Abstract
Objective:
To evaluate C-C chemokine receptor type 2 (CCR2) on monocyte subsets as a prognostic peripheral blood biomarker of HIV-associated neurocognitive disorders (HAND).
Methods:
We characterized monocyte populations in HIV-infected individuals with and without HAND from 2 cohorts and assessed their transmigration across an in vitro model of the human blood-brain barrier (BBB). We examined CCR2 expression among the monocyte populations as a prognostic/predictive biomarker of HAND and its functional consequences in facilitating monocyte diapedesis.
Results:
We determined that CCR2 was significantly increased on CD14+CD16+ monocytes in individuals with HAND compared to infected people with normal cognition. CCR2 remained elevated irrespective of the severity of cognitive impairment, combined antiretroviral therapy status, viral load, and current or nadir CD4 T-cell count. There was no association between CCR2 on other monocyte populations and HAND. There was a functional consequence to the increase in CCR2, as CD14+CD16+ monocytes from individuals with HAND transmigrated across our model of the human BBB in significantly higher numbers in response to its ligand chemokine (C-C) motif ligand 2 (CCL2) compared to the cell migration that occurred in people with no cognitive deficits. It should be noted that our study had the limitation of a smaller sample size of unimpaired individuals. In contrast, there was no difference in the transmigration of other monocyte subsets across the BBB in response to CCL2 in seropositive individuals with or without HAND.
Conclusions:
Our findings indicate CCR2 on CD14+CD16+ monocytes is a novel peripheral blood biomarker of HAND.
HIV enters the CNS within the first weeks of primary infection.1 A spectrum of cognitive deficits, termed HIV-associated neurocognitive disorders (HAND), manifests in >50% of seropositive individuals.2 Combined antiretroviral therapy (cART) does not treat or prevent HAND,3 although it reduced the incidence of the severest form of deficits.4 There is an increasing need for biomarkers that reflect HAND in all of its complexity.
A mature CD14+CD16+ monocyte subset contributes to HAND by bringing virus into the CNS and perpetuating low-level neuroinflammation.5 CD14+CD16+ monocytes are highly susceptible to HIV6 and represent a peripheral viral reservoir that presents an increased risk for the development of HAND.7 Chemokine (C-C) motif ligand 2 (CCL2) is a potent monocyte chemoattractant increased in the brain during HIV infection8 that remains elevated despite cART9 and facilitates monocyte diapedesis across the blood-brain barrier (BBB). C-C chemokine receptor type 2 (CCR2) is the only receptor for CCL2 present on monocytes10 and is of therapeutic interest to decrease chronic inflammation.11
In the current study, we characterized CCR2 on CD14+CD16+ monocytes in the peripheral blood of HIV-infected individuals with and without HAND. CCR2 was specifically increased on CD14+CD16+ monocytes in individuals with HAND. This elevation in CCR2 promoted the increased diapedesis of CD14+CD16+ monocytes across the BBB in those with HAND compared to cognitively normal seropositive controls. Our findings implicate CCR2 as a peripheral blood biomarker of HAND.
METHODS
Standard protocol approvals, registrations, and patient consents.
Blood samples from HIV-seropositive participants were obtained through the Manhattan HIV Brain Bank (MHBB; U01MH083501, U24MH100931) in New York, NY and the Women's Interagency HIV Study (WIHS; U01AI35004, U01 A142590) in Bronx, NY. All patients gave written informed consent for the provision of blood for the purpose of HIV research. The protocol under which these samples were obtained is approved by the Institutional Review Board at the Montefiore Medical Center, Albert Einstein College of Medicine, and the Mount Sinai Program for the Protection of Human Subjects Institutional Review Board. HIV-infected patient demographic and virologic information is listed in table e-1 at Neurology.org/nn. The inclusion criterion for participants in the study was HIV-positive individuals older than 18 years. An additional inclusion criterion for the MHBB participants was the agreement to be an organ donor for research purposes upon death. HIV-seropositive individuals with persistently low CD4 counts and neuropsychological impairment likely due to causes other than HIV were excluded from the study. The study was performed between June 25, 2012 and October 4, 2013, during which time samples were obtained from 60 participants that met the inclusion/exclusion criteria. The participants in this study were predominantly of a black and Hispanic racial/ethnic background and female, and the majority had undetectable viral loads. These individuals are representative of the population in East Harlem and the Bronx, NY, where the cohorts are located; however, our participants are oversampled with regard to female sex.
HIV-seropositive individuals underwent extensive neuromedical and psychiatric assessments, as described previously.12–14 The neuromedical examination included the collection of a detailed medical history and a structured medical evaluation. Collected blood samples were tested for liver diseases (hepatitis B and C antibodies), CD4+ lymphocyte counts, and HIV load. Diabetes status was ascertained through patient interview or review of the medical record and was confirmed in most cases by the documentation of oral or injectable hypoglycemic agents in the patient's medication list. Urine toxicology screened for amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, phencyclidine, methadone, and propoxyphene (illicit and prescribed).
Blood from HIV-seronegative participants was obtained from deidentified leukopaks from the New York Blood Center in accordance with protocols established at the Albert Einstein College of Medicine.
Neuropsychological testing.
MHBB participants were administered a battery of neuropsychological tests and functional status questionnaires that assessed a broad range of cognitive abilities sensitive to HIV impairment.15 Specific tests and their normative references can be found in Byrd et al.14 All individual tests were grouped according to the theoretically derived domains indicated in table e-2.15 Raw scores from all tests were converted to demographically adjusted T scores, which adjusted performance for the effects of age, education, sex, and ethnicity, where appropriate. Domain scores were derived from the mean T scores of the individual tests in that particular domain, and clinical ratings of severity of impairment (range = 1–9) were assigned from these.15 HAND diagnoses (HIV-associated dementia [HAD] and minor cognitive motor disorder [MCMD]) were assigned according to a modified American Academy of Neurology algorithm published by the Dana Consortium.16 Asymptomatic neurocognitive impairment (ANI) was diagnosed according to the clinical ratings system published by Woods et al.15 in 2004 and reflects the presence of cognitive impairment in the absence of functional impairment.
For the purposes of this study, cognitive data for the WIHS participants were extracted for those tests that were identical to those from the MHBB battery: Grooved Pegboard, Hopkins Verbal Learning Test-Revised, Controlled Oral Word Association Test F-A-S, Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Symbol, WAIS-III Symbol Search, and WAIS-III Letter Number Sequencing (see table e-2). Raw scores were converted to standard T scores using the same normative data applied to the MHBB test scores (see above). Functional status data were not available for these participants. Thus, HAND diagnoses could not be assigned. The presence of cognitive impairment in WIHS participants was determined by application of the cognitive criteria of the diagnostic algorithms referenced above.16
Statistical analysis.
Statistical analyses were performed using Prism 6.02 software (GraphPad Software, Inc., San Diego, CA) and the Statistical Package for Social Sciences (version 20.0 for Windows; IBM, Armonk, NY). Mann–Whitney tests were used to determine statistical significance for figures 1, A–C, 2A, and 2B (p ≤ 0.05). Linear regression was used to determine statistical significance for figure 2, E and F (p ≤ 0.05). Wilcoxon signed rank test was used to determine statistical significance for figure 3, B–D (p ≤ 0.05). Paired 2-tailed t test was used to determine statistical significance for figure 4, A–C (p ≤ 0.05). A logistic regression was performed to test the strength of comorbid clinical factors (presence of each condition coded as “1”) and cognitive status for the prediction of CCR2 high and low group classifications based on expression levels above 45 (p ≤ 0.05).
Figure 1. CCR2 on CD14+CD16+ monocytes correlates with HAND.
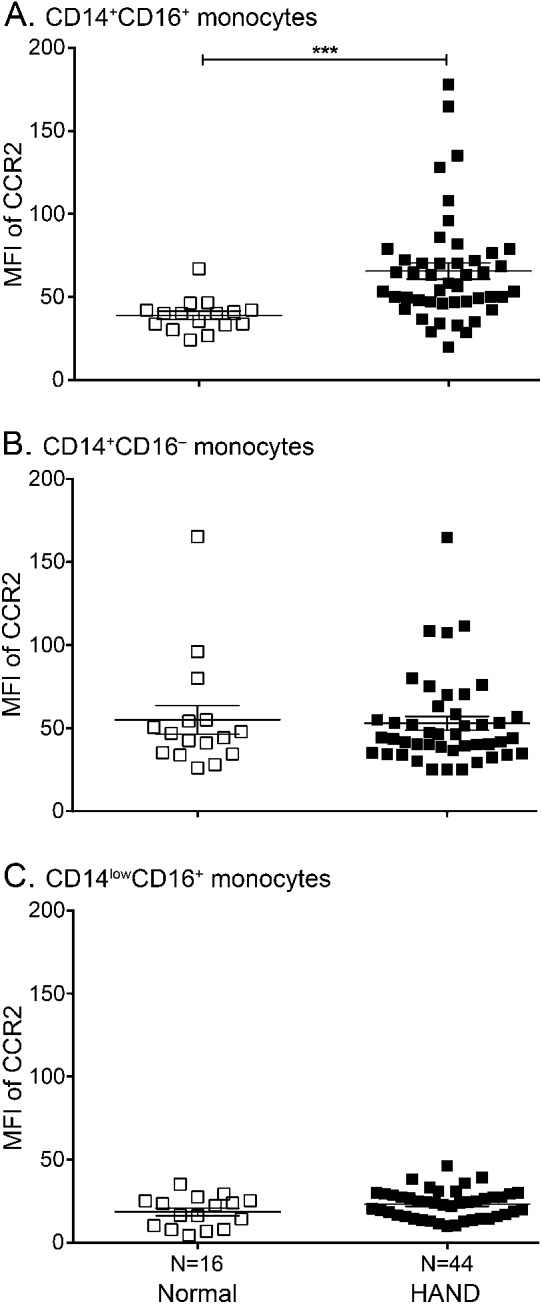
Peripheral blood mononuclear cells from 16 seropositive individuals with normal cognition and 44 with HIV-associated neurocognitive disorders (HAND) were stained with antibodies to CD14, CD16, and C-C chemokine receptor type 2 (CCR2) and analyzed by flow cytometry. After subtracting out the contribution of the isotype-matched negative control antibody, the mean fluorescence intensity (MFI) of CCR2 was determined for each monocyte subpopulation. (A) CCR2 is expressed on CD14+CD16+ monocytes from individuals with normal cognition (open squares) and is significantly increased in those with HAND (filled squares). (B) CCR2 is equally expressed on CD14+CD16− monocytes among people with normal cognition (open squares) and HAND (filled squares). (C) CD14lowCD16+ monocytes minimally express CCR2 irrespective of cognitive status. Statistical analyses were performed using the Mann–Whitney test. ***p < 0.001.
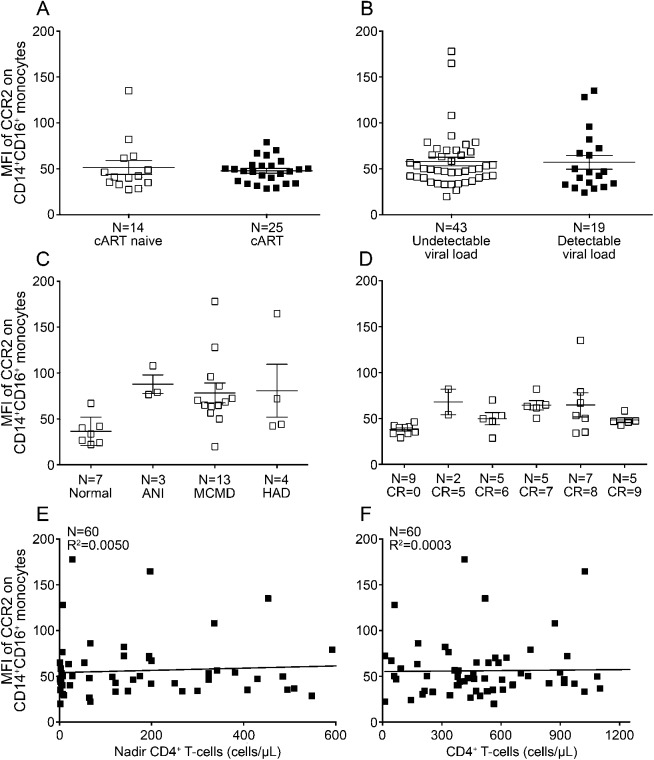
Figure 2. CCR2 on CD14+CD16+ monocytes does not correlate with HIV disease progression or severity of HIV-associated neurocognitive disorders.
The mean fluorescence intensity (MFI) of C-C chemokine receptor type 2 (CCR2) after subtracting out the isotype-matched negative control was determined for CD14+CD16+ monocytes from HIV-infected individuals, and its correlation to markers of immunovirologic status and severity of neurocognitive deficits was determined. (A) There was no difference in the MFI of CCR2 in people who were combined antiretroviral therapy (cART) naive (14 people, open squares) or experienced (25 people, filled squares). (B) The MFI of CCR2 did not vary among individuals with undetectable (43 people, open squares) or detectable (19 people, filled squares) viral loads. (C) CCR2 MFI was similarly increased on CD14+CD16+ monocytes isolated from individuals with asymptomatic neurocognitive impairment (ANI, 3 people), minor cognitive motor disorder (MCMD, 13 people), or HIV-associated dementia (HAD, 4 people) compared to seropositive individuals with normal cognition (7 people). (D) CCR2 MFI was similarly increased on CD14+CD16+ monocytes isolated from individuals with clinical ratings (CR) values in the impaired range of 5 (2 people), 6 (5 people), 7 (5 people), 8 (7 people), or 9 (5 people) compared to seropositive individuals with normal cognition with CR scores of 0 (9 people). There was no correlation with CCR2 and nadir (E) or current (F) CD4+ T-cell count. Statistical analyses were performed using the Mann–Whitney test or linear regression.
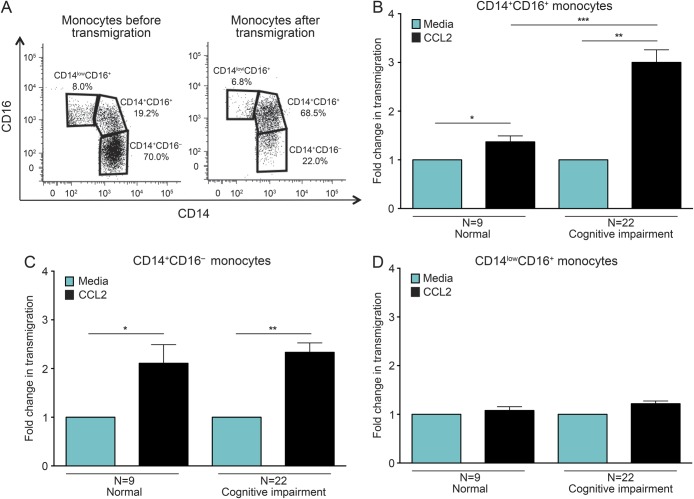
Figure 3. CD14+CD16+ monocytes from people with HAND are primed to transmigrate across the BBB.
Peripheral blood mononuclear cells from 9 seropositive individuals with normal cognition and 22 with cognitive deficits were added to our in vitro model of the human blood-brain barrier (BBB) and allowed to transmigrate in response to media or chemokine (C-C) motif ligand 2 (CCL2). The migrated cells were recovered and analyzed by flow cytometry for CD14 and CD16. (A) Fluorescence-activated cell sorting data represented as dot plots demonstrate the monocyte populations present before (monocytes before transmigration) and following (monocytes after transmigration) diapedesis across the BBB. Prior to transmigration, the cells consisted of all 3 monocyte subsets. Upon transmigration across the BBB, the monocytes were primarily comprised of CD14+CD16+ cells. (B–D) The pooled average transmigration in response to media (turquoise bars, set to 1) or CCL2 (black bars) was determined for each monocyte population. (B) CD14+CD16+ monocytes from those with normal cognition significantly migrated across the BBB in response to CCL2. There was a further significant increase in the CCL2-mediated transmigration of CD14+CD16+ monocytes for individuals with HIV-associated neurocognitive disorders (HAND) compared to those with normal cognition. (C) CD14+CD16− monocytes from people with normal cognition and HAND responded similarly to CCL2. (D) CCL2 did not promote transmigration of CD14lowCD16+ monocytes across the BBB. Statistical analyses were performed using Wilcoxon signed rank test.*p < 0.05, **p < 0.01, ***p < 0.001.
Figure 4. CCR2 high CD14+CD16+ monocytes selectively transmigrate across the BBB.
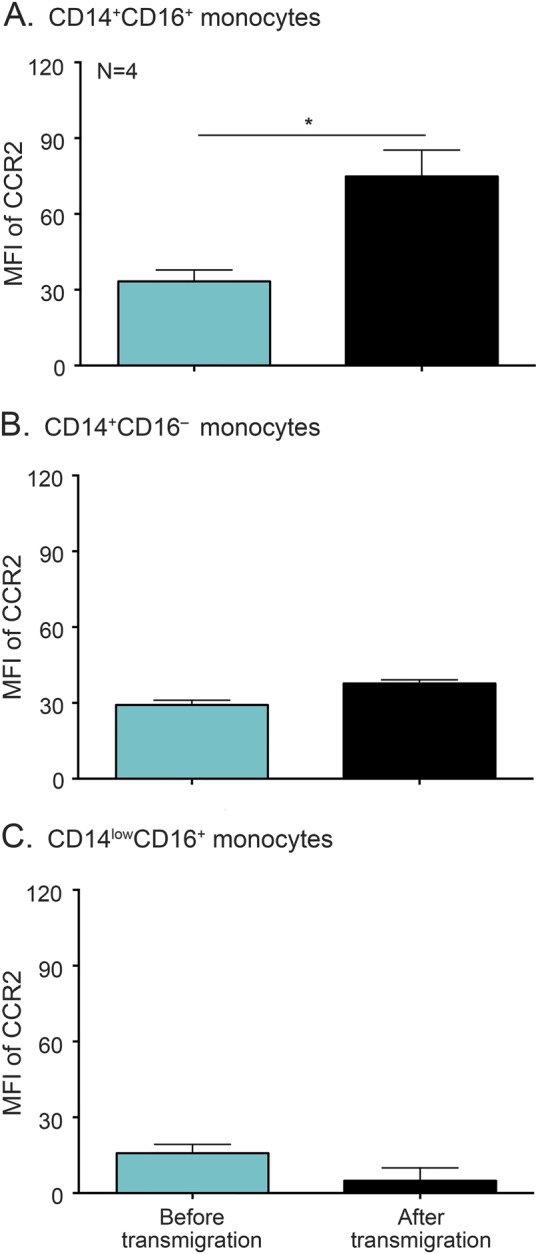
Peripheral blood mononuclear cells from 4 individuals were added to the blood-brain barrier (BBB) model and allowed to transmigrate for 24 hours in response to chemokine (C-C) motif ligand 2 (CCL2). A portion of the input cells was set aside and stained with antibodies specific for CD14, CD16, and C-C chemokine receptor type 2 (CCR2) or isotype-matched negative control. Following transmigration across the BBB, the migrated cells were recovered and analyzed by flow cytometry for CD14, CD16, and CCR2. After subtracting out the contribution of the isotype control, the mean fluorescence intensity (MFI) of CCR2 was determined on each monocyte subset before (turquoise bars) and after (black bars) transmigration. (A) There was a preferential selection for CD14+CD16+ monocytes with high amounts of CCR2 upon transmigration across the BBB. The expression of CCR2 remained the same on CD14+CD16− (B) and CD14lowCD16+ (C) monocytes prior to and following transmigration across the BBB. Statistical analyses were performed using paired 2-tailed t test. *p < 0.05.
Details on methods regarding cell isolation, monocyte identification by flow cytometry, CCR2 analysis by flow cytometry, and transmigration assays across the BBB model are provided in appendix e-1.
RESULTS
Participant characteristics.
As summarized in table e-1, the study consisted of 60 HIV-seropositive individuals from the MHBB (27 people) and the WHIS (33 people) cohorts. The participants in the cohorts did not differ with respect to race/ethnicity, CD4+ T-cell count, plasma viral load, the frequency of viral hepatitis, or rates of cognitive impairment. The WIHS cohort was comprised of women who were significantly younger than the MHBB cohort (49 ± 7 years compared to 55 ± 6 years, p < 0.001), a portion of whom were naive to cART.
CCR2 is increased on CD14+CD16+ monocytes in HIV-seropositive individuals with cognitive impairment.
To examine the monocyte subpopulations present in the peripheral blood of HIV-seropositive and seronegative individuals, peripheral blood mononuclear cells (PBMC) were isolated and analyzed by flow cytometry. Forward and side scatter characteristics were used to identify monocytes in the PBMC population (figure e-1A). Surface CD14 and CD16 were used to discriminate further among the CD14+CD16−, CD14+CD16+, and CD14lowCD16+ monocyte subsets (figure e-1B). We characterized CCR2 on the cell surface of each monocyte subset present in HIV-positive individuals to determine the mechanisms that may be mediating transmigration across the BBB and contributing to the neuropathogenesis of HIV. Multicolor flow cytometry was performed and CCR2 was characterized on the surface of each monocyte population from 16 seropositive donors with normal cognition and 44 individuals with HAND. CCR2 was present on CD14+CD16+ (figure 1A) and CD14+CD16− (figure 1B) monocytes isolated from those with normal cognition, but was minimally expressed on CD14lowCD16+ cells (figure 1C). There was a significant increase in the mean fluorescence intensity (MFI) of CCR2 on CD14+CD16+ monocytes from individuals with HAND (65.6 ± 4.8) compared to seropositive people with normal cognition (37.9 ± 2.5, p < 0.001), suggesting that it may be used to identify those at risk of developing cognitive deficits (figure 1A). There was no relationship between the presence of CCR2 on the other monocyte subsets and cognitive status (figure 1, B and C).
There was a “threshold of impairment” above which CCR2 was indicative of HAND. The MFI of CCR2 on CD14+CD16+ monocytes was below 45 for 88% of those with normal cognition (figure 1A). Conversely, the MFI of CCR2 on these cells was above 45 for 84% of individuals with HAND. While the majority of the MFIs of CCR2 were above the “threshold of impairment” for individuals with HAND, the expression pattern for the chemokine receptor was variable among donors and was not normally distributed. Future studies will evaluate host genetic contributors to the variability in CCR2 and the functional consequences of this heterogeneity. The expression of CCR2 on CD14+CD16+ monocytes was not significantly associated with cART status (figure 2A), viral load (figure 2B), the severity of cognitive deficits in the MHBB (figure 2C) or WIHS (figure 2D) participants, and nadir (figure 2E) or current (figure 2F) CD4 T-cell counts.
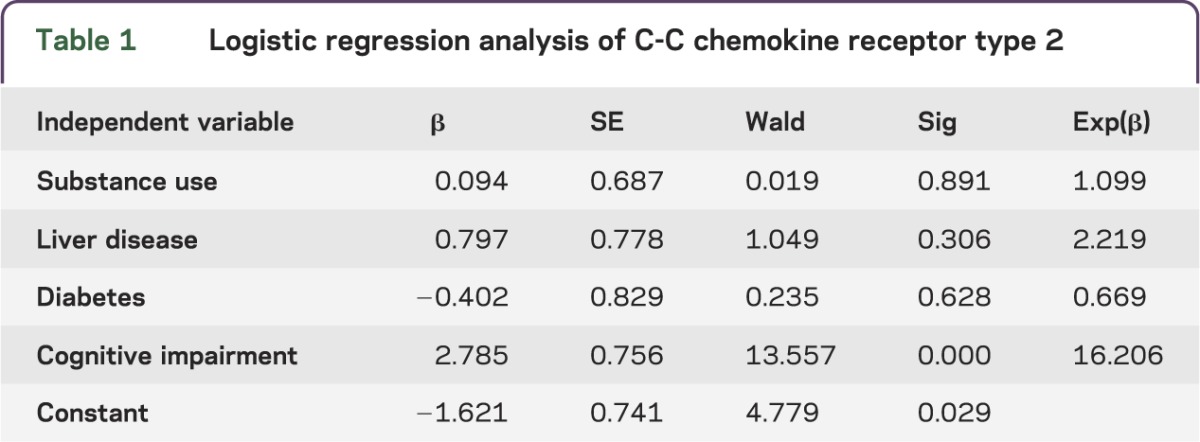
CCR2 on CD14+CD16+ monocytes is not associated with substance use, liver disease, or diabetes.
HAND may be present with increased incidence or severity due to comorbid conditions that often affect a large number of HIV-infected individuals. To examine the degree to which comorbid conditions may have influenced CCR2 expression, a logistic regression was performed with CCR2 group status above or below the threshold value of 45 as the outcome variable and the following as predictor variables: urine toxicology positive for substance use (see Methods for details), diabetes status, liver disease status, and cognitive impairment status. Results indicate that the overall model was significant (model χ2 = 20.584, p < 0.01), with cognitive status emerging as the only significant predictor variable associated with CCR2 (table 1). None of the comorbid conditions significantly predicted CCR2 status (all p > 0.05). These analyses suggest that CCR2 is predictive of cognitive impairment in HIV-infected individuals even in the context of comorbid conditions, and they provide further support for CCR2 on CD14+CD16+ monocytes as a potential prognostic biomarker of HAND.
Table 1.
Logistic regression analysis of C-C chemokine receptor type 2
CCR2 facilitates increased transmigration of CD14+CD16+ monocytes from individuals with HAND across the BBB in response to CCL2.
To determine whether the elevated amounts of CCR2 on CD14+CD16+ monocytes had functional consequences, PBMC from 9 HIV-infected individuals with normal cognition and 22 people with cognitive impairment were added to our in vitro model of the human BBB and allowed to transmigrate for 24 hours in response to media alone or CCL2. After the indicated time, the cells that transmigrated were recovered and stained with CD14, CD16, and CCR2 antibodies, and the monocyte populations were characterized and enumerated by flow cytometry. The CD14+CD16+ subset comprised the majority of monocytes that crossed the BBB (68.5% of transmigrating cells) (figure 3A). The migration of the other populations of monocytes was much lower in comparison. CD14+CD16− and CD14lowCD16+ cells comprised only 22.0% and 6.8% of transmigrating cells, respectively (figure 3A).
The CCL2-mediated transmigration of CD14+CD16+ monocytes across the BBB was significantly higher in participants with HAND (3- ± 0.3-fold higher than media alone, set to 1) compared to those with normal cognition (1.4- ± 0.1-fold higher than media alone, set to 1, p < 0.001) (figure 3B). This transmigration was not dependent on the severity of impairment, as cells isolated from individuals with ANI, MCMD, and HAD all responded similarly to CCL2 (data not shown). In contrast, there was no difference in the CCL2-mediated transmigration of CD14+CD16− (2.3- ± 0.2-fold compared to 2.1- ± 0.4-fold, p > 0.05) and CD14lowCD16+ (1.2- ± 0.1-fold compared to 1.1- ± 0.1-fold, p > 0.05) monocytes from seropositive individuals with HAND and those with normal cognition (figure 3, C and D).
CCR2 was characterized on each monocyte population after transmigration and compared to that present on the cells prior to migration. CCR2 was significantly increased on CD14+CD16+ monocytes following transmigration across the BBB (74.5 ± 10.5) compared to prior to migration (33.3 ± 4.5, p < 0.05) (figure 4A). There was no difference in CCR2 after transmigration for the other monocyte populations (figure 4, B and C). This suggests a preferential selection for CD14+CD16+ cells with high levels of CCR2, underscoring its importance in facilitating the entry of these cells into the brain.
DISCUSSION
HAND is a major consequence of HIV infection that greatly diminishes the quality of life of affected individuals and poses a risk for lower adherence to cART and a decreased lifespan.17 Although HAND may be characterized by decreased memory, psychomotor slowing, and loss of focus, the manifestations of cognitive deficits vary among individuals and formal neuropsychological testing is required to obtain a diagnosis.18 Screening for HAND is recommended for all HIV-infected patients at initial diagnosis and at repeated intervals for follow-up.19 However, this does not always translate into clinical practice because of time constraints and a less-than-clear consensus on the appropriate screening tools.15,20 A fast, standardized, and easily accessible biomarker is needed to identify individuals at risk for HAND. We demonstrated that the chemokine receptor CCR2 on the CD14+CD16+ monocyte subset is a novel biomarker of HAND. CCR2 on CD14+CD16+ monocytes represents an attractive biomarker because it can be identified by flow cytometry, a technique that is routinely performed to enumerate CD4+ T cells. Antibodies specific to CCR2, CD14, and CD16 can be added to the cocktail currently used to evaluate CD4+ T cells to identify those who may develop cognitive deficits.
CCR2 does not change with respect to cART status, viral load, or current and nadir CD4+ T-cell count, suggesting that it may be applicable to seropositive people with a wide range of virologic states. In addition, in this sample CCR2 is not associated with substance use, liver disease, or diabetes. HAND encompasses a spectrum of disorders greatly influenced by many comorbidities and risk factors.21,22 At times, these factors make it difficult to assign a HAND diagnosis because it is unclear whether the cognitive deficits are truly HIV-related. CCR2 may therefore be a useful means to diagnose HAND, as it remains predictive of cognitive impairment despite concomitant conditions with potential neurologic outcomes.
In addition to being used as a prognostic marker of disease, the expression of CCR2 on CD14+CD16+ monocytes is an attractive biomarker because it provides a more complete understanding of the mechanisms contributing to the neuropathogenesis of HIV. Monocytes serve as a “Trojan horse,” promoting viral seeding of the CNS upon their entry into the parenchyma and perivascular space.23 We identified the CD14+CD16+ subset as the specific monocyte population that may significantly contribute to HAND, as these cells selectively transmigrated across the BBB. CD14+CD16+ cells are the monocyte population with the highest susceptibility to HIV infection6 and pose the greatest risk for promoting viral seeding of the brain and contributing to neuroinflammatory processes associated with the sequelae of HAND.
In the current era of cART, the manifestations of HAND are often milder than previously seen. Severe cases of HAD and HIV encephalitis still occur,24 but less frequently. In addition, the contribution of neuroinflammation to the neuropathogenesis of HIV is less clear now. Some studies argue that neuroinflammation is minimal at best,25 while others find that “surprising” amounts persist.26–28 When present, neuroinflammation occurs even in those with milder forms of impairment.29 We determined that CCR2 on CD14+CD16+ monocytes remained elevated despite the extent of impairment, although our study was limited by small numbers of participants with ANI and HAD, and we did not have sufficient power to assess statistically the relationship with CCR2 for these HAND diagnoses. Future studies will be performed with additional participants to evaluate more clearly the expression of CCR2 with respect to the severity of cognitive impairment. The heightened response of monocytes to transmigrate across the BBB in response to CCL2 was mediated by CCR2 and remained similar among all individuals with HAND. These data suggest that monocyte transmigration across the BBB is a common mechanism that mediates all categorizations of HAND and that differences in severity of impairment may be due to other host factors.
Our data provide evidence as to why HAND persists despite cART. Antiretroviral therapy may quell viral replication, but it does not decrease chemokines, such as CCL2,9 within the CNS of HIV-infected individuals that recruit monocytes into the brain. This underscores that without adjunctive therapy, cellular influx into the CNS may continue to occur. cART also fails to limit monocyte/macrophage activation,30 which greatly contributes to HAND. Soluble CD163 is a marker of this activation and has been suggested as a potential means to identify those with HAND.31 Although it was not evaluated in this study, it provides further evidence of the importance of monocytes in mediating HAND.
We propose that the mature CD14+CD16+ monocyte population directly contributes to HAND by transmigrating across the BBB and initiating and perpetuating low-level neuroinflammation that mediates the neuropathogenesis of HIV. Neuropsychological testing is an extremely powerful and rigorous tool used to identify cognitive deficits in individuals with HIV. Our findings present an unbiased and objective means to diagnose HAND, which may be used in concert with neuropsychological testing, and to assess the efficacy of therapy used to limit HAND. CCR2 can be quickly assayed by simple flow cytometry and is an easily accessible peripheral blood marker that would facilitate diagnosis of HAND.
Supplementary Material
ACKNOWLEDGMENT
Data in this manuscript were collected by the Women's Interagency HIV Study Collaborative Study Group with Dr. Kathryn Anastos (New York City/Bronx Consortium) and the Manhattan HIV Brain Bank (member of the National NeuroAIDS Tissue Consortium) with Dr. Susan Morgello. The authors thank the MHBB and WIHS patients and staff, Drs. Eliseo A. Eugenin, Peter J. Gaskill, Jacqueline Coley, Brad Poulos, and Lydia Tesfa, and Bezawit Megra, Mike Veenstra, and Matias Jaureguiberry.
Glossary
- ANI
asymptomatic neurocognitive impairment
- BBB
blood-brain barrier
- cART
combined antiretroviral therapy
- CCL2
chemokine (C-C) motif ligand 2
- CCR2
C-C chemokine receptor type 2
- HAD
HIV-associated dementia
- HAND
HIV-associated neurocognitive disorders
- MCMD
minor cognitive motor disorder
- MFI
mean fluorescence intensity
- MHBB
Manhattan HIV Brain Bank
- PBMC
peripheral blood mononuclear cells
- WAIS
Wechsler Adult Intelligence Scale
- WIHS
Women's Interagency HIV Study
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
D.W.W. designed and performed experiments, analyzed data, and wrote the manuscript. D.B. and L.H.R. performed the neuropsychological testing, participated in data analysis, and edited the manuscript. K.A. and S.M. provided samples from HIV-seropositive participants, participated in data analysis, and edited the manuscript. J.W.B. participated in design of research, data analysis, and drafting and editing the manuscript.
STUDY FUNDING
This was supported by pilot funds from the Mount Sinai Institute for NeuroAIDS Disparities (R25 MH080663, D.W.W.), T32AI070117 (D.W.W.), UNCF/Merck Graduate Science Dissertation Fellowship (D.W.W.); K01MH098798 (L.H.R.); AI35004 (K.A.), A142590 (K.A.); MH080663 (S.M.), MH083501 (S.M.), MH100931 (S.M.); MH075679 (J.W.B.), MH090958 (J.W.B.), DA025567 (J.W.B.); and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (AI051519).
DISCLOSURE
D.W. Williams has received research support from UNCF/Merck and NIMH. D. Byrd has received research support from NIH. L.H. Rubin has received research support from NIMH. K. Anastos is on the scientific advisory board for Bristol Myers Squibb and has received research support from NIH. S. Morgello is on the advisory board for Temple University Comprehensive NeuroAids Center, is on the editorial board for Journal of Neurovirology, and has received research support from NIH. J.W. Berman holds a patent on the use of antibodies to junctional proteins to limit monocyte entry into the CNS and is a consultant for the Manhattan Brain Bank. Go to Neurology.org/nn for full disclosures.
REFERENCES
- 1.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012;206:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton R, Franklin D, Ellis R, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 2012;91:401–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 2007;178:6581–6589 [DOI] [PubMed] [Google Scholar]
- 7.Shiramizu B, Ananworanich J, Chalermchai T, et al. Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol 2012;18:69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cinque P, Vago L, Mengozzi M, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS 1998;12:1327–1332 [DOI] [PubMed] [Google Scholar]
- 9.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 2012;60:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpe S, Cameroni E, Moepps B, Thelen S, Apuzzo T, Thelen M. CCR2 acts as scavenger for CCL2 during monocyte chemotaxis. PLoS ONE 2012;7:e37208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter PH. Progress in the discovery of CC chemokine receptor 2 antagonists, 2009—2012. Expert Opin Ther Pat 2013;23:549–568 [DOI] [PubMed] [Google Scholar]
- 12.Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol 2004;61:546–551 [DOI] [PubMed] [Google Scholar]
- 13.Ryan E, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology 2004;62:957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd DA, Robinson-Papp J, Mindt MR, et al. Isolating cognitive and neurologic HIV effects in substance-dependent, confounded cohorts: a pilot study. J Int Neuropsychol Soc 2013;19:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 2004;26:759–778 [DOI] [PubMed] [Google Scholar]
- 16.Clinical confirmation of the American Academy of Neurology algorithm for HIV-1 associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology 1996;47:1247–1253 [DOI] [PubMed] [Google Scholar]
- 17.McArthur J, Smith B. Neurologic complications and considerations in HIV-infected persons. Curr Infect Dis Rep 2013;15:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 2007;45:174–182 [DOI] [PubMed] [Google Scholar]
- 19.Mind Exchange Working Group. Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clin Infect Dis 2013;56:1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morley D, McNamara P, Kennelly S, McMahon G, Bergin C. Limitations to the identification of HIV-associated neurocognitive disorders in clinical practice. HIV Med 2013;14:497–502 [DOI] [PubMed] [Google Scholar]
- 21.Winston A, Vera JH. Can antiretroviral therapy prevent HIV-associated cognitive disorders? Curr Opin HIV AIDS 2014;9:11–16 [DOI] [PubMed] [Google Scholar]
- 22.Manji H, Jäger HR, Winston A. HIV, dementia and antiretroviral drugs: 30 years of an epidemic. J Neurol Neurosurg Psychiatry 2013;84:1126–1137 [DOI] [PubMed] [Google Scholar]
- 23.González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol 2005;5:69–81 [DOI] [PubMed] [Google Scholar]
- 24.Gelman BB, Lisinicchia JG, Morgello S, et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr 2013;62:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everall I, Vaida F, Khanlou N, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol 2009;15:360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 2005;64:529–536 [DOI] [PubMed] [Google Scholar]
- 27.Roc AC, Ances BM, Chawla S, et al. Detection of human immunodeficiency virus induced inflammation and oxidative stress in lenticular nuclei with magnetic resonance spectroscopy despite antiretroviral therapy. Arch Neurol 2007;64:1249. [DOI] [PubMed] [Google Scholar]
- 28.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011;25:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desplats P, Dumaop W, Smith D, et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 2013;80:1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hearps A, Martin G, Rajasuriar R, Crowe S. Inflammatory co-morbidities in HIV+ individuals: learning lessons from healthy ageing. Curr HIV/AIDS Rep 2014;11:1–15 [DOI] [PubMed] [Google Scholar]
- 31.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 is a marker of neurocognitive impairment in HIV infection. AIDS 2013;27:1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.