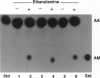
Abstract
Using RNA (Northern) blot hybridization and reverse transcription-PCR, we demonstrate that the brain-type cannabinoid receptor (CB1-R) mRNA, but not the spleen-type cannabinoid receptor (CB2-R) mRNA, is expressed in the mouse uterus and that this organ has the capacity to synthesize the putative endogenous cannabinoid ligand, anandamide (arachidonylethanolamide). The psychoactive cannabinoid component of marijuana--delta 9-tetrahydrocannabinol (THC)--or anandamide, but not the inactive and nonpsychoactive cannabidiol (CBD), inhibited forskolin-stimulated cyclic AMP formation in the mouse uterus, which was prevented by pertussis toxin pretreatment. These results suggest that uterine CB1-R is coupled to inhibitory guanine nucleotide-binding protein and is biologically active. Autoradiographic studies identified ligand binding sites ([3H]anandamide) in the uterine epithelium and stromal cells, suggesting that these cells are perhaps the targets for cannabinoid action. Scatchard analysis of the binding of [3H]WIN 55212-2, another cannabinoid receptor ligand, showed a single class of high-affinity binding sites in the endometrium with an apparent Kd of 2.4 nM and Bmax of 5.4 x 10(9) molecules per mg of protein. The gene encoding lactoferrin is an estrogen-responsive gene in the mouse uterus that was rapidly and transiently up-regulated by THC, but not by CBD, in ovariectomized mice in the absence of ovarian steroids. This effect, unlike that of 17 beta-estradiol (E2), was not influenced by a pure antiestrogen, ICI 182780, suggesting that the THC-induced uterine lactoferrin gene expression does not involve estrogen receptors. We propose that the uterus is a new target for cannabinoid ligand-receptor signaling.
Full text
PDF

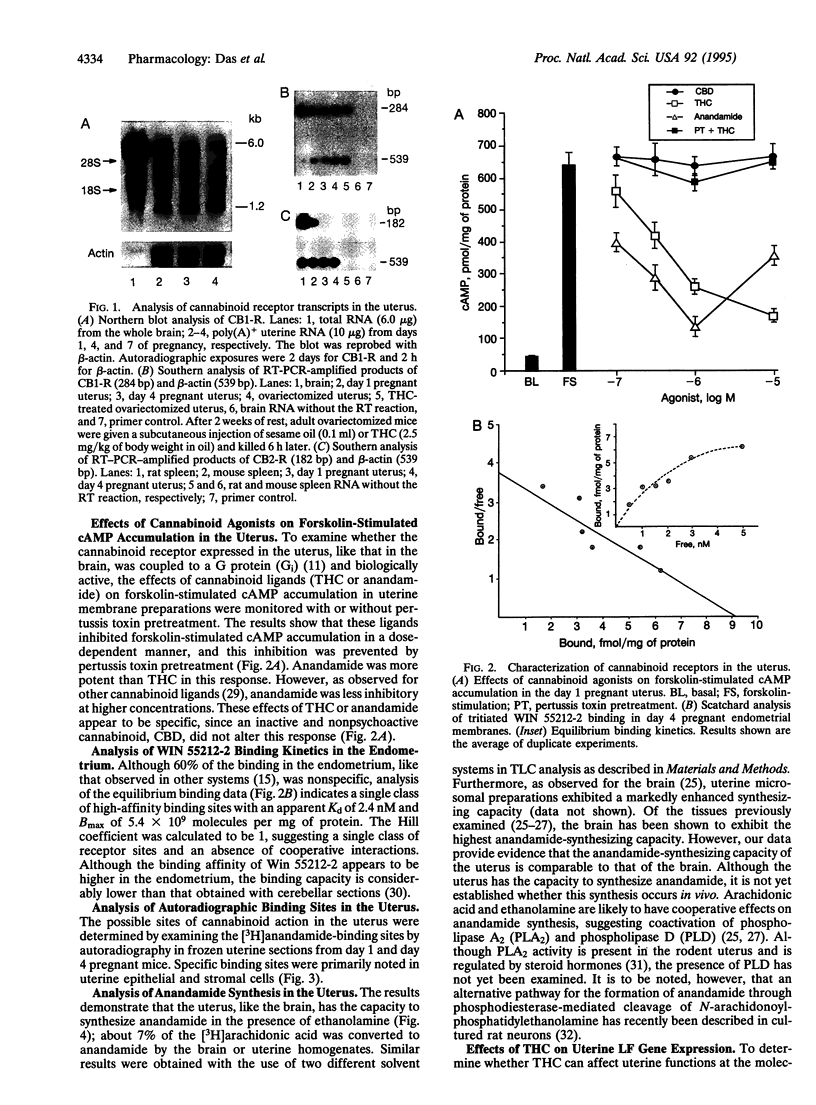
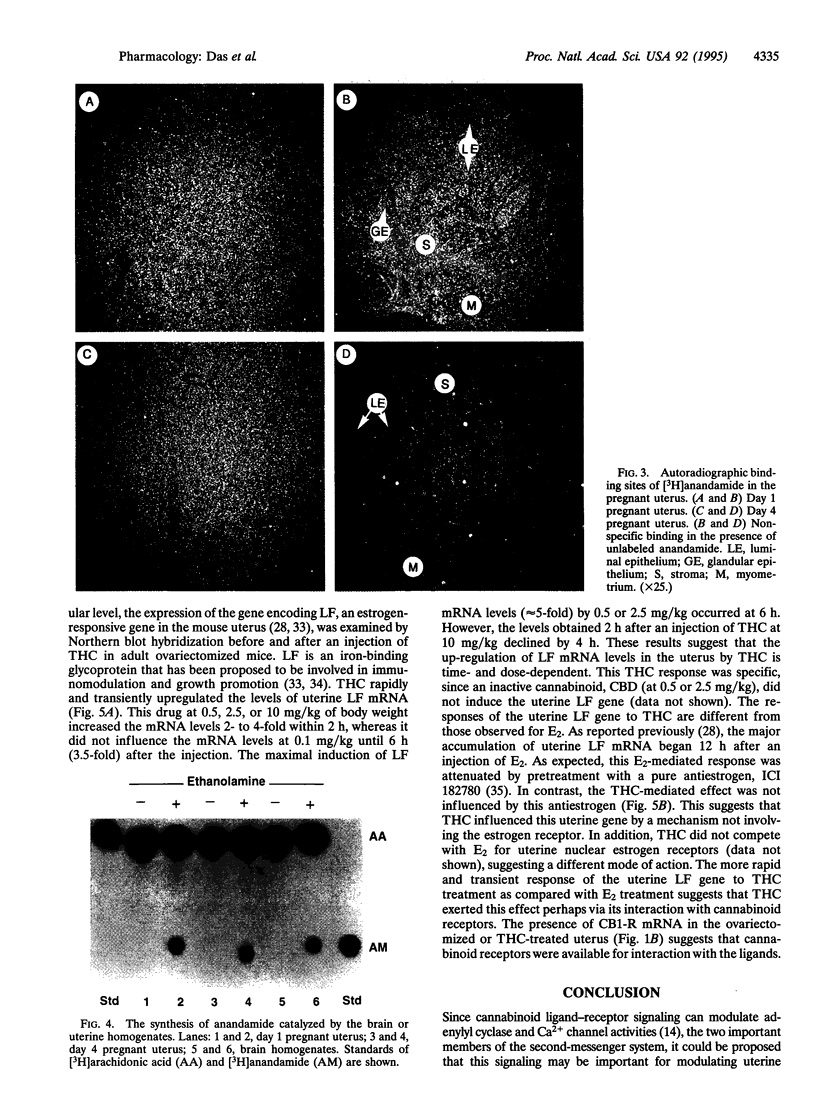

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch E., Thysen B., Morrill G. A., Gardner E., Fujimoto G. Effects of cannabinoids on reproduction and development. Vitam Horm. 1978;36:203–258. doi: 10.1016/s0083-6729(08)60985-1. [DOI] [PubMed] [Google Scholar]
- Bouaboula M., Rinaldi M., Carayon P., Carillon C., Delpech B., Shire D., Le Fur G., Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993 May 15;214(1):173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Chang M. C., Berkery D., Schuel R., Laychock S. G., Zimmerman A. M., Zimmerman S., Schuel H. Evidence for a cannabinoid receptor in sea urchin sperm and its role in blockade of the acrosome reaction. Mol Reprod Dev. 1993 Dec;36(4):507–516. doi: 10.1002/mrd.1080360416. [DOI] [PubMed] [Google Scholar]
- Das S. K., Wang X. N., Paria B. C., Damm D., Abraham J. A., Klagsbrun M., Andrews G. K., Dey S. K. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994 May;120(5):1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- Dauvois S., White R., Parker M. G. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993 Dec;106(Pt 4):1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Chin S. A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993 Sep 1;46(5):791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Devane W. A., Axelrod J. Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6698–6701. doi: 10.1073/pnas.91.14.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dewey W. L. Cannabinoid pharmacology. Pharmacol Rev. 1986 Jun;38(2):151–178. [PubMed] [Google Scholar]
- Dey S. K., Hoversland R. C., Johnson D. C. Phospholipase A2 activity in the rat uterus: modulation by steroid hormones. Prostaglandins. 1982 May;23(5):619–630. doi: 10.1016/s0090-6980(82)80002-6. [DOI] [PubMed] [Google Scholar]
- Dey S. K., Villanueva C., Abdou N. I. Histamine receptors on rabbit blastocyst and endometrial cell membranes. Nature. 1979 Apr 12;278(5705):648–649. doi: 10.1038/278648a0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Fontana A., Cadas H., Schinelli S., Cimino G., Schwartz J. C., Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994 Dec 15;372(6507):686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Briley E. M., Axelrod J., Simpson J. T., Mackie K., Devane W. A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G., Murad F. Assay of cyclic nucleotides by receptor protein binding displacement. Methods Enzymol. 1974;38:49–61. doi: 10.1016/0076-6879(74)38010-x. [DOI] [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A. C. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985 Apr;27(4):429–436. [PubMed] [Google Scholar]
- Howlett A. C., Fleming R. M. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol. 1984 Nov;26(3):532–538. [PubMed] [Google Scholar]
- Huet-Hudson Y. M., Andrews G. K., Dey S. K. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989 Sep;125(3):1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- Ince B. A., Montano M. M., Katzenellenbogen B. S. Activation of transcriptionally inactive human estrogen receptors by cyclic adenosine 3',5'-monophosphate and ligands including antiestrogens. Mol Endocrinol. 1994 Oct;8(10):1397–1406. doi: 10.1210/mend.8.10.7531820. [DOI] [PubMed] [Google Scholar]
- Jansen E. M., Haycock D. A., Ward S. J., Seybold V. S. Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Res. 1992 Mar 13;575(1):93–102. doi: 10.1016/0006-8993(92)90428-c. [DOI] [PubMed] [Google Scholar]
- Kaminski N. E., Abood M. E., Kessler F. K., Martin B. R., Schatz A. R. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol Pharmacol. 1992 Nov;42(5):736–742. [PMC free article] [PubMed] [Google Scholar]
- Kruszka K. K., Gross R. W. The ATP- and CoA-independent synthesis of arachidonoylethanolamide. A novel mechanism underlying the synthesis of the endogenous ligand of the cannabinoid receptor. J Biol Chem. 1994 May 20;269(20):14345–14348. [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Munirathinam G., Yoburn B. C. A simple procedure for assaying cAMP. Pharmacol Biochem Behav. 1994 Jul;48(3):813–816. doi: 10.1016/0091-3057(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K. L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993 Sep 2;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nelson K. G., Takahashi T., Bossert N. L., Walmer D. K., McLachlan J. A. Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):21–25. doi: 10.1073/pnas.88.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey A. B., Bondy G. P. Is delta-9-tetrahydrocannabinol estrogenic. Science. 1977 Mar 4;195(4281):904–906. doi: 10.1126/science.841319. [DOI] [PubMed] [Google Scholar]
- Paria B. C., Das S. K., Andrews G. K., Dey S. K. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):55–59. doi: 10.1073/pnas.90.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentecost B. T., Teng C. T. Lactotransferrin is the major estrogen inducible protein of mouse uterine secretions. J Biol Chem. 1987 Jul 25;262(21):10134–10139. [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R. F., Mani S. K., Codina J., Conneely O. M., O'Malley B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991 Dec 13;254(5038):1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Rawitch A. B., Schultz G. S., Ebner K. E., Vardaris R. M. Competition of delta 9-tetrahydrocannabinol with estrogen in rat uterine estrogen receptor binding. Science. 1977 Sep 16;197(4309):1189–1191. doi: 10.1126/science.897662. [DOI] [PubMed] [Google Scholar]
- Schatz A. R., Kessler F. K., Kaminski N. E. Inhibition of adenylate cyclase by delta 9-tetrahydrocannabinol in mouse spleen cells: a potential mechanism for cannabinoid-mediated immunosuppression. Life Sci. 1992;51(6):PL25–PL30. doi: 10.1016/0024-3205(92)90414-k. [DOI] [PubMed] [Google Scholar]
- Schuel H., Goldstein E., Mechoulam R., Zimmerman A. M., Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7678–7682. doi: 10.1073/pnas.91.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. G., Asch R. H. Drug abuse and reproduction. Fertil Steril. 1987 Sep;48(3):355–373. doi: 10.1016/s0015-0282(16)59400-x. [DOI] [PubMed] [Google Scholar]
- Solomon J., Cocchia M. A., DiMartino R. Effect of delta-9-tetrahydrocannabinol on uterine and vaginal cytology of ovariectomized rats. Science. 1977 Mar 4;195(4281):875–877. doi: 10.1126/science.841311. [DOI] [PubMed] [Google Scholar]
- Solomon J., Cocchia M. A., Gray R., Shattuck D., Vossmer A. Uterotrophic effect of delta-9-tetrahydrocannabinol in ovariectomized rats. Science. 1976 May 7;192(4239):559–561. doi: 10.1126/science.1257790. [DOI] [PubMed] [Google Scholar]
- Temeles G. L., Ram P. T., Rothstein J. L., Schultz R. M. Expression patterns of novel genes during mouse preimplantation embryogenesis. Mol Reprod Dev. 1994 Feb;37(2):121–129. doi: 10.1002/mrd.1080370202. [DOI] [PubMed] [Google Scholar]