ABSTRACT
CD8+ T cells specific for pp65, IE1, and IE2 are present at high frequencies in human cytomegalovirus (HCMV)-seropositive individuals, and these have been shown to have phenotypes associated with terminal differentiation, as well as both cytokine and proliferative dysfunctions, especially in the elderly. However, more recently, T cell responses to many other HCMV proteins have been described, but little is known about their phenotypes and functions. Consequently, in this study, we chose to determine the diversity of HCMV-specific CD8+ T cell responses to the products of 11 HCMV open reading frames (ORFs) in a cohort of donors aged 20 to 80 years old as well as the ability of the T cells to secrete gamma interferon (IFN-γ). Finally, we also tested their functional antiviral capacity using a novel viral dissemination assay. We identified substantial CD8+ T cell responses by IFN-γ enzyme-linked immunospot (ELISPOT) assays to all 11 of these HCMV proteins, and across the cohort, individuals displayed a range of responses, from tightly focused to highly diverse, which were stable over time. CD8+ T cell responses to the HCMV ORFs were highly differentiated and predominantly CD45RA+, CD57+, and CD28−, across the cohort. These highly differentiated cells had the ability to inhibit viral spread even following direct ex vivo isolation. Taken together, our data argue that HCMV-specific CD8+ T cells have effective antiviral activity irrespective of the viral protein recognized across the whole cohort and despite viral immune evasion.
IMPORTANCE Human cytomegalovirus (HCMV) is normally carried without clinical symptoms and is widely prevalent in the population; however, it often causes severe clinical disease in individuals with compromised immune responses. HCMV is never cleared after primary infection but persists in the host for life. In HCMV carriers, the immune response to HCMV includes large numbers of virus-specific immune cells, and the virus has evolved many mechanisms to evade the immune response. While this immune response seems to protect healthy people from subsequent disease, the virus is never eliminated. It has been suggested that this continuous surveillance by the immune system may have deleterious effects in later life. The study presented in this paper examined immune responses from a cohort of donors and shows that these immune cells are effective at controlling the virus and can overcome the virus' lytic cycle immune evasion mechanisms.
INTRODUCTION
The betaherpesvirus human cytomegalovirus (HCMV) is a common pathogen worldwide (1). After primary infection, the virus establishes lifelong persistence in individuals, at least in part due to its ability to undergo latent infection in pluripotent CD34+ stem cells in the bone marrow and the myeloid cell lineages derived from them (2). Both primary infection with HCMV and its long-term persistence are largely subclinical for the majority of individuals. However, infection, whether due to primary infection, reactivation from latency, or superinfection in the immunocompromised or immature (such as HIV/AIDS patients, transplant patients, and the fetus in utero, respectively), can be life threatening (1). Primary HCMV infection of an otherwise healthy host elicits responses from both the innate and adaptive arms of the immune system, and evidence from patients undergoing bone marrow and stem cell transplantations has shown that the generation of HCMV-specific CD4+ and CD8+ T cell responses is crucial for successful control of virus infection (3–7). This importance of T cell responses is further supported by evidence from the murine model of murine cytomegalovirus (MCMV) infection, where transfer of immediate early (IE) antigen-specific CD8+ T cells into animals with an ablated immune system was protective against viral challenge (8). Other murine studies have also shown that removal of CD4+ T cells from mice leads to reactivation of virus, resulting in disease (9). Additionally, CD8+ T cells also prevent lethal infection of mice with MCMV in the absence of CD4+ T cells (10, 11).
It is now well established that there are high-frequency cytomegalovirus-specific CD8+ T cell responses directed toward the viral proteins pp65 (UL83) and IE1 (UL123) in the majority of HCMV-seropositive individuals (4, 12–15). Although the responses to pp65 and IE1 are immunodominant and large, in most individuals there are specific CD8+ T cell responses to numerous other HCMV proteins (16), such that the frequency of the total CD8+ T cell response to HCMV in infected individuals has been estimated to comprise up to 10% of the total CD8+ T cell compartment in peripheral blood (16).
As a consequence of HCMV infection, it has been established that the composition of the T cell repertoire is altered, resulting from virus-specific cells undergoing major expansions, which has been linked to the concept of “memory inflation” described for the murine model of MCMV infection (17, 18). The CD8+ IE and pp65-specific T cells that have been studied have a phenotype that has been linked to terminal differentiation as well as dysfunction, defined by the re-expression of CD45RA (19–24), the loss of expression of the costimulatory molecules CD27 and CD28 (13, 21, 25–27), and expression of CD57, a marker of activation associated with differentiated T cells (21, 28–30). It has also been concluded, from these studies, that enlarged HCMV-specific CD8+ T cell populations accumulate with age and include increasing numbers of “dysfunctional” T cells, as defined by their highly differentiated phenotype, loss of cytokine secretion ability, and limited proliferation capacity (21, 27, 31). These observations have led to the suggestion that the HCMV-induced changes to the T cell immune system may become detrimental to individuals during their lifetime. These HCMV-influenced changes to the phenotypes of host CD8+ T cells have been used to suggest on the basis of association that older seropositive individuals are more susceptible to infection, respond poorly to vaccinations, and have an increased risk of mortality compared to age-matched HCMV-seronegative individuals (32–36). However, older individuals do not appear to suffer from overt HCMV disease resulting from reactivating virus or reinfection, which suggests that HCMV-specific T cells in these elderly donors do retain the ability to control the virus infection (37). Other studies have also challenged the dogma that these expansions of HCMV-specific T cells are dysfunctional and shown that the CD45RAhi HCMV-specific CD8+ T cells found in elderly donors proliferate well when given the correct costimulation signals (38) and that the accumulating HCMV-specific CD8+ population tends to be polyfunctional, as they secrete multiple antiviral cytokines and are highly cytotoxic (39).
In this study, we wished to analyze the diversity of HCMV-specific CD8+ T cell responses to 11 of the highest-ranked T cell ORFs in a cohort of donors with wide age variation and to determine the stability of these responses over a period of years. Additionally, we wished to determine if T cells specific to HCMV antigens other than pp65 and IE displayed alternative differentiation phenotypes and, if so, how this correlated with gamma interferon (IFN-γ) production, cytotoxicity, and antiviral activity. To address this, we screened a donor cohort aged 24 to 80 years old and measured the CD8+ T cell response to the proteins encoded by the ORFs UL83 (pp65), UL82 (pp71), UL123 (IE-1), UL122 (IE-2), UL99, UL28, UL48, US29, US32, UL55 (gB), and US3 by IFN-γ enzyme-linked immunospot (ELISPOT) assays. The results showed substantial CD8+ T cell responses to all of these proteins and showed that, over a period of 3 years, the diversity of HCMV protein responses in individual donors was stable. Of the 11 ORFs analyzed, we identified 6 which were recognized by the majority of the donor cohort (UL83, UL82, UL123, UL122, UL28, and US3) and performed both a functional and phenotypic analysis of the CD8+ T cell responses to these ORFs. While many of the HCMV-specific CD8+ T cells identified secreted IFN-γ, there were a large proportion of antigen-specific T cells that did not secrete the cytokine. These analyses also revealed a highly differentiated memory T cell population common to all the ORFs studied and displayed by all individuals in the cohort. Finally, T cells with all the HCMV specificities tested were able to prevent dissemination of a clinical isolate of HCMV through indicator fibroblasts.
MATERIALS AND METHODS
Donor sample collection and isolation.
Heparinized peripheral blood was collected from healthy donors, and HCMV serostatus was determined using an IgG enzyme-linked immunosorbent assay (Trinity Biotech, Didcot, United Kingdom). Eighteen HCMV-seropositive and four HCMV-seronegative donors were included in this study. Ethical approval was obtained from the Addenbrookes National Health Service Hospital Trust institutional review board (Cambridge Research Ethics Committee) for this study. Informed written consent was obtained from all recipients in accordance with the Declaration of Helsinki (LREC 97/092). The age range of the HCMV-seropositive donors was 24 to 80 years; 5 donors were female (37.2 ± 12.3 years), and 13 were male (47.5 ± 16.9 years). All donors were HLA typed by genotyping by the Diabetes and Inflammation Laboratory, CIMR, University of Cambridge, or the NHS Tissue Typing Service, Addenbrookes Hospital, Cambridge, United Kingdom. Peripheral blood mononuclear cells (PBMC) were isolated using Lymphoprep (Axis-Shield, Oslo, Norway) density gradient centrifugation. PBMC were used fresh or frozen in liquid nitrogen in a 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Poole, United Kingdom) and 90% fetal calf serum (FCS) (PAA, Linz, Austria) solution. Frozen PBMC were rapidly thawed in a 37°C water bath, and the freezing medium was diluted into 25 ml of fresh RPMI 1640 with 10% FCS (RPMI-10), centrifuged, and resuspended in fresh RPMI-10 before use.
HCMV ORF peptide mixes.
Eleven HCMV ORFs (UL28, UL48, UL55 [gB], UL82 [pp71], UL83 [pp65], UL99, UL122 [IE2], UL123 [IE1], US3, US29, and US32) were selected, and consecutive 15-mer peptides overlapping by 10-amino-acid libraries were synthesized by ProImmune PEPScreen (Oxford, United Kingdom) from sequences detailed in the study by Sylwester et al. (16). The individual lyophilized peptides from each ORF library were reconstituted in 80% DMSO–20% RPMI 1640 (PAA Laboratories, Austria) to give a 40-mg/ml master stock; the individual peptides were then diluted 1/40 in RPMI 1640 (unsupplemented) to give a 1-mg/ml (2% DMSO) working stock. Peptide pools were used as either entire ORF mixes at a concentration of 5 μg/ml/peptide or (in the case of the UL48 ORF) 10 pools of 48 peptides at a concentration of 20 μg/ml/peptide in this study. Additionally, ProMix HCMVA (pp65) and Promix HCMVA (US3) peptide pools (ProImmune) diluted to the equivalent of 20 μg/ml/peptide were also used.
Individual HLA-typed HCMV and Epstein-Barr virus (EBV) peptides.
Individual HLA-restricted peptides from HCMV pp65 and IE1 used in this study were HLA-A2 (NLVPMVATV; pp65 amino acids [aa] 495 to 504), HLA-A1 (YSEHPTFTSQY; pp65 aa 363 to 373), HLA-A3 (KLGGALQAK; IE1 aa 184 to 192), HLA-B7 (TPRVTGGGAM; pp65 aa 417 to 426), HLA-B8 (QIKVRVDMV; IE1 aa 88 to 96), and HLA-A2 (VLEETSVML; IE1 aa 316 to 324). Additionally, individual HLA-restricted peptides from EBV BMLF-1, EBNA 3A, and BZLF-1 were also used in the study: HLA-A2 (GLCTLVAML; BMLF-1 aa 259 to 267), HLA-A3 (RLRAEAQVK; EBNA 3A aa 603 to 611), HLA-B7 (RPPIFIRRL; EBNA-3A aa 247 to 255), and HLA-B8 (RAKFKQLL; BZLF-1 aa 190 to 197) (all from ProImmune).
ELISPOT assays.
PBMC or PBMC that had been depleted of CD4+ T cells using magnetic cell separation and anti-CD4-conjugated beads (Miltenyi Biotech, Bisley, United Kingdom) (2 × 105 to 3 × 105) were resuspended in supplemented RPMI 1640 with 10% FCS, and IFN-γ ELISPOT assays were performed according to the manufacturer's instructions (eBioscience, San Diego, CA, USA) in 96-well PVDF membrane plates (Millipore, Billerica, MA, USA). Cells were stimulated with entire ORF mix peptides (final peptide concentration, 2 μg/ml/peptide) or ORF pool mix peptides (final peptide concentration, 5 μg/ml/peptide) at 37°C in a humidified CO2 atmosphere for 48 h. All the ELISPOT assays were performed on fresh PBMC on the day the blood was taken, and assays were all performed according to a fixed protocol and using antibody sets from the same company as well as ELISPOT plates with identical incubation periods. Phytohemagglutinin (PHA) was used as a positive control in every assay. Developed plates were read with a ELISPOT plate reader (AID, Strassberg, Germany) and counted using ImageJ software (National Institutes of Health, USA).
Intracellular cytokine and phenotype staining.
PBMC (2 × 106) resuspended in RPMI plus 10% FCS were stimulated with ORF peptide mixes or individual mapped peptides overnight. After 2 h incubation, 5 μg/ml brefeldin A (BD Biosciences, San Jose, CA, USA) was added for the remainder of the incubation period. Cells were then washed and stained with a combination of surface antibodies, including anti-CD3 eFluor 650NC (eBioscience), anti-CD3 Qdot655 (Invitrogen, Paisley, United Kingdom), anti-CD45RA phycoerythrin (PE)-conjugated Cy7, anti-CD27 allophycocyanin (APC)-conjugated eFluor 780 (eBioscience), anti-CD8 Alexa Fluor 700, anti-CD57 Alexa Fluor 647 (BioLegend, San Diego, USA), anti-CD28 peridinin chlorophyll protein (PerCP)-conjugated Cy5.5, anti-CD45RO fluorescein isothiocyanate (FITC) (BD Biosciences), and LIVE/DEAD fixable yellow dead-cell stain (Invitrogen). Cells were then fixed and permeabilized using Fix&Perm (ADG, Kaumberg, Austria). Peptide-specific T cells were identified by the coexpression of CD69 and 4-1BB according to established published protocols (40–43). In brief, cells were stained intracellularly with CD69 Pacific Blue, 4-1BB PE-Cy5 (BioLegend), and IFN-γ PE (BD Biosciences) at 4°C in the dark. Samples were washed and fixed in a final 1% paraformaldehyde solution and acquired on a BD LSR Fortessa cytometer using FACSDiva software (BD Biosciences). Data were analyzed using FlowJo software (Treestar, OR, USA).
Virus.
HCMV strain TB40/e UL32-GFP (gift of Christian Sinzger, University of Tübingen, Tübingen, Germany) grown in human foreskin fibroblasts (HFFs) as previously described (44) was used in this study.
Expansion of HCMV-specific CD8+ T cells.
CD8+ T cells were isolated from PBMC using magnetically activated cell sorting (MACS) with anti-CD8 direct beads (Miltenyi Biotec, Bisley, United Kingdom) and then resuspended in supplemented RPMI plus 10% fetal bovine serum (FBS) (Invitrogen) and 10% heat-inactivated autologous donor serum. Cells were stimulated with peptide-pulsed irradiated autologous PBMC in the presence of 2.5 IU/ml human recombinant interleukin 2 (IL-2) (National Institute for Biological Standards and Control, Potters Bar, United Kingdom) in round-bottom 96-well plates at 37°C in 5% CO2 for 10 to 14 days, and the medium was replenished with fresh medium every 5 days. The specificity of expanded CD8+ T cell cultures using mapped peptides was determined by specific pentamer staining; cells were harvested, washed, stained with the specific unlabeled pentamer (ProImmune), washed again, and then further stained with a pentamer-specific PE fluorophore (ProImmune) and anti-CD8 and anti-CD3 antibodies conjugated to PerCP-Cy5.5 and FITC, respectively. Cells were then fixed and acquired on a FACSort using CellQuest software (BD Biosciences), and data were analyzed using FlowJo software. All expanded T cell lines were also tested for specificity using an IFN-γ ELISPOT assays. Briefly, resting CD8+ T HCMV-specific cells were harvested and washed, and then 2,000 T cells were incubated in IFN-γ-coated PVDF membrane plates with 50,000 peptide-pulsed autologous B-lymphoblastoid cells (LCLs) and with unpulsed and mitogen-stimulated controls for 48 h at 37°C plus 5% CO2. ELISPOT assays were again developed according to the manufacturer's instructions.
Measurement of cytotoxicity.
The cytotoxic capability of the expanded HCMV-specific CD8+ T cell lines was assessed using a chromium release assay. Briefly, the rested CD8+ T cell lines were harvested, washed in acidified RPMI-10, and plated at effector-to-target cell (E:T) ratios of 15:1 to 80:1 in triplicate. Target cells used comprised autologous LCLs; the cells were washed in phosphate-buffered saline (PBS), and Na2Cr57O4 (PerkinElmer) was added to the cell pellet, which was then incubated at 37°C for 45 min. The appropriate peptide or no peptide was then added to the target cells, which were incubated for an additional 45 min at 37°C. Target cells were washed three times in acidified RPMI plus 10% FBS and added to the assay plate (including wells containing effectors, medium-only wells, and wells containing Nonidet P-40, as described above) at 2,000 to 4,000 cells/well in acidified medium. Plates were incubated at 37°C for 5 h, after which 70 μl of supernatant was harvested from each well and used to quantify radioactive emission. Percent specific lysis was calculated using the following formula: 100 × [(target release − spontaneous release)/(maximum release − spontaneous release)].
In vitro viral dissemination assay.
The ability of specific HCMV CD8 T cells to control the spread of HCMV in vitro was measured. Autologous or partial HLA-matched dermal fibroblasts were seeded in a 24- or 48-well plate to be 80 to 90% confluent when they were infected with TB40e UL32-GFP virus at a multiplicity of infection (MOI) of 0.03. Rested HCMV-specific CD8+ T cells were harvested, washed, resuspended in supplemented RPMI 1640 plus 10% FBS, and then added to the infected fibroblasts 24 h postinfection at T cell-to-fibroblast ratios of 5:1, 2.5:1, 1.2:1, 0.6:1, and 0.3:1; each experiment included a CD8+ T cell line specific to an HLA-matched individual peptide from EBV (listed above) as a control. In further experiments, total CD8+ T cells isolated directly ex vivo from HLA-matched CMV-seropositive and -seronegative donors were added to infected fibroblasts 24 h postinfection at T cell-to-fibroblast ratios of 5, 2.5, and 1.2, or NLV and VLE major histocompatibility complex (MHC) class I pentamer FACS-sorted CD8+ T cells, obtained from PBMC directly ex vivo, were added at T cell-to-fibroblast ratios of 0.14 and 0.08. The viral dissemination assay was incubated at 37°C plus 5% CO2, and viral dissemination was assessed at 14 and 21 days by detection of green fluorescent protein (GFP) expression by both fluorescence microscopy and flow cytometry. The data were analyzed, and the percentage of GFP-positive fibroblasts was expressed as a proportion of the infected control for each time point.
Statistics.
Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). The correlation between age and the T cell response to HCMV was assessed by Pearson's or Spearman's correlation according to the distribution of the data. The Wilcoxon matched-pairs test was used to compare two groups of matched data, and a 1-way analysis of variance (ANOVA) with a paired Friedman's test compared three groups of matched data.
RESULTS
HCMV-specific T cell responses vary widely in their diversity between individuals.
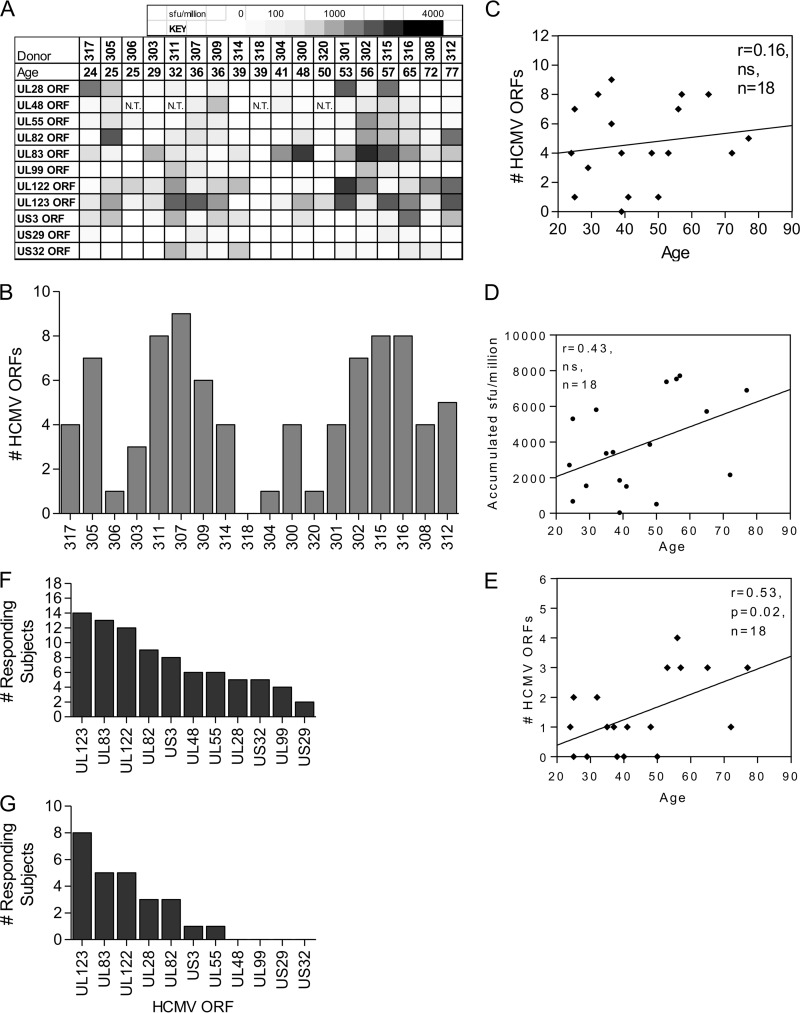
The whole-proteome screen described by Sylwester et al. in 2005 (16) examined the CD8+ and CD4+ T cell responses in a cohort of 33 individuals chosen to represent a wide variety of ethnic and HLA backgrounds. Based on this analysis, we selected 11 of the HCMV ORFs most frequently recognized by CD8+ T cells, which included 6 corresponding to the highest-frequency CD8+ T cell responses derived from a meta-analysis of data from a number of independent studies (45). We determined the frequency of the CD8+ T cell response to each of these ORFs in a cohort of 18 CMV-seropositive donors and 4 CMV-seronegative donors using IFN-γ ELISPOT assays. The 18 CMV-seropositive donors represented a wide range of ages (24 to 77 years at the start of the study) and included 5 older donors, aged 56 to 77 years. Table 1 presents the age, gender, and HLA information for each donor and lists the numbers of HCMV ORFs responded to by CD8+ T cells (defined by a response of >100 spot-forming units [SFU]/million cells above background [unstimulated], as determined by IFN-γ ELISPOT assay).
TABLE 1.
Donor information and summary of HCMV CD8+ T cell responses
| Donor | Age (yrs) | Gender | Serostatus | HLA alleles |
No. of ORFs eliciting responsea | HCMV ORF(s) eliciting response of: |
|||
|---|---|---|---|---|---|---|---|---|---|
| HLA-A | HLA-B | HLA-C | >100 SFU/million | >1,000 SFU/million | |||||
| CMV300 | 48 | F | + | 02, 02 | 44, 44 | 05, 07 | 4/11 | UL82, UL123, US3 | UL83 |
| CMV301 | 53 | M | + | 02, 02 | 07, 40 | 03, 07 | 4/11 | UL83 | UL28, UL122, UL123 |
| CMV302 | 56 | M | + | 02, 03 | 07, 37 | 07, 06 | 7/11 | UL48, UL99, UL123 | UL55, UL82, UL83, UL122 |
| CMV303 | 29 | M | + | 01, 24 | 08, 35 | 04, 07 | 3/11 | UL83, UL122, UL123 | |
| CMV304 | 41 | M | + | 02, 03 | 15, 56 | 01, 15 | 1/11 | UL83 | |
| CMV305 | 25 | M | + | 03, 26 | 44, 51 | 05, 14 | 7/11 | UL28, UL48, UL55, UL122, US3 | UL82, UL123 |
| CMV306 | 25 | F | + | 01, 29 | 08, 27 | 07, 16 | 1/10 | UL122 | |
| CMV307 | 36 | M | + | 01, 26 | 08, 27 | 01, 07 | 9/11 | UL48, UL55, UL82, UL83, UL99, UL122, US29 | UL123 |
| CMV308 | 72 | M | + | 01, 01 | 40, 58 | 03, 07 | 4/11 | UL83, UL123, US32 | UL122 |
| CMV309 | 36 | M | + | 01, 23 | 35, 37 | 04, 06 | 6/11 | UL48, UL55, UL82, UL122, US3 | UL123 |
| CMV311 | 32 | M | + | 25, 25 | 18, 18 | 12, 12 | 8/10 | UL28, UL82, UL83, UL99, US3, US32 | UL122, UL123 |
| CMV312 | 77 | M | + | 02, 29 | 35, 44 | 04, 16 | 5/11 | UL83, US3 | UL82, UL122, UL123 |
| CMV314 | 39 | F | + | 30, 74 | 14, 50 | 08, 06 | 4/11 | UL83, UL122, US3, US32 | |
| CMV315 | 57 | M | + | 01, 32 | 07, 13 | 07, 06 | 8/11 | UL48, UL55, UL82, UL99, US29 | UL28, UL83, UL123 |
| CMV316 | 65 | M | + | 01, 03 | 08, 14 | 07, 08 | 8/11 | UL48, UL55, UL82, UI122, US32 | UL83, UL123, US3 |
| CMV317 | 24 | F | + | 01, 31 | 35, 51 | 07, 14 | 4/11 | UL83, UL123, US3 | UL28 |
| CMV318 | 39 | M | + | 03, 30 | 13, 38 | 06, 12 | 0/10 | ||
| CMV320 | 50 | F | + | 01, 32 | 14, 27 | 08, 03 | 1/10 | UL123 | |
| CMV400 | 55 | M | − | 02, 33 | 44, 44 | 05, 07 | 0/10 | ||
| CMV401 | 45 | M | − | 24, 25 | 08, 35 | 07, 12 | 0/10 | ||
| CMV405 | 35 | F | − | 02, 03 | 14, 40 | 08, 03 | 0/10 | ||
| CMV406 | 24 | M | − | 02, 26 | 14, 40 | 08, 03 | 0/10 | ||
Number of ORFs eliciting response/number tested.
The results showed considerable interdonor variation in the pattern of HCMV ORF products which CD8+ T cells responded to and showed that some donors mounted a more diverse response while other donors had a more focused HCMV response (Fig. 1A and B). Comparison of donor age and the number of HCMV ORF products recognized also showed that the age of the donor had little impact on the number of HCMV ORF products recognized (Fig. 1C). We also calculated the cumulative T cell response in each donor and correlated this with the donor age. There was a trend toward an increase in the magnitude of the CD8+ T cell response with age, but this did not reach significance for this cohort (Spearman r = 0.43; n = 18) (Fig. 1D). However, when the analysis was performed on the number of ORF products that elicited high-frequency responses (>1,000 SFU/million) (Fig. 1E), this strongly correlated with age (Pearson r = 0.53; n = 18; P = 0.02).
FIG 1.
The HCMV ORF-specific diversity of CD8+ T cell responses varies widely between donors. The frequency of the CD8+ T cell responses to 11 HCMV ORF products in 18 donors is shown. The responses were measured by IFN-γ ELISPOT assay and are shown as SFU/million cells following the subtraction of background counts from unstimulated cells. (A) The donor cohort is arranged according to the ages of the donors, and the size of the response to each HCMV ORF product is shown as a heat map. (B) Number of ORF products each donor responded to (>100 SFU/million); (C) the same numbers plotted according to the ages of the donors. There was no statistical correlation between age and the number of ORF products an individual recognized by Pearson's correlation. (D) The cumulative IFN-γ response to all HCMV ORF products for each donor was plotted according to donor age, and this was not significantly correlated with age by Spearman's correlation. (E) The number of ORF products each donor responded to at high frequency (>1,000 SFU/million) was also correlated with age by Pearson's correlation; there is a significant increase in high-frequency responses to ORF products in older donors (P = 0.02). (F and G) The frequency of recognition by CD8+ T cells of each HCMV ORF product is shown for all responses of >100 SFU/million (F) and the high-frequency responses (>1,000 SFU/million) (G; a subset of the data in panel F). The HCMV ORFs were ranked according to the number of subjects responding.
The frequency of the CD8+ T cell response of each individual donor to each HCMV ORF was also tallied, ranked, and then subdivided into responders (those exhibiting responses of >100 SFU/million by IFN-γ ELISPOT assay) (Fig. 1F) and the subset of high-frequency responders (>1,000 SFU/million by IFN-γ ELISPOT assay) (Fig. 1G). We identified 4 HCMV ORFs whose products the majority of the donors in the cohort responded to: UL123 (IE1), UL83 (pp65), UL122 (IE2), and UL82 (pp71), which many donors also recognized at high frequency, in addition to UL28 and US3, which were also recognized at high frequency (Fig. 1F and G). The CD8+ T cell responses to these selected ORF products were further characterized in this study.
The diversity and magnitude of HCMV-specific T cell responses were not significantly changed over time.
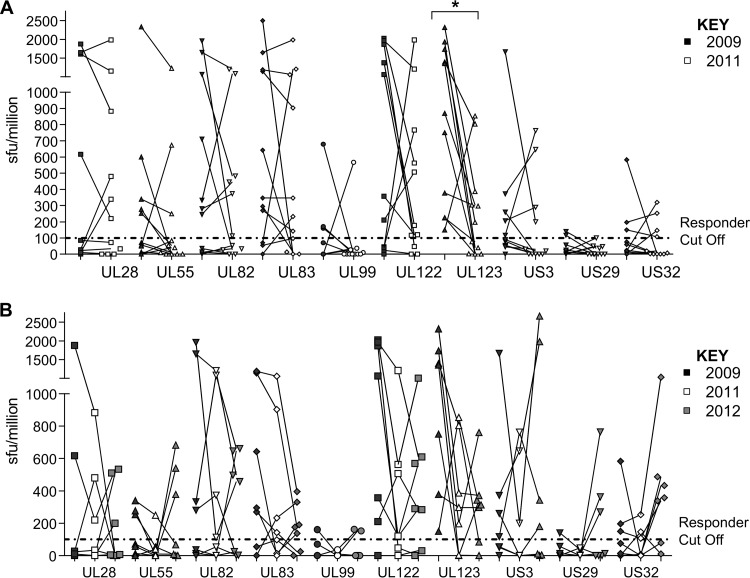
T cell responses to HCMV in some individuals in the cohort were clearly less diverse than the HCMV-specific T cell responses of others. In order to determine if donors with less diverse responses generated responses to additional HCMV ORF products or if donors with diverse responses could lose responses, we determined the stability of the CD8+ T cell responses by repeating the HCMV ORF screen analysis at 24 months (n = 11) (Fig. 2A) and 36 months (n = 7) (Fig. 2B) after the original analysis. While the magnitude of individual responses to ORF products does vary with individual donors over the three time points at 24 months, 9/10 ORF products showed no statistically significant differences in the magnitude of the response for each donor. Although a decrease in the magnitude of the IE1 (UL123) response was observed over this 2-year period (P = 0.01; Wilcoxon matched pairs test), a further examination of this response at 36 months (1-way ANOVA with a paired Friedman's test) revealed that, overall, there was no statistically significant difference in the size of the IFN-γ response for any of the ORFs examined. The data also shows that no new CD8+ T cell responses to ORF products were observed for any individual donor in the cohort at either 24 or 36 months following the original analysis.
FIG 2.
The frequency and diversity of HCMV ORF CD8+ T cell responses were not significantly changed over time. HCMV ORF-specific T cell frequency was determined for 18 donors in 2009 by IFN-γ ELISPOT and then determined again for 11 donors in 2011 (24 months) and again for 7 donors in 2012 (36 months). The responses for each ORF are shown for the 24-month (A) and the 36-month (B) data. The positive-response cutoff (dashed line) is 100 SFU/million. The 24-month paired data were tested using a Wilcoxon matched-pairs test; significant results (*, P < 0.05) are indicated. The 3 time points in the 36-month data group were tested using a 1-way ANOVA with a paired Friedman's test, which showed no significant change in the variance of the data.
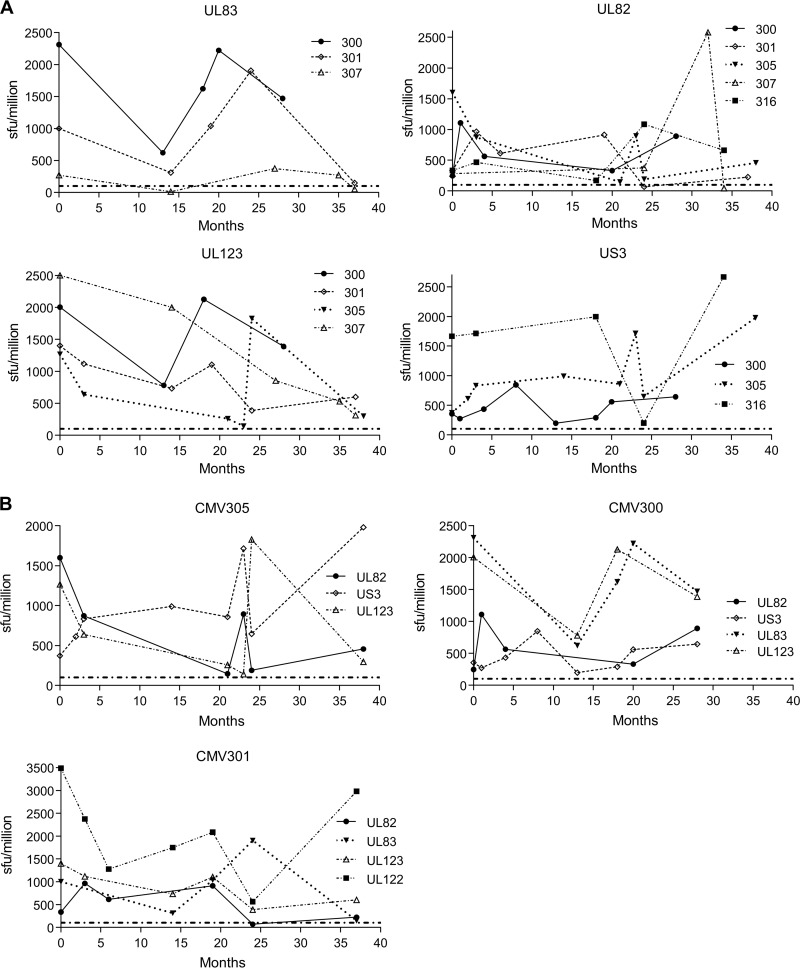
We noted that within the data presented in Fig. 2 there was an obvious change in the frequency of the response to individual HCMV ORF products for some donors. To clarify this observation, we further analyzed the responses to the products of the ORFs UL83 (pp65), UL82 (pp71), UL123 (IE1), and US3 in 5 donors within the cohort which had been sampled at multiple time points over a 40-month period by IFN-γ ELISPOT assay (Fig. 3A). There was a 4-fold difference in the magnitude of the detectable response to the HCMV ORF product in an individual over this time period. The specific responses for donors CMV 305, CMV 300, and CMV 301 were also collated (Fig. 3B). We observed a decrease or increase in a number of responses at selected time points for each donor.
FIG 3.
There is fluctuation in the magnitude of an individual donor's CD8+ T cell response to particular HCMV ORFs. HCMV ORF-specific T cell frequency was determined by IFN-γ ELISPOT at multiple time points over a 40-month period. (A) The responses of 5 different donors corresponding to HCMV ORFs UL83 (pp65), UL82 (pp71), UL123 (IE1), and US3 show that for an individual, the magnitude of the response both decreases and increases during the period of time observed. (B) Responses of donors CMV305, CMV300, and CMV301 to selected HCMV ORFs for the same period. The positive-response cutoff (dashed line) is 100 SFU/million.
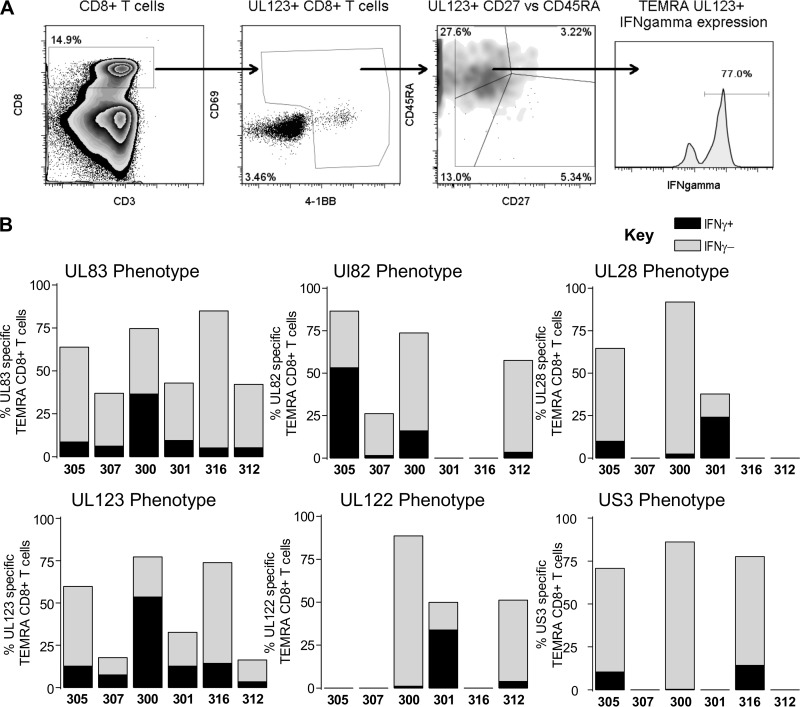
HCMV-specific T cells are predominantly T effector memory CD45RA+ (TEMRA) cells, irrespective of their antigen specificity.
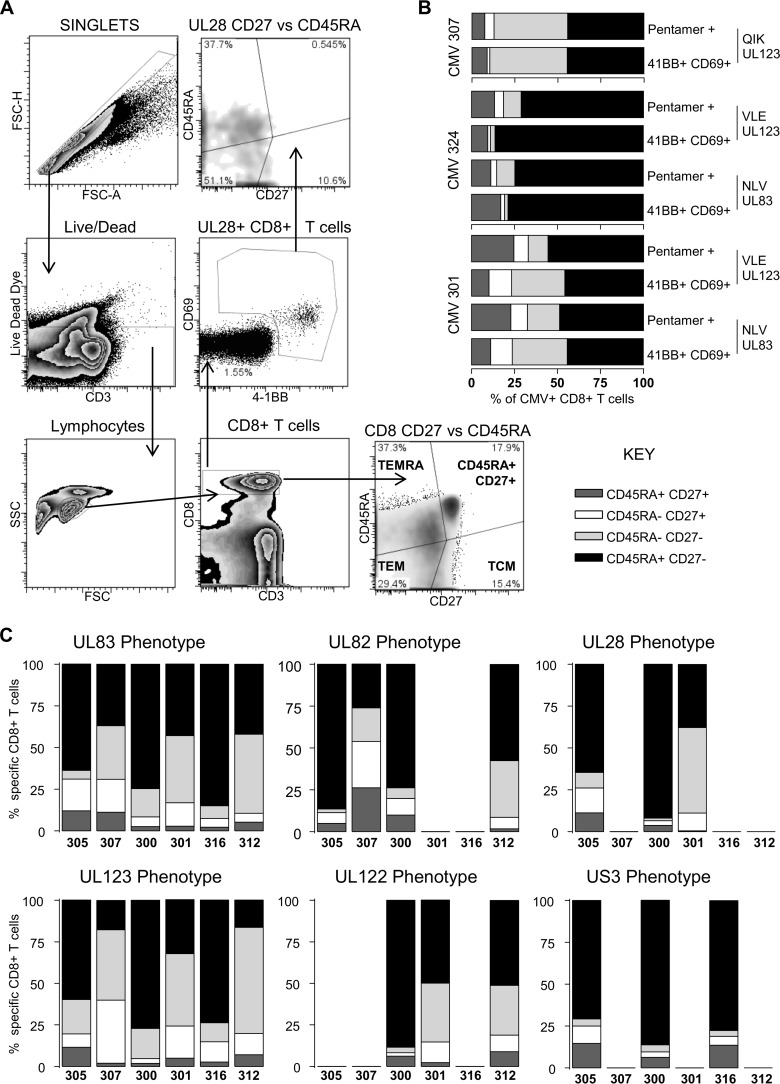
Numerous studies of pp65 (UL83)- and IE1 (UL123)-T cells in the peripheral blood of healthy donors have reported that HCMV pp65-specific CD8+ T cells have a differentiated memory phenotype compared to other virus-specific T cells; this is characterized by the loss of expression of the costimulatory molecule CD27 and re-expression of CD45RA (19–23, 28, 46). Within our donor cohort, we identified 4 additional HCMV ORFs (UL122, UL28, UL82, and US3), besides UL83 and UL123, whose products generated CD8+ T cell responses in a large proportion of subjects examined (Fig. 1F and G). Consequently, we asked whether these ORF-specific CD8+ T cells directed against these other HCMV ORF products had a memory phenotype similar to that previously described for pp65- and IE-specific T cells. The phenotype of HCMV-specific CD8+ T cells from a range of donors (encompassing the age range of the donor cohort) was determined by coexpression of 4-1BB, CD69, CD45RA, and CD27 after ORF stimulation. Following the exclusion of doublet and dead cells, four memory T cell subsets were defined (Fig. 4A). A representative activation and phenotype analysis following UL28 stimulation is illustrated (Fig. 4A). To demonstrate that CD69 and 4-1BB coexpression identifies antigen-specific T cells in combination with CD45RA and CD27 as memory subset markers, we analyzed the CD8+ T cell response from three donors in the cohort; in parallel, cells were stained with MHC class I pentamers containing either the pp65 epitope NLV or IE epitopes (VLE and QIK) or stimulated with these peptides, and we identified antigen-specific cells by CD69 and 4-1BB expression in combination with CD45RA and CD27 expression. The results show that the phenotypic distribution of naive, T effector memory (TEM), T central memory (TCM), and TEMRA cells identified by MHC class I pentamers was very similar to the proportions identified by CD69 and 4-1BB expression (Fig. 4B). The size of the antigen-specific T cell population identified by pentamer staining compared to that identified by CD69/4-1BB coexpression was variable between the donors. The NLV response was very similar in donor CMV324 (pentamer versus CD69/4-1BB, 2.5% versus 2.3%); in donor CMV307, the pentamer population was larger than the CD69/4-1BB population (6% versus 2.4%); and in donor CMV301, the pentamer population was smaller than the CD69/4-1BB population, e.g., for NLV (1% versus 1.9%).
FIG 4.
HCMV-specific CD8+ T cells have a predominantly CD45RA+ CD27− (TEMRA) phenotype. PBMC were stimulated overnight with mapped HCMV ORF peptides or HCMV ORF peptide pools in the presence of brefeldin A. (A) Identification of HCMV-specific CD8+ T cell responses was as shown in the example of gating strategy. (B) Antigen-specific CD8+ populations were identified by expression of 4-1BB and CD69, and 4 memory populations were defined according to the expression of CD27 and CD45RA (CD27+ CD45RA+, CD27+ CD45RA− [TCM], CD27− CD45RA− [TEM], and CD27− CD45RA+ [TEMRA] cells). (B) Comparison of the CD27 and CD45RA phenotype of mapped peptide-stimulated 4-1BB- and CD69-positive CD8+ T cells with matched pentamer identified CD8+ T cells. (C) The memory phenotypes of T cells specific for the products of 6 HCMV ORFs (pp65, pp71, UL28, IE1, IE2, and US3) identified by expression of CD69 and 4-1BB for 6 donors were examined. The data for each ORF are arranged in order of increasing donor age from left to right on the x axis (C).
Six donors were analyzed using 6 HCMV ORF stimulations (Fig. 4C). As expected, the results confirmed previous analyses that pp65- and IE-T cells were predominantly TEMRA cells (CD45RA+ CD27−) (22, 38, 47, 48). However, we were also able to show that T cells specific for another immediate early antigen, US3, and another late antigen, pp71, were also dominated by TEMRA cells, as well as subpopulations of TEM (CD45RA− CD27−) and TCM (CD45RA− CD27+) cells. CD8+ T cells specific for the products of two further ORFs, UL28 and IE2, also had a very similar pattern of T cell subset distribution.
HCMV-specific CD8+ T cells can secrete cytokines and are cytotoxic.
We also assessed the ability of the different antigen-specific CD8+ T cell populations we had identified in our cohort to secrete IFN-γ following ORF stimulation. T cells reactive to specific ORF products were, again, identified by expression of 4-1BB and CD69 following the exclusion of doublet and dead cells; in addition, the T cells were permeabilized and stained for intracellular IFN-γ expression as a control. The percent IFN-γ expression in cells not expressing 4-1BB and CD69 was also determined; in most cases, this was negative or <0.1%. The results show that all ORF-specific T cells from all donors had populations of T cells which were IFN-γ positive as well as negative. In every donor, we could identify individual ORF responses in which the T cells had predominantly lost the ability to make IFN-γ, as well as other ORF responses where the ability to make IFN-γ was predominantly retained (Table 2).
TABLE 2.
Frequency of HCMV ORF-specific CD8+ T cell responses (4-1BB+ and CD69+) and proportion that secrete IFN-γa
| Donor | % of cells recognizing product of ORF |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UL28 |
UL82 |
UL83 |
UL122 |
UL123 |
US3 |
|||||||||||||
| CD8+ UL28+ | Unact IFN-γ+ | UL28+ IFN-γ+ | CD8+ UL82+ | Unact IFN-γ+ | UL82+ IFN-γ+ | CD8+ UL83+ | Unact IFN-γ+ | UL83+ IFN-γ+ | CD8+ UL122+ | Unact IFN-γ+ | UL122+ IFN-γ+ | CD8+ UL123+ | Unact IFN-γ+ | UL123+ IFN-γ+ | CD8+ US3+ | Unact IFN-γ+ | US3+ IFN-γ+ | |
| CMV305 | 0.24 | 0.05 | 11.80 | 0.24 | 0.00 | 59.60 | 0.08 | 0.07 | 10.30 | 0.08 | 0.00 | 21.80 | 0.07 | 0.05 | 10.40 | |||
| CMV307 | 0.06 | 0.00 | 4.62 | 0.19 | 0.00 | 17.30 | 4.06 | 0.04 | 25.60 | |||||||||
| CMV300 | 1.00 | 0.00 | 2.67 | 0.97 | 0.29 | 19.40 | 1.45 | 0.08 | 54.80 | 0.68 | 0.00 | 1.90 | 2.73 | 0.09 | 71.70 | 0.63 | 0.00 | 0.41 |
| CMV301 | 1.18 | 0.00 | 59.80 | 0.16 | 0.00 | 24.80 | 0.59 | 0.00 | 45.40 | 0.06 | 0.00 | 32.60 | ||||||
| CMV316 | 0.11 | 0.02 | 12.10 | 0.87 | 0.09 | 33.20 | 4.79 | 0.05 | 17.80 | |||||||||
| CMV312 | 0.54 | 0.00 | 11.10 | 0.03 | 0.03 | 13.20 | 0.34 | 0.00 | 8.90 | 0.09 | 0.02 | 9.33 | ||||||
ORF-specific T cells were identified by 4-1BB and CD69 coexpression in 6 donors; IFN-γ secretion was determined by intracellular staining. The percentage of HCMV ORF-specific CD8+ T cells from each donor which secrete IFN-γ was determined. IFN-γ secretion by cells not expressing 4-1BB and unactivated CD69 (unact) cells was also determined.
As TEMRA cells are considered more highly differentiated, we also determined the ability of UL82-, UL83-, UL28-, US3-, UL123-, and UL122-specific TEMRA (CD27− CD45RA+) cells to secrete IFN-γ following stimulation (Fig. 5). The results show that only a proportion of these cells retained the ability to make IFN-γ; however, this was observed in all the donors tested.
FIG 5.
CD8+ T cells specific to products of 6 different HCMV ORFs had very similar patterns of IFN-γ secretion. PBMC from 6 donors were stimulated overnight with mapped HCMV ORF peptides or HCMV ORF peptide pools in the presence of brefeldin A. (A) Antigen-specific populations were identified as described in the legend to Fig. 5. (B) IFN-γ production by the TEMRA antigen-specific CD8+ T cell subset was determined. The proportion of the HCMV-specific TEMRA population which secreted IFN-γ (black) as a proportion of the total percentage of antigen-specific TEMRA cells (gray) for the different HCMV ORFs examined (pp65, pp71, UL28, IE1, IE2, and US3) are shown; the data for each ORF are arranged in order of increasing donor age from left to right on the x axis. The proportion of HCMV-specific TEMRA cells which secreted IFN-γ varied between donors, but there was no relationship with either age or HCMV ORF specificity.
The gain of CD57 and loss of CD28 expression are also well recognized markers of the late differentiation status of TEMRA cells. Consequently, we determined the percentage of CD28− and CD57+ HCMV ORF-specific TEMRA cells in each of the donors (Table 3). The results clearly show that, irrespective of antigen specificity, the majority of the TEMRA cells had lost CD28 expression and gained CD57 expression and that this occurred to the same extent in all of the donors.
TABLE 3.
Percentage of HCMV ORF-specific CD8+ TEMRA cells with CD28− and CD57+ phenotypesa
| Donor | % of cells recognizing ORF |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UL28 |
UL82 |
UL83 |
UL122 |
UL123 |
US3 |
|||||||
| CD28− | CD57+ | CD28− | CD57+ | CD28− | CD57+ | CD28− | CD57+ | CD28− | CD57+ | CD28− | CD57+ | |
| CMV305 | 90.40 | 19.20 | 69.70 | 32.80 | 59.50 | 40.50 | 65.40 | 32.70 | 90.40 | 35.30 | ||
| CMV307 | 94.10 | 29.40 | 96.70 | 60.00 | 89.10 | 64.10 | ||||||
| CMV300 | 73.90 | 77.50 | 73.70 | 61.40 | 78.60 | 58.80 | 76.60 | 80.40 | 75.90 | 86.50 | 75.70 | 68.10 |
| CMV301 | 86.10 | 53.50 | 80.00 | 71.10 | 79.30 | 83.50 | 88.10 | 69.50 | ||||
| CMV316 | 62.00 | 75.10 | 61.10 | 77.90 | 62.30 | 68.80 | ||||||
| CMV312 | 0.86 | 100.00 | 0.00 | 81.20 | 2.08 | 97.90 | 7.14 | 92.90 | ||||
ORF-specific CD8+ T cells were identified by 4-1BB and CD69 expression in 6 donors. The proportion of HCMV-specific TEMRA cells that were CD57+ and CD28− were enumerated.
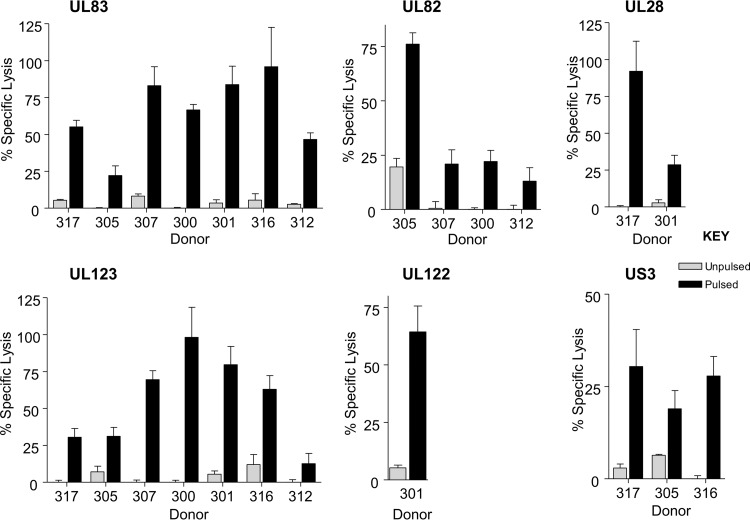
In addition to assessing IFN-γ function of T cells specific to different HCMV ORFs, we also measured their cytotoxic capability. T cells lines specific to UL82-, UL28-, US3-, and UL122-encoded proteins were expanded in vitro. The specificity of these T cell lines was confirmed by IFN-γ ELISPOT or MHC class I pentamer-specific staining (data not shown), and then they were used in chromium release cytotoxicity assays. As expected, CD8+ T cells specific for pp65 and IE1 from all donors tested elicited cytotoxicity (Fig. 6). Similarly, UL82-, UL28-, US3-, and UL122-specific CD8+ T cells also demonstrated good cytotoxic function.
FIG 6.
HCMV-CD8+ T cells specific for pp65, IE1, pp71, UL28, US3, and IE2 mediated cytotoxicity. HCMV-specific CD8+ T cells were used as effector cells in chromium release assays to determine the cytotoxicity function of cells specific for pp65, IE1, pp71, UL28, US3, and IE2. Target cells were donor-matched lymphoblastoid B cell lines which were pulsed with mapped peptides for each donor's known HCMV ORF responses or not pulsed. CD8+ T cells specific to all the HCMV ORFs examined show specific lysis of peptide-pulsed target cells compared to activity against unpulsed target cells.
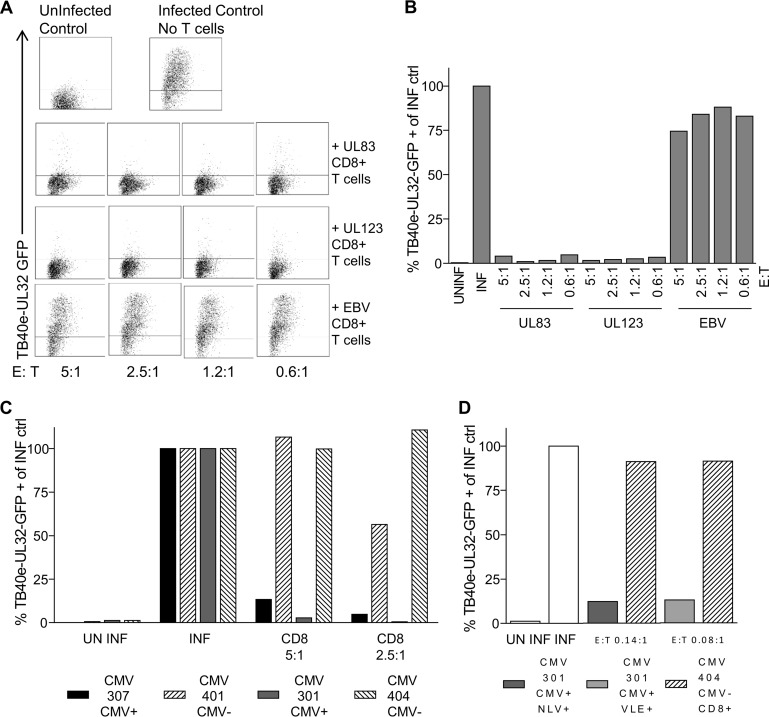
HCMV-specific CD8+ T cells are able to control viral dissemination in vitro.
We next wanted to assess the ability of individual HCMV ORF-specific CD8+ T cells to recognize virus-infected cells. Consequently, we developed a novel in vitro assay to measure the ability of virus-specific CD8+ T cells to control the spread of HCMV in culture. Primary dermal fibroblasts derived from specific donors were infected with a low MOI of a GFP-tagged clinical strain of HCMV (TB40e UL32GFP) and then cocultured with individual ORF-specific CD8+ T cells at a range of effector-to-target cell ratios. After 10 to 28 days of coculture, the spread of the virus through the fibroblast layer was measured by flow-cytometric analysis of GFP expression in the indicator fibroblasts. The specificity of inhibition of HCMV spread by HCMV-specific T cells was controlled for by including T cell lines specific for EBV derived from each donor in the assays. Additionally, we included a positive control consisting of infected fibroblasts with no T cell coculture as well as a negative control consisting of uninfected fibroblasts in each assay.
A representative assay comparing UL83 (pp65)- and UL123 (IE1)-specific CD8+ T cells derived from donor CMV307 is illustrated in Fig. 7A and B and shows that both IE- and pp65-specific T cells are highly effective at controlling spread of HCMV even at the lowest E:T ratio of 0.6:1. Importantly, EBV-specific T cells from the same donor could not inhibit HCMV spread. We repeated this analysis on pp65 and IE1 CD8+ T cell lines derived from four additional donors with similar results (Table 4).
FIG 7.
Both pp65- and IE1-specific CD8+ T cells control viral dissemination in an in vitro assay. Donor-matched dermal fibroblasts were infected with TB40e-UL32-GFP virus at a low MOI, and HCMV-specific in vitro-expanded CD8+ T cells were cocultured with the virus-infected fibroblasts at a range of effector-to-target cell ratios. The percentage of GFP-positive fibroblasts was measured by flow cytometry at 14, 21, and 28 days in comparison to an uninfected control, an infected control, and a nonspecific CD8+ T cell line (EBV specific). (A and B) A representative example from donor CMV307 at day 14 of the assay was used to generate dot plots (A) and a summarized bar chart (B), with data expressed as a proportion of the infected control for pp65- and IE1-specific CD8+ T cells, which are both equally able to control dissemination of virus. (C) CD8+ T cells obtained directly ex vivo from HLA-matched CMV-seropositive and -seronegative donors were cocultured with the virus-infected fibroblasts, and the percentages of GFP-positive fibroblasts as a proportion of the infected control at day 21 of the assay are shown. (D) HCMV-specific CD8+ pentamer-sorted cells obtained directly ex vivo at effector-to-target cell ratios of 0.14:1 and 0.08:1 were able to control the dissemination of virus. Data shown are from day 21 of the assay.
TABLE 4.
pp65 and IE1 specific CD8+ T cells control viral dissemination in an in vitro assay
| Donor | % TB40e-UL32-GFP+ fibroblastsa |
|||||||
|---|---|---|---|---|---|---|---|---|
| Uninfected | Infected | Specific (at E:T ratio) for: |
||||||
| pp65 | IE1 | EBV | ||||||
| 1.2:1 | 0.6:1 | 1.2:1 | 0.6:1 | 1.2:1 | 0.6:1 | |||
| CMV305 | 0.10 | 100.00 | 0.18 | 13.47 | 0.52 | 12.18 | 104.15 | 96.63 |
| CMV307 | 0.57 | 100.00 | 2.66 | 14.76 | 0.74 | 0.71 | 28.46 | 63.05 |
| CMV300 | 0.28 | 100.00 | 4.08 | 19.17 | 8.04 | 8.14 | 79.72 | 88.75 |
| CMV316 | 0.87 | 100.00 | 20.22 | 26.05 | 7.87 | 0.41 | 93.08 | 96.74 |
| CMV312 | 0.05 | 100.00 | 5.90 | 1.98 | 44.00 | 23.75 | 104.25 | 88.25 |
Percentage of TB40eUL32-GFP+ fibroblasts present at day 21 for UL83 (pp65)- and UL123 (IE1)-specific cells compared to EBV-specific cells, expressed as a percentage of the infected control.
The effector T cells used in these assays were generated from resting memory cells, previously expanded in vitro, and while the analysis clearly shows that these in vitro-generated cell lines have specificity and were able to prevent viral dissemination, the in vitro manipulation might not represent effector cells generated in vivo. To address this, we isolated total CD8+ T cells directly ex vivo from two different HCMV-seropositive and two HCMV-seronegative donors for which we had also derived an autologous fibroblast cell line. Total CD8 T cells were added at E:T ratios of 5:1 and 2.5:1 to the viral dissemination assays. The results show that T cells from HCMV-seropositive donors prevented viral dissemination, while T cells from HCMV-seronegative donors did not (Fig. 7C). We also performed this experiment using defined antigen-specific T cells; NLV pp65-specific or VLE (IE)-specific T cells were isolated directly ex vivo, using MHC class I pentamers and FACS, from an HLA-A2-seropositive donor, and total CD8 T cells were obtained from an HLA-A2-seronegative donor; the T cells were then used in viral dissemination assays. The results again show that the directly ex vivo-isolated HCMV antigen-specific T cells exhibited direct antiviral function, preventing viral dissemination in these assays (Fig. 7D).
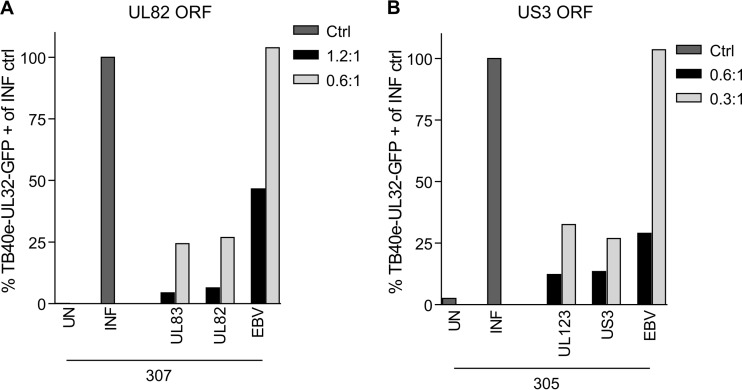
Finally, we also examined the ability of CD8+ T cells specific for UL82 (Fig. 8A) and US3 (Fig. 8B) to prevent viral dissemination, and we clearly observed that both US3- and UL82-specific CD8+ T cells were also able to control the spread of a nonattenuated, clinical strain of HCMV. It was noted that the EBV-specific T cell line did show some nonspecific viral control at the higher E:T ratio, but this was not observed at the lower E:T ratio (Fig. 8B).
FIG 8.
UL82- and US3 -CD8+ T cells control viral dissemination in an in vitro assay. Donor-matched dermal fibroblasts were infected with TB40e-UL32-GFP at a low MOI. HCMV-specific in vitro-expanded CD8+ T cells were cocultured with the virus-infected fibroblasts at a range of effector-to-target cell ratios as described for Fig. 6. (A and B) Summary bar charts showing the percentages of GFP-positive fibroblasts present at day 21 for UL82 (A)- and US3 (B)-specific CD8+ T cells compared to UL83 (pp65)- and UL123 (IE1)-specific cells, respectively (expressed as a proportion of the infected control). Both UL82- and US3-specific CD8+ T cells are able to control dissemination of virus in this assay.
DISCUSSION
In this study, we investigated the T cell response to 11 HCMV ORF products in a cohort of 18 donors ranging from 20 to 80 years of age. Within this cohort, some donors had only a small diversity of responses to HCMV proteins, whereas other donors responded to a much broader range of HCMV antigens. There was a significant correlation between age and the number of ORFs that elicited high-frequency (>1,000 SFU/million) T cell responses. The memory phenotype of CD8+ T cells specific for products of 6 HCMV ORFs recognized by most donors, including pp65 and IE, showed that the predominant phenotype of CD8+ T cells for all the HCMV ORFs was CD27− CD45RA+ TEMRA cells across the cohort tested. Likewise, the distribution of IFN-γ-expressing T cells and markers of late differentiation (presence of CD57 and loss of CD28) was similar. Similarly, pp65- and IE-specific T cells were both effective antiviral T cells.
Memory T cell “inflation” was originally observed in MCMV infection of mice, where the magnitude of memory and long-term memory T cell responses was measured following primary MCMV infection (17, 49, 50). Based on these studies, it has been suggested that the same phenomenon also occurs in the latently infected human host. However, there have been no extensive longitudinal studies on latently infected individuals, and therefore, memory T cell inflation in the human host has only been inferred by analysis of donors over a range of ages and, almost exclusively, by examining pp65- and IE-specific T cell responses (21, 31, 34, 51, 52). It is clear that elderly individuals often have high-frequency pp65- and IE-specific T cell responses (21, 47, 53–57), although it is not unusual to also observe this in young individuals (39). Clearly, an important issue is the use of the age of a donor to infer the length of time that individual has been carrying HCMV. However, it is important to note that infection in early childhood would mean that a 30-year-old (classified as young) would have been carrying virus for just as long as a 75-year-old who was infected in his/her midforties. Given this caveat, our data show that the cumulative T cell frequency, including that of cells with HCMV specificities other than pp65 and IE, tends to increase with age and that the number of ORFs that elicit responses at high frequency (>1,000 SFU/million) was strongly correlated with age, thus supporting the idea that periodic HCMV reactivation provides antigenic stimulation to drive increased T cell responses.
Analysis of the frequency of T cells specific to 11 different HCMV ORF products, in a number of donors at multiple time points for up to 36 months, showed that the frequency of T cells which recognized these ORF products did not show inflation and in fact demonstrated both increases and decreases. A detailed analysis of some of the cohorts sampled at multiple time points over 40 months confirmed that the frequency of T cells specific to an individual ORF product in an individual donor varies with time. These observations are in complete agreement with a study published by Crough and colleagues (58), who first described this periodic fluctuation in both HCMV- and EBV-specific CD8+ T cells over a 9- to 25-week period and provided evidence of subclinical viral reactivation driving expansions. Clearly, this fluctuation continues to occur over years; however, as an important caveat, the antigen-specific frequency was determined using a functional assay (IFN-γ ELISPOT), and therefore, the fluctuations noted could be due to changes in the proportion of cells with this function rather than changes in the absolute size of the population. Interestingly, ORFs that were not recognized in the original analysis were not recognized in analyses at later time points. Consequently, a longitudinal study in humans over a much longer period of time will be required if the process of memory inflation is to be observed, and the use of a more absolute measure of frequency, such as MHC class I multimers, in conjunction with functional measures would be most informative.
The expansion of a TEMRA CD8+ HCMV-specific population has been reported previously for pp65- and IE-specific T cells (14, 19, 20, 22, 23, 28, 46). Our observations also show that T cells specific for other HCMV ORF products underwent a similar expansion of this phenotype across the cohort tested. It was also noted that the distribution of memory T cell phenotypes in a given donor was very similar irrespective of the ORF specificity; this was also observed in the two donors who were analyzed by MHC class I pentamers to either IE- or pp65-specific peptides. Both donors had similar T cell subset distributions regardless of the epitope specificity (Fig. 4). Although accumulation of TEMRA CD8+ T cells in HCMV-infected individuals has been associated in some studies with a loss of T cell functionality (21, 34), more recent studies by us and others have shown that these T cells are actually polyfunctional as long as they are correctly costimulated and are able to proliferate and secrete multiple antiviral cytokines as well as markers of cytotoxic potential (38, 39).
The CD8+ HCMV-specific TEMRA cells for all the HCMV ORF specificities identified in this study, despite having a highly differentiated CD57+ CD28− phenotype, were also able to secrete IFN-γ following antigen stimulation. The low percentage of IFN-γ secretion by CMV ORF-specific CD8+ T cells by some donors has been observed in other studies (34, 59, 60). The changing pattern of cytokine secretion by antigen-specific T cells has been proposed as a measure of the differentiation status of the memory T cell population (61–64). In particular, the loss of IFN-γ secretion capacity coupled with the secretion of CC chemokines (MIP-1β, MIP-1α, and RANTES) has been associated with very-late-stage-differentiated CD8+ T cells and has been observed principally in CMV-specific CD8+ T cells (65).
The relationship between HCMV T cell antigen specificity, the frequency of highly differentiated T cells, and their overall antiviral functionality with respect to the changes that are observed in elderly individuals remains unclear. HCMV infection in the elderly has been associated with a detrimental impact on the health of elderly individuals (32–36), and it is suggested that this may result from the T cell response to HCMV itself becoming dysfunctional, with a subsequent loss of control of the virus as well as a concomitant degradation of the host immune system in general, to the point where the generation of new responses is impaired (66). Despite the association of HCMV with a loss of immune function, older seropositive individuals do not appear to suffer from overt HCMV disease from either reactivating virus or new infections, suggesting that HCMV-specific T cells do retain the ability to control the virus (37). T cell functional fitness is assessed as the ability of T cells to mount appropriate polyfunctional responses by multianalyte analysis or even mediate cytotoxicity. However, this is an indirect measure of antiviral activity, and previously described assays have not, so far, used HCMV-infected cells as targets. Consequently, they do not assess functionality in a background of, for instance, the well-described MHC class I evasion mechanisms which occur during HCMV lytic infection.
In our analyses, we have developed a viral dissemination assay, with T cell coculture, in order to provide a direct measure of antiviral activity. This now allows an assessment of T cells with different HCMV ORF specificities from the same donor for their antiviral potential. Interestingly, such assays showed that T cells specific for the late antigen pp65 were as effective as IE-specific T cells at preventing viral dissemination. All the pp65- and IE-specific T cells derived from individual members of the cohort were effective at preventing viral dissemination.
HCMV is a paradigm for viral immune evasion of T cell recognition, yet it has always been somewhat paradoxical that a virus with such an extensive array of immune evasion mechanisms elicits a very strong T cell response following primary infection (25, 28) and, likely, reactivation, as immunosuppressed HCMV-seropositive patients often lose the ability to control viral replication, resulting in end organ disease. These observations suggest that the T cell immunity must also be effective at preventing disease following reactivation in normal healthy individuals. The data presented in this article support this conjecture, as T cells specific for both early and late gene products are highly effective at preventing viral dissemination in an in vitro model, and the viral immune evasion strategies appear to be unable to allow avoidance of T cell immune responses in the context of lytic infection. Our view is that the real value of the T cell immune evasion functions of HCMV is to allow a latent virus in an individual cell sufficient time to undergo reactivation and assemble new virus rather than to allow unrestricted viral replication that ends up causing disease in the host upon a primary infection or following reactivation.
ACKNOWLEDGMENTS
This work was funded by British Medical Research Council grant G0701279 and supported by the NIHR Cambridge BRC Cell Phenotyping hub.
Footnotes
Published ahead of print 9 July 2014
REFERENCES
- 1.Gandhi MK, Khanna R. 2004. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect. Dis. 4:725–738. 10.1016/S1473-3099(04)01202-2 [DOI] [PubMed] [Google Scholar]
- 2.Sinclair J, Sissons P. 2006. Latency and reactivation of human cytomegalovirus. J. Gen. Virol. 87:1763–1779. 10.1099/vir.0.81891-0 [DOI] [PubMed] [Google Scholar]
- 3.Riddell SR, Kathe SW, Goodrich JM, Li CR, Agha ME, Greenberg PD. 1992. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 257:238–241. 10.1126/science.1352912 [DOI] [PubMed] [Google Scholar]
- 4.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038–1044. 10.1056/NEJM199510193331603 [DOI] [PubMed] [Google Scholar]
- 5.Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H. 2002. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99:3916–3922. 10.1182/blood.V99.11.3916 [DOI] [PubMed] [Google Scholar]
- 6.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S. 2003. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 362:1375–1377. 10.1016/S0140-6736(03)14634-X [DOI] [PubMed] [Google Scholar]
- 7.Cwynarski K, Ainsworth J, Cobbold M, Wagner S, Mahendra P, Apperley J, Goldman J, Craddock C, Moss PAH. 2001. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood 97:1232–1240. 10.1182/blood.V97.5.1232 [DOI] [PubMed] [Google Scholar]
- 8.Reddehase MJ, Mutter W, Munch K, Buhring HJ, Koszinowski UH. 1987. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J. Virol. 61:3102–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Luccaronin P, Jonjic S, Koszinowski UH. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podlech J, Holtappels R, Pahl-Seibert MF, Steffens HP, Reddehase MJ. 2000. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J. Virol. 74:7496–7507. 10.1128/JVI.74.16.7496-7507.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddehase MJ, Jonjic S, Weiland F, Mutter W, Koszinowski UH. 1988. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J. Virol. 62:1061–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borysiewicz LK, Morris S, Page JD, Sissons JG. 1983. Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity. Eur. J. Immunol. 13:804–809. 10.1002/eji.1830131005 [DOI] [PubMed] [Google Scholar]
- 13.Kern F, Surel IP, Faulhaber N, Frommel C, Schneider-Mergener J, Schonemann C, Reinke P, Volk HD. 1999. Target structures of the CD8+-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J. Virol. 73:8179–8184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan N, Cobbold M, Keenan R, Moss P-A. 2002. Comparative analysis of CD8+ T cell responses against human cytomegalovirus proteins pp65 and immediate early 1 shows similarities in precursor frequency, oligoclonality, and phenotype. J. Infect. Dis. 185:1025–1034. 10.1086/339963 [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin-Taylor E, Pande H, Forman SJ, Tanamachi B, Li CR, Zaia JA, Greenberg PD, Riddell SR. 1994. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J. Med. Virol. 43:103–110. 10.1002/jmv.1890430119 [DOI] [PubMed] [Google Scholar]
- 16.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673–685. 10.1084/jem.20050882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022–2029. 10.4049/jimmunol.170.4.2022 [DOI] [PubMed] [Google Scholar]
- 18.Northfield J, Lucas M, Jones H, Young NT, Klenerman P. 2005. Does memory improve with age? CD85j (ILT-2/LIR-1) expression on CD8+ T cells correlates with ‘memory inflation' in human cytomegalovirus infection. Immunol. Cell Biol. 83:182–188. 10.1111/j.1440-1711.2005.01321.x [DOI] [PubMed] [Google Scholar]
- 19.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379–385. 10.1038/nm0402-379 [DOI] [PubMed] [Google Scholar]
- 20.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106–111. 10.1038/35065118 [DOI] [PubMed] [Google Scholar]
- 21.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth J, Sinclair A, Nayak L, Moss P. 2002. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 169:1984–1992. 10.4049/jimmunol.169.4.1984 [DOI] [PubMed] [Google Scholar]
- 22.Wills MR, Carmichael AJ, Weekes MP, Mynard K, Okecha G, Hicks R, Sissons JGP. 1999. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J. Immunol. 162:7080–7087 [PubMed] [Google Scholar]
- 23.Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJG, Carmichael AJ. 2002. Identification of naive or antigen-experienced human CD8+ T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T cell response. J. Immunol. 168:5455–5464. 10.4049/jimmunol.168.11.5455 [DOI] [PubMed] [Google Scholar]
- 24.Weekes MP, Wills MR, Sissons JGP, Carmichael AJ. 2004. Long-term stable expanded human CD4+ T cell clones specific for human cytomegalovirus are distributed in both CD45RAhigh and CD45ROhigh populations. J. Immunol. 173:5843–5851. 10.4049/jimmunol.173.9.5843 [DOI] [PubMed] [Google Scholar]
- 25.Gamadia LE, Remmerswaal EBM, Weel JF, Bemelman F, van Lier RAW, ten Berge IJM. 2003. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 101:2686–2692. 10.1182/blood-2002-08-2502 [DOI] [PubMed] [Google Scholar]
- 26.van Leeuwen EMM, Remmerswaal EBM, Vossen MTM, Rowshani AT, Wertheim-van Dillen PME, van Lier RAW, ten Berge IJM. 2004. Emergence of a CD4+CD28− granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J. Immunol. 173:1834–1841. 10.4049/jimmunol.173.3.1834 [DOI] [PubMed] [Google Scholar]
- 27.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. 2005. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 175:8218–8225. 10.4049/jimmunol.175.12.8218 [DOI] [PubMed] [Google Scholar]
- 28.Day EK, Carmichael AJ, ten Berge IJM, Waller ECP, Sissons JGP, Wills MR. 2007. Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. J. Immunol. 179:3203–3213. 10.4049/jimmunol.179.5.3203 [DOI] [PubMed] [Google Scholar]
- 29.Gillespie GMA, Wills MR, Appay V, O'Callaghan C, Murphy M, Smith N, Sissons P, Rowland-Jones S, Bell JI, Moss PAH. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J. Virol. 74:8140–8150. 10.1128/JVI.74.17.8140-8150.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miles DJC, van der Sande M, Jeffries D, Kaye S, Ismaili J, Ojuola O, Sanneh M, Touray ES, Waight P, Rowland-Jones S, Whittle H, Marchant A. 2007. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J. Virol. 81:5766–5776. 10.1128/JVI.00052-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. 2007. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J. Virol. 81:7759–7765. 10.1128/JVI.01262-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. 2006. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 176:2645–2653. 10.4049/jimmunol.176.4.2645 [DOI] [PubMed] [Google Scholar]
- 33.Olsson J, Wikby A, Johansson B, Löfgren S, Nilsson BO, Ferguson FG. 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121:187–201 [DOI] [PubMed] [Google Scholar]
- 34.Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G. 2004. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp. Gerontol. 39:607–613. 10.1016/j.exger.2003.11.016 [DOI] [PubMed] [Google Scholar]
- 35.Wikby A, Johansson B, Olsson J, Löfgren S, Nilsson BO, Ferguson F. 2002. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol. 37:445–453. 10.1016/S0531-5565(01)00212-1 [DOI] [PubMed] [Google Scholar]
- 36.Wikby A, Mansson IA, Johansson B, Strindhall J, Nilsson SE. 2008. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology 9:299–308. 10.1007/s10522-008-9138-6 [DOI] [PubMed] [Google Scholar]
- 37.Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. 2007. Chronic herpesvirus reactivation occurs in aging. Exp. Gerontol. 42:563–570. 10.1016/j.exger.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waller EC, McKinney N, Hicks R, Carmichael AJ, Sissons JG, Wills MR. 2007. Differential costimulation through CD137 (4 1BB) restores proliferation of human virus-specific “effector memory” (CD28 CD45RAHI) CD8+ T cells. Blood 110:4360–4366. 10.1182/blood-2007-07-104604 [DOI] [PubMed] [Google Scholar]
- 39.Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A, Kern F. 2012. Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J. Virol. 86:1001–1009. 10.1128/JVI.00873-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wehler T, Karg M, Distler E, Konur A, Nonn M, Meyer R, Huber C, Hartwig U, Herr W. 2008. Rapid identification and sorting of viable virus-reactive CD4(+) and CD8(+) T cells based on antigen-triggered CD137 expression. J. Immunol. Methods 339:23–37. 10.1016/j.jim.2008.07.017 [DOI] [PubMed] [Google Scholar]
- 41.Wölfl M, Kuball J, Eyrich M, Schlegel P, Greenberg P. 2008. Use of CD137 to study the full repertoire of CD8+ T cells without the need to know epitope specificities. Cytometry A 73:1043–1049. 10.1002/cyto.a.20594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfl M, Kuball J, Ho W, Nguyen H, Manley T, Bleakley M, Greenberg P. 2007. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 110:201–210. 10.1182/blood-2006-11-056168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe K, Suzuki S, Kamei M, Toji S, Kawase T, Takahashi T, Kuzushima K, Akatsuka Y. 2008. CD137-guided isolation and expansion of antigen-specific CD8 cells for potential use in adoptive immunotherapy. Int. J. Hematol. 88:311–320. 10.1007/s12185-008-0134-z [DOI] [PubMed] [Google Scholar]
- 44.Wills MR, Ashiru O, Reeves MB, Okecha G, Trowsdale J, Tomasec P, Wilkinson GWG, Sinclair J, Sissons JGP. 2005. Human cytomegalovirus encodes an MHC class I-like molecule (UL142) that functions to inhibit NK cell lysis. J. Immunol. 175:7457–7465. 10.4049/jimmunol.175.11.7457 [DOI] [PubMed] [Google Scholar]
- 45.Crough T, Khanna R. 2009. Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 22:76–98. 10.1128/CMR.00034-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauce D, Larsen M, Leese AM, Millar D, Khan N, Hislop AD, Rickinson AB. 2007. IL-7Ralpha versus CCR7 and CD45 as markers of virus-specific CD8+ T cell differentiation: contrasting pictures in blood and tonsillar lymphoid tissue. J. Infect. Dis. 195:268. 10.1086/510248 [DOI] [PubMed] [Google Scholar]
- 47.Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P. 2009. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 155:423–432. 10.1111/j.1365-2249.2008.03785.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kern F, Khatamzas E, Surel I, Frommel C, Reinke P, Waldrop SL, Picker LJ, Volk HD. 1999. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 29:2908–2915. [DOI] [PubMed] [Google Scholar]
- 49.Holtappels R, Pahl-Seibert M-F, Thomas D, Reddehase MJ. 2000. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62Llo memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J. Virol. 74:11495–11503. 10.1128/JVI.74.24.11495-11503.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 177:450–458. 10.4049/jimmunol.177.1.450 [DOI] [PubMed] [Google Scholar]
- 51.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. 2005. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79:3675–3683. 10.1128/JVI.79.6.3675-3683.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pita-Lopez M, Gayoso I, DelaRosa O, Casado J, Alonso C, Munoz-Gomariz E, Tarazona R, Solana R. 2009. Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun. Ageing 6:11. 10.1186/1742-4933-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. 2004. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 173:7481–7489. 10.4049/jimmunol.173.12.7481 [DOI] [PubMed] [Google Scholar]
- 54.Schwanninger A, Weinberger B, Weiskopf D, Herndler-Brandstetter D, Reitinger S, Gassner C, Schennach H, Parson W, Wurzner R, Grubeck-Loebenstein B. 2008. Age-related appearance of a CMV-specific high-avidity CD8+ T cell clonotype which does not occur in young adults. Immun. Ageing 5:14. 10.1186/1742-4933-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vescovini R, Biasini C, Fagnoni FF, Telera AR, Zanlari L, Pedrazzoni M, Bucci L, Monti D, Medici MC, Chezzi C, Franceschi C, Sansoni P. 2007. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J. Immunol. 179:4283–4291. 10.4049/jimmunol.179.6.4283 [DOI] [PubMed] [Google Scholar]
- 56.Vescovini R, Telera A, Fagnoni FF, Biasini C, Medici MC, Valcavi P, Di Pede P, Lucchini G, Zanlari L, Passeri G, Zanni F, Chezzi C, Franceschi C, Sansoni P. 2004. Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8+ T cells. Exp. Gerontol. 39:1233–1243. 10.1016/j.exger.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 57.Vescovini R, Biasini C, Telera AR, Basaglia M, Stella A, Magalini F, Bucci L, Monti D, Lazzarotto T, Dal Monte P, Pedrazzoni M, Medici MC, Chezzi C, Franceschi C, Fagnoni FF, Sansoni P. 2010. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J. Immunol. 184:3242–3249. 10.4049/jimmunol.0902890 [DOI] [PubMed] [Google Scholar]
- 58.Crough T, Burrows J, Fazou C, Walker S, Davenport M, Khanna R. 2005. Contemporaneous fluctuations in T cell responses to persistent herpes virus infections. Eur. J. Immunol. 35:139–149. 10.1002/eji.200425548 [DOI] [PubMed] [Google Scholar]
- 59.Gamadia LE, Rentenaar RJ, Baars PA, Remmerswaal EB, Surachno S, Weel JF, Toebes M, Schumacher TN, ten Berge IJ, van Lier RA. 2001. Differentiation of cytomegalovirus-specific CD8+ T cells in healthy and immunosuppressed virus carriers. Blood 98:754–761. 10.1182/blood.V98.3.754 [DOI] [PubMed] [Google Scholar]
- 60.Bronke C, Palmer NM, Jansen CA, Westerlaken GH, Polstra AM, Reiss P, Bakker M, Miedema F, Tesselaar K, van Baarle D. 2005. Dynamics of cytomegalovirus (CMV)-specific T cells in HIV-1-infected individuals progressing to AIDS with CMV end-organ disease. J. Infect. Dis. 191:873–880. 10.1086/427828 [DOI] [PubMed] [Google Scholar]
- 61.Ellefsen K, Harari A, Champagne P, Bart PA, Sekaly RP, Pantaleo G. 2002. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur. J. Immunol. 32:3756–3764. [DOI] [PubMed] [Google Scholar]
- 62.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. 2006. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211:236–254. 10.1111/j.0105-2896.2006.00395.x [DOI] [PubMed] [Google Scholar]
- 63.Harari A, Zimmerli SC, Pantaleo G. 2004. Cytomegalovirus (CMV)-specific cellular immune responses. Hum. Immunol. 65:500–506. 10.1016/j.humimm.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 64.Pantaleo G, Harari A. 2006. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 6:417–423. 10.1038/nri1840 [DOI] [PubMed] [Google Scholar]
- 65.Kim TK, St John LS, Wieder ED, Khalili J, Ma Q, Komanduri KV. 2009. Human late memory CD8+ T cells have a distinct cytokine signature characterized by CC chemokine production without IL-2 production. J. Immunol. 183:6167–6174. 10.4049/jimmunol.0902068 [DOI] [PubMed] [Google Scholar]
- 66.Moss P. 2010. The emerging role of cytomegalovirus in driving immune senescence: a novel therapeutic opportunity for improving health in the elderly. Curr. Opin. Immunol. 22:529–534. 10.1016/j.coi.2010.07.001 [DOI] [PubMed] [Google Scholar]