Abstract
Regular physical activity is known to decrease the risk of cardiovascular disease, but the risk of ischemic stroke immediately following moderate or vigorous physical activity remains unclear. The authors evaluated the risk of acute ischemic stroke immediately following physical activity and examined whether the risk was modified by regular physical activity. In a multicenter case-crossover study, the authors interviewed 390 ischemic stroke patients (209 men, 181 women) at 3 North American hospitals between January 2001 and November 2006. Physical activity during the hour before stroke symptoms arose was compared with usual frequency of physical activity over the prior year. Of the 390 subjects, 21 (5%) reported having engaged in moderate or vigorous physical activity during the hour before ischemic stroke onset, and 6 subjects had lifted an object weighing at least 50 pounds (≥23 kg) during that hour. The rate ratio for ischemic stroke was 2.3 (95% confidence interval (CI): 1.5, 3.7; P < 0.001) for moderate or vigorous physical activity in the previous hour and 2.6 (95% CI: 1.1, 5.9; P = 0.02) for lifting 50 pounds or more. People who reported engaging in moderate or vigorous physical activity at least 3 times per week experienced a 2-fold increased risk (95% CI: 1.2, 3.3) with each bout of physical activity, as compared with a 6.8-fold risk (95% CI: 2.5, 18.8) among more sedentary subjects (P for homogeneity = 0.03).
Keywords: case-control studies, exercise, physical exertion, stroke
Habitual physical activity has been shown to decrease the risk of coronary heart disease (1) and stroke (2, 3). Among the benefits of regular aerobic activity are lower long-term mean arterial blood pressure (4, 5), an improved lipid profile (4, 6), enhanced capacity for fat oxidation (6), less inflammation (7), and better glycemic control (4, 8). On the other hand, each bout of physical activity causes increased sympathetic nervous system activity, leading to an increased heart rate and a surge in systolic blood pressure, with resultant shear stress and a shift in the endogenous thrombotic-fibrinolytic balance (3). Investigators have previously reported that bouts of rigorous physical activity can trigger myocardial infarction (9–15), subarachnoid hemorrhage (16), or sudden cardiac death (11, 17, 18), even among people who regularly exercise vigorously (9, 10, 14, 15). However, little is known about the risk of ischemic stroke onset following physical activity and heavy lifting and whether the association between bouts of physical activity and stroke is lower among people who exercise regularly.
MATERIALS AND METHODS
Study population
Between January 2001 and November 2006, 390 patients (209 men and 181 women) were interviewed a median of 3 days (range, 0–14) after sustaining an acute ischemic stroke at 3 North American medical centers (Beth Israel Deaconess Medical Center, Boston, Massachusetts; University of North Carolina Hospitals, Chapel Hill, North Carolina; and Vancouver Island Health Authority, Victoria, British Columbia, Canada). Trained research staff identified eligible patients by reviewing charts of patients admitted to each hospital’s stroke service and daily hospital admission logs, and patients with new-onset acute neurologic syndrome compatible with stroke were screened upon admission to emergency departments. Presumed stroke etiology was categorized according to an abbreviated form of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system (19) after review of the diagnostic workup.
Study personnel used standardized abstraction forms to record data on demographic factors, medical history, and admission laboratory results. For inclusion in the study, patients were required to have a neurologist-confirmed diagnosis of acute ischemic stroke, either by clinical diagnosis or appropriate imaging studies, to be English-speaking, and to have been free of dementia prior to the index event. Patients were excluded if they could not identify the time of onset of their stroke symptoms or if the treating clinician deemed them unable to complete the structured interview (which lasted 30–45 minutes) because of cognitive impairment, poor memory of the stroke, aphasia, or illness. Across all study sites, 43% of patients with confirmed ischemic stroke met all inclusion criteria. Of these, 83% agreed to participate, 5.5% refused, and the remaining 12.5% were discharged from the hospital before the interviewers were able to approach them. The protocol was approved by the institutional review board at each participating medical center, and informed consent was obtained from each subject.
Interviewers used a structured questionnaire and asked subjects to report the date and time of the first symptoms heralding their stroke. Subjects were asked about the frequency and timing of “moderate exertion, with deep breathing, such as: normal walking, golfing on foot, slow biking, raking leaves, cleaning windows, or fishing” and the frequency and timing of “vigorous exertion, with panting and overheating, such as: slow jogging, fast biking, shoveling snow, mowing with a push mower, or heavy gardening.” Subjects who reported any moderate or vigorous physical activity were asked to specify the last time they had reached that level of exertion and their usual frequency of physical activity over the prior week and the prior year. Similar questions were asked about lifting at least 25, 50, or 100 pounds (≥11, ≥23, or ≥45 kg). Subjects were also asked to report the timing of their last exposure to other potential triggers—including stress, anger, and use of caffeine, alcohol, cigarettes, marijuana, and cocaine—and their usual frequency of exposure to these factors during the year preceding the onset of stroke symptoms. Other information collected during the interview included information on medication use and symptoms experienced on the day of the stroke.
Reliability of the questionnaire
The test-retest reliability of the Stroke Onset Study questionnaire was assessed in a subgroup of 25 subjects who were reinterviewed up to 6 days after their initial interview and were asked again about their physical activity in the past year and the time of their last bout of physical activity before the onset of their stroke symptoms. The intraclass correlation for the usual frequency of moderate or vigorous physical activity was found to be excellent (intraclass correlation coefficient = 0.99), and there was perfect agreement for reporting of any moderate or vigorous physical activity during the past year and during the hour preceding stroke onset (κ = 1.0). The intraclass correlation coefficient for the usual frequency of lifting 50 pounds or more (≥23 kg) was 0.99, with a high level of agreement (κ = 1.0).
Study design
The Stroke Onset Study used a case-crossover design, a variation of a case-control design that is appropriate when a brief exposure (physical activity) causes a transient change in the risk of an acute outcome (ischemic stroke) (20, 21). We compared a subject's physical activity during the hour prior to onset of stroke symptoms (the hazard period) with the same subject's usual frequency of physical activity during the prior year (the control period). Because control information for each subject in a case-crossover study is based on his or her own past exposure, self-matching eliminates confounding by risk factors that are constant within individuals over the sampling period but often differ between study subjects. For each case, moderate or vigorous physical activity during the 1-hour hazard period was compared with the expected frequency in an average 1-hour period based on the control data of self-reported usual frequency of physical activity in the past year.
Statistical analysis
In a case-crossover study, each subject forms his or her own stratum and thus serves as his or her own control (22). The analysis of “usual frequency” data from case-crossover studies follows standard methods for stratified person-time data (20, 21), with the individual subject serving as the stratifying variable. In essence, the data for each subject form a separate 2 × 2 table and the Mantel-Haenszel incidence rate ratio is calculated (23). The ratio of the observed exposure frequency in the hazard period to the expected frequency based on control information about physical activity or lifting in the previous year was used to calculate the rate ratio. We multiplied the usual annual frequency of physical activity by its median duration (1 hour) to estimate the amount of person-time of exposure to physical activity. The amount of person-time unexposed was calculated by subtracting this value from the number of hours in 1 year. The data were analyzed using methods for cohort studies with sparse data in each stratum.
We conducted stratified analyses to evaluate effect modification by sex, age (<65 years vs. ≥65 years), smoking status (current smokers vs. never smokers), hypertension status (yes/no), stroke etiology (large vessel, small vessel, cardioembolic, or other/undetermined), and habitual physical activity (≥3 times per week vs. <3 times per week) and compared the rate ratios by means of a test for homogeneity (24).
Because people are more likely to engage in physical activity during typical waking hours, we conducted a sensitivity analysis in which we restricted the data to subjects who experienced a stroke between 6 AM and 10 PM. We made the conservative assumption that the patients had only engaged in moderate and vigorous physical activity in the past year during that time of day, which should have resulted in a conservative estimate of the rate ratio.
To evaluate whether exposure to other triggering behaviors could account for the observed association, we conducted a sensitivity analysis excluding subjects who were exposed to other potential triggers during the hour preceding their stroke (e.g., alcohol, marijuana, and anger). In another sensitivity analysis, we used the reported usual frequency of physical activity in the past week as the control information. All analyses were carried out using SAS 9.1 (SAS Institute, Inc., Cary, North Carolina). Two-sided P values less than 0.05 were considered statistically significant.
RESULTS
Physical activity
Table 1 shows the characteristics of the study subjects by level of physical activity during the past year. Of the 390 patients with acute ischemic stroke who were interviewed, 287 (74%) reported having engaged in moderate or vigorous physical activity during the past year. The types of activities reported were either mostly dynamic, such as walking, running, or swimming (47%); mostly static, such as lifting groceries and household cleaning (8%); or mixed, such as gardening and shoveling snow (42%). Compared with persons who reported no physical activity in the past year, subjects who reported moderate or vigorous physical activity were more likely to be younger and more likely to be male. One subject did not provide information on the usual frequency of activity and was therefore excluded from further analysis. Among the 286 subjects who reported engaging in moderate or vigorous physical activity during the past year, 117 (41%) reported engaging in such activity at least daily, 132 (46%) 1 or more times per week, 22 (8%) 1 or more times per month, and 15 (5%) less than once per month. The median frequency of physical activity was 4 times per week, with an average duration of 64 minutes (interquartile range, 20–60) for each episode. There were 166 subjects who reported exposure to moderate or vigorous physical activity during the week prior to stroke, and 21 reported having engaged in moderate or vigorous physical activity within 1 hour before the onset of stroke symptoms.
Table 1.
Clinical Characteristics of Ischemic Stroke Patients According to Level of Moderate or Vigorous Physical Activity in the Past Year (n = 390), Stroke Onset Study, 2001–2006a
| Moderate or Vigorous Physical Activity in Past Year |
||||
| Yesb (n = 286) |
Noc (n = 103) |
|||
| No. | % | No. | % | |
| Male sex | 166 | 58 | 43 | 42 |
| Smoking status | ||||
| Never smoker | 93 | 32 | 35 | 34 |
| Former smoker | 135 | 47 | 49 | 48 |
| Current smoker | 58 | 20 | 19 | 18 |
| Obesity (body mass indexd ≥30) | 64 | 23 | 26 | 26 |
| Diabetes mellitus | 66 | 23 | 28 | 27 |
| Hypercholesterolemia | 114 | 40 | 32 | 31 |
| Hypertension | 188 | 66 | 63 | 61 |
| Atrial fibrillation | 37 | 13 | 17 | 17 |
| Medical history | ||||
| Myocardial infarction | 36 | 13 | 13 | 13 |
| Stroke | 47 | 16 | 25 | 24 |
| Transient ischemic attack | 33 | 12 | 14 | 14 |
| Coronary revascularization | 29 | 10 | 13 | 13 |
| Carotid endarterectomy | 4 | 1 | 3 | 3 |
| Stroke etiologye | ||||
| Small vessel | 76 | 29 | 35 | 38 |
| Large vessel | 52 | 20 | 15 | 16 |
| Cardioembolic | 62 | 23 | 19 | 20 |
| Other/undetermined | 75 | 28 | 24 | 26 |
One subject did not provide information on the usual frequency of activity and was therefore excluded from further analysis.
The mean age of subjects with any moderate or vigorous physical activity in the past year was 67 years (standard deviation, 14.0).
The mean age of subjects with no moderate or vigorous physical activity in the past year was 73 years (standard deviation, 13.9).
Weight (kg)/height (m)2. There were no available data on body mass index for 6 subjects.
At one of the study centers, stroke etiology was not determined (n = 31).
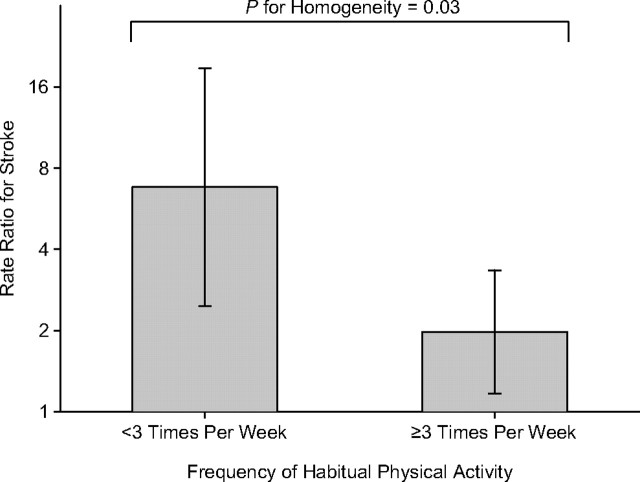
Within 1 hour after moderate or vigorous physical activity, the risk of stroke onset was 2.3 times higher (95% confidence interval (CI): 1.5, 3.7; P < 0.001) compared with periods of lower activity or rest. The rate ratios for recent physical activity did not vary by sex, age, smoking status, hypertension status, or stroke etiology (Table 2). Figure 1 shows that the rate ratio for ischemic stroke following an isolated bout of moderate or vigorous physical activity was attenuated by habitual physical activity. Subjects who reported being physically active at least 3 times per week (n = 187) experienced only a 2-fold (95% CI: 1.2, 3.3) increased risk with each bout of activity as compared with a 6.8-fold (95% CI: 2.5, 18.8) risk among more sedentary subjects (P for homogeneity = 0.03).
Table 2.
Rate Ratio for Stroke Onset Within 1 Hour of Moderate or Vigorous Physical Activity Among Ischemic Stroke Patients (n = 390), According to Patient Characteristics, Stroke Onset Study, 2001–2006a
| No. Exposed to MVPA in Past Year | No. Exposed to MVPA in Past Hour | Mean No. of MVPA Episodes in Past Year | Mantel-Haenszel Rate Ratio | 95% Confidence Interval | P Valueb | P for Homogeneityc | |
| All patients | 286 | 21 | 308.7 | 2.3 | 1.5, 3.7 | <0.001 | |
| Sex | 0.89 | ||||||
| Female | 120 | 9 | 313.6 | 2.4 | 1.1, 5.1 | 0.02 | |
| Male | 166 | 12 | 305.2 | 2.3 | 1.2, 4.1 | 0.008 | |
| Age, years | 0.83 | ||||||
| <65 | 119 | 10 | 340.5 | 2.4 | 1.2, 4.9 | 0.01 | |
| ≥65 | 167 | 11 | 286.1 | 2.2 | 1.2, 4.2 | 0.02 | |
| Smoking status | |||||||
| Current smoker | 58 | 2 | 309.0 | 1.0 | 0.2, 5.1 | 0.97 | 0.25 |
| Never smoker | 228 | 19 | 308.7 | 2.6 | 1.6, 4.3 | <0.001 | |
| Hypertension | 0.56 | ||||||
| Yes | 188 | 13 | 320.8 | 2.1 | 1.1, 3.8 | 0.02 | |
| No | 98 | 8 | 285.6 | 2.8 | 1.3, 5.9 | 0.008 | |
| Stroke etiology | 0.89 | ||||||
| Large vessel | 52 | 3 | 263.5 | 2.0 | 0.6, 6.3 | 0.25 | |
| Small vessel | 76 | 5 | 374.2 | 1.8 | 0.6, 5.3 | 0.29 | |
| Cardioembolic | 62 | 5 | 323.8 | 2.3 | 0.9, 5.7 | 0.07 | |
| Other/undetermined | 75 | 6 | 275.3 | 2.7 | 1.2, 6.1 | 0.02 | |
| Habitual physical activity, times/week | 0.03 | ||||||
| ≥3 | 187 | 17 | 442.9 | 2.0 | 1.2, 3.3 | 0.01 | |
| <3 | 99 | 4 | 55.2 | 6.8 | 2.5, 18.8 | <0.001 |
Abbreviation: MVPA, moderate or vigorous physical activity.
One subject did not provide information on the usual frequency of activity and was therefore excluded from further analysis.
P value for the stratum-specific Mantel-Haenszel rate ratio.
P value for the χ2 test for homogeneity.
Figure 1.
Impact of habitual physical activity on the rate ratio for stroke onset following an isolated bout of moderate or vigorous physical activity, Stroke Onset Study, 2001–2006. Bars, 95% confidence interval.
In a sensitivity analysis using the conservative assumption that moderate or vigorous physical activity occurred only during typical waking hours (6 AM–10 PM), moderate or vigorous activity was still associated with an increased risk of ischemic stroke onset (rate ratio = 2.8, 95% CI: 1.7, 4.6; P < 0.001).
Among the 286 subjects who reported having engaged in moderate or vigorous physical activity in the past year, 78 had been exposed to other potential triggers during the hour prior to stroke onset. Of the 21 subjects who engaged in physical activity in the hour prior to stroke onset, 4 also drank alcohol, 4 drank a caffeinated beverage, and 1 had influenza. When we conducted an analysis excluding the 78 subjects exposed to any potential trigger in the hour preceding stroke onset, the results remained similar (rate ratio = 1.9, 95% CI: 1.0, 3.4; P = 0.04).
On average, subjects reported lower levels of physical activity during the week preceding the stroke than in the prior year. Among the 286 subjects who reported engaging in moderate or vigorous physical activity during the past year, the mean usual frequency of physical activity during the past year was 5.9 times per week, whereas the mean frequency of reported physical activity during the past week was 3.4. However, this included 120 subjects who reported engaging in no moderate or vigorous physical activity during the week before stroke symptoms. Among the 166 subjects reporting moderate or vigorous physical activity in the past week, the mean frequency of reported physical activity during the past week was 5.8. We conducted a sensitivity analysis using each subject's reported frequency of physical activity in the past week as the control period, and found that the risk of ischemic stroke onset increased 4.5-fold (95% CI: 2.8, 7.4; P < 0.001) within 1 hour of moderate or vigorous physical activity as compared with periods of lower activity or rest.
Heavy lifting
Among the 106 patients who reported having lifted 50 pounds or more (≥23 kg) at least once during the year prior to the stroke, 6 (6%) reported such lifting within 1 hour prior to stroke symptom onset. Within 1 hour after lifting an object weighing 50 pounds or more, the risk of stroke onset was 2.6 times higher (95% CI: 1.1, 5.9; P = 0.02) compared with periods without lifting. Among the 106 subjects who had lifted 50 pounds or more at least once during the year prior to the stroke, the mean usual frequency of lifting ≥50 pounds during the past year was 4.1 times per week, whereas the mean frequency of lifting ≥50 pounds during the past week was 2.5. The sensitivity analysis based on reported exposure during the past week resulted in a higher stroke rate ratio of 4.7 (95% CI: 2.0, 11.1; P < 0.001) for lifting 50 pounds or more. We did not have sufficient statistical power to assess whether the risk of ischemic stroke associated with lifting 50 pounds or more was altered by habitual physical activity.
DISCUSSION
In this study, we found a 2.3-fold increased risk of stroke within 1 hour of an episode of moderate or vigorous physical activity. We also found that risk was greater among sedentary subjects than among those who had exercised at least 3 times per week in the prior year. Previous studies found that the role of vigorous physical activity as a trigger of acute myocardial infarction was limited to the time of exertion and extended up to 30–60 minutes after the activity (9, 15). These findings suggest that the increased risk persists for 1 hour following the episode of physical activity.
Our findings are similar to the results of the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS), in which Anderson et al. (16) found a 3-fold increased risk of hemorrhagic stroke in the 2 hours following moderate or extreme physical activity as compared with periods of no physical activity. In that study, the rate ratio was not affected by habitual physical activity, suggesting a possible difference in the impact of habitual physical activity for hemorrhagic and ischemic stroke. In a study of ischemic stroke in 200 patients, Koton et al. (25) found an approximately 2-fold increased risk of ischemic stroke within 2 hours of physical activity that did not reach nominal statistical significance (rate ratio = 2.1, 95% CI: 0.9, 5.6). They did not assess whether this risk varied by habitual physical activity. In a matched case-control study of participants in the Copenhagen City Heart Study, Krarup et al. (26) compared the level of physical activity performed during the week preceding an ischemic stroke with that of community controls and found that for each 10-point increase in physical activity score, the odds ratio for ischemic stroke was 0.86 (95% CI: 0.82, 0.90). Our finding of a higher risk among less active persons lends support to the hypothesis that habitual physical activity is beneficial, lowering the risk that an individual bout of exertion might trigger the onset of ischemic stroke.
There are several possible mechanisms that may explain the higher risk of ischemic stroke immediately following physical activity. Physical activity acutely increases sympathetic nervous system output and decreases parasympathetic activity. These shifts in autonomic nervous control may result in hemodynamic stress, including increased heart rate and blood pressure, resulting in increased shear stress. Increased norepinephrine levels may lead to increased platelet aggregation and oxygen demand (15), which may lead to an increased risk of thrombotic occlusion (27) in the setting of disrupted endothelial surface secondary to shear stress from hemodynamic factors. Circulating levels of catecholamines correlate more closely with the relative intensity of physical activity than with absolute exercise intensity. Therefore, platelet activation may be related to the relative intensity of the physical activity exposure (15). Heavy lifting may alter levels of intrathoracic pressure resulting from expiratory strain and hence produce a transient elevation in blood pressure (28).
Previous studies have shown that the acute risk of myocardial infarction following vigorous physical activity is higher among sedentary persons than among those who regularly engage in vigorous physical activity (9, 10, 14, 15). Our results indicate that habitual activity also protects against ischemic stroke. The stroke protection derived from regular exercise might be due to lower heart rate, peak systolic pressure, and circulating catecholamine levels at a given workload (4, 5), improved lipid profiles (4, 6), and lower levels of arteriosclerosis (11) and inflammation (7) and thereby decreased plaque vulnerability and enhanced capacity for fat oxidation (6). Physical activity may also have antithrombotic results, enhancing fibrinolysis (3) and reducing blood viscosity, fibrinogen levels, and platelet aggregation. Less catecholamine release after exertion in physically fit individuals may explain the observation that a bout of physical activity was associated with a greater increase in risk in sedentary persons than in active persons.
There were some limitations to our study. Since each subject served as his or her own control, there was no confounding by characteristics that are stable over time, but confounding could still have occurred if multiple exposures occurred concurrently within an individual. However, the results remained similar when we excluded subjects who had engaged in other potentially triggering activities during the hour preceding stroke onset. In an effort to minimize reporting bias, we made efforts to ensure the subjects’ privacy. We used a standardized structured interview, and subjects were not informed of the duration of the hypothesized hazard period. Some (29) but not all (30) validation studies have shown that subjects may overreport their habitual frequency of vigorous physical activity and underestimate moderate-level activities. This appeared to have occurred in our study, as suggested by the fact that recall of actual activity in the 7 days prior to stroke onset was lower than the reported frequency of activity over the past year. Alternatively, this may have been due to decreased activity in response to prodromal stroke symptoms. Because we restricted our sample to relatively healthy stroke patients and not all eligible persons agreed to participate, our results may not be generalizable to patients who experience severe or fatal stroke. Previous studies have shown that the acute impact of physical activity depends on the type, intensity, and duration of the exercise (31). However, we did not have enough participants exposed within the past hour to examine differences by level or type of activity. Therefore, further research is necessary to identify the type and duration of physical activity that triggers ischemic stroke and how much is necessary to prevent a stroke.
In conclusion, we found that isolated episodes of physical activity could transiently increase the risk of ischemic stroke and that the rate ratio was greatly attenuated by habitual physical activity. This study reinforces the importance of habitual physical activity in stroke prevention. Not only does habitual physical activity lower the baseline risk of stroke, it also appears to lower the risk of having an ischemic stroke triggered by an episode of physical activity.
Acknowledgments
Author affiliations: Cardiovascular Epidemiology Research Unit, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts (Elizabeth Mostofsky, Eva Laier, Emily B. Levitan, Murray A. Mittleman); Stroke Clinic, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts (Gottfried Schlaug); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Elizabeth Mostofsky, Murray A. Mittleman); and Department of Epidemiology, School of Public Health, University of North Carolina, Chapel Hill, North Carolina (Wayne D. Rosamond).
This work was supported by an Established Investigator Grant from the American Heart Association (grant 0140219N) and a training grant from the National Institutes of Health (grant T32-A1007535-11).
The authors thank Cindy Aiello for outstanding administrative assistance.
Preliminary results were presented in poster form at the American Heart Association Conference on Cardiovascular Disease Epidemiology and Prevention, Phoenix, Arizona, March 4, 2006.
Dr. Murray A. Mittleman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis.
Conflict of interest: none declared.
Glossary
Abbreviations
- ACROSS
Australasian Cooperative Research on Subarachnoid Hemorrhage Study
- CI
confidence interval
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Wendel-Vos GC, Schuit AJ, Feskens EJ, et al. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33(4):787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 3.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34(10):2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 4.Jennings G, Nelson L, Nestel P, et al. The effects of changes in physical activity on major cardiovascular risk factors, hemodynamics, sympathetic function, and glucose utilization in man: a controlled study of four levels of activity. Circulation. 1986;73(1):30–40. doi: 10.1161/01.cir.73.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Kohno K, Matsuoka H, Takenaka K, et al. Depressor effect by exercise training is associated with amelioration of hyperinsulinemia and sympathetic overactivity. Intern Med. 2000;39(12):1013–1019. doi: 10.2169/internalmedicine.39.1013. [DOI] [PubMed] [Google Scholar]
- 6.Pruchnic R, Katsiaras A, He J, et al. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004;287(5):E857–E862. doi: 10.1152/ajpendo.00459.2003. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13(5):561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Praet SF, van Rooij ES, Wijtvliet A, et al. Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2008;51(5):736–746. doi: 10.1007/s00125-008-0950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittleman MA, Maclure M, Tofler GH, et al. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329(23):1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 10.Willich SN, Lewis M, Löwel H, et al. Physical exertion as a trigger of acute myocardial infarction. Triggers and Mechanisms of Myocardial Infarction Study Group. N Engl J Med. 1993;329(23):1684–1690. doi: 10.1056/NEJM199312023292302. [DOI] [PubMed] [Google Scholar]
- 11.Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin. 1996;14(2):263–270. doi: 10.1016/s0733-8651(05)70279-4. [DOI] [PubMed] [Google Scholar]
- 12.Giri S, Thompson PD, Kiernan FJ, et al. Clinical and angiographic characteristics of exertion-related acute myocardial infarction. JAMA. 1999;282(18):1731–1736. doi: 10.1001/jama.282.18.1731. [DOI] [PubMed] [Google Scholar]
- 13.Hallqvist J, Möller J, Ahlbom A, et al. Does heavy physical exertion trigger myocardial infarction? A case-crossover analysis nested in a population-based case-referent study. Am J Epidemiol. 2000;151(5):459–467. doi: 10.1093/oxfordjournals.aje.a010231. [DOI] [PubMed] [Google Scholar]
- 14.Baylin A, Hernandez-Diaz S, Siles X, et al. Triggers of nonfatal myocardial infarction in Costa Rica: heavy physical exertion, sexual activity, and infection. Ann Epidemiol. 2007;17(2):112–118. doi: 10.1016/j.annepidem.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Thompson PD, Franklin BA, Balady GJ, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 16.Anderson C, Ni Mhurchu C, Scott D, et al. Triggers of subarachnoid hemorrhage: role of physical exertion, smoking, and alcohol in the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Stroke. 2003;34(7):1771–1776. doi: 10.1161/01.STR.0000077015.90334.A7. [DOI] [PubMed] [Google Scholar]
- 17.Albert CM, Mittleman MA, Chae CU, et al. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343(19):1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 18.Whang W, Manson JE, Hu FB, et al. Physical exertion, exercise, and sudden cardiac death in women. JAMA. 2006;295(12):1399–1403. doi: 10.1001/jama.295.12.1399. [DOI] [PubMed] [Google Scholar]
- 19.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 21.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. Am J Epidemiol. 1995;142(1):91–98. doi: 10.1093/oxfordjournals.aje.a117550. [DOI] [PubMed] [Google Scholar]
- 22.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 23.Sorock GS, Lombardi DA, Hauser R, et al. A case-crossover study of transient risk factors for occupational acute hand injury. Occup Environ Med. 2004;61(4):305–311. doi: 10.1136/oem.2002.004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 25.Koton S, Tanne D, Bornstein NM, et al. Triggering risk factors for ischemic stroke: a case-crossover study. Neurology. 2004;63(11):2006–2010. doi: 10.1212/01.wnl.0000145842.25520.a2. [DOI] [PubMed] [Google Scholar]
- 26.Krarup LH, Truelsen T, Pedersen A, et al. Level of physical activity in the week preceding an ischemic stroke. Cerebrovasc Dis. 2007;24(2-3):296–300. doi: 10.1159/000105683. [DOI] [PubMed] [Google Scholar]
- 27.Lee KW, Lip GY. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: a systematic review. Arch Intern Med. 2003;163(19):2368–2392. doi: 10.1001/archinte.163.19.2368. [DOI] [PubMed] [Google Scholar]
- 28.Palatini P, Mos L, Munari L, et al. Blood pressure changes during heavy-resistance exercise. J Hypertens Suppl. 1989;7(6):S72–S73. doi: 10.1097/00004872-198900076-00032. [DOI] [PubMed] [Google Scholar]
- 29.Ainsworth BE, Bassett DR, Jr, Strath SJ, et al. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc. 2000;32(suppl 9):S457–S464. doi: 10.1097/00005768-200009001-00004. [DOI] [PubMed] [Google Scholar]
- 30.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 31.von Klot S, Mittleman MA, Dockery DW, et al. Intensity of physical exertion and triggering of myocardial infarction: a case-crossover study. Eur Heart J. 2008;29(15):1881–1888. doi: 10.1093/eurheartj/ehn235. [DOI] [PubMed] [Google Scholar]