Abstract
In Escherichia coli, the ribosome-associated chaperone Trigger Factor (TF) promotes the folding of newly synthesized cytosolic proteins. TF is composed of three domains: an N-terminal domain (N), which mediates ribosome binding; a central domain (P), which has peptidyl-prolyl cis/trans isomerase activity and is involved in substrate binding in vitro; and a C-terminal domain (C) with unknown function. We investigated the contributions of individual domains (N, P, and C) or domain combinations (NP, PC, and NC) to the chaperone activity of TF in vivo and in vitro. All fragments comprising the N domain (N, NP, NC) complemented the synthetic lethality of Δtig ΔdnaK in cells lacking TF and DnaK, prevented protein aggregation in these cells, and cross-linked to nascent polypeptides in vitro. However, ΔtigΔdnaK cells expressing the N domain alone grew more slowly and showed less viability than ΔtigΔdnaK cells synthesizing either NP, NC, or full-length TF, indicating beneficial contributions of the P and C domains to TF's chaperone activity. In an in vitro system with purified components, none of the TF fragments assisted the refolding of denatured d-glyceraldehyde-3-phosphate dehydrogenase in a manner comparable to that of wild-type TF, suggesting that the observed chaperone activity of TF fragments in vivo is dependent on their localization at the ribosome. These results indicate that the N domain, in addition to its function to promote binding to the ribosome, has a chaperone activity per se and is sufficient to substitute for TF in vivo.
The ribosome-associated chaperone Trigger Factor (TF) cooperates with the DnaK system to promote the folding of newly synthesized proteins in the Escherichia coli cytosol, as indicated by genetic data (5, 21). While DnaK is essential for viability only at temperatures above 37°C and below 15°C and TF is not essential at any temperature, the combined lack of TF and DnaK functions in ΔtigΔdnaK double-mutant cells is synthetically lethal for E. coli at 30°C and at higher temperatures (5, 21). In Δtig cells grown at 37°C, the depletion of DnaK resulted in the aggregation of approximately 340 cytosolic protein species (4, 5).
TF associates in a 1:1 stoichiometry with ribosomes via the ribosomal protein L23 located at the polypeptide exit channel of the large ribosomal subunit (8, 13, 14). At the ribosome, TF interacts with virtually all nascent polypeptides (2, 8, 24). The ribosomal attachment of TF is a prerequisite for its interaction with nascent polypeptide chains and its activity in the folding of newly synthesized proteins (13).
In vitro, uncomplexed TF acts as a chaperone to prevent the aggregation and promote the refolding of denatured rabbit d-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (11). Moreover, TF displays peptidyl-prolyl cis/trans isomerase (PPIase) activity, i.e., it isomerizes peptidyl-prolyl peptide bonds in tetrapeptides and protein substrates such as unfolded RNaseT1 (10, 19, 20).
TF (432 amino acids [aa]) is a dimer in solution and consists of three domains. The N-terminal domain (N domain, aa 1 to 144) mediates ribosome binding, while the central domain (P domain, aa 145 to 247) has PPIase and substrate binding activity and is homologous to the PPIase family of FK506-binding proteins (3, 8-10, 20). The function of TF's large C-terminal domain (C domain, aa 248 to 432) is unknown (9, 17). The contribution of the individual TF domain to its chaperone activity in vivo and in vitro is poorly understood. Moreover, for full-length TF there exist no structural data that would provide insights into TF's mechanism of chaperone action. It is likely that the N or C domain contributes, or that both domains contribute, to the folding activity of TF in vitro since the refolding of the model substrate RNase T1 is efficiently catalyzed only by full-length TF but not by the isolated PPIase domain (19).
Here, we set out to thoroughly characterize the contributions of each of the three TF domains to its chaperone activity in vivo and in vitro. Therefore, we analyzed the ability of separated TF domains and TF domain combinations to (i) complement the lethality of the mutation for cells lacking TF and DnaK, (ii) prevent the aggregation of newly synthesized proteins in vivo, (iii) associate with nascent polypeptide chains in vitro, and (iv) prevent the aggregation and promote the refolding of denatured GAPDH in vitro.
MATERIALS AND METHODS
Growth conditions.
Strains were grown in Luria broth (LB) medium containing IPTG (isopropyl-β-d-thiogalactopyranoside) as indicated and supplemented with ampicillin (100 μg/ml), tetracycline (5 μg/ml), or kanamycin (40 μg/ml) when appropriate.
Strains and plasmids.
E. coli strains were derivatives of MC4100. The ΔdnaK strain GK2 used in this study, carrying a chromosomal deletion of the entire dnaK open reading frame and expressing wild-type levels of DnaJ, was constructed as described previously (12).
Cloning and purification of TF fragments.
The Vector pTrc99B (1) was used to construct plasmids encoding TF or TF fragments. pTrc99B was digested with NcoI, blunt ended by use of T4-DNA-polymerase (New England Biolabs, Inc.), and finally digested with BamHI, resulting in a linearized vector DNA fragment that comprises the ATG start codon at the blunt-ended site and a 5′ overhang generated by BamHI digestion on the other side. This linearized vector fragment was used to clone all tig constructs described below.
All PCR fragments described below contained a BamHI restriction site at the 3′ end and a 5′ blunt end as generated by proof-reading polymerases. Primer P5′-N (5′-CAAGTTTCAGTTGAAACCACTC-3′) was used to amplify tig fragments encoding the N-terminal ribosome binding domain of TF (pTrc-TF, pTrc-N, pTrc-NP, and pTrc-NC). Primer P5′-P (5′-CGTAAACAGCAGGCGACCTGG-3′) was used to amplify the tig fragments in plasmid pTrc-P and pTrc-PC, and P5′-C (5′-CTGACTGCAGAATTCATCAAAC-3′) was used to generate the tig fragment encoding the C-terminal domain of TF in plasmid pTrc-C. Primer P3′-N (5′-GGCCGGATCCTTACAGAGTATCCAGCATGCCGTC-3′) was used to generate the tig fragment encoding the N-terminal domain of TF in plasmid pTrc-N. Primer P3′-P (5′-GGCCGGATCCTTATTCCGGCAGTTCACGCTCTTC-3′) was used to generate pTrc-NP and pTrc-P, whereas primer P3′-C (5′-GGCCGGATCCTTACGCCTGCTGGTTCATCAGCTC-3′) was used to generate plasmids pTrc-TF, pTrc-PC, pTrc-NC, and pTrc-C.
The plasmid pTrc-NC encoding a hybrid protein containing the N domain of TF fused to the C domain was constructed as follows: in two independent PCRs the DNA fragment encoding the N domain of TF was amplified by using P5′-N and P3′-fus (5′-CGTTTGATGAATTCTGCAGTCAGCAGAGTATCCAGCATGCCGTC-3′), and the DNA fragment encoding the C domain of TF was amplified by using P5′-fus (5′-GACGGCATGCTGGATACTCTGCTGACTGCAGAATTCATCAAACG-3′) and P3′-C. Both PCR fragments were gel purified, and 0.1-μg amounts of each fragment were mixed and used as a template for a second PCR using the primers P5′-N and P3′-C. This PCR resulted in a fusion of the DNA fragments encoding the N domain including the linker sequence (aa 1 to 144) and the C-terminal domain (aa 248 to 432).
For purification of C-terminally His-tagged TF or TF fragments, E. coli cells carrying the appropriate plasmid were grown at 30°C in LB with 100 μg of ampicillin/ml to an optical density at 600 nm of 0.6 and before adding 500 μM IPTG for induction of expression from plasmid. Two hours after induction, cells were harvested and the cell pellet was resuspended in 25 ml of ice-cold French press buffer (50 mM Tris-HCl [pH 7.5], 20 mM imidazole [pH 7.5], 200 mM NaCl, 1 mM EDTA) supplemented with 1 mM phenylmethylsulfonyl fluoride. Cells were lysed with a French press two times at a pressure of 8,000 lb/in2. Cell debris was separated from the soluble fraction by centrifugation at 20,000 × g for 30 min. The Ni-nitrilotriacetic (Ni-NTA) purification was done as a batch purification. Six to eight milliliters of Ni-NTA agarose (Qiagen) was equilibrated with 5 volumes of cold French press buffer in a suction filter. The supernatant from the centrifugation step was supplemented with 10 mM MgCl2, incubated with the equilibrated Ni-NTA agarose on ice for 15 min, and then passed through the suction filter. After being washed with at least 0.5 liter of ice-cold washing buffer (50 mM Tris-HCl [pH 7.5], 20 mM imidazole [pH 7.5], 500 mM NaCl) and 5 volumes of ice-cold low-salt buffer (50 mM Tris-HCl [pH 7.5], 20 mM imidazole [pH 7.5], 25 mM NaCl), the protein was eluted from the Ni-NTA agarose with 3 volumes of cold elution buffer (50 mM Tris-HCl [pH 7.5], 250 mM imidazole [pH 7.5], 25 mM NaCl), directly applied to an anion exchange column (ResourceQ6; Pharmacia) at 4°C, and eluted with a salt gradient (going from a low-salt buffer consisting of 50 mM Tris-HCl [pH 7.5], 25 mM NaCl, and 1 mM EDTA to a high-salt buffer consisting of 50 mM Tris-HCl [pH 7.5], 1 M NaCl, and 1 mM EDTA). Under these conditions, Trigger Factor elutes at 150 mM NaCl (15% high-salt buffer). The times of elution of TF fragments or mutants may vary, and all elution fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Appropriate fractions were pooled and dialyzed against 2 liters of storage buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA) overnight at 4°C.
In vivo complementation analysis.
E. coli ΔtigΔdnaK strains were constructed by P1 transduction by using ΔdnaK GK2 cells as recipient and a P1vir lysate prepared from E. coli Δtig::kan zba-3054::Tn10 (5). Transductants were selected at 30°C on tetracycline-containing LB plates (5 μg/ml) and screened for deletion of the tig gene by screening on kanamycin-containing LB plates (20 μg/ml). P1 lysates, P1 transductions, and disruption of chromosomal genes were done according to the method described in reference 15.
Preparation of aggregates.
For quantitative isolation of aggregates, 20 ml of cultures grown to log phase in LB liquid media was divided into aliquots of 10 ml, harvested, and lysed (5). Aggregated material was isolated as reported previously (22). Appropriate amounts were withdrawn for protein determination by the Bradford assay (Bio-Rad) and the remainder was centrifuged (30 min, 10,000 × g, 4°C) and subjected to SDS-PAGE (16).
In vitro transcription-translation and chemical cross-linking.
Preparation of extracts and generation of arrested nascent chains were performed as described previously (18). For generation of nascent chains from PykF and IcdH, transcription was started with 0.4 ng of pET-PykF/μl or 2 ng of pET-IcdH/μl (4). Arrested nascent chains were produced by the addition of 40 ng of antisense oligonucleotide/μl (CCTTCAATGGCGGTAACTTCC for PykF and CCCCCATCTCTTCACGCAGG for IcdH). Translation extracts were additionally supplemented with 0.3 U of T7 polymerase/μl and 0.3 μCi of [35S]-methionine/μl and 300 nM TF or TF fragments. After 30 min, the cross-linker disuccinimidyl-suberate (DSS) (final concentration, 25 mM) was added, and samples were incubated for 30 min at room temperature. The reaction was quenched with 50 mM Tris-HCl (pH 7.5) for 15 min at room temperature, and ribosomal complexes were purified (9). The pellet was resolubilized in phosphate-buffered saline, and coimmunoprecipitation was performed with TF-specific antisera (5).
Prevention of GAPDH aggregation and GAPDH refolding assay.
Aggregation and refolding of denatured GAPDH from rabbit muscle (Sigma catalogue no. G-2267) was measured in the presence or the absence of 2.5 μM TF or 2.5 to 40 μM TF fragments as described earlier (11, 12). It is to be noted that the refolding activity of denatured GAPDH in the presence of equimolar concentrations of TF varies between 60 and 80%, depending on the specific GAPDH batch purchased from the manufacturer, although the overall behavior of the chaperone remains consistent (11, 12; this study).
RESULTS
The N-terminal domain of TF is sufficient to complement synthetic lethality of ΔtigΔdnaK cells.
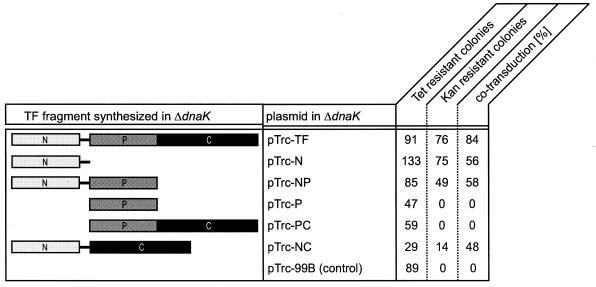
To investigate which domains of TF are necessary for its in vivo function, we constructed plasmids allowing the expression of single TF domains or combinations of TF domains (Fig. 1). To test their ability to complement the synthetic lethality for ΔtigΔdnaK cells, we performed cotransduction experiments using a ΔdnaK strain as the recipient. In the first step, we introduced the plasmids encoding different TF fragments under the control of an IPTG-inducible promoter into the ΔdnaK strain (Fig. 1). Subsequently, transformants were used to perform P1vir cotransduction experiments using a P1vir lysate raised on a strain with a Tn10::tet-selective marker (zba-3054::Tn10) adjacent to the Δtig::kan allele. Screening for cells lacking both tig and dnaK was done in the presence of various concentrations of IPTG. The cotransduction frequency of the tetracycline and kanamycin resistance markers in wild-type cells was approximately 80%, and in ΔdnaK cells it was 0% at 30°C (Fig. 1) (5).
FIG. 1.
TF fragments complement synthetic lethality of the ΔtigΔdnaK double mutation. (Left panel) Schematic outline of the TF fragments synthesized in ΔdnaK cells based on the IPTG-regulated expression of the tig gene or tig fragments from the vector pTrc-99B (middle panel) (for details, see Materials and Methods). (Right panel) Cotransduction frequencies of ΔdnaK cells as an indicator of synthetic lethality at 30°C. P1 transduction was performed by using ΔdnaK cells synthesizing various TF fragments as recipient and a P1vir lysate prepared from E. coli Δtig::kan zba-3054::Tn10 (5). Transductants were selected at 30°C on tetracycline-containing LB plates and subsequently screened for deletion of the tig gene on kanamycin-containing LB plates.
In a first series of experiments, transductants were selected on LB plates with tetracycline and 50 μM IPTG at 30°C. Subsequently, tetracycline-resistant clones were screened for kanamycin resistance in the presence of 50 μM IPTG. All cells expressing the N domain of TF or combinations thereof (N, NP, or NC) (Fig. 1) revealed cotransduction, although the frequencies were lower than those of cells expressing wild-type TF. Neither the expression of the isolated P domain nor that of the PC fragment encoding both the P domain and the C domain of TF allowed the cotransduction of the Tn10::tet-selective marker (zba-3054::Tn10) and the Δtig::kan allele at 30°C, even when cotransduction experiments were performed at higher or lower concentrations of IPTG. The expression levels of all TF fragments from plasmids were comparable to those of wild-type TF (see Fig. 3A; also data not shown). This result demonstrates that the N domain of TF is necessary and sufficient to at least partially rescue lethality of the double mutation ΔtigΔdnaK.
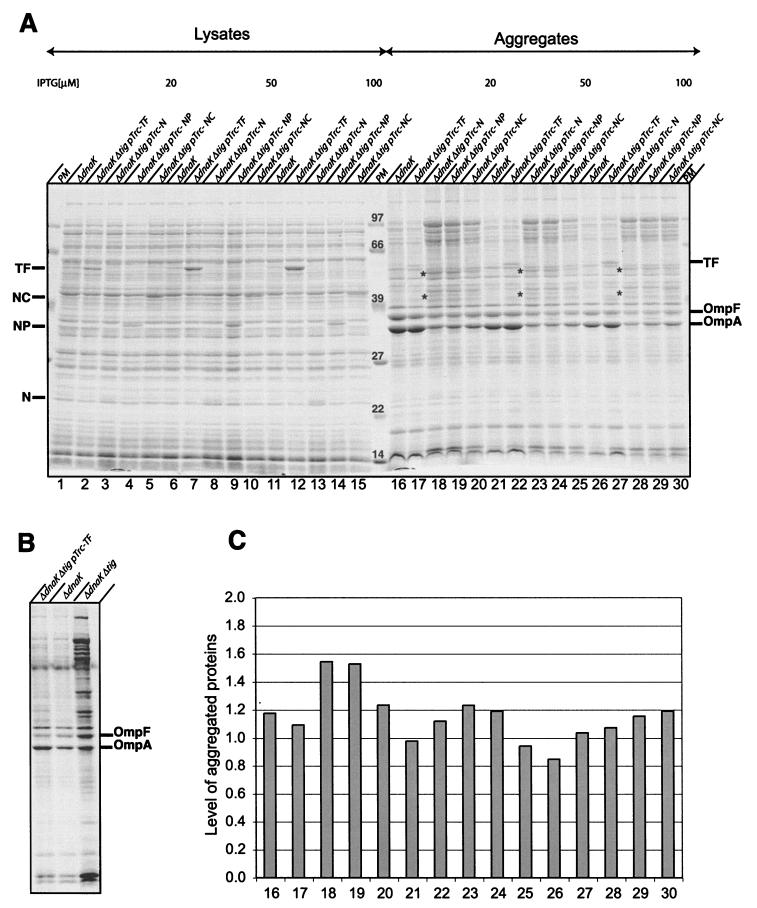
FIG. 3.
Aggregation analyses. (A) Cells were grown at 30°C in LB media in the presence of different concentrations of IPTG. At log phase, cells were harvested and aggregates were isolated and analyzed by SDS-PAGE and Coomassie blue staining. Total lysates (left) and isolated aggregates (right) are shown. The asterisks indicate two aggregated proteins that were found exclusively in ΔtigΔdnaK cells expressing N, NP, or NC independent of the amount of IPTG inducer. The nature of these proteins is unknown. Note that the outer membrane proteins OmpF and OmpA copurified with aggregated cytoslic proteins. The reason for their partial disappearance in ΔtigΔdnaK cells synthesizing N, NP, or NC is unknown. (B) The Coomassie blue-stained SDS-PAGE shows controls. Aggregated proteins were isolated from ΔdnaK and ΔtigΔdnaK (pTrc-TF) cells grown in LB in the presence of 20 μM IPTG and ΔtigΔdnaK cells grown at 30°C in LB for a few generations (25). (C) Quantification of aggregates. Aggregated proteins were directly quantified from the Coomassie blue-stained SDS gel shown in panel A by use of the MacBasV2.5 program. The bar numbers correspond to the lanes in panel A. The average of the levels of aggregates isolated from ΔdnaK cells from three independent experiments (lanes 16, 21, and 26) was set as 1.
Viability of ΔtigΔdnaK cells complemented with TF fragments.
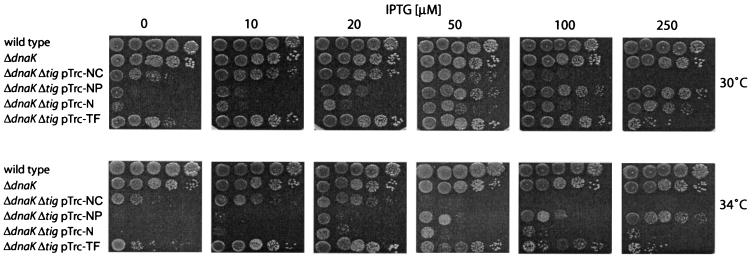
To investigate the growth behaviors of ΔtigΔdnaK cells expressing different TF fragments, freshly generated cotransductants were grown at 30°C to log phase in LB liquid media in the presence of 50 μM IPTG, subsequently spotted in serial dilutions on LB plates containing different concentrations of IPTG, and incubated for 24 h at different temperatures (Fig. 2). At a given inducer concentration and a given temperature, the expression levels of all TF variants were similar as judged by Coomassie blue-stained SDS gels and Western blotting (data not shown). Double-mutant cells expressing full-length TF showed growth even in the absence of IPTG. This is due to the leakiness of the IPTG-regulated promoter, which drives the expression of the tig gene from the pTrc-plasmid (data not shown). In the presence of 10 μM IPTG at 30°C and of 20 μM IPTG at 34°C, the growth of these cells was fully restored and was similar to that of ΔdnaK cells (Fig. 2). As reported earlier, stronger overproduction of TF (at 100 to 250 μM IPTG) is lethal at 30 and 34°C, and this is likely due to cell division defects (7, 18). None of the tested TF fragments complemented synthetic lethality for ΔtigΔdnaK cells in a manner comparable to that of wild-type TF. The N domain restored growth only at very high levels of inducer (250 μM IPTG) at 30°C and only partially at 34°C. Furthermore, the viability of ΔtigΔdnaK cells expressing the N domain was reduced at these temperatures, as judged by the decreased number of CFU compared to those of cells expressing moderate amounts of TF. The TF fragments NP and NC were more efficient than the N domain in complementation of growth of ΔtigΔdnaK cells at both temperatures (Fig. 2) but still less efficient than wild-type TF. Interestingly, the NC fragment, which lacks the central PPIase domain, complemented the loss of TF more efficiently than did N and NP, indicating that the C-terminal domain significantly contributes to TF's in vivo activity.
FIG. 2.
Growth analysis of ΔtigΔdnaK cells expressing TF fragments. Growth of wild-type MC4100, ΔdnaK, and ΔtigΔdnaK cells expressing different TF fragments at different temperatures and in the presence of different amount of IPTG was analyzed. Cells grown overnight at 30°C in the presence of 50 μM IPTG were diluted (to concentrations corresponding to 104, 103, 102, or 10 cells/5 μl). Cells were spotted on LB plates and incubated for 24 h at the indicated temperatures.
In summary, we conclude that the N domain can partially substitute for TF in vivo; however, ΔtigΔdnaK cells producing the N domain grow more slowly and show less viability than do cells synthesizing either NP, NC, or full-length TF. Neither the PPIase domain nor the C-terminal domain is essential for TF's in vivo function; however, they appear to synergistically contribute to TF's chaperone activity when attached to the ribosome via the N-terminal domain.
Analysis of protein aggregation in ΔtigΔdnaK cells.
Next, we analyzed the ability of TF fragments to suppress protein aggregation in vivo. Therefore, we grew ΔtigΔdnaK cells expressing TF fragments at 30°C to logarithmic phase in the presence of either 20, 50, or 100 μM IPTG and subsequently isolated aggregated proteins (Fig. 3A). It was reported earlier that at 30°C only mild folding defects exist in ΔdnaK cells and approximately 1% of total proteins are aggregated, in contrast to the massive (>10%) protein aggregation observed in cells lacking both DnaK and TF (4, 25) (Fig. 3B). We now find that the overproduction of TF fragments (N, NP, and NC) in ΔtigΔdnaK cells compensates for the loss of TF comparably well at all IPTG concentrations tested. The pattern of aggregated proteins was rather similar, and the total amount of aggregated proteins isolated from ΔtigΔdnaK cells complemented with N, NP, or NC was slightly higher (up to 1.5-fold) than that of cells expressing full-length TF (Fig. 3C). However, the prevention of aggregation of proteins of >40 kDa seemed less efficient in cells expressing these TF fragments. We conclude that all TF fragments that are competent to suppress synthetic lethality of the ΔtigΔdnaK double mutation also prevent the aggregation of newly synthesized proteins in vivo, albeit less efficiently than wild-type TF.
Interaction with nascent polypeptides.
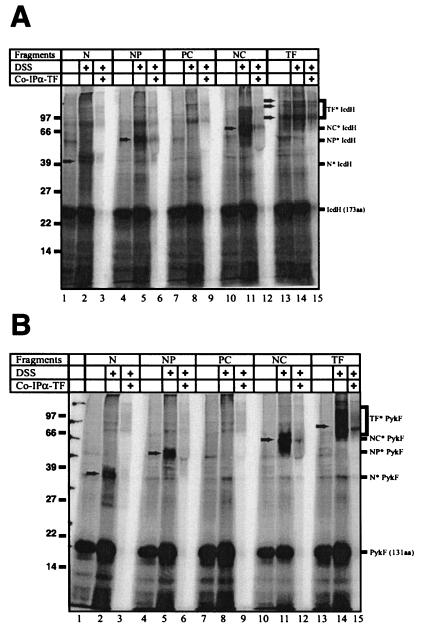
One striking characteristic of TF is its ability to associate with nascent polypeptides. Interaction with nascent substrates can be monitored by in vitro cross-linking of TF to arrested nascent polypeptide chains by use of the chemical cross-linker DSS. To test the TF fragments for their ability to associate with nascent chains, we generated arrested nascent polypeptides of two different TF substrates, isocitrate dehydrogenase (IcdH [173 aa]) and pyruvate kinase (PykF [131 aa]) in an E. coli-based in vitro transcription-translation system (4). The translation-competent extract was derived from a Δtig strain and thus allowed the exogenous addition of various TF fragments. Translation was carried out in the presence of [35S]methionine to label the nascent polypeptides and at a physiological 1:3 molar ratio of ribosomes to TF or TF fragments. In the presence of wild-type TF, addition of DSS led to the appearance of multiple cross-linking products of about 80, 100, and 110 kDa for nascent IcdH (Fig. 4 A, lane 14) and of 70 and 110 kDa for nascent PykF (Fig. 4B, lane 14). By coimmunoprecipitation TF was identified as a cross-linking partner of both nascent polypeptides. All fragments containing the N domain (N, NP, and NC) revealed cross-linking to nascent IcdH and PykF (Fig. 4A and B, lanes 2, 5, and 11), whereas the P and the C domains (data not shown) and the PC fragment (Fig. 4A and B) could not be cross-linked as reported earlier (17). To control the cross-linking efficiency in these samples, wild-type TF was added in substoichiometric amounts (1:10 TF to ribosomes), giving rise to minor amounts of cross-linking products between TF and nascent PykF or IcdH (Fig. 4A and B).
FIG. 4.
Interaction of nascent polypeptides with TF and TF fragments. We generated arrested 35S-labeled nascent polypeptides of IcdH (aa 1 to 173) (A) or PykF (aa 1 to 131) (B) in an in vitro TF-free transcription-translation system supplemented with TF or TF fragments. Chemical cross-linking of TF or TF fragments was performed by the addition of DSS (added where indicated) and sucrose cushion centrifugation; ribosome-nascent chain complexes were coimmunoprecipitated to identify cross-links of TF and TF variants. Cross-linking products are indicated by arrows and brackets. Antibodies raised against TF reach less efficiently with TF fragments.
Taken together, all TF fragments that showed ribosome association (N, NP, and NC) were able to complement synthetic lethality of the ΔtigΔdnaK double mutation and revealed cross-linking to nascent polypeptides.
In vitro chaperone activity of TF fragments.
To obtain more-detailed information on the ability of the TF fragments to act as a chaperone, we tested them in an in vitro chaperone assay using denatured GAPDH as a substrate. It was shown earlier that TF efficiently prevents the aggregation and promotes the refolding of chemically denatured GAPDH in vitro independently of peptidyl-prolyl isomerization (11).
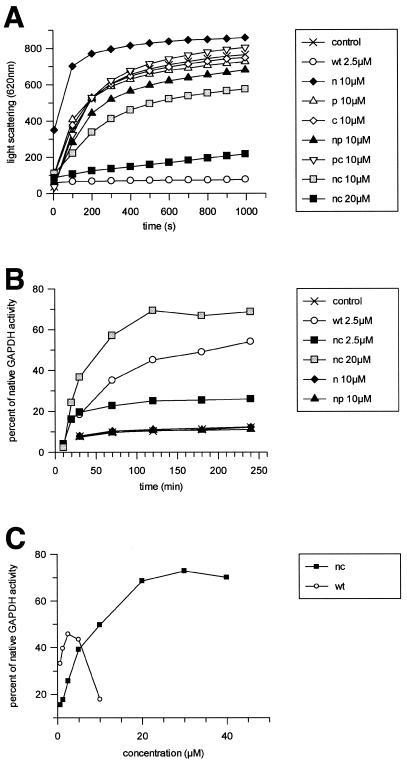
Upon 50-fold dilution, the chemically denatured GAPDH aggregated rapidly (Fig. 5A). Stoichiometric amounts of TF efficiently prevented GAPDH aggregation, since no increase of light-scattering signal at 620 nm was observed. Neither the isolated TF domains (N, P, or C) nor the fragment NP or PC prevented the aggregation of GAPDH even when present in fourfold excess over GAPDH (Fig. 5A). Remarkably, the presence of the N domain led to increased maximal light-scattering values compared to GAPDH alone, indicating that the non-ribosome-associated N domain by itself tends to aggregate in this assay. The presence of the NC fragment prevented aggregation in a concentration-dependent manner, but in contrast to full-length TF a 10-fold-higher concentration of NC was required for almost complete suppression of GAPDH aggregation (Fig. 5A). Addition of the P domain in trans did not enhance the activity of NC (data not shown), indicating that the refolding activity of full-length TF is not just the sum of single domain activities but rather a coordinated process.
FIG. 5.
Prevention of GAPDH aggregation and refolding of denatured GAPDH. (A) Aggregation of GAPDH is followed by an increase of the light-scattering signal at 620 nm after a 50-fold dilution of the denatured enzyme (final concentration, 2.5 μM). Addition of 2.5 μM TF (wt) or 20 μM TF fragment (nc) significantly inhibits aggregation. (B and C) Refolding of denatured GAPDH is monitored by measuring the enzymatic activity at different time points after a 50-fold dilution (final concentration, 2.5 μM) in the absence or the presence of TF or TF fragments. (B) Refolding activities of TF fragments N, NP, and NC in comparison to wild-type TF (wt). (C) By titration of TF and the NC fragment, activity of GAPDH was determined after 4 h.
GAPDH activity can be monitored by observing the reduction of NAD+ to NADH at 340 nm in a photometer. This assay was used to determine the activities of TF and TF fragments in the refolding of denatured GAPDH. The chemically denatured enzyme was diluted 50-fold in the presence or absence of TF or TF fragments, and after different time points the restoration of GAPDH enzymatic activity was determined. In the absence of TF, GAPDH remained inactive over a 4-h time period (Fig. 5B). In contrast, the presence of wild-type TF restored up to 60% of nondenatured GAPDH activity, such results being similar to those reported earlier (11). Complementing the results above, all TF fragments that were inactive in preventing GAPDH aggregation (N, P, C, NP, and PC) also lacked refolding activity at all concentrations tested (Fig. 5B and data not shown). Only NC displayed significant activity when present in stoichiometric amounts. However, the shape of the GAPDH refolding curve differed from that of the wild-type TF, indicating a different substrate interaction of this artificial TF fragment. The rate of refolding was higher for NC (0.0547/min versus 0.0131/min), but the maximum yield of recovered GAPDH activity was significantly lower (25 versus 50%, approximately). However, increasing the concentration of NC up to 20 μM (Fig. 5C) led to a even higher total yield of activity (70% refolding) compared to that obtained with wild-type TF. Interestingly, in contrast to wild-type TF, which showed a concentration-dependent refolding activity with a narrow maximum at 1:1 stoichiometry compared to GAPDH (2.5 μM TF monomer), the activity of the NC fragment reached a stable maximum at concentrations above ∼20 μM (Fig. 5C). This finding indicates differences between full-length TF and NC in the interaction with unfolded protein substrate and complements well the results obtained in the GAPDH prevention of aggregation assay.
In summary, we conclude the following: (i) none of the fragments tested revealed a chaperone activity comparable to that of wild-type TF in vitro; and (ii) of the fragments complementing for the loss of TF in vivo (N, NP, and NC), only the artificial NC fragment displayed residual chaperone activity. Thus, although neither P nor C is essential in vivo, the full chaperone activity cannot be restored by single domains or domain combinations but needs the coordinated assembly within the entire TF molecule.
DISCUSSION
In this study we determined the contributions of individual TF domains to TF's in vivo function, its association with nascent polypeptide chains, and its in vitro chaperone activity.
Surprisingly, expression of the isolated N domain of TF is sufficient to partially complement the synthetic lethality of the ΔtigΔdnaK double mutation at 30°C. It is important to note that the viability of these cells is significantly reduced compared to that of ΔtigΔdnaK cells expressing plasmid-encoded wild-type TF, as judged by a 10-fold reduction in the number of CFU even when this domain is more highly overexpressed. Consistently, enhanced protein aggregation was observed in ΔtigΔdnaK cells expressing the N domain compared to that of cells expressing full-length TF. In vitro, the N domain of TF could be cross-linked to nascent IcdH and PykF polypeptide chains. In contrast to other TF fragments investigated, the separated N domain could not be cross-linked to the secretory nascent proOmpA (17; E. Deuerling and B. Bukau, unpublished results), suggesting differences between full-length TF and the N-domain fragment in nascent chain recognition. Furthermore, in contrast to full-length TF, the isolated N domain did not show any detectable chaperone activity in the GAPDH assay in vitro. Some caution is needed with respect to this result, because the N-domain fragment has a tendency to aggregate in vitro. However, a similar lack of chaperone activity was found for the fully soluble NP fragment.
Our data suggest that the N domain fulfills a specific function at the ribosomal exit tunnel that is distinct from a typical substrate-chaperone interaction but that is sufficient to partially complement the synthetic lethality of the ΔtigΔdnaK double mutation. One possible explanation would be that the association of the N domain with the ribosome protects nascent polypeptides against unproductive association with the ribosomal surface. Accordingly, it was recently shown that in the absence of TF nascent polypeptides cross-link to the ribosomal protein L23, supporting the model of a shielding function of TF (6, 23). The N domain may thus not only target TF to the ribosome but also have chaperone activity for the nascent chains, perhaps by a shielding effect at the ribosomal exit site.
Expression of the NP fragment in the ΔtigΔdnaK mutant increased viability of the cells more efficiently than the N domain itself. NP furthermore cross-linked to all nascent polypeptides tested (ICDH, PykF, and proOmpA) but failed to show any chaperone activity in vitro. These findings led us to conclude that either the PPIase activity contributes to the fitness of the cell or the PPIase domain contributes to the chaperone activity of the N domain per se.
Expression of NC also led to a viability of ΔtigΔdnaK cells at 30 and 34°C that was increased compared to that of cells just expressing N, and the NC fragment was cross-linked in vitro to all nascent chains tested. An unexpected finding was that in contrast to all other fragments tested, NC was the only TF fragment that displayed chaperone activity towards chemically unfolded GAPDH. These results show that the C-terminal domain makes a major contribution to the chaperone activity of TF. However, the NC fragment and full-length TF showed strong differences in the efficiency and kinetics of GAPDH refolding, clearly demonstrating the importance of the PPIase domain for the chaperone activity of TF in consistency with the proposed function of the PPIase domain in substrate binding and recognition (17). It is clear from these experiments, though, that the presence of the PPIase domain is not a strict requirement for the chaperone activity of TF. Interestingly, neither the P domain nor the PC fragment substituted for TF in vivo; nor did these fragments cross-link to nascent polypeptides. We therefore speculate that either the potential chaperone function of the N domain at the ribosomal exit site is absolutely essential for the TF function or the P or C domain of TF must be tethered to the ribosome in order to prevent the misfolding of newly synthesized proteins in cells lacking TF and DnaK. The latter hypothesis is strongly supported by the finding that even full-length TF does not prevent the misfolding of newly synthesized proteins when its ribosomal docking is inhibited (13).
Taken together, our data indicate that the isolated domains or pairwise combinations of TF maintain a surprisingly high degree of activities, even though full-length TF is more active than the sum of its separated domains.
Acknowledgments
We thank members of the Bukau lab for comments on the manuscript and discussions.
This work was supported by grants from the DFG (SFB352, Leibnizprogramm) to B.B. and E.D., the Fonds der Chemischen Industrie to B.B., the HFSP (Human Frontier Science Program) to E.D., a Heisenberg fellowship of the DFG to E.D., and a fellowship of the Boehringer Ingelheim Fonds to T.R.
REFERENCES
- 1.Amann, E., B. Ochs, and K. J. Abel. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301-315. [DOI] [PubMed] [Google Scholar]
- 2.Beck, K., L.-F. Wu, J. Brunner, and M. Müller. 2000. Discrimination between SRP- and SecA/SecB-dependent substrates involves selective recognition of nascent chains by SRP and trigger factor. EMBO J. 19:134-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callebaut, I., and J. P. Mornon. 1995. Trigger factor, one of the Escherichia coli chaperone proteins, is an original member of the FKBP family. FEBS Lett. 374:211-215. [DOI] [PubMed] [Google Scholar]
- 4.Deuerling, E., H. Patzelt, S. Vorderwülbecke, T. Rauch, G. Kramer, E. Schaffitzel, A. Mogk, A. Schulze-Specking, H. Langen, and B. Bukau. 2003. Trigger Factor and DnaK possess overlapping substrate pools and binding specificities. Mol. Microbiol. 47:1317-1328. [DOI] [PubMed] [Google Scholar]
- 5.Deuerling, E., A. Schulze-Specking, T. Tomoyasu, A. Mogk, and B. Bukau. 1999. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 400:693-696. [DOI] [PubMed] [Google Scholar]
- 6.Eisner, G., H. G. Koch, K. Beck, J. Brunner, and M. Muller. 2003. Ligand crowding at a nascent signal sequence. J. Cell Biol. 163:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guthrie, B., and W. Wickner. 1990. Trigger factor depletion or overproduction causes defective cell division but does not block protein export. J. Bacteriol. 172:5555-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesterkamp, T., and B. Bukau. 1996. Identification of the prolyl isomerase domain of Escherichia coli trigger factor. FEBS Lett. 385:67-71. [DOI] [PubMed] [Google Scholar]
- 9.Hesterkamp, T., E. Deuerling, and B. Bukau. 1997. The amino-terminal 118 amino acids of Escherichia coli trigger factor constitute a domain that is necessary and sufficient for binding to ribosomes. J. Biol. Chem. 272:21865-21871. [DOI] [PubMed] [Google Scholar]
- 10.Hesterkamp, T., S. Hauser, H. Lütcke, and B. Bukau. 1996. Escherichia coli trigger factor is a prolyl isomerase that associates with nascent polypeptide chains. Proc. Natl. Acad. Sci. USA 93:4437-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, G. C., Z. Y. Li, J. M. Zhou, and G. Fischer. 2000. Assisted folding of d-glyceraldehyde-3-phosphate dehydrogenase by trigger factor. Protein Sci. 9:1254-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer, G., H. Patzelt, T. Rauch, T. A. Kurz, S. Vorderwulbecke, B. Bukau, and E. Deuerling. 2004. Trigger factor peptidyl-prolyl cis/trans isomerase activity is not essential for the folding of cytosolic proteins in Escherichia coli. J. Biol. Chem. 279:14165-14170. [DOI] [PubMed] [Google Scholar]
- 13.Kramer, G., T. Rauch, W. Rist, S. Vorderwülbecke, H. Patzelt, A. Schulze-Specking, N. Ban, E. Deuerling, and B. Bukau. 2002. L23 protein functions as a chaperone docking site on the ribosome. Nature 419:171-174. [DOI] [PubMed] [Google Scholar]
- 14.Lill, R., E. Crooke, B. Guthrie, and W. Wickner. 1988. The “Trigger factor cycle” includes ribosomes, presecretory proteins and the plasma membrane. Cell 54:1013-1018. [DOI] [PubMed] [Google Scholar]
- 15.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 16.Mogk, A., T. Tomoyasu, P. Goloubinoff, S. Rüdiger, D. Röder, H. Langen, and B. Bukau. 1999. Identification of thermolabile E. coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 18:6934-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patzelt, H., S. Rudiger, D. Brehmer, G. Kramer, S. Vorderwulbecke, E. Schaffitzel, A. Waitz, T. Hesterkamp, L. Dong, J. Schneider-Mergener, B. Bukau, and E. Deuerling. 2001. Binding specificity of Escherichia coli trigger factor. Proc. Natl. Acad. Sci. USA 98:14244-14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaffitzel, E., S. Rüdiger, B. Bukau, and E. Deuerling. 2001. Functional dissection of Trigger Factor and DnaK: interactions with nascent polypeptides and thermally denatured proteins. Biol. Chem. 382:1235-1243. [DOI] [PubMed] [Google Scholar]
- 19.Scholz, C., G. Stoller, T. Zarnt, G. Fischer, and F. X. Schmid. 1997. Cooperation of enzymatic and chaperone functions of trigger factor in the catalysis of protein folding. EMBO J. 16:54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoller, G., K. P. Ruecknagel, K. H. Nierhaus, F. X. Schmid, G. Fischer, and J.-U. Rahfeld. 1995. A ribosome-associated peptidyl-prolyl cis/trans isomerase identified as the trigger factor. EMBO J. 14:4939-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teter, S. A., W. A. Houry, D. Ang, T. Tradler, D. Rockabrand, G. Fischer, P. Blum, C. Georgopoulos, and F. U. Hartl. 1999. Polypeptide flux through bacterial Hsp70: DnaK cooperates with Trigger Factor in chaperoning nascent chains. Cell 97:755-765. [DOI] [PubMed] [Google Scholar]
- 22.Tomoyasu, T., A. Mogk, H. Langen, P. Goloubinoff, and B. Bukau. 2001. Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol. Microbiol. 40:397-413. [DOI] [PubMed] [Google Scholar]
- 23.Ullers, R. S., E. N. Houben, A. Raine, C. M. ten Hagen-Jongman, M. Ehrenberg, J. Brunner, B. Oudega, N. Harms, and J. Luirink. 2003. Interplay of signal recognition particle and trigger factor at L23 near the nascent chain exit site on the Escherichia coli ribosome. J. Cell Biol. 161:679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valent, Q. A., D. A. Kendall, S. High, R. Kusters, B. Oudega, and J. Luirink. 1995. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 14:5494-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vorderwulbecke, S., G. Kramer, F. Merz, T. A. Kurz, T. Rauch, B. Zachmann-Brand, B. Bukau, and E. Deuerling. 2004. Low temperature or GroEL/ES overproduction permits growth of Escherichia coli cells lacking trigger factor and DnaK. FEBS Lett. 559:181-187. [DOI] [PubMed] [Google Scholar]