Abstract
The mammalian testis possesses a special immunological environment because of its properties of remarkable immune privilege and effective local innate immunity. Testicular immune privilege protects immunogenic germ cells from systemic immune attack, and local innate immunity is important in preventing testicular microbial infections. The breakdown of local testicular immune homeostasis may lead to orchitis, an etiological factor of male infertility. The mechanisms underlying testicular immune privilege have been investigated for a long time. Increasing evidence shows that both a local immunosuppressive milieu and systemic immune tolerance are involved in maintaining testicular immune privilege status. The mechanisms underlying testicular innate immunity are emerging based on the investigation of the pattern recognition receptor-mediated innate immune response in testicular cells. This review summarizes our current understanding of testicular defense mechanisms and identifies topics that merit further investigation.
Keywords: immune privilege, innate immunity, testis
Introduction
Testicular defense mechanisms have two aspects: protection of auto-antigens from detrimental immune responses and counteraction of invading microbial pathogens. The mammalian testis represents an immune privileged organ where both allo- and auto-antigens can be tolerated without evoking immune rejection. The initial discovery of the testis as an immunoprivileged site was over 40 years ago when it was observed that allografts in the rat testis enabled long or indefinite survival.1 Testicular immune privilege is maintained through the coordination of systemic immune tolerance, the local physical structure and active local immunosuppression. The local immune modulatory milieu has been intensively investigated, but the mechanisms underlying systemic immune tolerance to male germ cell antigens are less understood. This review briefly outlines the properties of testicular immune privilege. Several recently published comprehensive reviews should be consulted for more in-depth information.2,3,4
The testis can be infected by various microbial pathogens derived from circulating blood or that ascend the genitourinary tract. To elicit an appropriate and effective local response against invading pathogens, testicular cells have to overcome immune privilege. This is accomplished by adopting effective antimicrobial innate immune responses. The roles of the pattern recognition receptors (PRRs) in initiating testicular innate immune responses are beginning to emerge. Testicular innate immunity is particularly critical when systemic immunity is reduced. Here, we discuss the local cellular innate immune defense system of the testis.
Impairment of immune homeostasis in the testis can result in orchitis, an etiological factor of male infertility. Orchitis is characterized by the infiltration of leukocytes into the testis and damage of the seminiferous epithelium.5 Although clinical orchitis is defined as an inflammatory disease due to microbial infection, testicular inflammation due to noninfectious factors, such as chemical and physical factors, trauma and neoplastic processes, should also be included.6 Notably, orchitis can perturb testicular functions and male fertility in humans, but natural infectious orchitis has not been observed in mice. Experimental autoimmune orchitis (EAO) that is induced by immunizing animals with testicular antigens is used as a model for elucidating the pathogenic mechanisms that are involved in testicular damage.7 The testicular immune components of EAO are documented in this review.
Immune privilege in the testis
Immune privilege implies a special immunological status found in several mammalian tissues, where allografts and xenografts have long survival rates.8 Immune privilege was discovered via tissue transplantation experiments that were conducted over a century ago involving transplantation of a tumor into the rabbit eye or rodent brain. Subsequent studies revealed that several mammalian tissues beyond the tissues of the eye and brain exhibit immune privilege. These tissues included the pregnant uterus and testis.8 The testis represents a distinct immunoprivileged site where both allo-antigens and immunogenic auto-antigens can be tolerated without evoking detrimental immune responses.9
Properties of testicular immune privilege
The phenomenon of testicular immune privilege emerged as early as 1767 when John Hunter transplanted a cock testis into the belly of a hen and subsequently recovered a testis of normal structure from the hen.10 Testicular transplantation was broadly performed among animals and humans between the 1910s to the 1930s.10 As a recipient site, the testis was initially found to protect follicle development in transplanted ovaries for a period of months.11 In the 1970s to the 1980s, a variety of allografts and xenografts were found to function in the testis for an extended amount of time.12 Notably, the survival time of insulin-secreting xenogeneic islets is significantly prolonged in the testis compared to other recipient sites.13
The testicular properties that provide immune privilege can also protect auto-antigenic germ cells from detrimental immune responses. During the development of an individual's immune system, the ability to tolerate self-antigens is acquired. A large number of auto-antigens, which are recognized as foreign molecules by the immune system, are produced by developing germ cells after immune competence is established. These auto-antigens induce strong autoimmune responses when they are injected into non-testicular sites.14 Based on this property, the transplantation of allo- and xeno-genetic germ cells into the testis has been a popular approach not only to study germ cell development but also to breed commercially viable and endangered species.15,16 Testicular immune privilege is not consistent among different species. Prolonged graft survival in the testis has been convincingly demonstrated in small laboratory animals, such as rats, mice and guinea pigs.1 However, the same studies conducted in other large species, such as sheep and monkeys, have been less successful for unknown reasons.17,18
Mechanisms underlying testicular immune privilege
Testicular immune privilege was initially proposed to be attributed to the absence of lymphatic drainage, which was challenged by the discovery of the afferent lymphatic vessels in the testis.19,20 The sequestration of auto-antigens from the immune system by the blood–testis barrier (BTB) was believed to be critical for testicular immune privilege. However, the interstitial spaces and early-stage germ cells that localize outside the BTB, including spermatogonia and preleptotene spermatocytes, also benefit from immune privilege.10,21 These observations suggest that other mechanisms are involved in the maintenance of testicular immune privilege. Multiple mechanisms and factors, including the physical structure, the local active immunosuppressive milieu and systemic immune tolerance, coordinate to regulate the immunoprivileged state in the testis.3,22
Testicular structure contributes to immune privilege
The testis is a complex organ with a unique physical structure and a large number of cell types. The mammalian testis consists of two distinct compartments: the seminiferous tubules and the interstitial spaces between the tubules (Figure 1). Spermatogenesis occurs within the seminiferous tubules and steroidogenesis is achieved by Leydig cells that are located in the interstitial spaces. These two processes are the dual functions of the testis.
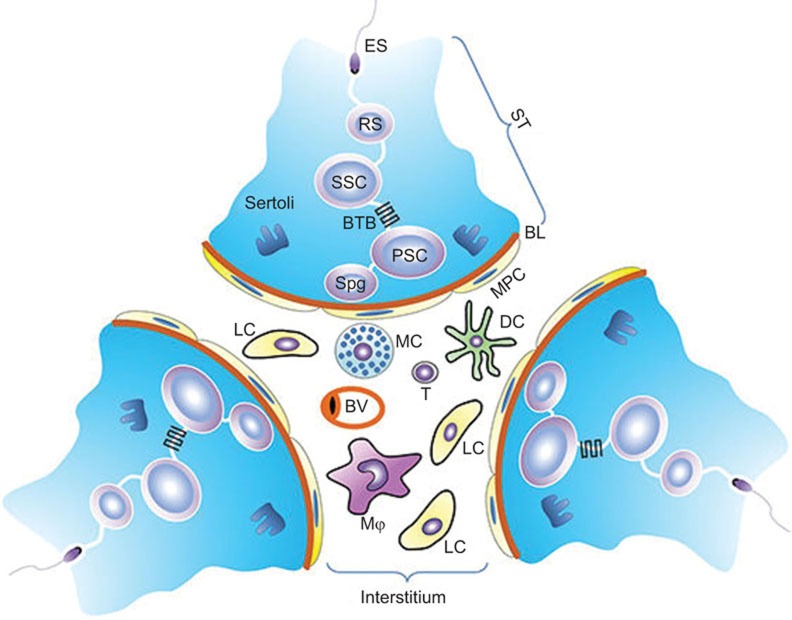
Figure 1.
Schematic of the mammalian testicular structure. The testis consists of two compartments: the ST and the interstitial space. The ST is surrounded by MPC, which together with Sertoli cells secrete substances to form the BL that encloses the seminiferous epithelium. The seminiferous epithelium is composed of different stages of developing germ cells, including SPG, PSCs, SSCs, RSs and ESs, which are surrounded by columnar Sertoli cells extending from the BL to the lumen of the seminiferous tubules. The BTB is formed by the junctions between neighboring Sertoli cells near the BL. BL, basal lamina; BTB, blood−testis barrier; ES, elongated spermatid; MPC, myoid peritubular cell; PSC, primary spermatocyte; RS, round spermatid; SPG, spermatogonia; SSC, secondary spermatocyte; ST, seminiferous tubule.
Seminiferous tubules
Seminiferous tubules are surrounded by myoid peritubular cells (MPCs). MPCs, together with Sertoli cells (SCs), secrete substances that form the basal lamina that encloses the seminiferous epithelium. The tubular wall comprises both MPCs and the basal lamina. The seminiferous epithelium is composed of columnar SCs extending from the basal lamina to the tubular lumen and developing germ cells that are encompassed by SCs. This epithelium forms the microenvironment for spermatogenesis (Figure 1). Although the tubular wall is arguably believed to contribute to the immune privilege within the tubules, the BTB formed by two adjacent SCs near the basal lamina certainly plays a role in separating the majority of germ cell antigens in the tubular lumen from the immunological components in the interstitial spaces. The BTB is created by several types of junctions, including the tight junction, the basal ectoplasmic specialization, the gap junction and the desmosome-like junction between two SCs, which divide the seminiferous epithelium into two parts: the basal and adluminal compartments.23 The BTB limits the access of systemic immune contents to the adluminal compartment and sequesters most of the auto-antigens of germ cells within the adluminal compartment. Therefore, the BTB has important functions in maintaining the immunoprivileged state of the testis, at least within the adluminal compartment.
In addition to their role in BTB formation, SCs have inherent immunosuppressive properties. Suppression of the immune responses by SCs through the secretion of immunosuppressive factors was determined more than two decades ago.24,25 Tissue transplantation studies confirmed that SCs provide immune protection resulting in prolonged survival of grafts after co-transplantation with SCs.26,27 Most cells undergo apoptosis during spermatogenesis, and the cytoplasmic compartments of sperm form residual bodies that are shed before maturation. Phagocytic removal of the apoptotic germ cells and residual bodies is critical in maintaining testicular homeostasis and normal spermatogenesis.28 Phagocytosis of apoptotic cells is a well-known process that regulates immunity and supports self-tolerance.29 We recently demonstrated that damaged germ cells induce inflammatory responses in the testis.30 Therefore, timely removal of apoptotic germ cells and residual bodies by the SCs is important to avoid autoimmune responses.
Male germ cells secrete various cytokines, including IL-1α and TNF-α, suggesting that germ cells may function in regulating the immune response.31,32 Fas ligand (FasL) is abundantly expressed in male germ cells.33 FasL-induced apoptosis of Fas-bearing lymphocytes is an important mechanism for suppression of immune responses.34 However, whether the FasL expressed in germ cells contributes to testicular immune privilege remains to be elucidated. Although SCs and germ cells may be important in suppressing immune responsiveness and contributing to immune privilege within the seminiferous tubules, the interstitial spaces are also immunoprivileged microenvironments.
Immunological contents in the interstitial spaces
The interstitial spaces represent only a small part of the testis, but they are composed of a large number of cell types. In particular, most types of immune cells can be found in the interstitial spaces. Macrophages are a major population of cells that represent approximately 20% of the total testicular interstitial cells in mice under physiological conditions.35 The macrophages have an important function in regulating the development and steroidogenesis of Leydig cells in rats.36 Macrophages belong to the family of antigen-presenting cells. However, testicular macrophages exhibit relatively low inflammatory responses and high immunosuppressive properties compared with the macrophages located in other tissues.37 Moreover, testicular macrophages in rats are less activated in response to pathogen stimulation, and they constitutively produce anti-inflammatory cytokines.38,39 These phenotypes support testicular immune privilege. By contrast, circulating macrophages significantly infiltrate the testis in orchitis and are detrimental to spermatogenesis.40,41 Clinical observation revealed high macrophage numbers in the testis of patients with aspermatogenesis and infertility indicating a negative correlation between circulating macrophages and spermatogenesis.42
Dendritic cells (DCs) exist in the testicular interstitial spaces and represent a minor population of the interstitial cells in the normal testis, reaching one-tenth of the numbers of macrophages. DC numbers significantly increase in EAO,43 suggesting that these cells may be involved in the testicular autoimmune response. DCs, the most powerful antigen-presenting cells, induce activation and differentiation of lymphocytes in response to allo-antigens, but also minimize the autoimmune response by tolerating T cells to auto-antigens under physiological conditions.44 The functions of DCs in the testis are not yet understood because of their small numbers. The DCs in the normal testis exhibit immature phenotypes.45 Testicular DCs and DCs from testicular draining lymph nodes do not activate lymphocytes under physiological conditions. This suggests that the testicular DCs have adopted tolerant status. In rat EAO, DCs exhibit mature properties.46
Lymphocytes are always found in the interstitial spaces of the rat testis under physiological conditions.47 Most testicular lymphocytes are T cells, with CD8+ cells being more predominant and CD4+ cells more rare. B cells are not found in the normal testis. In EAO and infertile patients with sperm autoimmunity, the number of lymphocytes is significantly high,48,49 suggesting that lymphocytes are involved in testicular pathogenesis under inflammatory conditions. The rat testis also contains immunoregulatory T cells, including natural killer (NK) T cells and CD4+CD25+ regulatory T cells (Tregs). Tregs are powerful immunosuppressive cells that promote peripheral tolerance and control the tolerogenic versus autoimmune response to sperm antigens in vasectomy models.50 Lymphocyte subsets shift in a rat testis undergoing autoimmune-related orchitis.51 Mouse testes that receive pancreatic islet cell allografts destroy memory T cells and recruit Tregs.52,53 These observations suggest that Tregs contribute to testicular immune privilege. The roles of NK cells have not yet been reported in the testis.
Mast cells are among the most significant immune cell populations in the testis. Mast cell increases are associated with male infertility.54 Mast cells increased more than 10-fold in number in EAO models.55 These cells secrete serine protease tryptase, which induces fibroblast proliferation and collagen synthesis by fibroblasts, thus resulting in tissue fibrosis and sclerosis.56 Accordingly, granuloma formation, a type of fibrosis in the testis, is frequently observed in infertile patients and in EAO models.57 In addition to the inflammatory regulation, mast cells are essential intermediaries for regulatory T-cell tolerance.58 However, the functions of the mast cells in maintaining testicular immune privilege remain unclear.
In addition to the immune cells, steroidogenic Leydig cells represent the majority of interstitial cells. Leydig cells synthesize androgens for both spermatogenesis and extratesticular androgen target organs.59 Previous studies demonstrated that rat Leydig cells exhibit innate antiviral ability in response to viral infection.60,61 Human Leydig cells show relatively weak antiviral activity compared with rat cells.62 These observations raise an interesting question whether the human testis has a weaker innate defense system against viral infection compared with the murine testis. Notably, microbial infection frequently results in orchitis in humans. By contrast, natural orchitis due to microbial infection has not been found in mice. Given that most studies of testicular immunity have been performed in murine models, the innate defense mechanisms in the human testis are relevant topics for future research. Leydig cells also regulate immune responses by affecting testicular macrophage and lymphocyte numbers.63 Androgens suppress autoimmune responses, which is associated with the immunological differences between the sexes.61 Androgens also regulate the testicular immunoprivileged status.64,65,66 Notably, androgens would not directly affect testicular immune cells because these cells lack the androgen receptor.
MPCs are located outside of the BTB and conveniently communicate with the interstitial cells. The contractile ability of MPCs to facilitate transport of spermatozoa from the testis to the epididymis is their best-characterized function.67 Based on their localization, MPCs are believed to regulate the testicular immune environment. MPCs secrete numerous cytokines, including pro-inflammatory and anti-inflammatory factors, under physiological and inflammatory conditions.22,68 The functions of MPCs that are involved in regulating testicular immunity are worthy of further investigation.
Endocrine and paracrine controls of testicular immune privilege
Both endocrine and paracrine networks coordinate to regulate testicular immune privilege (Figure 2). The androgens synthesized by Leydig cells suppress both systemic and testicular immune responses to auto-antigens. Moreover, several negative regulatory immune systems have been found in the testis. In particular, numerous paracrine cytokines, including various anti-inflammatory factors, would contribute to the maintenance of testicular immune privilege.
Figure 2.
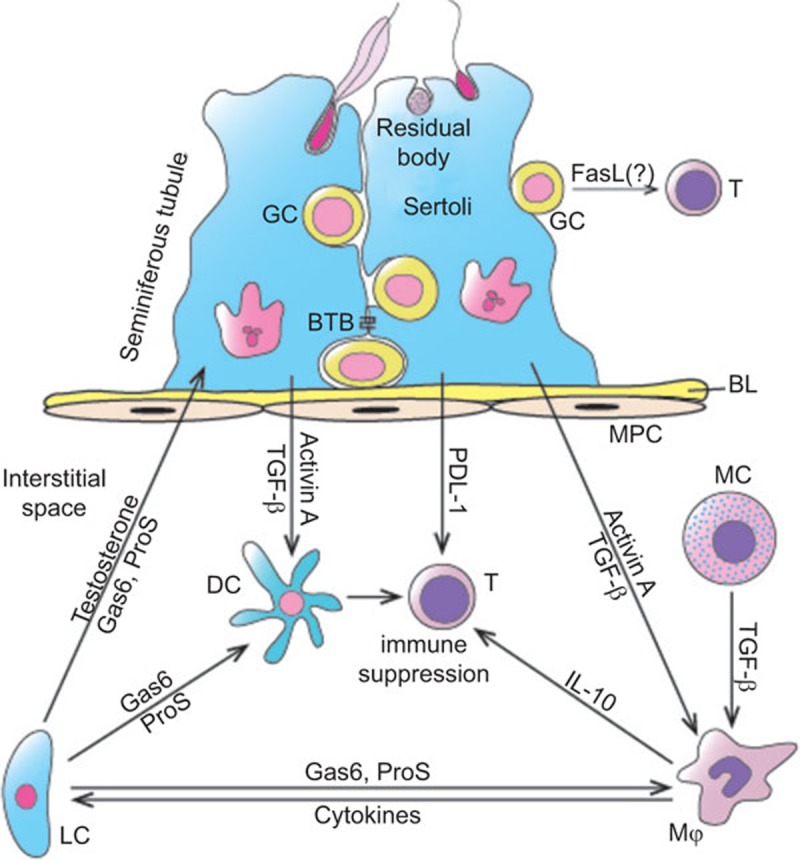
Schematic of immunosuppressive molecules that support immune privilege in the testis. SCs and LCs secrete multiple immunosuppressive factors, including activin A, TGF-β, PDL-1, Gas6, ProS and testosterone, which directly or indirectly suppress immune cell activation. Mϕ and MCs exhibit immunosuppressive properties by producing anti-inflammatory factors, such as IL-10 and TGF-β. Germ cells abundantly express FasL. Whether FasL induces apoptosis of T lymphocytes that may infiltrate the seminiferous tubules under inflammatory conditions remains unclear. FasL, Fas ligand; Gas6, growth arrest-specific gene 6; LC, Leydig cell; MC, mast cell; Mϕ, testicular macrophages; PDL-1, programmed death ligand-1; ProS, protein S; SC, Sertoli cell; TGF-β, transforming growth factor β.
Endocrine hormones
Leydig cells synthesize androgens upon luteinizing hormone regulation. Androgen administration suppresses autoimmune disease.69 Luteinizing hormone antagonists reduce the levels of Tregs and increase the levels of NK cells in men.70 Testosterone inhibits EAO induction in rats.66 Within the testis, the androgens act on SCs that express the androgen receptor. Conditional knockout of the androgen receptor in SCs in mice impairs testicular immune privilege, possibly due to the impairment of BTB permeability.65,71 Taken together, androgens would contribute to testicular immune privilege by negatively regulating the local immune responses and systemic tolerance to auto-antigens.
Negative immunoregulatory systems
Several immunosuppressive systems have been identified in the murine testis. The Fas/FasL system suppresses immune responses by inducing the apoptosis of Fas-bearing activated lymphocytes.72 FasL is abundantly expressed in the testis.73 This system was demonstrated to be critical in maintaining testicular immune privilege by inducing lymphocyte apoptosis via FasL expression in SCs.74 This conclusion had been challenged by the observation that neutralizing antibodies to FasL did not reduce the survival of islets in diabetic mice after cotransplantation with SCs.75 FasL is predominantly expressed in male germ cells, but not in SCs.33 Whether FasL that is expressed in the germ cells induces lymphocyte apoptosis and contributes to immune privilege within the seminiferous tubules remains to be investigated.
Programmed death receptor-1/programmed death ligand-1 (PD-1/PD-L1) is another T-cell tolerance system. PD-L1 inhibits T-cell activation through PD-1.76 It is constitutively expressed in the testis and involved in the survival of islet allografts, suggesting that the PD-1/PD-L1 system is a mechanism that underlies testicular immune privilege.77
The growth arrest-specific gene 6 (Gas6)/Protein S (ProS)-Tyro3, Axl and Mer (TAM) system is a negative regulatory immune system.78 We showed that TAM receptor tyrosine kinases and their common ligands, Gas6 and ProS, regulate immune homeostasis in the mouse testis. TAM receptors are abundantly expressed in SCs and Leydig cells, whereas Gas6 and ProS are predominantly produced by Leydig cells.79 Male TAM triple knockout (TAM−/−) mice are infertile and develop chronic orchitis.80,81,82 Multiple mechanisms can be involved in the regulation of testicular immunity by the Gas6/ProS-TAM system. TAM receptors are negative regulators of systemic innate immunity.83 We also demonstrated that TAM signaling inhibits the innate immune responses in SCs and Leydig cells.84,85 Moreover, the Gas6/ProS-TAM system facilitates phagocytic clearance of apoptotic germ cells by SCs.86 The removal of apoptotic germ cells by phagocytes facilitates the elimination of the auto-antigens, which may reduce endogenous inflammation. We recently demonstrated that damaged germ cells induce endogenous inflammatory responses in the testis.30 Therefore, the Gas6/ProS-TAM system plays roles in maintaining testicular homeostasis by inhibiting local innate immune responses and facilitating the clearance of auto-antigens.
Immunosuppressive factors
Numerous local immunoregulatory cytokines, including both pro-inflammatory and anti-inflammatory factors, are involved in the regulation of the testicular immune environment.22 The anti-inflammatory factors can actively suppress the immune response in the testis. The transforming growth factors (TGF) β1−β3 are constitutively expressed in the testis and predominantly produced by SCs.87,88 As an anti-inflammatory factor, TGF-β1 is implicated in the protection of islet β-cell grafts after co-transplantation with SCs.27 SCs also abundantly express activin A and B.89 The activins have functions in SC development and initiation of spermatogenesis.90,91 Activin A inhibits the expression of pro-inflammatory cytokines, including IL-1 and IL-6, thus suppressing the testicular inflammatory responses.92 IL-10 is also a well-defined anti-inflammatory factor in the testis. IL-10 can be induced by orchitis and is predominantly produced by testicular macrophages.93 Overexpression of IL-10 in the testis significantly reduces the levels of inflammation in EAO models.94 Therefore, multiple immunosuppressive factors are involved in the maintenance of immune homeostasis in testicular tissue.
Testicular innate immunity
Although the testis is an immunoprivileged site where systemic immune responses are remarkably reduced, microbial pathogens that invade from both the circulating blood and via the ascending male genitourinary tract are usually eliminated. This phenomenon suggests that the testis has effective local innate immunity against invading pathogens. The testicular innate antiviral responses have been previously demonstrated based on a series of pioneering studies on the expression and regulation of both interferons (IFNs) and antiviral proteins in the testis by Professor Jégou's group.60,61,62,95,96 These investigators also showed that several defensins are expressed in murine and human testes, and may contribute to the innate antimicrobial defense.97 Recent studies have revealed that various PRRs are abundantly expressed in testicular cells and initiate testicular innate immune responses. The PRR-initiated innate responses would be important for testicular cells to overcome immune privilege and elicit an appropriate local response against pathogen invasion. PRRs are a superfamily of receptors that can be activated by conserved molecular structures of microbial pathogens, termed pathogen-associated molecule patterns (PAMPs). PRR activation initiates the innate immune response and subsequently drives the adaptive immune response involved in counteracting microbes.98 PRRs can also be activated by endogenous auto-antigens released from damaged tissues and necrotic cells, termed damage-associated molecular patterns (DAMPs) and trigger endogenous inflammation.99 Several subfamilies of PRRs have been identified.100 Toll-like receptors (TLRs) are the best characterized, and 13 TLR members have been found in mammals. The cytosolic double-stranded RNA (dsRNA) sensors, termed retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), include two functional members, namely, melanoma differentiation-associated protein 5 (MDA5) and RIG-I. The NOD-like receptor (NLR) subfamily contains a large number of cytoplasmic PRRs that recognize a broad spectrum of PAMPs and DAMPs. Moreover, intracellular DNA sensors have recently emerged.101 Since the first investigation of TLRs in testicular SCs,102 several PRRs that initiate the testicular innate immune responses have been described, and their signaling within testicular cells has been documented (Figure 3). Table 1 shows a summary of the expression and function of PRRs in testicular cells.
Figure 3.
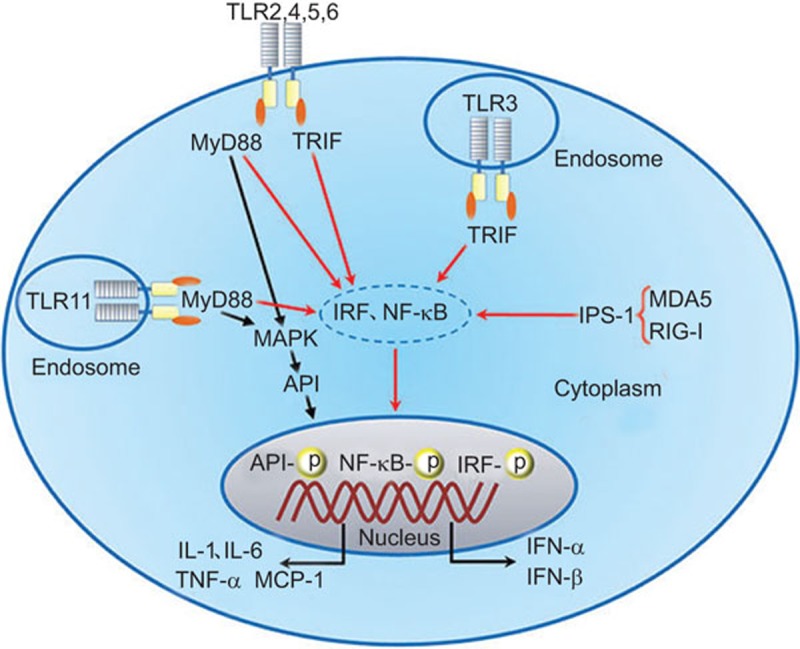
PRR signaling in testicular cells. Several TLRs, including TLR2–TLR6, are expressed and functional in Sertoli cells.102,106,107 TLR3, TLR4, MDA5 and RIG-I initiate the innate immune response in Leydig cells.85,112 TLR3, TLR11 and MDA5 are functional in germ cells.108,109,112 After ligand recognition, the TLR conformation changes due to the recruitment of adaptors, including the MyD88 and/or the TRIF. All other TLRs exclusively initiate the MyD88-dependent pathway, except for TLR3 and TLR4. TLR3 exclusively triggers the TRIF-dependent pathway. TLR4 initiates both the MyD88- and TRIF-dependent pathways. MDA5 and RIG-I activation recruits IPS-1, thus initiating the IPS-1-dependent signaling pathway. Different PRR signaling pathways activate multiple transcription factors, including AP1 and NF-κB, to induce pro-inflammatory cytokine expression, as well as IRFs, for induction of type 1 IFN-α/β. AP1, activator protein 1; IFN, interferon; IPS-1, IFN-β promoter stimulator 1; IRF, interferon regulatory factor; MDA5, melanoma differentiation-associated protein 5; MyD88, myeloid differentiation protein 88; PRR, pattern recognition receptor; RIG-I, retinoic acid-inducible gene I; TLR, Toll-like receptor; TRIF, Toll/IL-1 receptor domain-containing adaptor protein-inducing interferon-β.
Table 1. Functions of PRRs in initiating innate immune response in mouse testicular cells.
| Cell types | PRRs | Ligands | Signaling | Immune molecules | Ref. |
|---|---|---|---|---|---|
| Sertoli cells | TLR2 | LipoproteinDAMPs | NF-κBMAPKs | TNF-α, IL-1β, IL-6, MCP-1 | 27,99,103 |
| TLR3 | Poly(I:C) | IRF3/NF-κB | TNF-α, IL-6, IFN-α, IFN-β | 81,104 | |
| TLR4 | LPSDAMPs | NF-κB, MAPKs, IRF3 | TNF-α, IL-1β, IL-6, MCP-1, IFN-α, IFN-β | 27,99,103 | |
| TLR5 | Flagellin | NF-κB, MAPKs | TNF-α, IL-1, IL-6, MCP-1 | 103 | |
| TLR6 | Peptidoglycan | NF-κB, MAPKs | TNF-α, IL-1, IL-6 | 103 | |
| Leydig cells | TLR3 | Poly(I:C) | IRF3NF-κB | TNF-α, IL-6, IFN-α, IFN-βAntiviral proteins | 82 |
| TLR4 | LPS | NF-κBIRF3 | TNF-α, IL-1β, IL-6IFN-α, IFN-β | 82 | |
| RIG-I | Poly(I:C) | IRF3NF-κB | IFN-β, TNF-α, IL-6Antiviral proteins | 109 | |
| MDA5 | Poly(I:C) | IRF3NF-κB | IFN-β, TNF-α, IL-6Antiviral proteins | 109 | |
| SpermatogniaSpermatocytes | TLR3 | poly(I:C) | IRF3NF-κB | IFN-α, IFN-β, TNF-α, IL-6Antiviral proteins | 105 |
| Spermatids | TLR11 | ProfillinUPEC | NF-κB, MAPKsIRF3 | TNF-α, IL-6, MCP-1IL-12, IFN-β, IFN-γ | 106 |
| MDA5 | Poly(I:C) | IRF3 | IFN-β, TNF-α, IL-6Antiviral proteins | 109 |
Abbreviations: DAMP, damage-associated molecular pattern; IFN, interferon; IRF, interferon regulatory factor; LPS, lipopolysaccharide; MDA5, melanoma differentiation-associated protein 5; PRR, pattern recognition receptor; RIG-I, retinoic acid-inducible gene I; TLR, Toll-like receptor.
TLRs in testicular cells
TLRs are the best-characterized PRRs in the testis.103 Functional TLRs were initially demonstrated within murine SCs, showing that TLR2 and TLR4 signaling can be initiated by their ligands.102 TLR expression was then further defined in male rat reproductive tracts and testicular cells.104,105 The functions of more TLRs, including TLR3, TLR5 and TLR6, were investigated in mouse SCs by our group and by other researchers.106,107 TLRs initiate the innate immune response in SCs by inducing immunoregulatory cytokines, including TNF-α, IL-1, IL-6, MCP-1 and type 1 IFNs (Table 1). In addition to SCs, we also found that TLR3 and TLR4 are expressed and are functional in murine Leydig cells.85 Interestingly, TLR-initiated innate immune responses in Sertoli and Leydig cells are negatively regulated by Gas6/ProS-TAM signaling, which may play roles in avoiding sustained inflammatory conditions that could impair testicular functions.84,85 Different stages of germ cells also express TLRs.105 We recently demonstrated that TLR3 in spermatogonia and spermatocytes and TLR11 in spermatids initiate innate immune responses.108,109 The TLR-initiated innate immune responses in male germ cells are particularly interesting because the germ cells represent a majority of the testicular cell population. Moreover, the adluminal compartments of the seminiferous tubules are separated from the interstitial immune components by the BTB. Therefore, the innate immune responses in the germ cells and SCs within the seminiferous tubules would be critical to counteracting the invading pathogens from the ascending genitourinary tract. The innate defense function of male germ cells is worthy of further investigation.
The RLR-initiated innate antiviral response
RLRs are cytosolic dsRNA sensors, which recognize viral dsRNA that are produced by many types of viruses during replication, thus initiating antiviral immune responses.110 RLRs can also be activated by the synthetic dsRNA analog, polyinosinic–polycytidylic acid (Poly(I:C)). The RLR subfamily contains three members: RIG-I, MDA5 and laboratory of genetics and physiology 2. Laboratory of genetics and physiology 2 does not induce an innate immune response due to its lack of the domain responsible for triggering signaling.111 By contrast, both RIG-I and MDA5 initiate innate antiviral responses following recognition of viral dsRNA. We recently demonstrated that RIG-I and MDA5 are abundantly expressed in mouse Leydig cells, and MDA5 is also expressed in spermatids.112 Poly(I:C) triggers innate antiviral responses in both Leydig cells and spermatids through RIG-I/MDA5-mediated signaling. Poly(I:C) induces the expression of IFN-α/β and several antiviral proteins in Leydig cells and germ cells (Table 1). The innate antiviral state in mouse testicular cells is interesting because viral infection can cause chronic orchitis and perturb testicular functions and male fertility in humans.6 However, natural viral orchitis has not been observed in mice. Whether human testicular cells possess weaker antiviral capacities compared with their murine counterparts is worthy of investigation via analysis of human samples. Understanding the different mechanisms underlying the innate defense system between murine and human testes would aid in the development of therapeutic and preventive strategies for orchitis.
Other PRRs in the testis
In addition to TLRs and RLRs, NLRs belong to another subfamily of intracellular PRRs that are characterized by a common NOD motif.113 More than 20 NLR members have been identified in humans, and these NLRs recognize a broad spectrum of PAMPs and DAMPs. NLRs exhibit different functions in the defense against pathogens. Some of these NLRs, such as NOD1 and NOD2, induce inflammatory cytokine expression.113 Other NLRs are involved in the processing and activation of inflammatory cytokines, including IL-1β and IL-18, which are activators of inflammasomes.114 Both NOD1 and NOD2 mRNAs were detected in some testicular cells, including SCs and germ cells,105 but their functions in these cells have not been examined. Many NLR members are involved in immune modulation as inflammasome activators.115 The functions of the inflammasomes in the testis remain to be clarified. The antiviral functions of cytosolic DNA sensors have been determined.101 The functions of DNA sensors in the testis are worthy of investigation in order to ascertain the full contributions of the PRR-mediated testicular innate immune responses to the testicular defense against different pathogens.
Concluding remarks
The testis exhibits special defense mechanisms considering its remarkable immunoprivileged status and effective local innate immunity. Disruption of the testicular immunological environment may lead to chronic orchitis, which is a significant etiological factor of male infertility. Further understanding of the mechanisms underlying testicular immune homeostasis has important implications for the intervention of male immunological subfertility. The function of PRRs and their role in negatively regulating systemic tolerance to testicular auto-antigens are relevant areas for future research. In PRR-initiated testicular innate immunity, the functions of inflammasomes and cytosolic DNA sensors need further elucidation. The innate defense functions of germ cells deserve great attention because of the large number of these unique cells within the testis. Notably, the innate immune response is a ‘double-edge sword'. Defense against microbial pathogens is critical for hosts to recover from infection. However, the inflammatory milieu may cause damage to the host. The detrimental effects of inflammatory cytokines that are secreted by the testicular cells need to be clarified. The investigations on these topics will further improve our understanding of the testicular defense mechanisms and may provide novel clues that will be useful for translational medicine.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants: 31171445, 31261160491 and 31371518).
References
- Head JR, Neaves WB, Billingham RE. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation. 1983;36:423–431. doi: 10.1097/00007890-198310000-00014. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Immune Privilege of the Testis: Meaning, Mechanisms, and Manifestations. Infection, Immune Homeostasis and Immune Privilege. New York: Springer; 2012. pp. 31–52. [Google Scholar]
- Li N, Wang T, Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol. 2012;3:152. doi: 10.3389/fimmu.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Mital P, Dufour J. Testis immune privilege—assumptions versus facts. Anim Reprod. 2013;10:3–15. [PMC free article] [PubMed] [Google Scholar]
- Weidner W, Krause W.OrchitisIn: Knobil E, Neill JD (ed.)Encyclopedia of Reproduction San Diego, CA: Academic Press; 1998524–527. [Google Scholar]
- Schuppe HC, Meinhardt A, Allam JP, Bergmann M, Weidner W, Haidl G. Chronic orchitis: a neglected cause of male infertility. Andrologia. 2008;40:84–91. doi: 10.1111/j.1439-0272.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- Jacobo P, Guazzone VA, Theas MS, Lustig L. Testicular autoimmunity. Autoimmun Rev. 2011;10:201–204. doi: 10.1016/j.autrev.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Simpson E. A historical perspective on immunological privilege. Immunol Rev. 2006;213:12–22. doi: 10.1111/j.1600-065X.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- Setchell BP. The testis and tissue transplantation: historical aspects. J Reprod Immunol. 1990;18:1–8. doi: 10.1016/0165-0378(90)90020-7. [DOI] [PubMed] [Google Scholar]
- Sand K. Experiments on the internal secretion of the sexual glands, especially on experimental hermaphroditism. J Physiol. 1919;53:257–263. doi: 10.1113/jphysiol.1919.sp001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010;139:495–504. doi: 10.1530/REP-09-0384. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Chick WL. Pancreatic Islet Transplantation: Immunomodulation of Pancreatic Islets. Georgetown, TX: R.G. Landes; 1994. [Google Scholar]
- Tung KS, Teuscher C, Meng AL. Autoimmunity to spermatozoa and the testis. Immunol Rev. 1981;55:217–255. doi: 10.1111/j.1600-065x.1981.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RC, Costa GM, Lacerda SM, Batlouni SR, Soares JM, Avelar GF, et al. Germ cell transplantation in felids: a potential approach to preserving endangered species. J Androl. 2012;33:264–276. doi: 10.2164/jandrol.110.012898. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Granholm T, Ritzen EM. Failure of thyroid allografts to function in the testes of cynomolgous monkeys. J Reprod Immunol. 1995;28:75–80. doi: 10.1016/0165-0378(94)00897-g. [DOI] [PubMed] [Google Scholar]
- Maddocks S, Setchell BP. The rejection of thyroid allografts in the ovine testis. Immunol Cell Biol. 1988;66 Pt 1:1–8. doi: 10.1038/icb.1988.1. [DOI] [PubMed] [Google Scholar]
- Fijak M, Bhushan S, Meinhardt A. Immunoprivileged sites: the testis. Methods Mol Biol. 2011;677:459–470. doi: 10.1007/978-1-60761-869-0_29. [DOI] [PubMed] [Google Scholar]
- Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol. 1988;141:1161–1167. [PubMed] [Google Scholar]
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–68. doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The blood–testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CR, Law L, Magnuson JA, Griswold MD, Magnuson NS. Suppression of lymphocyte proliferation by proteins secreted by cultured Sertoli cells. J Reprod Immunol. 1988;14:27–40. doi: 10.1016/0165-0378(88)90033-2. [DOI] [PubMed] [Google Scholar]
- de Cesaris P, Filippini A, Cervelli C, Riccioli A, Muci S, Starace G, et al. Immunosuppressive molecules produced by Sertoli cells cultured in vitro: biological effects on lymphocytes. Biochem Biophys Res Commun. 1992;186:1639–1646. doi: 10.1016/s0006-291x(05)81596-7. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Borlongan CV, Saporta S, Cameron DF. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat Biotechnol. 1996;14:1692–1695. doi: 10.1038/nbt1296-1692. [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon W, Korbutt GS, Power R, Hooton J, Rajotte RV, Rabinovitch A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes. 2000;49:1810–1818. doi: 10.2337/diabetes.49.11.1810. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Shiratsuchi A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biol Pharm Bull. 2004;27:13–16. doi: 10.1248/bpb.27.13. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Asano K, Qiu CH. Immune regulation by apoptotic cell clearance. Ann NY Acad Sci. 2010;1209:37–42. doi: 10.1111/j.1749-6632.2010.05746.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang T, Deng T, Xiong W, Lui P, Li N, et al. Damaged spermatogenic cells induce inflammatory gene expression in mouse Sertoli cells through the activation of Toll-like receptors 2 and 4. Mol Cell Endocrinol. 2013;365:162–173. doi: 10.1016/j.mce.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Haugen TB, Landmark BF, Josefsen GM, Hansson V, Hogset A. The mature form of interleukin-1 alpha is constitutively expressed in immature male germ cells from rat. Mol Cell Endocrinol. 1994;105:R19–R23. doi: 10.1016/0303-7207(94)90177-5. [DOI] [PubMed] [Google Scholar]
- De SK, Chen HL, Pace JL, Hunt JS, Terranova PF, Enders GC. Expression of tumor necrosis factor-alpha in mouse spermatogenic cells. Endocrinology. 1993;133:389–396. doi: 10.1210/endo.133.1.8319585. [DOI] [PubMed] [Google Scholar]
- D'Alessio A, Riccioli A, Lauretti P, Padula F, Muciaccia B, de Cesaris P, et al. Testicular FasL is expressed by sperm cells. Proc Natl Acad Sci USA. 2001;98:3316–3321. doi: 10.1073/pnas.051566098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Macrophages and the immune responsiveness of the testis. J Reprod Immunol. 2002;57:19–34. doi: 10.1016/s0165-0378(02)00016-5. [DOI] [PubMed] [Google Scholar]
- Hutson JC. Physiologic interactions between macrophages and Leydig cells. Exp Biol Med (Maywood) 2006;231:1–7. doi: 10.1177/153537020623100101. [DOI] [PubMed] [Google Scholar]
- Kern S, Robertson SA, Mau VJ, Maddocks S. Cytokine secretion by macrophages in the rat testis. Biol Reprod. 1995;53:1407–1416. doi: 10.1095/biolreprod53.6.1407. [DOI] [PubMed] [Google Scholar]
- Winnall WR, Muir JA, Hedger MP. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J Leukoc Biol. 2011;90:133–143. doi: 10.1189/jlb.1010557. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Hossain H, Lu Y, Geisler A, Tchatalbachev S, Mikulski Z, et al. Uropathogenic E. coli induce different immune response in testicular and peritoneal macrophages: implications for testicular immune privilege. PLoS ONE. 2011;6:e28452. doi: 10.1371/journal.pone.0028452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theas MS, Rival C, Jarazo-Dietrich S, Jacobo P, Guazzone VA, Lustig L. Tumour necrosis factor-alpha released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum Reprod. 2008;23:1865–1872. doi: 10.1093/humrep/den240. [DOI] [PubMed] [Google Scholar]
- Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, van Rooijen N, et al. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J Pathol. 2008;215:108–117. doi: 10.1002/path.2328. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Calandra RS, Lustig L, Meineke V, Kohn FM, Vogt HJ, et al. Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil Steril. 2002;78:298–306. doi: 10.1016/s0015-0282(02)03206-5. [DOI] [PubMed] [Google Scholar]
- Rival C, Lustig L, Iosub R, Guazzone VA, Schneider E, Meinhardt A, et al. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006;324:311–318. doi: 10.1007/s00441-005-0129-5. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Rival C, Guazzone VA, von Wulffen W, Hackstein H, Schneider E, Lustig L, et al. Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol Hum Reprod. 2007;13:853–861. doi: 10.1093/molehr/gam067. [DOI] [PubMed] [Google Scholar]
- Guazzone VA, Hollwegs S, Mardirosian M, Jacobo P, Hackstein H, Wygrecka M, et al. Characterization of dendritic cells in testicular draining lymph nodes in a rat model of experimental autoimmune orchitis. Int J Androl. 2011;34:276–289. doi: 10.1111/j.1365-2605.2010.01082.x. [DOI] [PubMed] [Google Scholar]
- Hedger MP, Meinhardt A. Local regulation of T cell numbers and lymphocyte-inhibiting activity in the interstitial tissue of the adult rat testis. J Reprod Immunol. 2000;48:69–80. doi: 10.1016/s0165-0378(00)00071-1. [DOI] [PubMed] [Google Scholar]
- Lustig L, Lourtau L, Perez R, Doncel GF. Phenotypic characterization of lymphocytic cell infiltrates into the testes of rats undergoing autoimmune orchitis. Int J Androl. 1993;16:279–284. doi: 10.1111/j.1365-2605.1993.tb01192.x. [DOI] [PubMed] [Google Scholar]
- el-Demiry MI, Hargreave TB, Busuttil A, Elton R, James K, Chisholm GD. Immunocompetent cells in human testis in health and disease. Fertil Steril. 1987;48:470–479. doi: 10.1016/s0015-0282(16)59421-7. [DOI] [PubMed] [Google Scholar]
- Wheeler K, Tardif S, Rival C, Luu B, Bui E, del Rio R, et al. Regulatory T cells control tolerogenic versus autoimmune response to sperm in vasectomy. Proc Natl Acad Sci USA. 2011;108:7511–7516. doi: 10.1073/pnas.1017615108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo P, Guazzone VA, Jarazo-Dietrich S, Theas MS, Lustig L. Differential changes in CD4+ and CD8+ effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J Reprod Immunol. 2009;81:44–54. doi: 10.1016/j.jri.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Dai Z, Nasr IW, Reel M, Deng S, Diggs L, Larsen CP, et al. Impaired recall of CD8 memory T cells in immunologically privileged tissue. J Immunol. 2005;174:1165–1170. doi: 10.4049/jimmunol.174.3.1165. [DOI] [PubMed] [Google Scholar]
- Nasr IW, Wang Y, Gao G, Deng S, Diggs L, Rothstein DM, et al. Testicular immune privilege promotes transplantation tolerance by altering the balance between memory and regulatory T cells. J Immunol. 2005;174:6161–6168. doi: 10.4049/jimmunol.174.10.6161. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Abou-Deif ES, Bedaiwy MA, Said TM, Mustafa MG, Nada E, et al. Phenotypic characterization of the immune and mast cell infiltrates in the human testis shows normal and abnormal spermatogenesis. Fertil Steril. 2005;83:1447–1453. doi: 10.1016/j.fertnstert.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Iosub R, Klug J, Fijak M, Schneider E, Frohlich S, Blumbach K, et al. Development of testicular inflammation in the rat involves activation of proteinase-activated receptor-2. J Pathol. 2006;208:686–698. doi: 10.1002/path.1938. [DOI] [PubMed] [Google Scholar]
- Abe M, Kurosawa M, Ishikawa O, Miyachi Y, Kido H. Mast cell tryptase stimulates both human dermal fibroblast proliferation and type I collagen production. Clin Exp Allergy. 1998;28:1509–1517. doi: 10.1046/j.1365-2222.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- Apa DD, Cayan S, Polat A, Akbay E. Mast cells and fibrosis on testicular biopsies in male infertility. Arch Androl. 2002;48:337–344. doi: 10.1080/01485010290099183. [DOI] [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Diemer T, Hales DB, Weidner W. Immune-endocrine interactions and Leydig cell function: the role of cytokines. Andrologia. 2003;35:55–63. doi: 10.1046/j.1439-0272.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- Dejucq N, Lienard MO, Guillaume E, Dorval I, Jegou B. Expression of interferons-alpha and -gamma in testicular interstitial tissue and spermatogonia of the rat. Endocrinology. 1998;139:3081–3087. doi: 10.1210/endo.139.7.6083. [DOI] [PubMed] [Google Scholar]
- Melaine N, Lienard MO, Guillaume E, Ruffault A, Dejucq-Rainsford N, Jegou B. Production of the antiviral proteins 2′5′oligoadenylate synthetase, PKR and Mx in interstitial cells and spermatogonia. J Reprod Immunol. 2003;59:53–60. doi: 10.1016/s0165-0378(02)00061-x. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A, Denis H, Satie AP, Patard JJ, Ruffault A, Jegou B, et al. Antiviral responses of human Leydig cells to mumps virus infection or poly I:C stimulation. Hum Reprod. 2008;23:2095–2103. doi: 10.1093/humrep/den207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raburn DJ, Coquelin A, Reinhart AJ, Hutson JC. Regulation of the macrophage population in postnatal rat testis. J Reprod Immunol. 1993;24:139–151. doi: 10.1016/0165-0378(93)90016-b. [DOI] [PubMed] [Google Scholar]
- De Carolis S, Botta A, Fatigante G, Garofalo S, Ferrazzani S, Gasbarrini A, et al. Celiac disease and inflammatory bowel disease in pregnancy. Lupus. 2004;13:653–658. doi: 10.1191/0961203304lu1096oa. [DOI] [PubMed] [Google Scholar]
- Meng J, Greenlee AR, Taub CJ, Braun RE. Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice. Biol Reprod. 2011;85:254–260. doi: 10.1095/biolreprod.110.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, et al. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011;186:5162–5172. doi: 10.4049/jimmunol.1001958. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Kamimura K, Nagano T. Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol. 1996;59:1–13. doi: 10.1679/aohc.59.1. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A. Human testicular peritubular cells: more than meets the eye. Reproduction. 2013;145:R107–R116. doi: 10.1530/REP-12-0497. [DOI] [PubMed] [Google Scholar]
- Cutolo M. Androgens in rheumatoid arthritis: when are they effectors. Arthritis Res Ther. 2009;11:126. doi: 10.1186/ar2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood–testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- Korbutt GS, Suarez-Pinzon WL, Power RF, Rajotte RV, Rabinovitch A. Testicular Sertoli cells exert both protective and destructive effects on syngeneic islet grafts in non-obese diabetic mice. Diabetologia. 2000;43:474–480. doi: 10.1007/s001250051331. [DOI] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Dai H, Wan N, Moore Y, Vankayalapati R, Dai Z. Interaction of programmed death-1 and programmed death-1 ligand-1 contributes to testicular immune privilege. Transplantation. 2009;87:1778–1786. doi: 10.1097/TP.0b013e3181a75633. [DOI] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen Y, Ge Y, Ma P, Ma Q, Ma J, et al. Immunoexpression of Tyro 3 family receptors—Tyro 3, Axl, and Mer—and their ligand Gas6 in postnatal developing mouse testis. J Histochem Cytochem. 2005;53:1355–1364. doi: 10.1369/jhc.5A6637.2005. [DOI] [PubMed] [Google Scholar]
- Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang H, Qi N, Wu H, Xiong W, Ma J, et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction. 2009;138:655–666. doi: 10.1530/REP-09-0101. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li N, Chen Q, Yan K, Liu Z, Zhang X, et al. Breakdown of immune homeostasis in the testis of mice lacking Tyro3, Axl and Mer receptor tyrosine kinases. Immunol Cell Biol. 2013;91:416–426. doi: 10.1038/icb.2013.22. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Sun B, Qi N, Shang T, Wu H, Deng T, Han D. Sertoli cell-initiated testicular innate immune response through Toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology. 2010;151:2886–2897. doi: 10.1210/en.2009-1498. [DOI] [PubMed] [Google Scholar]
- Shang T, Zhang X, Wang T, Sun B, Deng T, Han D. Toll-like receptor-initiated testicular innate immune responses in mouse Leydig cells. Endocrinology. 2011;152:2827–2836. doi: 10.1210/en.2011-0031. [DOI] [PubMed] [Google Scholar]
- Xiong W, Chen Y, Wang H, Wang H, Wu H, Lu Q, et al. Gas6 and the Tyro 3 receptor tyrosine kinase subfamily regulate the phagocytic function of Sertoli cells. Reproduction. 2008;135:77–87. doi: 10.1530/REP-07-0287. [DOI] [PubMed] [Google Scholar]
- Mullaney BP, Skinner MK. Transforming growth factor-beta (beta 1, beta 2, and beta 3) gene expression and action during pubertal development of the seminiferous tubule: potential role at the onset of spermatogenesis. Mol Endocrinol. 1993;7:67–76. doi: 10.1210/mend.7.1.8446109. [DOI] [PubMed] [Google Scholar]
- Avallet O, Vigier M, Leduque P, Dubois PM, Saez JM. Expression and regulation of transforming growth factor-beta 1 messenger ribonucleic acid and protein in cultured porcine Leydig and Sertoli cells. Endocrinology. 1994;134:2079–2087. doi: 10.1210/endo.134.5.8156908. [DOI] [PubMed] [Google Scholar]
- Buzzard JJ, Loveland KL, O'Bryan MK, O'Connor AE, Bakker M, Hayashi T, et al. Changes in circulating and testicular levels of inhibin A and B and activin A during postnatal development in the rat. Endocrinology. 2004;145:3532–3541. doi: 10.1210/en.2003-1036. [DOI] [PubMed] [Google Scholar]
- Barakat B, O'Connor AE, Gold E, de Kretser DM, Loveland KL. Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction. 2008;136:345–359. doi: 10.1530/REP-08-0140. [DOI] [PubMed] [Google Scholar]
- Meehan T, Schlatt S, O'Bryan MK, de Kretser DM, Loveland KL. Regulation of germ cell and Sertoli cell development by activin, follistatin, and FSH. Dev Biol. 2000;220:225–237. doi: 10.1006/dbio.2000.9625. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, de Kretser DM, Hedger MP. Activin and related proteins in inflammation: not just interested bystanders. Cytokine Growth Factor Rev. 2009;20:153–164. doi: 10.1016/j.cytogfr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- O'Bryan MK, Gerdprasert O, Nikolic-Paterson DJ, Meinhardt A, Muir JA, Foulds LM, et al. Cytokine profiles in the testes of rats treated with lipopolysaccharide reveal localized suppression of inflammatory responses. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1744–R1755. doi: 10.1152/ajpregu.00651.2004. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kashiwakura Y, Kusumi N, Tamayose K, Nasu Y, Nagai A, et al. Adeno-associated virus-mediated human IL-10 gene transfer suppresses the development of experimental autoimmune orchitis. Gene Ther. 2005;12:1126–1132. doi: 10.1038/sj.gt.3302463. [DOI] [PubMed] [Google Scholar]
- Dejucq N, Dugast I, Ruffault A, van der Meide PH, Jegou B. Interferon-alpha and -gamma expression in the rat testis. Endocrinology. 1995;136:4925–4931. doi: 10.1210/endo.136.11.7588226. [DOI] [PubMed] [Google Scholar]
- Dejucq N, Chousterman S, Jegou B. The testicular antiviral defense system: localization, expression, and regulation of 2′5′ oligoadenylate synthetase, double-stranded RNA-activated protein kinase, and Mx proteins in the rat seminiferous tubule. J Cell Biol. 1997;139:865–873. doi: 10.1083/jcb.139.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Com E, Bourgeon F, Evrard B, Ganz T, Colleu D, Jegou B, et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003;68:95–104. doi: 10.1095/biolreprod.102.005389. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta S, Castellani P, Delfino L, Tassi S, Vene R, Rubartelli A. DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol. 2009;86:549–555. doi: 10.1189/jlb.1008598. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Keating SE, Baran M, Bowie AG. Cytosolic DNA sensors regulating type I interferon induction. Trends Immunol. 2011;32:574–581. doi: 10.1016/j.it.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Riccioli A, Starace D, Galli R, Fuso A, Scarpa S, Palombi F, et al. Sertoli cells initiate testicular innate immune responses through TLR activation. J Immunol. 2006;177:7122–7130. doi: 10.4049/jimmunol.177.10.7122. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation—a perspective. J Reprod Immunol. 2011;88:130–141. doi: 10.1016/j.jri.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MA, Johnson TA, Gupta R, Chapman JL, Ojha P. Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod. 2007;76:958–964. doi: 10.1095/biolreprod.106.059410. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Tchatalbachev S, Klug J, Fijak M, Pineau C, Chakraborty T, et al. Uropathogenic Escherichia coli block MyD88-dependent and activate MyD88-independent signaling pathways in rat testicular cells. J Immunol. 2008;180:5537–5547. doi: 10.4049/jimmunol.180.8.5537. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang H, Xiong W, Chen S, Tang H, Han D. Expression patterns and functions of toll-like receptors in mouse Sertoli cells. Endocrinology. 2008;149:4402–4412. doi: 10.1210/en.2007-1776. [DOI] [PubMed] [Google Scholar]
- Starace D, Galli R, Paone A, de Cesaris P, Filippini A, Ziparo E, et al. Toll-like receptor 3 activation induces antiviral immune responses in mouse Sertoli cells. Biol Reprod. 2008;79:766–775. doi: 10.1095/biolreprod.108.068619. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang X, Chen Q, Deng T, Zhang Y, Li N, et al. Toll-like receptor 3-initiated antiviral responses in mouse male germ cells in vitro. Biol Reprod. 2012;86:106. doi: 10.1095/biolreprod.111.096719. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhu W, Liu Z, Yan K, Zhao S, Han D. Toll-like receptor 11-initiated innate immune response in male mouse germ cells. Biol Reprod. 2014;90:308. doi: 10.1095/biolreprod.113.114421. [DOI] [PubMed] [Google Scholar]
- Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen Q, Yan K, Liu Z, Li N, Zhang X, et al. RIG-I-like receptors mediate innate antiviral response in mouse testis. Mol Endocrinol. 2013;27:1455–1467. doi: 10.1210/me.2013-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Lupfer C, Kanneganti TD. The expanding role of NLRs in antiviral immunity. Immunol Rev. 2013;255:13–24. doi: 10.1111/imr.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]