Abstract
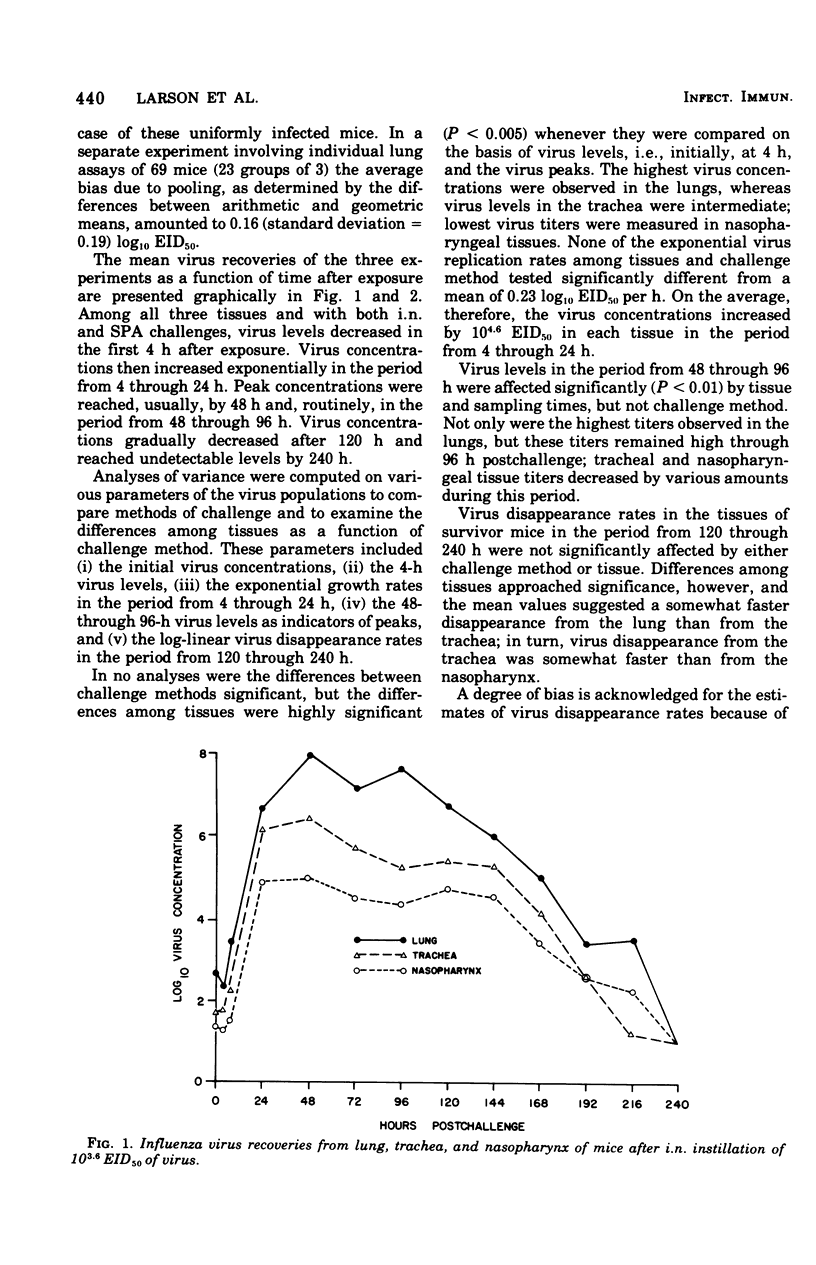
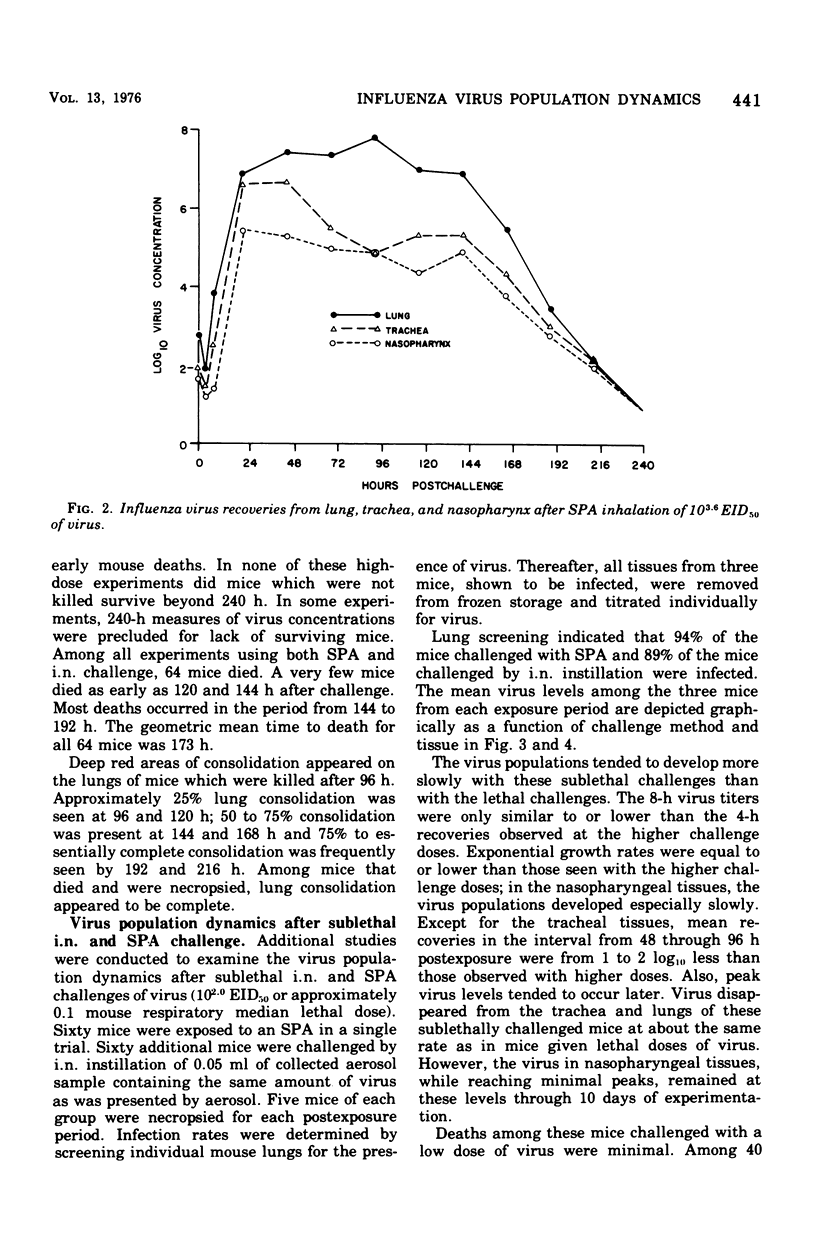
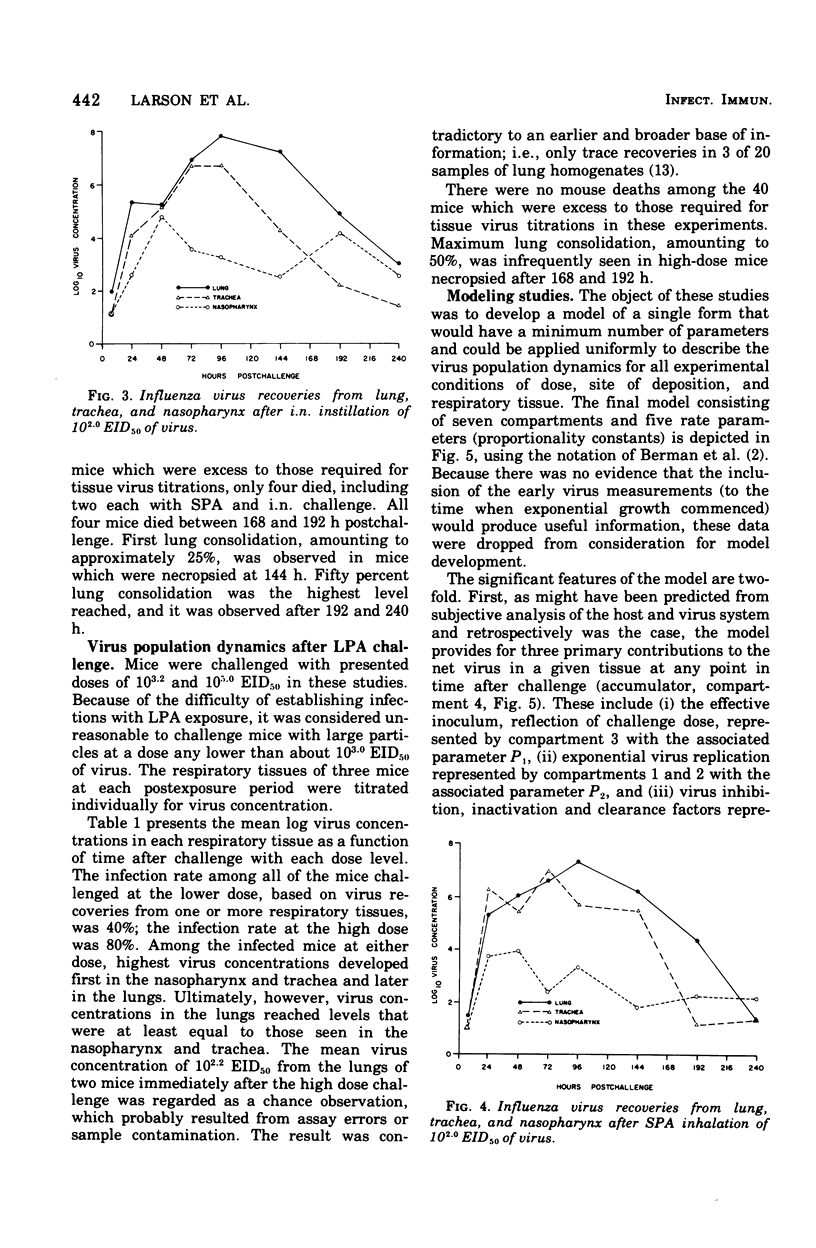
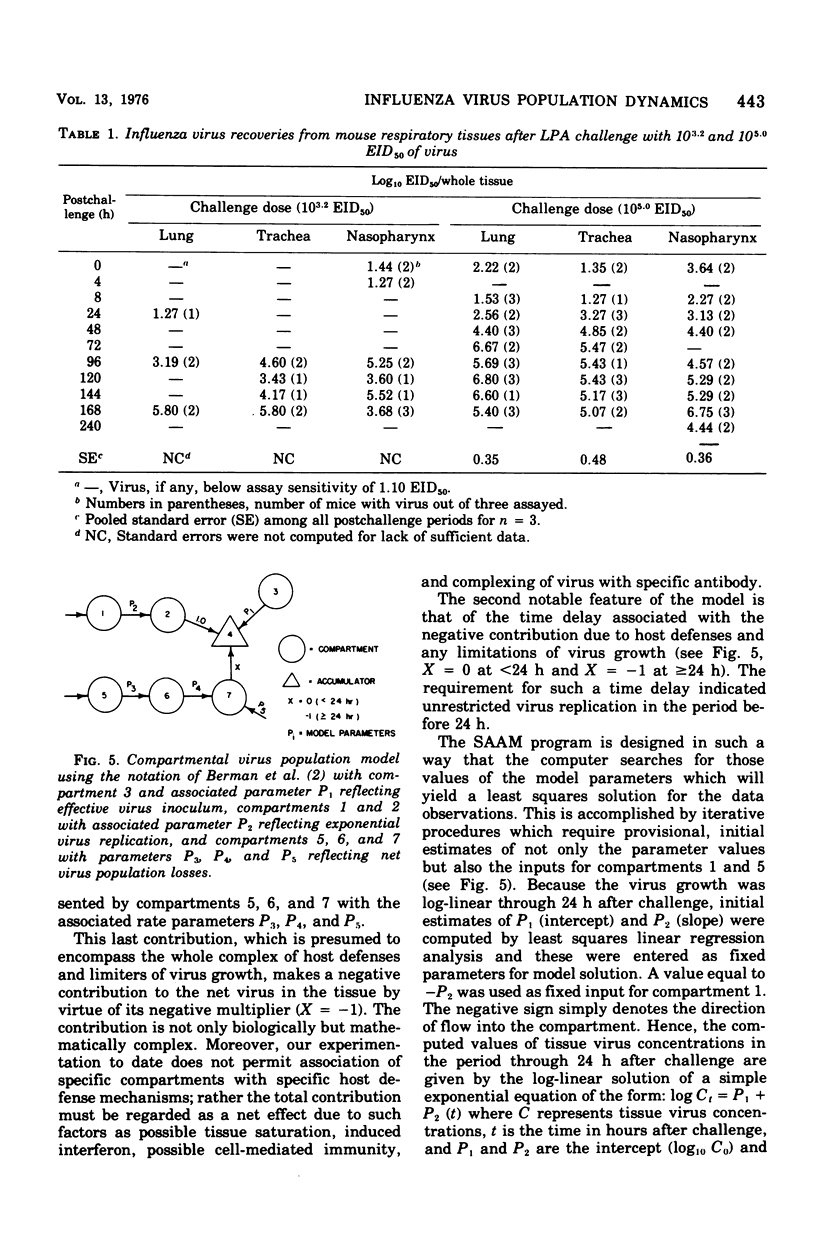
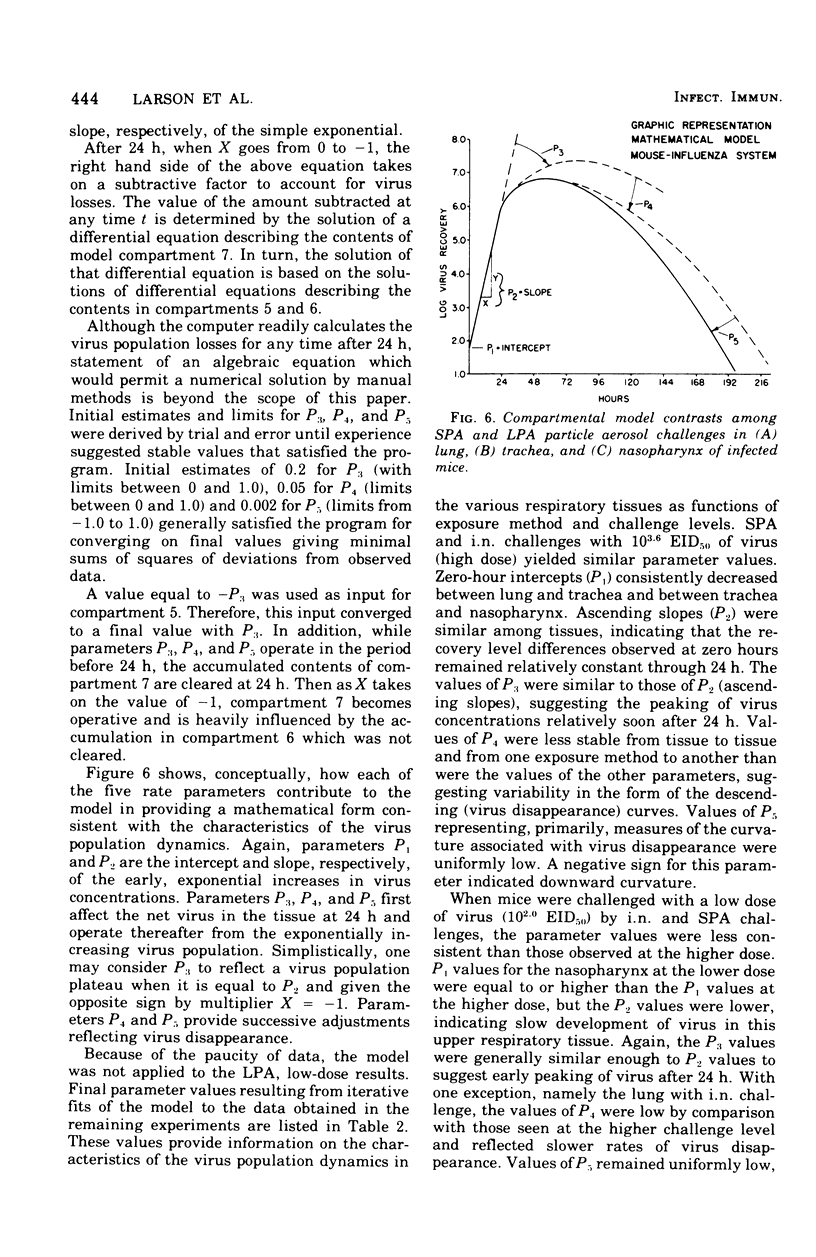
Virus population dynamics in the lungs, trachea, and nasopharynx of Swiss-ICR mice were studied after respiratory challenge with mouse-adapted preparations of strain A2/Aichi/2/68 influenza virus. Markedly higher doses of virus were required to produce infection with nasopharyngeal challenge than with bronchoalveolar challenge. In all of the infections, the highest virus concentrations were observed in the lungs. Peak concentrations in the trachea were lower than in the lungs but higher than in the nasopharynx. Decreasing virus levels were observed by 120 h after challenge and were generally below detectable levels by the end of 10 days. A compartmental model of a single mathematical form was developed which provided close fits of the virus concentration measurements regardless of the challenge dose, site of initial deposition, or respiratory tissue considered. The model includes seven compartments with five associated rate parameters. The application of compartmental modeling techniques and expression of the virus population dynamics in mathematical terms is regarded as a new approach to the study of the pathogenesis of infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERMAN M., WEISS M. F., SHAHN E. Some formal approaches to the analysis of kinetic data in terms of linear compartmental systems. Biophys J. 1962 May;2:289–316. doi: 10.1016/s0006-3495(62)86856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt R. F. Simian model for the evaluation of immunity to influenza. Infect Immun. 1974 Jan;9(1):101–105. doi: 10.1128/iai.9.1.101-105.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERONE P. J., WARD T. G., CHAPPELL W. A. Combined infections in mice with influenza virus and Diplococcus pneumoniae. Am J Hyg. 1957 Nov;66(3):331–341. doi: 10.1093/oxfordjournals.aje.a119906. [DOI] [PubMed] [Google Scholar]
- HENDERSON D. W. An apparatus for the study of airborne infection. J Hyg (Lond) 1952 Mar;50(1):53–68. doi: 10.1017/s0022172400019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERS J. F., MULDER J. Broad aspects of the pathology and pathogenesis of human influenza. Am Rev Respir Dis. 1961 Feb;83(2):84–97. doi: 10.1164/arrd.1961.83.2P2.84. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Pierce A. K., Sanford J. P. Pulmonary function in uncomplicated influenza. Am Rev Respir Dis. 1969 Aug;100(2):141–146. doi: 10.1164/arrd.1969.100.2.141. [DOI] [PubMed] [Google Scholar]
- Young H. W., Larson E. W., Dominik J. W. Modified spinning top homogeneous spray apparatus for use in experimental respiratory disease studies. Appl Microbiol. 1974 Dec;28(6):929–934. doi: 10.1128/am.28.6.929-934.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


