachment from endothelial cells in vitro and is seen in post-mortem tissue from patients with AD(Ozen et al., 2012). Pericyte number and coverage are decreased in AD patients; this reduction correlates with increases in both fibrin (a sign of increased BBB permeability) and Aβ accumulation(Sengillo et al., 2013). A recent study using a transgenic mouse line with overexpression of both human Aβ and PDGF-Rβ+/− demonstrated increased Aβ pathology with pericyte loss, and that pericyte loss also triggered tau pathology and early neuronal loss(Sagare et al., 2013). It has been hypothesized(Winkler et al., 2011b) that defects in LRP-1, and subsequent diminished clearing of Aβ from the CNS, contribute to the progression of AD pathology. It is unclear whether pericytes play a major role in LRP-1-associated Aβ clearing, but diminished pericyte function is known to increase BBB permeability and influx of inflammatory mediators leading to neurodegeneration.
Stroke
Ischemic stroke within the CNS results from vascular blockage, which halts cerebral blood flow and induces local hypoxia. Reperfusion of oxygenated blood flow to the immediate area is paramount to attenuate neuronal damage following ischemia. However, reperfusion has been found to have its own challenges. CNS microvascular pericytes contract as soon as one hour after artery blockage and remain contracted even after blockage of the vessel is removed(Yemisci et al., 2009). Pericyte contraction during ischemia was determined to result from oxidative and nitrosative stress and that this stress is the primary cause of BBB breakdown during reperfusion(Yemisci et al., 2009). Pericyte contraction is diminished when oxidative and nitric stress mechanisms are inhibited, and improved microvascular flow promotes cerebral tissue survival, yet positive effects are miniscule in comparison to total blockage of microvascular blood flow(Yemisci et al., 2009). It was recently revealed that constricted pericytes readily die after cerebral ischemia(Hall et al., 2014). This death is not reduced by free radical scavenging and contributes to ongoing neuronal damage that would explain why treatment after ischemic stress with oxidative and nitric stress inhibitors is only partially successful(Hall et al., 2014). Increases in oxidative and nitrosative stress during acute ischemia also disrupt the BBB through numerous other mechanisms including activation of MMPs, upregulation of inflammatory cytokines, and disruption of TJ(Pun et al., 2009). After the immediate damage, the BBB becomes leaky again, leading to neutrophil infiltration and continuation of the inflammatory response(Pillai et al., 2009). Understanding this biphasic damage mechanism will undoubtedly prove key in preventing microcirculatory failure and developing anti-inflammatory treatments for acute ischemia.
MS
MS is an inflammatory disease in which immune cells attack the myelin sheath that surrounds axons within the CNS(Zlokovic, 2011). MS is considered an autoimmune disease due to its inflammatory autoimmune responses and breach of the immunological privilege of the CNS. This hypothesis indicates reactivity of T cell receptors on CD4+ T lymphocytes with myelin antigens presented by macrophages, microglia, or astrocytes leading to acute focal inflammatory demyelination and oligodendrocyte death(Hartung et al., 2014). MS is associated with chronic BBB abnormalities resulting in chronic inflammation and neurodegeneration. The persistent BBB abnormalities seen in MS are: decrease in TJ protein expression (occludin, ZO1, and claudin-5), BM abnormalities, and ECM modifications(Kirk et al., 2003). These alterations are present in chronic active and inactive lesions, and in MS normal-appearing white matter, indicating BBB dysfunction(Kirk et al., 2003). Transplantation of mesenchymal stem cells (MSCs) has been tested as a possible treatment option for experimental autoimmune encephalomyelitis, a murine model of MS. It is hypothesized that MSCs are closely related to pericytes, and their normal function is to maintain angiogenic properties and immunologic surveillance(da Silva Meirelles et al., 2008). This treatment is proposed due to the ability of MSCs to inhibit both innate and adaptive immunity with immunosuppressive effects on T-cells, B-cells, natural killer cells, and antigen-presenting cells(Darlington et al., 2010). These data point to a significant role of pericyte dysfunction in MS and the chronic infiltration of activated leukocytes through the BBB. Further studies are needed to elucidate the true function of pericytes in MS.
Diabetic retinopathy (DR)
DR is a common and debilitating complication of diabetes affecting nearly all type 1 diabetes patients and 80% of type 2 diabetes patients who have had the disease for longer than 20 years(Wohlfart et al., 2014). DR is characterized by two distinct phases of pericyte loss: nonproliferative and proliferative retinopathy(Hammes et al., 2011). The nonproliferative phase of DR includes pericyte apoptosis, BM thickening, altered blood flow in the retinal capillaries, microaneurysms of the vessels, and vasoregression(Armulik et al., 2011b; Winkler et al., 2011a). Proliferative DR shows enhanced proliferation of abnormal microvessels, which are prone to rupture, leading to retinal detachment(Armulik et al., 2011b; Winkler et al., 2011a). The cause of the switch from nonproliferative to proliferative DR is still unknown, but Enge et al.(Enge et al., 2002) noted that when pericyte coverage of ECs with ablated PDGF-B fell below 50%, enhanced proliferation of ECs was seen suggesting a control mechanism of pericytes on EC proliferation. Vascular changes of both proliferative and nonproliferative DR can be induced both by ablation of PDGF-B or overexpression of Ang-2(Pfister et al., 2010).
Inflammation may be an important disease mechanism in DR. Inflammatory cells play a role in retinal vessels early in disease pathology due to an increase in expression of ICAM-1 by ECs(Noda et al., 2014). This may not be the case though, as there is evidence for both increased and unaltered levels of ICAM-1 expression in animal models of DR(Hughes and Chan-Ling, 2004). An upregulation of the ICAM-1 gene was shown in a rat model of type 2 diabetes resulting in DR(Wohlfart et al., 2014). However, another recent study describes unaltered levels of ICAM-1 during the early phase of DR and decreased expression in the later stages(Noda et al., 2014). This decrease in expression is thought to be due to endothelial dysfunction. It is still unknown if an inflammatory response element facilitates pericyte dysfunction and apoptosis during DR.
HIV-1 infection
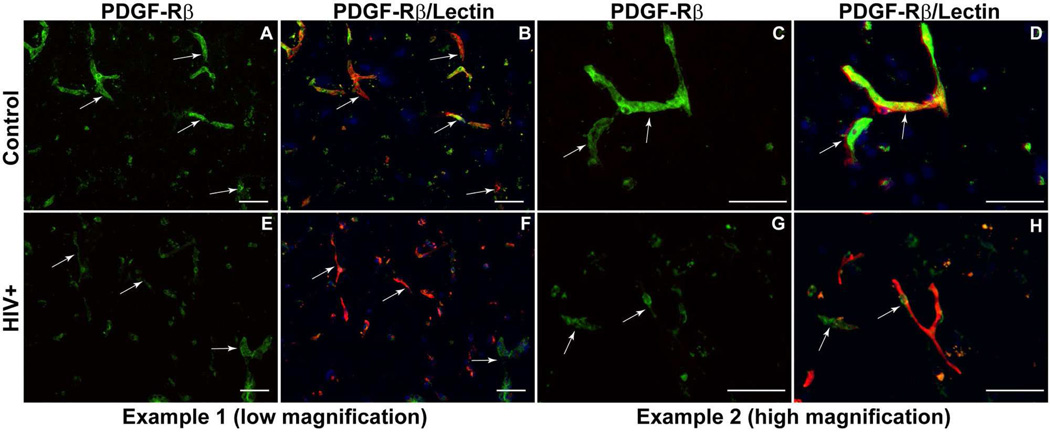
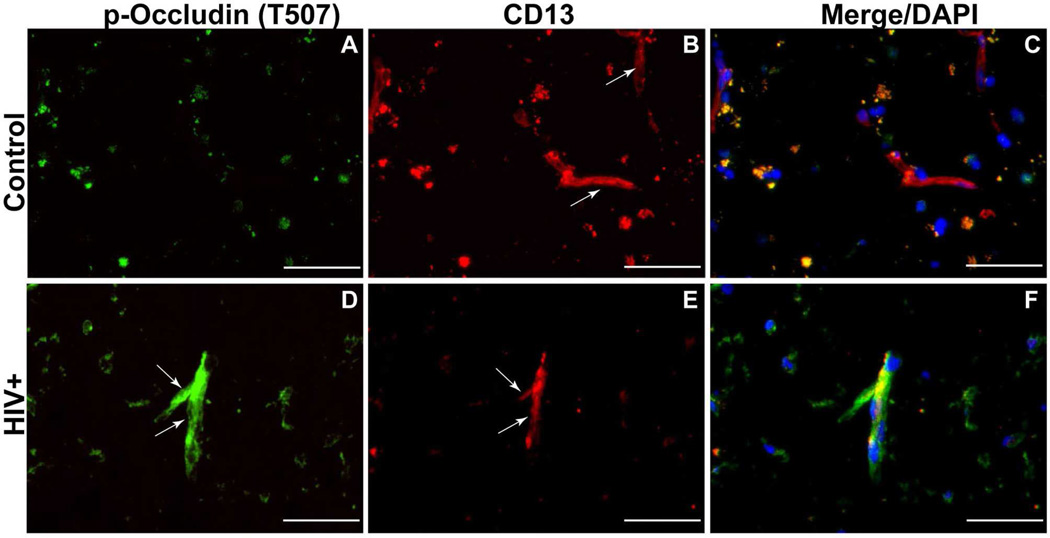
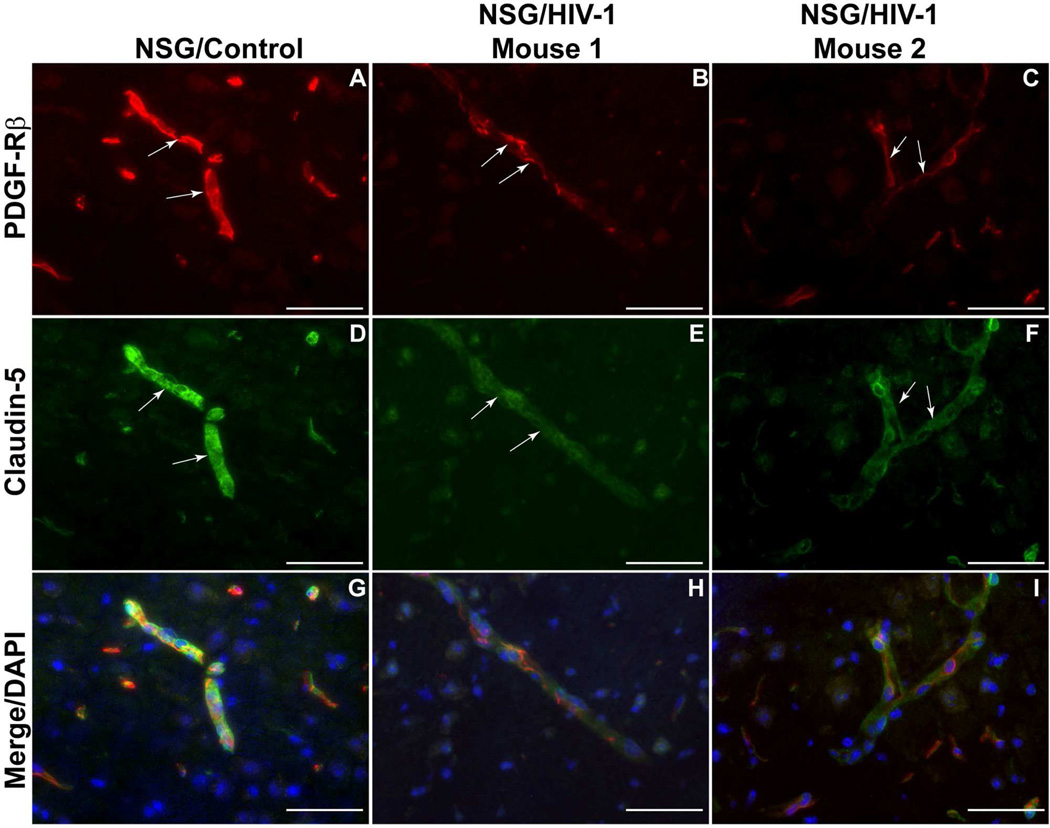
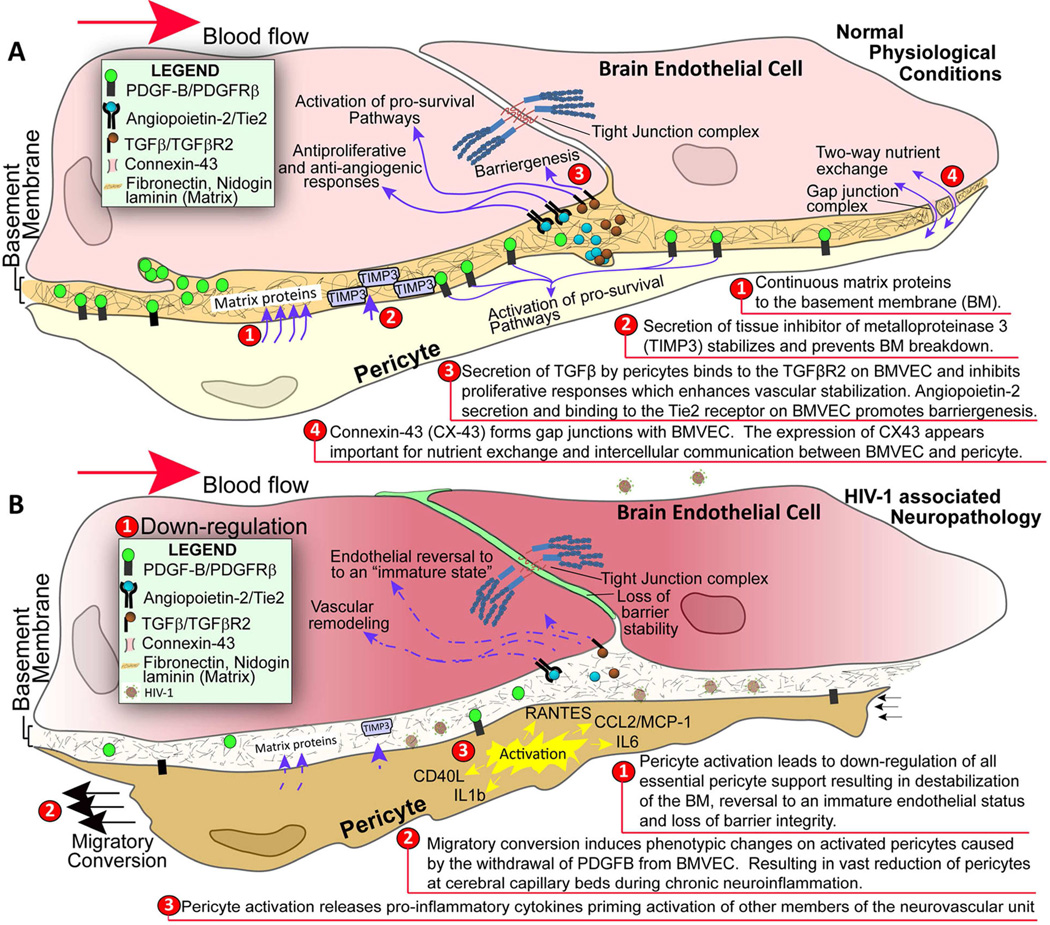
As mentioned previously, HIV-1 infection is associated with impaired BBB stability and alteration of TJ(Persidsky et al., 2006). This injury, along with chronic inflammatory responses and low-level monocyte/lymphocyte migration across the BBB, is linked to prevalence of neurocognitive impairment among patients with HIV-1 infection(Kusao et al., 2012). BMVEC injury and its underlying mechanisms have been explored during the last few years; however, changes in other cells constituting the BBB (like pericytes) have not been studied in detail in the context of HIV-1 infection and related neurocognitive deficits. Recent studies have established that HIV-1 can replicate at low levels in pericytes, and that infected pericytes produce IL-6 and disrupt endothelial monolayers(Nakagawa et al., 2012). Treatment of BMVEC/pericyte co-cultures with LPS shows increased penetration of cell-free HIV-1 as compared to BMVECs cultured alone(Dohgu and Banks, 2013). Enhanced penetration is presumed to occur through LPS acting on the luminal surface of BMVECs, which stimulates release of soluble factors from the abluminal surface modulating pericytic release of chemokines(Dohgu and Banks, 2013). Unpublished results from our lab show a decrease of pericyte coverage of BBB (Fig. 1) as well as modifications of TJ proteins (associated with increased BBB permealility) both in HIV-1 infected patients (even without evidence of encephalitis) (Fig. 2) and in an animal model of chronic HIV-1 infection in humanized NSG mice (Fig. 3). These analyses indicate that chronic BBB impairment occurs in HIV-1 infection. Future studies are needed for better understanding of functions of pericyte subsets in BBB biology and leukocyte migration during neuroinflammation associated with HIV-1 infection and associated neurocognitive decline. Figure 4 summarizes the important role of pericytes in BBB maintenance under physiologic conditions (A) and HIV-1 infection/neuroinflammation (B).
Figure 1.
Decrease of pericyte coverage of the BBB in HIV-1 infected patients. (A,C) Strong contiguous staining for PDGF-Rβ (green) was found in control brains corresponding to the endothelial lining (Ulex europeus, red) (B,D). (E,G) In HIV cases, there was noticeable diminution in PDGF-Rβ labeling of pericytes (green), confirmed by double staining for endothelial cells (Ulex europeus, red) (F,H). Arrows indicate the same microvessels in double-stained PDGF-Rβ (green)/Ulex (red) panels: A/B, C/D, E/F, G/H. Original magnification: A,B,E,F ×200; C,D,G,H ×400. Scale bar =50 mm
Figure 2.
Altered tight junctions (TJ) and diminished pericyte coverage in HIV-1-infected patients. (A) There was no p-occludin staining (green) detecting compromised TJ in microvessels of control brain tissue, with strong CD13 (pericyte marker, red, B). (D) Microvessels in HIV-1 brains (even without HIVE) featured p-occludin (green) and diminution of CD13 (red, E). Panels C and F are overlays of A/B and D/E. Original magnification: A-F ×400. Scale bar =50 mm
Figure 3.
Decreased claudin-5 staining and diminished pericyte coverage in HIV-infected ‘humanized’ NSG mice. NOD.Cg-Prkdcscid Il2rgtm1Wjl /SzJ, huNSG mice (reconstituted with CD34+ cells at birth) were infected with HIV-1 demonstrating sustained viremia (350,000 copies/mL, over 3 months period). (A) Contiguous strong staining for PDGF-Rβ (red) was found in pericytes in control mice without HIV-1 infection (A,G) and paralleling uniform expression of claudin-5 (green, D,G). There was obvious down-regulation or even disappearance of PDGF-Rβ (red) in HIV-infected animals (B,C,H,I) and uneven staining for claudin 5 (green) in the same microvessels (E,F,H,I). Panel G is the overlay for A/D, H for panels B/E, and I for panels C/F. Original magnification: A-I ×400. Scale bar =50 mm. Arrows indicate the same microvessels in double-stained panels
Figure 4.
Pericyte-brain endothelial cell interactions under physiologic conditions (A) and HIV-1 infection/chronic inflammation (B)
Future directions
Information presented in this review indicates the importance of the involvement of pericytes in physiologic maintenance and pathologic changes of the BBB in diverse neurologic conditions. Several areas garnering future investigation include covering basic mechanisms of pericyte participation in barrier function, BBB formation (barrier genesis) and its functional support, regulation of blood flow, and inflammatory responses (leukocyte migration/perivascular retention, effects of pro-inflammatory factors altering pericyte functions). Rapidly expanding studies in these areas will allow better understanding of how optimal functioning of these unique perivascular cells can protect the BBB and prevent barrier injury via targeted interventions in pericytes.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants NS086570 (SHR), MH65151 and AA015913 (YP), DA013429 (SHR, YP), and The Shriners Hospital for Children grant 85110-PHI-14 (SHR).
The authors express their grateful acknowledgement for proofreading and editing to Nancy L. Reichenbach.
Footnotes
Conflict of Interest
The authors of this manuscript declare that there are no actual or potential conflicts of interest
References
- Abramsson A, Kurup S, Busse M, Yamada S, Lindblom P, Schallmeiner E, Stenzel D, Sauvaget D, Ledin J, Ringvall M, Landegren U, Kjellen L, Bondjers G, Li JP, Lindahl U, Spillmann D, Betsholtz C, Gerhardt H. Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes & development. 2007;21:316–331. doi: 10.1101/gad.398207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochimica et biophysica acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochimica et biophysica acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Mae M, Betsholtz C. Pericytes and the blood-brain barrier: recent advances and implications for the delivery of CNS therapy. Therapeutic delivery. 2011a;2:419–422. doi: 10.4155/tde.11.23. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011b;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Ayres-Sander CE, Lauridsen H, Maier CL, Sava P, Pober JS, Gonzalez AL. Transendothelial migration enables subsequent transmigration of neutrophils through underlying pericytes. PloS one. 2013;8:e60025. doi: 10.1371/journal.pone.0060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. Journal of neurocytology. 2001;30:35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, Colton AM, Ingles JL, Jhamandas K, Boegman RJ. Picolinic acid blocks the neurotoxic but not the neuroexcitant properties of quinolinic acid in the rat brain: evidence from turning behaviour and tyrosine hydroxylase immunohistochemistry. Neuroscience. 1994;61:603–612. doi: 10.1016/0306-4522(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids and barriers of the CNS. 2011;8:8. doi: 10.1186/2045-8118-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachvogel B, Pausch F, Farlie P, Gaipl U, Etich J, Zhou Z, Cameron T, von der Mark K, Bateman JF, Poschl E. Isolated Anxa5+/Sca-1+ perivascular cells from mouse meningeal vasculature retain their perivascular phenotype in vitro and in vivo. Experimental cell research. 2007;313:2730–2743. doi: 10.1016/j.yexcr.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stankovic R, Cullen KM, Meininger V, Garner B, Coggan S, Grant R, Brew BJ, Guillemin GJ. The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotoxicity research. 2010;18:132–142. doi: 10.1007/s12640-009-9129-7. [DOI] [PubMed] [Google Scholar]
- Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney international. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Maxeiner S, Kruger O, Theis M, Willecke K, Petit C. Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear. Cell and tissue research. 2004;316:15–22. doi: 10.1007/s00441-004-0861-2. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem cells (Dayton, Ohio) 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta neuropathologica. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington PJ, Boivin MN, Renoux C, Francois M, Galipeau J, Freedman MS, Atkins HL, Cohen JA, Solchaga L, Bar-Or A. Reciprocal Th1 and Th17 regulation by mesenchymal stem cells: Implication for multiple sclerosis. Annals of neurology. 2010;68:540–545. doi: 10.1002/ana.22065. [DOI] [PubMed] [Google Scholar]
- DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circulation research. 1997;80:444–451. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histology and histopathology. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- Dimova I, Hlushchuk R, Makanya A, Styp-Rekowska B, Ceausu A, Flueckiger S, Lang S, Semela D, Le Noble F, Chatterjee S, Djonov V. Inhibition of Notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis. 2013;16:921–937. doi: 10.1007/s10456-013-9366-5. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Banks WA. Brain pericytes increase the lipopolysaccharide-enhanced transcytosis of HIV-1 free virus across the in vitro blood-brain barrier: evidence for cytokine-mediated pericyte-endothelial cell crosstalk. Fluids and barriers of the CNS. 2013;10:23. doi: 10.1186/2045-8118-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain research. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Current pharmaceutical design. 2008;14:1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods in molecular biology (Clifton, NJ) 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- Eberth CJ. Handbuch der Lehre von der Gewegen des Menschen und der Tiere. Leipzig. 1871 [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. The EMBO journal. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Seminars in immunopathology. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, Oliner JD, McDonald DM. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. The American journal of pathology. 2009;175:2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell death and differentiation. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- Fang JS, Dai C, Kurjiaka DT, Burt JM, Hirschi KK. Connexin45 regulates endothelial-induced mesenchymal cell differentiation toward a mural cell phenotype. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:362–368. doi: 10.1161/ATVBAHA.112.255950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nature medicine. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxidants & redox signaling. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. Journal of immunology (Baltimore, Md : 1950) 1998;160:3543–3554. [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell and tissue research. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. The EMBO journal. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Liu XY, Fagan A, Gonzalez-Toledo ME, Zhao LR. Ultrastructural changes in cerebral capillary pericytes in aged Notch3 mutant transgenic mice. Ultrastructural pathology. 2012;36:48–55. doi: 10.3109/01913123.2011.620220. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. Journal of leukocyte biology. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ. Characterization of the kynurenine pathway in human neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Frontiers in neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung HP, Aktas O, Menge T, Kieseier BC. Immune regulation of multiple sclerosis. Handbook of clinical neurology. 2014;122:3–14. doi: 10.1016/B978-0-444-52001-2.00001-7. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. The Journal of cell biology. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circulation research. 2003;93:429–437. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- Hosaka K, Yang Y, Seki T, Nakamura M, Andersson P, Rouhi P, Yang X, Jensen L, Lim S, Feng N, Xue Y, Li X, Larsson O, Ohhashi T, Cao Y. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nature communications. 2013;4:2129. doi: 10.1038/ncomms3129. [DOI] [PubMed] [Google Scholar]
- Hu B, Hissong BD, Carlin JM. Interleukin-1 enhances indoleamine 2,3-dioxygenase activity by increasing specific mRNA expression in human mononuclear phagocytes. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1995;15:617–624. doi: 10.1089/jir.1995.15.617. [DOI] [PubMed] [Google Scholar]
- Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Investigative ophthalmology & visual science. 2004;45:2795–2806. doi: 10.1167/iovs.03-1312. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfield DA, Banks WA. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain, behavior, and immunity. 2009;23:507–517. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. The Journal of clinical investigation. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F, Baumann M, Kalimo H, Lendahl U. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circulation research. 2008;102:1483–1491. doi: 10.1161/CIRCRESAHA.107.167965. [DOI] [PubMed] [Google Scholar]
- Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. The Journal of pathology. 2003;201:319–327. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development (Cambridge, England) 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- Kume T. Ligand-dependent Notch signaling in vascular formation. Advances in experimental medicine and biology. 2012;727:210–222. doi: 10.1007/978-1-4614-0899-4_16. [DOI] [PubMed] [Google Scholar]
- Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, Velasco VN, Marshall A, Whitenack N, Shikuma C, Valcour V. Cognitive performance related to HIV-1-infected monocytes. The Journal of neuropsychiatry and clinical neurosciences. 2012;24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwidzinski E, Bechmann I. IDO expression in the brain: a double-edged sword. Journal of molecular medicine (Berlin, Germany) 2007;85:1351–1359. doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circulation research. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Developmental cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science (New York, NY) 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B. Notch3 is critical for proper angiogenesis and mural cell investment. Circulation research. 2010;107:860–870. doi: 10.1161/CIRCRESAHA.110.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. The Journal of clinical investigation. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science (New York, NY) 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- McCullough PA, Olobatoke A, Vanhecke TE. Galectin-3: a novel blood test for the evaluation and management of patients with heart failure. Reviews in cardiovascular medicine. 2011;12:200–210. doi: 10.3909/ricm0624. [DOI] [PubMed] [Google Scholar]
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. The Journal of experimental medicine. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Castro V, Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. Journal of cellular and molecular medicine. 2012;16:2950–2957. doi: 10.1111/j.1582-4934.2012.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M, Weyrich P, Juchem G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. American journal of physiology Heart and circulatory physiology. 2012;302:H69–H84. doi: 10.1152/ajpheart.00359.2011. [DOI] [PubMed] [Google Scholar]
- Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. Journal of pharmacological sciences. 2010;112:251–254. doi: 10.1254/jphs.09292sc. [DOI] [PubMed] [Google Scholar]
- Noda K, Nakao S, Zandi S, Sun D, Hayes KC, Hafezi-Moghadam A. Retinopathy in a novel model of metabolic syndrome and type 2 diabetes: new insight on the inflammatory paradigm. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.12-215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. The Journal of cell biology. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owe-Young R, Webster NL, Mukhtar M, Pomerantz RJ, Smythe G, Walker D, Armati PJ, Crowe SM, Brew BJ. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance and neurotoxicity. Journal of neurochemistry. 2008;105:1346–1357. doi: 10.1111/j.1471-4159.2008.05241.x. [DOI] [PubMed] [Google Scholar]
- Ozen I, Boix J, Paul G. Perivascular mesenchymal stem cells in the adult human brain: a future target for neuroregeneration? Clinical and translational medicine. 2012;1:30. doi: 10.1186/2001-1326-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes & development. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patan S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvascular research. 1998;56:1–21. doi: 10.1006/mvre.1998.2081. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Age-related changes in the morphology of cerebral capillaries do not correlate with cognitive decline. The Journal of comparative neurology. 2012;520:1339–1347. doi: 10.1002/cne.22809. [DOI] [PubMed] [Google Scholar]
- Pfister F, Wang Y, Schreiter K, vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes HP, Feng Y. Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta diabetologica. 2010;47:59–64. doi: 10.1007/s00592-009-0099-2. [DOI] [PubMed] [Google Scholar]
- Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain research. 2014;1550:1–8. doi: 10.1016/j.brainres.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schuierer G, Bogdahn U, Schlachetzki F. Cerebral ischemia-reperfusion injury in rats--a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:1846–1855. doi: 10.1038/jcbfm.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquer-Gil M, Garcia-Verdugo JM, Zipancic I, Sanchez MJ, Alvarez-Dolado M. Cell fusion contributes to pericyte formation after stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:480–485. doi: 10.1038/jcbfm.2008.150. [DOI] [PubMed] [Google Scholar]
- Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE, Persidsky Y. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood. 2005;106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. The Journal of experimental medicine. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free radical research. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Friman T, Kowanetz M, van Wieringen T, Gustafsson R, Sundberg C. Phenotypical differences in connective tissue cells emerging from microvascular pericytes in response to overexpression of PDGF-B and TGF-beta1 in normal skin in vivo. The American journal of pathology. 2013;182:2132–2146. doi: 10.1016/j.ajpath.2013.01.054. [DOI] [PubMed] [Google Scholar]
- Rouget C. Memoire sur le developpement, la structures et les proprietes des capillaires sanguins et lymphatiques. Archs Physiol Norm Pathol. 1873;5:603–633. [Google Scholar]
- Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends in cell biology. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Molecular neurobiology. 2012;45:327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nature communications. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schlondorff D, Banas B. The mesangial cell revisited: no cell is an island. Journal of the American Society of Nephrology : JASN. 2009;20:1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. Journal of the American Society of Nephrology : JASN. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain pathology (Zurich, Switzerland) 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Guillemin GJ, Pemberton L, Kerr S, Nath A, Smythe GA, Brew BJ. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. Journal of neurovirology. 2001;7:56–60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- Smith SW, Eardley KS, Croft AP, Nwosu J, Howie AJ, Cockwell P, Isacke CM, Buckley CD, Savage CO. CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney international. 2011;80:199–207. doi: 10.1038/ki.2011.103. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes & development. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Stefanska A, Peault B, Mullins JJ. Renal pericytes: multifunctional cells of the kidneys. Pflugers Archiv : European journal of physiology. 2013;465:767–773. doi: 10.1007/s00424-013-1263-7. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Jones N, Dumont DJ, Puri MC, Bernstein A. Selective role of a distinct tyrosine residue on Tie2 in heart development and early hematopoiesis. Molecular and cellular biology. 2005;25:4693–4702. doi: 10.1128/MCB.25.11.4693-4702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. The Journal of biological chemistry. 1988;263:2041–2048. [PubMed] [Google Scholar]
- Takikawa O, Tagawa Y, Iwakura Y, Yoshida R, Truscott RJ. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Advances in experimental medicine and biology. 1999;467:553–557. doi: 10.1007/978-1-4615-4709-9_68. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS biology. 2003;1:E52. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geest RJ, Klaassen I, Vogels IM, Van Noorden CJ, Schlingemann RO. Differential TGF-{beta} signaling in retinal vascular cells: a role in diabetic retinopathy? Investigative ophthalmology & visual science. 2010;51:1857–1865. doi: 10.1167/iovs.09-4181. [DOI] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Experimental cell research. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, Sheibani N, Burridge K. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PloS one. 2012;7:e45499. doi: 10.1371/journal.pone.0045499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. The Journal of experimental medicine. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development (Cambridge, England) 2014;141:307–317. doi: 10.1242/dev.096107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nature neuroscience. 2011a;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Lack of Smad or Notch leads to a fatal game of brain pericyte hopscotch. Developmental cell. 2011b;20:279–280. doi: 10.1016/j.devcel.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Wohlfart P, Lin J, Dietrich N, Kannt A, Elvert R, Herling AW, Hammes HP. Expression patterning reveals retinal inflammation as a minor factor in experimental retinopathy of ZDF rats. Acta diabetologica. 2014 doi: 10.1007/s00592-013-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Larbi KY, Young RE, Nourshargh S. Migration of leukocytes through the vessel wall and beyond. Thrombosis and haemostasis. 2003;90:598–606. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nature medicine. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Zimmerman KW. Der feinere bau der blutcapillares. Z Anat Entwicklungsgesch. 1923;68:3–109. [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature reviews Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]