Abstract
Utilizing a human cytomegalovirus-specific fusion inhibitor and an antiglycoprotein H antibody, we studied the role of virion fusion in interferon-stimulated gene (ISG) induction. Our results indicate that ISG induction does not occur when virion-mediated, post-high-affinity attachment events are inhibited by either reagent. Thus, virion-mediated postattachment events, such as fusion, are required for ISG induction.
A large number of interferon-stimulated genes (ISGs) are strongly activated following human cytomegalovirus (HCMV) infection (19). In early studies, UV-irradiated HCMV was shown to induce ISGs (19). Subsequent studies revealed that virion envelope proteins, specifically glycoprotein B (gB), were responsible for the induction of ISGs (3, 17). Soluble gB applied to human fibroblasts resulted in the induction of ISGs. Because the latter experiment was done under nonphysiological conditions and because gB has been shown to have roles in both virion attachment and fusion, it was unclear whether virion attachment alone was sufficient to induce ISG expression. Alternatively, other HCMV envelope glycoproteins or perhaps the fusion process itself is required for ISG activation during natural infection, as is the case for ISG induction by herpes simplex virus (14). In this study, we examined the requirement of postattachment steps of HCMV entry for the induction of ISGs.
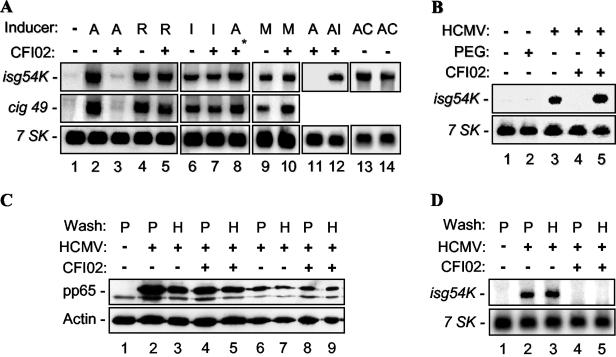
To characterize the role of virion fusion in HCMV-mediated ISG induction, an HCMV-specific fusion inhibitor, CFI02, was used (2). It was previously shown that this inhibitor has no effect on the attachment of virus to cells, but that it efficiently blocks subsequent viral-cell fusion at a 2 μM concentration (7). CFI02 was used to determine if the compound also blocks induction of ISGs by HCMV (Fig. 1A). Human foreskin fibroblast (HFF) cells were either mock infected or infected with HCMV in the presence of dimethyl sulfoxide (control) or CFI02 for 8 h. Induction of the ISGs isg54K and cig49 was determined by RNA blot analysis. A cellular pseudogene, 7SK (13), was used as an internal control. Induction of ISGs by HCMV was inhibited in the presence of CFI02 (Fig. 1A, compare lanes 2 and 3). These results indicated that a postattachment step of HCMV infection, presumably virion fusion with the cellular membrane, was required for the induction of ISG expression. The effect of CFI02 inhibition on HCMV fusion was specific because (i) it had no effect on CFI02-resistant, HCMV-mediated ISG induction (the resistance phenotype was mapped to gB) (7) (lanes 4 and 5); (ii) it did not block murine-CMV-mediated ISG induction (lanes 9 and 10); (iii) it had no effect on alpha interferon (IFN-α)-induced ISG expression (lanes 6 and 7); and (iv) it was not able to block induction of ISGs when added 2 h after infection (lanes 3 and 8). Furthermore, inhibition of HCMV-induced ISG expression by CFI02 had no effect on IFN-α-mediated induction of ISGs (lanes 11 and 12). In addition, as shown previously (19), HCMV is able to induce isg54K at 12 and 24 h postinfection (hpi) in the presence of cycloheximide, indicating that protein synthesis is not required for this activation (Fig. 1A, lanes 13 and 14, respectively). Since CFI02 has no effect on the initial attachment of HCMV to the cell membrane (2), postattachment events, such as interactions between envelope glycoproteins, formation of stable interactions with cellular receptor(s), and/or subsequent fusion, appear to be required for triggering the induction of ISGs.
FIG. 1.
Fusion is required for the induction of ISGs by HCMV. (A) HFF cells were either mock infected (lane 1), infected at an MOI of 3 with different cytomegaloviridae (AD169 [A], a CFI02-resistant strain of AD169 [R], and murine CMV [M]) for 8 h (lanes 1 to 12), 12 h (lane 13), or 24 h (lane 14), or treated with IFN-α (I) for 4 h in the presence of 2 μM CFI02 (+) or 0.3% dimethyl sulfoxide (−). In lane 8, CFI02 was added 2 hpi (*); in lane 12, IFN-α was added 4 hpi; and in lanes 13 and 14, cycloheximide (C; 100 μg/ml) was added during infection. The expression of isg54K, cig49, and 7SK was measured by RNA blot hybridization analysis. (B) HFF cells were either mock infected or infected with HCMV at an MOI of 3 in the presence (+) or absence (−) of 2 μM CFI02 for 2 h. The cells were then either left untreated (−) or briefly (5 to 10 s) treated with 50% (wt/vol) PEG 8000 (+). After removal of the PEG, the cells were further incubated for 18 h for analysis of ISG induction by RNA blot hybridization. (C and D) HFF cells were incubated with AD169 (MOI, 3) or mock infected at 4°C for 30 min. Inocula were then removed, and cells were incubated for another 30 min at 4°C in the presence (+) or absence (−) of CFI02 (3 μM). The cells were then washed with either PBS (P) or 10 μg of heparin (H) per ml, as described previously (6). One set of samples was used to measure viruses bound to cells by immunoblot analysis with anti-pp65 antibody (C), with lanes 1 to 5 containing 30 μl of cell lysate each and lanes 6 to 9 containing 10 μl of cell lysate each. Anti-pp65 antibody cross-reacted with a cellular protein (just below the pp65 band) that serves as an internal control. Another internal control, actin, was detected by an anti-actin antibody (Cl-80). The second set of samples was shifted to 37°C for 8 h to determine isg54K induction by RNA blot hybridization analysis (D).
To further determine if the inhibition of ISG induction by CFI02 was secondary to an inhibition of virion-cell fusion, a protocol adapted to study the effect of CFI02 on HCMV infectivity was utilized (7). It was shown previously that inhibition of HCMV virion fusion by CFI02 could be reversed by the treatment of virion-adsorbed cells with a fusion-inducing polymer, polyethylene glycol (PEG) (7). HFF cells were infected with HCMV in the absence or presence of a fusion inhibitor, CFI02, and then treated with PEG (Fig. 1B). As expected, HCMV-induced ISG expression was blocked by CFI02 (Fig. 1B, lanes 3 and 4). This inhibition was reversed by the addition of PEG, which resulted in a strong activation of ISG (lane 5). In contrast, PEG itself had no effect on ISG expression (lane 2), indicating that possible cell-cell fusion alone is not sufficient to induce ISGs, but that HCMV virion-cell fusion is a requirement. These data further support the conclusion that ISG induction following HCMV infection requires postattachment events that occur during, or as result of, virion fusion.
HCMV attachment to the cell surface occurs in two steps. Initially, there is a low-affinity interaction between HCMV glycoproteins (gB and gM) and cellular surface heparan sulfate proteoglycans (6, 8). This low-affinity interaction is then rapidly converted to stable high-affinity binding that may involve additional interactions between HCMV glycoproteins (including gB) and receptors on the cell surface (5, 6), including the epidermal growth factor (EGF) receptor in some cell types (18). The low-affinity, but not the high-affinity, interaction can be disrupted by soluble heparin, causing virion dissociation from the cell (6). It is known that CFI02 does not block HCMV attachment but does block HCMV fusion when CFI02 is added after virus binding to high-affinity receptors (i.e., after an adsorption period at 4°C) (6, 7). Thus, a relevant question is whether virion binding to high-affinity receptors is sufficient for ISG induction. To address this question specifically, three similar sets of experiments were performed, using the following protocol: HCMV (multiplicity of infection [MOI], 3) was incubated with HFF cells at 4°C for 30 min to allow high-affinity binding of virus to the cells. After removal of the inoculum, CFI02 was added to the culture for 30 min at 4°C. The cells were then washed with phosphate-buffered saline (PBS) or heparin (as described in reference 6). In the control set of plates, cell culture was continued for 3 days at 37°C to verify the inhibitory activity of CFI02. CFI02 blocked HCMV infection in both PBS- and heparin-washed samples (data not shown). In the second set, cell lysates were made immediately after the PBS or heparin wash and used for immunoblot analysis of the abundant virion tegument protein pp65 (Fig. 1C). The results demonstrated that equivalent amounts of virus remained attached to cellular high-affinity receptors, since there was no detectable differential dissociation of virus from untreated or CFI02-treated cells with either the PBS or the heparin wash. Specifically, CFI02 did not affect the stable high-affinity interaction between HCMV and cellular receptors because heparin did not dissociate bound virus in the presence of CFI02 (Fig. 1C, compare lanes 4 and 5 and lanes 8 and 9). In the third set, after the PBS or heparin wash, cells were cultured at 37°C for 8 h and induction of isg54K was determined by RNA blot analysis (Fig. 1D). The data showed that isg54K induction was completely blocked by CFI02 under these conditions (Fig. 1D, compare lanes 2 and 3 with lanes 4 and 5). These crucial experiments prove that CFI02 blocks HCMV induction of ISGs not by inhibiting high-affinity binding of virion to cellular receptor but by preventing a required post-high-affinity attachment event, including gB-mediated fusion.
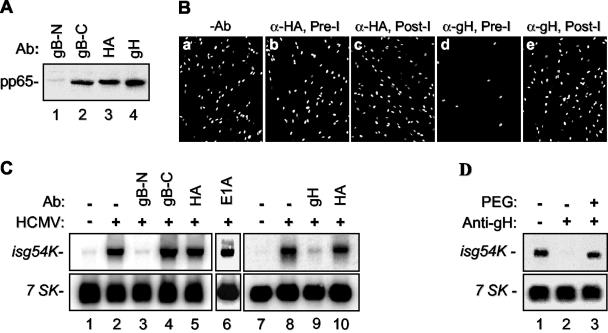
To assess the possible role of virion envelope glycoproteins other than gB, particularly those glycoproteins believed to be responsible for virion-host cell fusion, in the induction of ISGs, we investigated the role of HCMV gH with a virus-neutralizing antibody reactive with it. It is known that gH is expressed together with gL and gO as a glycoprotein complex and that gH and gL are required for virion fusion during entry (9, 10, 12). Neutralizing monoclonal antibodies against gH have been reported to inhibit virion-cell fusion but not attachment (9, 15). If a postattachment step in virus infection is required for ISG induction, as suggested by the CFI02 experiments, then inhibiting fusion (but not attachment) with an anti-gH antibody should block induction of ISGs by HCMV. Initially, the step of the viral infection cycle at which the anti-gH antibody 14-4B (11) neutralizes infectious virus was confirmed (Fig. 2A). Gradient-purified HCMV infectious virions were preincubated with several monoclonal antibodies. These antibodies included the HCMV-neutralizing antibodies anti-gB-N (7-17) (4) and anti-gH, the non-HCMV-neutralizing antibody anti-gB-C (58-15) (1), and the negative-control antibodies anti-HA (12CA5) and anti-E1A (M73). After incubation, antibody-treated virus was used to infect HFF cells for 1 h at 4°C, a procedure that allows attachment but not fusion. Following washing to remove unbound particles, bound viral particles were assayed by immunoblotting using antibody against the abundant HCMV tegument protein pp65 (65-8) (16). As expected, neutralizing anti-gB-N antibody blocked binding (no cell-associated pp65 was detected), while negative-control antibody anti-HA did not block binding (Fig. 2A, compare lanes 1 and 3). In contrast to results with anti-gB-N antibody, cellular adsorption of anti-gB-C- and anti-gH-treated virions was not inhibited (lanes 2 and 4). Thus, the anti-gH neutralizing antibody did not inhibit viral attachment.
FIG. 2.
Induction of ISGs requires gH. (A) Gradient-purified infectious particles of AD169 (3 × 105 PFU) were incubated with one of four monoclonal antibodies (Ab) (anti-gB-N, anti-gB-C, anti-gHA, or anti-gH) for 1 h at 37°C. These mixtures were then used to infect HFF cells at an MOI of 1 at 4°C for 1 h. The cells were then washed twice in ice-cold PBS, followed by Western blot analysis for HCMV pp65 with monoclonal antibody 65-8. (B) HCMV infectious virions (106 PFU) were incubated with one of several different monoclonal antibodies for 1 h at 37°C. These mixtures were then used to infect HFF cells at an MOI of 0.1 at 37°C. Immunofluorescence staining of HCMV-infected cells with anti-IE1 antibody was done at 48 hpi. The samples were either preincubated (Pre-I) with no antibody (−Ab) (a), with anti-HA (α-HA) (b), or with anti-gH (d) or incubated beginning 1 hpi (Post-I) with anti-HA (c) or anti-gH (e). The results showed that HCMV infection was neutralized by anti-gH antibody under preincubation conditions. (C) Under the same conditions as described for panel B, induction of isg54K by HCMV was measured. Infection was carried out for 18 h in order to obtain a higher level of isg54 expression. It showed that isg54 expression was blocked by anti-gB-N (lane 3) and anti-gH (lane 9) but not by anti-gB-C (lane 4), anti-HA (lanes 5 and 10), or anti-E1A (lane 6). 7SK is an internal control. (D) HCMV-anti-gH-inhibited isg54K induction by HCMV (lane 2) can be reversed by PEG (lane 3).
To monitor the efficiency of neutralization, gradient-purified HCMV was incubated with different antibodies and then used to infect HFF cells at 37°C. Infectivity was judged by immunofluorescent staining for HCMV IE1-positive cells. Consistent with the studies cited above, preincubation of virions with anti-gB-N and anti-gH antibodies almost completely blocked viral infection, while preincubation with anti-gB-C, anti-HA, and anti-E1A antibodies had little effect on infection (Fig. 2B and data not shown). When added to infected cells at 1 hpi, anti-gH had no effect on infection, confirming that anti-gH neutralizing activity is due to inhibition of a postattachment step of virus infection, such as virion fusion (Fig. 2B, panel e). To assess the effects of these five antibodies on ISG induction, antibody-treated virions were used to infect HFF cells, and RNA blot analysis was used to measure ISG expression (Fig. 2C). As predicted, anti-gB-N antibody blocked the induction of ISGs (Fig. 2C, lane 3) because this neutralizing antibody blocks virion adsorption to cells. In contrast, nonneutralizing anti-gB-C antibody failed to inhibit induction of ISGs presumably because it recognizes the cytoplasmic portion of gB (which is within the virion) and does not block viral infection (lane 4). Two additional negative-control antibodies, anti-HA (lanes 5 and 10) and anti-E1A (lane 6), had no effect on the induction of ISGs by HCMV. Most importantly, anti-gH antibody 14-4B strongly blocked the induction of ISGs by HCMV (lane 9). This anti-gH-mediated inhibition of ISG induction was also reversed by PEG treatment (Fig. 2D). Together with our findings for the fusion inhibitor CFI02, these results provide additional evidence that the induction of ISGs requires postattachment fusion-related events that may be mediated not only by gB but also by gH. Herpes simplex virus utilizes a similar mechanism for the induction of ISGs (14).
In this study, the role of HCMV entry in ISG induction was analyzed. Under conditions that are normally utilized for HCMV infection of susceptible human fibroblasts, HCMV adsorption to the cell surface was not sufficient for the induction of ISGs. Postattachment events, most likely fusion mediated by virion envelope glycoproteins, are required for this activity. This conclusion is based on several observations: (i) CFI02, an HCMV-specific fusion inhibitor, blocks ISG induction; (ii) the block of ISG induction by CFI02 is reversed by a fusion-inducing agent, PEG; (iii) binding of HCMV to high-affinity cellular receptors is not sufficient for ISG induction; (iv) HCMV-neutralizing antibody against gH that blocks virion fusion prevents the induction of ISGs; and (v) the anti-gH inhibitory activity can be reversed by PEG.
While it is not clear how HCMV virions activate ISGs under the physiological conditions described herein, we propose two possible mechanisms. In the first, HCMV glycoprotein complex interacts with cellular receptors or mediators during the fusion process, resulting in the initiation of a signal transduction pathway that leads to the activation of ISGs. For example, it was shown recently that the EGF receptor serves as an HCMV gB receptor and is required for a number of HCMV-triggered signal transduction pathways (18). Interestingly, an inhibitor of the EGF-receptor kinase also prevented HCMV virion fusion but not attachment (18). In the second, the fusion process, although required, may not be directly involved in this induction, but postfusion events, such as activation by virion-associated tegument proteins which are released intracellularly after fusion, are required. Further studies are necessary to elucidate the nature of ISG induction by HCMV virions.
Acknowledgments
We thank T. Shenk for reagents and advice and C. Patterson and V. Bellofatto for critically reading the manuscript.
This work was supported by NIH grants AI50709-2A1 (H.Z.) and AI35602 (W.J.B.), New Jersey Commission on Cancer Research grant 702021 (H.Z.), and UMDNJ Foundation grant 104727 (H.Z.).
REFERENCES
- 1.Billstrom, M. A., and W. J. Britt. 1995. Postoligomerization folding of human cytomegalovirus glycoprotein B: identification of folding intermediates and importance of disulfide bonding. J. Virol. 69:7015-7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom, J. D., M. J. DiGrandi, R. G. Dushin, K. J. Curran, A. A. Ross, E. B. Norton, E. Terefenko, T. R. Jones, B. Feld, and S. A. Lang. 2003. Thiourea inhibitors of herpes viruses. Part 1: bis-(aryl)thiourea inhibitors of CMV. Bioorg. Med. Chem. Lett. 13:2929-2932. [DOI] [PubMed] [Google Scholar]
- 3.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 5.Compton, T. 1995. Towards a definition of the HCMV entry pathway. Scand. J. Infect. Dis. Suppl. 99:30-32. [PubMed] [Google Scholar]
- 6.Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834-841. [DOI] [PubMed] [Google Scholar]
- 7.Jones, T. R., S.-W. Lee, S. V. Johann, V. Razinkov, R. J. Visalli, B. Feld, J. D. Bloom, and J. O'Connell. 2004. Specific inhibition of human cytomegalovirus glycoprotein B-mediated fusion by a novel thiourea small molecule. J. Virol. 78:1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kari, B., and R. Gehrz. 1993. Structure, composition and heparin binding properties of a human cytomegalovirus glycoprotein complex designated gC-II. J. Gen. Virol. 74:255-264. [DOI] [PubMed] [Google Scholar]
- 9.Keay, S., and B. Baldwin. 1991. Anti-idiotype antibodies that mimic gp86 of human cytomegalovirus inhibit viral fusion but not attachment. J. Virol. 65:5124-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinzler, E. R., R. N. Theiler, and T. Compton. 2002. Expression and reconstitution of the gH/gL/gO complex of human cytomegalovirus. J. Clin. Virol. 25(Suppl. 2):S87-S95. [DOI] [PubMed] [Google Scholar]
- 11.Li, L., J. A. Nelson, and W. J. Britt. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J. Virol. 71:3090-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milne, R. S., D. A. Paterson, and J. C. Booth. 1998. Human cytomegalovirus glycoprotein H/glycoprotein L complex modulates fusion-from-without. J. Gen. Virol. 79:855-865. [DOI] [PubMed] [Google Scholar]
- 13.Murphy, S., F. Altruda, E. Ullu, M. Tripodi, L. Silengo, and M. Melli. 1984. DNA sequences complementary to human 7 SK RNA show structural similarities to the short mobile elements of the mammalian genome. J. Mol. Biol. 177:575-590. [DOI] [PubMed] [Google Scholar]
- 14.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen, L., S. Resta, and T. Merigan. 1991. Human cytomegalovirus glycoprotein-receptor interactions. Transplant. Proc. 23:60-63. [PubMed] [Google Scholar]
- 16.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 19.Zhu, H., J. P. Cong, and T. Shenk. 1997. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. USA 94:13985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]