Abstract
Human herpesvirus 8 (HHV-8) encodes multiple proteins that disrupt the host antiviral response, including viral interferon (IFN) regulatory factor 1 (vIRF-1). The product of the vIRF-1 gene blocks responses to IFN when overexpressed by transfection, but the functional consequence of vIRF-1 that is expressed during infection with HHV-8 is not known. These studies demonstrate that BCBL-1 cells that were latently infected with HHV-8 expressed low levels of vIRF-1 that were associated with PML bodies, whereas much higher levels of vIRF-1 were transiently expressed during the lytic phase of HHV-8 replication. The low levels of vIRF-1 that were associated with PML bodies were insufficient to block alpha IFN (IFN-α)-induced alterations in gene expression, whereas cells that expressed high levels of vIRF-1 were resistant to some changes induced by IFN-α, including the expression of the double-stranded-RNA-activated protein kinase. High levels of vIRF-1 were expressed for only a short period during the lytic cascade, so many cells with HHV-8 in the lytic phase responded to IFN-α with increased expression of antiviral genes and enhanced apoptosis. Furthermore, the production of infectious virus was severely compromised when IFN-α was present early during the lytic cascade. These studies indicate that the transient expression of high levels of vIRF-1 is inadequate to subvert many of the antiviral effects of IFN-α so that IFN-α can effectively induce apoptosis and block production of infectious virus when present early in the lytic cascade of HHV-8.
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma (KS) herpesvirus, is a large double-stranded DNA virus belonging to the gammaherpesvirus subfamily (11, 43). HHV-8 is present in KS lesions from all risk groups worldwide and appears to be essential in the pathogenesis of KS (11, 42, 43). HHV-8 is also present in primary effusion lymphomas (PELs) (6-8), in many cases of multicentric Castleman's disease (62), and in HHV-8-associated germinotropic lymphoproliferative disorder (19), strongly suggesting an etiologic linkage to these diseases as well.
Much of our knowledge about HHV-8 replication comes from studies done with cell lines derived from PELs, including BCBL-1 cells that are infected with HHV-8 but not with Epstein-Barr virus (EBV) (52). Under standard culture conditions for BCBL-1 cells, HHV-8 is latent in most cells. Virions are not produced during latency, and only a limited number of viral genes are expressed (15, 35, 50, 73). Pharmacological agents such as the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) can induce HHV-8 in BCBL-1 cells to enter the lytic replicative cycle (52, 72). Lytic replication is characterized by a temporal cascade of viral gene expression that includes expression of viral proteins required for replicating HHV-8 DNA and packaging it into infectious virions (29, 48, 56).
The host response to viral infection has evolved to restrict the production of infectious virus and eradicate virally infected cells (1, 28). An important component of the host response is the production of interferons (IFNs). These secreted proteins enhance the cellular and host antiviral responses by inducing the expression of multiple genes involved in the innate and acquired immune responses, including the double-stranded-RNA-activated protein kinase (PKR), 2′,5′-oligoadenylate synthetase (2′,5′-OAS), and major histocompatibility complex class I (17, 57-59). To overcome cellular defenses, most viruses express products that disrupt responses to IFNs and/or to IFN-induced genes (34, 65). For example, HHV-8 encodes at least four proteins that resemble the interferon regulatory factor (IRF) family of transcription factors (3-5, 24, 39, 53, 74). Each of these inhibits responses to IFNs when overexpressed through transfection. The most extensively studied is viral IRF-1 (vIRF-1). Overexpression of vIRF-1 inhibits the ability of alpha IFN (IFN-α), IFN-γ, IRF-1, and IRF-3 to induce gene expression (24, 38, 74). In addition, vIRF-1 inhibits apoptosis and p53-mediated gene expression and alters chromatin structure and the function of transcriptional coactivator proteins (4, 37, 38, 55). These diverse functions have been demonstrated primarily through constitutive expression of vIRF-1 in non-HHV-8 infected cells, whereas less is known about vIRF-1 expressed as a consequence of HHV-8 infection.
Despite the existence of multiple HHV-8-encoded genes that can disrupt responses to IFNs, not all IFN-induced responses are blocked in HHV-8-infected cells. IFN-α inhibits reactivation of HHV-8 in PELs and reduces the HHV-8 load in peripheral blood mononuclear cells (41). IFN-α also induces apoptosis in HHV-8-infected PELs (16). Most of the vIRFs are expressed during lytic replication (14, 39, 69), yet IFN-α induces more apoptosis when HHV-8 has entered the lytic phase than when HHV-8 is primarily latent (16). This raises concerns that vIRFs might not be expressed at sufficient levels or for sufficient durations to inhibit IFN-α-induced antiviral responses. Alternatively, vIRFs might inhibit antiviral responses during vulnerable stages of viral replication but not block IFN-α-induced changes after key events in viral production have been completed.
In this study, we explore vIRF-1 expression during the course of viral infection in BCBL-1 cells and examine whether vIRF-1 is able to protect HHV-8-infected cells from changes induced by IFN-α. We demonstrate that vIRF-1 colocalizes to PML bodies in all HHV-8-infected BCBL-1 cells, including cells that are latently infected. In addition, vIRF-1 is transiently expressed at high levels in cells that have entered the lytic cascade of HHV-8 replication. The vIRF-1 protein has a short half-life and does not remain at high levels throughout the lytic cascade, in contrast to polymerase processivity factor (PPF) (open reading frame [ORF] 59), which remains elevated for much longer. The cells that express high levels of vIRF-1 are more resistant to IFN-α-induced expression of PKR than neighboring cells that lack high levels of vIRF-1, yet high vIRF-1 levels are not present during much of the lytic cascade, and IFN-α effectively blocks the production of most infectious virus when added early in the lytic cascade.
MATERIALS AND METHODS
Cell culture.
BCBL-1 (an HHV-8-positive, EBV-negative PEL cell line), JS-EBV (an EBV-immortalized B-lymphoblastoid cell line), and BL-41 (an HHV-8-negative, EBV-negative B cell line) (NIH AIDS Research and Reference Program, Rockville, Md.) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 10 U of streptomycin per ml. A reporter cell line for HHV-8, T1H6 (27), was maintained in Dulbecco's modified Eagle's medium (Cellgro, Herndon, Va.) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin per ml, 10 U of streptomycin per ml, and 50 μg of hygromycin B per ml and split 1:10 every 3 to 4 days.
Preparation of rabbit antiserum to vIRF-1.
Rabbit antiserum to vIRF-1 was generated against full-length His-tagged bacterially expressed vIRF-1 at the Pocono Rabbit Farm and Laboratory (Canadensis, Pa.). By Western blot analysis, the polyclonal antiserum to vIRF-1 at a 1:1,000 dilution did not cross-react with recombinant IRF-1, IRF-2, or extracts from cells not infected with HHV-8 (data not shown). However, it reacted with in vitro translated-vIRF-1 and with a protein of identical molecular mass that corresponded to the predicted molecular mass of vIRF-1 in HHV-8-positive BCBL-1 cells (the predicted molecular mass of vIRF-1 is 48,465 kDa).
Northern blot analysis.
Total cellular RNA was isolated from BCBL-1 cells by using Trizol (GibcoBRL, Grand Island, N.Y.) and was size fractionated on a 1% agarose-formaldehyde gel. RNA was transferred to nitrocellulose and covalently linked by UV irradiation with a Stratalinker (Stratagene, La Jolla, Calif.) and by baking in vacuo for 2 h at 80°C. DNA probes for vIRF-1 (74), Rta (54), viral interleukin-6 (vIL-6) (72), v-cyclin (72), 2′,5′-OAS (2), and PKR (26) were radiolabeled by using an oligolabeling kit (Stratagene) with [32P]dCTP according to the manufacturer's recommendations. Hybridization was performed at 42°C in 5 ×SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1% sodium dodecyl sulfate (SDS), 5× Denhardt's solution, 100 μg of denatured salmon sperm DNA per ml, 50% formamide, 10% dextran sulfate, and radiolabeled DNA probe. The nitrocellulose was washed with a final stringency of 0.2× SSC in 0.1% SDS at 55°C. Blots were serially probed for the genes indicated, and the nitrocellulose was stripped with boiling water prior to rehybridization with other probes.
Western blot analysis.
Western blot analysis was performed with total cellular lysate from BCBL-1 cells that were harvested and lysed in cell lysis buffer (Cell Signaling Technology, Beverly, Mass.) with protease inhibitor cocktail (BD PharMingen, San Diego, Calif.). Total protein was size fractionated by SDS-12.5% polyacrylamide gel electrophoresis. Proteins were transferred from the acrylamide gel to polyvinylidene difluoride membranes. The membranes were blocked with 5% dry nonfat milk and then probed with antibodies against vIRF-1 at a 1:1,000 dilution, against vIL-6 at 1:80, and against α-tubulin at 1:1,000. After the secondary antibody reaction, the membranes were washed in TBS-Tween (0.02 M Tris [pH 7.6], 0.1 M sodium chloride, 0.05% Tween 20), visualized by using the enhanced chemiluminescence reaction (Amersham Pharmacia) as specified by the manufacturer, and subjected to autoradiography. Semiquantitative analysis of proteins was performed with National Institutes of Health image software. The antibody to α-tubulin was from Sigma (St. Louis, Mo.) and the vIL-6 antibody was a rabbit polyclonal antibody kindly provided by Patrick Moore and Yuan Chang.
ISH.
In situ hybridization (ISH) was performed on sections from cell pellets that were fixed overnight in 4% paraformaldehyde-0.01 M phosphate-buffered saline (pH 7.4) and embedded in paraffin. The sections were digested with proteinase K (75 μg/ml) for 10 min at 37°C. Both sense and antisense riboprobes were prepared from linearized pcDNA3/vIRF-1 (74) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (American Type Culture Collection, Manassas, Va.) plasmids by using digoxigenin-UTP (Roche Molecular Diagnostics/Boehringer Mannheim, Indianapolis, Ind.) according to the manufacturer's protocol. Antisense GAPDH, detecting an endogenous RNA target, was used as a positive control probe, while sense vIRF-1 and sense GAPDH were negative control probes. Hybridization was performed at 50°C for 18 to 24 h with riboprobes (10 ng/ml) in 50% formamide hybridization cocktail (Ameresco, Solon, Ohio). Sections were washed with a final stringency of 0.1× SSC at 37°C and then treated with RNase A (2.5 μg/ml) (Sigma) for 20 min. Alkaline phosphatase-conjugated antidigoxigenin antibody (1:500; Roche Molecular Diagnostics/Boehringer Mannheim) and nitroblue tetrazolium chloride grade III/5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt (Sigma) substrate were used for detection, followed by nuclear red counterstain. JS-EBV was used as a negative cellular control.
IFA.
Cytospin preparations from BCBL-1 cells were fixed in ice-cold acetone and air dried. Both single and dual immunofluorescence (IFA) analyses were done with the anti vIRF-1 (1:1,000), anti-PKR (B-10, 1:100; Santa Cruz Biotechnology), anti-PML (PG-M3, 1:500; Santa Cruz Biotechnology), anti-PPF (ORF 59 clone 11D1, 1:500; Advanced Biotechnologies, Columbia, Md.), anti-γ-tubulin (GTU-88, 1:1000; Sigma), anti-p53 (DO-1, 1:100, Santa Cruz Biotechnology), and anti-LANA (ORF 73, clone LN53, 1:500; Advanced Biotechnologies) antibodies. Secondary antibodies were goat anti-mouse, goat anti-rat, and goat anti-rabbit conjugated to Alexa Fluor 568 or Alexa Fluor 488 (Molecular Probes Inc., Eugene, Oreg.). The nuclei were counterstained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) (Molecular Probes Inc.), and cells were mounted with the ProLong antifade kit (Molecular Probes). Imaging was performed with a Nikon Eclipse E-800 microscope equipped with an Optronics MagnaFire S99800 digital camera or with a Zeiss axioplasm laser-scanning confocal microscope with a Zeiss X100 1.3 oil emersion objective. The emission patterns of the two or three fluorescent probes were collected separately and were overlaid by use of MagnaFire software to create two- and three-color images.
Fluorescence ISH (FISH) analysis.
BCBL-1 cells were cytospun onto positively charged glass slides (ThermoShandon, Pittsburgh, Pa.), fixed in ice-cold acetone, air dried, and stained with vIRF-1 antibodies as described for IFA. The cells were then fixed with 10% paraformaldehyde at room temperature for 10 min; washed with phosphate-buffered saline twice; dehydrated sequentially for 1 min in ice-cold 70, 85, and 100% ethanol; and air dried. To denature the DNA, the cells were treated with 70% formamide in 2× SSC (pH 7.0 to 8.0) at 73°C for 7 min. The specimens were dehydrated at room temperature in the ethanol series and left to air dry. The CEP8 SpectrumOrange DNA probe (10 μl) to AT-rich alpha satellite sequences in the centromere region of chromosome 8 from the CEP8 SpectrumOrange direct-labeled fluorescent DNA probe kit (Vysis, Inc., Downers Grove, Ill.) was placed directly on the samples and covered with a glass coverslip that was sealed with rubber cement. The hybridization was carried out at 42°C in a humidified chamber for 4 h. The cells were washed with 0.4× SSC for 5 min at 73°C and with 2× SSC-0.1% NP-40 for 1 min at room temperature, followed by counterstaining with DAPI. Images were captured and processed as described for IFA.
Assessment of cell viability and apoptosis.
Trypan blue exclusion and direct counting with a hemacytometer were used to determine cell viability. Apoptosis was determined by using the DeadEnd fluorometric terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) system (Promega Corp., Madison, Wis.). As a negative control, BCBL-1 cells were incubated in the absence of terminal deoxynucleotidyltransferase enzyme. Cells were counterstained with propidium iodide containing DNase-free RNase A. For each sample, 10,000 events were acquired on a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.).
Assay for infectious HHV-8 production with the T1H6 cell line.
HHV-8 infection of T1H6 cells activates the lacZ gene under control of the PAN promoter in a sensitive and quantitative manner (27). By using this cell line, the amounts of infectious HHV-8 in medium obtained from BCBL-1 cultures under different conditions were measured as described previously (27). Briefly, 8 × 104 T1H6 cells were seeded in each well of a 48-well plate. The following day, medium from BCBL-1 cultures (200 μl) was added to each well in the presence of 8 μg of Polybrene per ml. Three days later, cells were harvested, and their β-galactosidase activities were measured by luminescent β-galactosidase assay (Clontech, Palo Alto, Calif.) with a LUMIstar Galaxy luminometer (BMG LabTechnologies, Durham, N.C.). A dilution series of infectious virus was used as a standard curve, and recombinant β-galactosidase was also used to determine the linear range of the assay.
RESULTS
Time course of vIRF-1 expression.
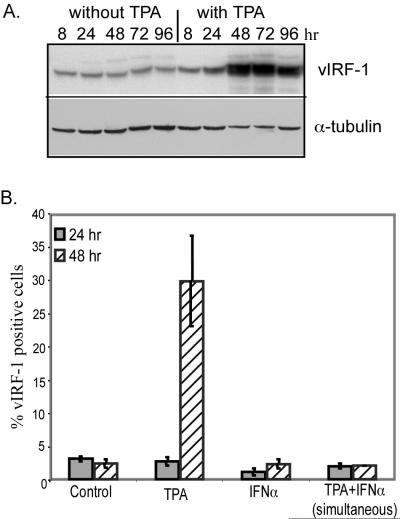
To elucidate the effects of vIRF-1 on IFN-induced changes during the course of viral infection, we first analyzed the time course of vIRF-1 expression by Western blot analysis. BCBL-1 cells that were not incubated with TPA expressed low levels of vIRF-1 at all time points examined (Fig. 1A). There was a slight increase in vIRF-1 expression at 8 and 24 h after TPA treatment, whereas peak levels were achieved by 48 h following incubation with TPA and remained elevated for at least 96 h. As a control, levels of α-tubulin were assessed and found to decrease slightly at the time points at which vIRF-1 increased.
FIG. 1.
Changes in expression of vIRF-1. (A) Total cellular extracts (50 μg/lane) were prepared from BCBL-1 cells that were cultured in the absence or the presence of TPA (20 ng/ml) for the indicated durations. Levels of vIRF-1 and α-tubulin in these extracts were determined by Western blot analysis with the antibodies described in Materials and Methods. (B) The percentages of BCBL-1 cells that expressed high levels of vIRF-1 after incubation for 24 and 48 h with the indicated agents were assessed by IFA. BCBL-1 cells from nine random fields were photographed at a magnification of ×200 for each experimental condition in duplicate. The number of intensely vIRF-1-positive cells within each field was determined with Image Pro Plus software and was compared to the total number of cells. Error bars indicate standard deviations.
To assess changes at an individual cell level, the numbers of cells that expressed high levels of vIRF-1 were counted by IFA. The percentage was not above the control level of 1 to 4% when cells were incubated with TPA for 24 h, but the percentage increased to approximately 30% of cells when incubation with TPA was continued for 48 h (Fig. 1B). When IFN-α was present during incubation with TPA, there was no increase in vIRF-1 expression, indicating that IFN-α blocked the ability of TPA to induce high-level vIRF-1 expression.
Unique localization of vIRF-1.
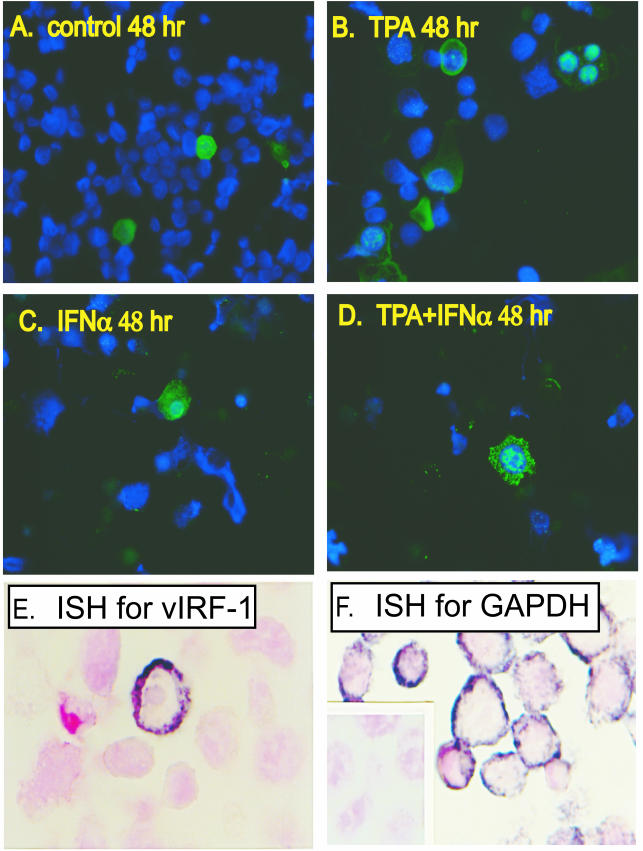
The cellular localization and morphological appearance of vIRF-1 within BCBL-1 cells varied among the conditions examined. Intense immunoreactivity for vIRF-1 was most frequently localized in the nucleus, but some cells contained high levels of vIRF-1 in the cytoplasm, including cells with high levels of nuclear and cytoplasmic vIRF-1 and others with high levels of vIRF-1 predominately in the cytoplasm (Fig. 2A to D). Some cells had multiple nuclear elements, each expressing vIRF-1 at high levels (Fig. 2B). The pattern of vIRF-1 expression in cells that were incubated with IFN-α alone did not differ from that in untreated cells (Fig. 2A and C), whereas cells that were simultaneously incubated with TPA and IFN-α had a distinct pattern of vIRF-1 that was characterized by large clumps of nuclear vIRF-1 and extensive punctuate vIRF-1 in the cytoplasm (Fig. 2D). In situ hybridization of nonstimulated cells demonstrated that only a few cells expressed very high levels of vIRF-1 mRNA, whereas vIRF-1 mRNA was below the level of detection in the majority of the population (Fig. 2E).
FIG. 2.
Morphology of BCBL-1 cells that express vIRF-1. (A to D) BCBL-1 cells were incubated for 48 h in standard medium (A) or in medium supplemented with TPA (20 ng/ml) (B), with IFN-α (1,000 U/ml) (C), or with both TPA and IFN-α simultaneously (D). IFA was done with a polyclonal antibody to vIRF-1 and a secondary antibody conjugated with Alexa Fluor 488 (green). The nuclei of all cells were labeled with DAPI (blue). (E and F) In situ hybridization was performed on uninduced BCBL-1 cells by using digoxigenin-labeled antisense riboprobes to vIRF-1 (E) and to GAPDH (F). The specificity of the riboprobes was demonstrated by using sense vIRF-1 (data not shown) and sense GAPDH (inset in panel F), both of which were nonreactive. Magnifications, ×200 (A to D) and ×400 (E and F).
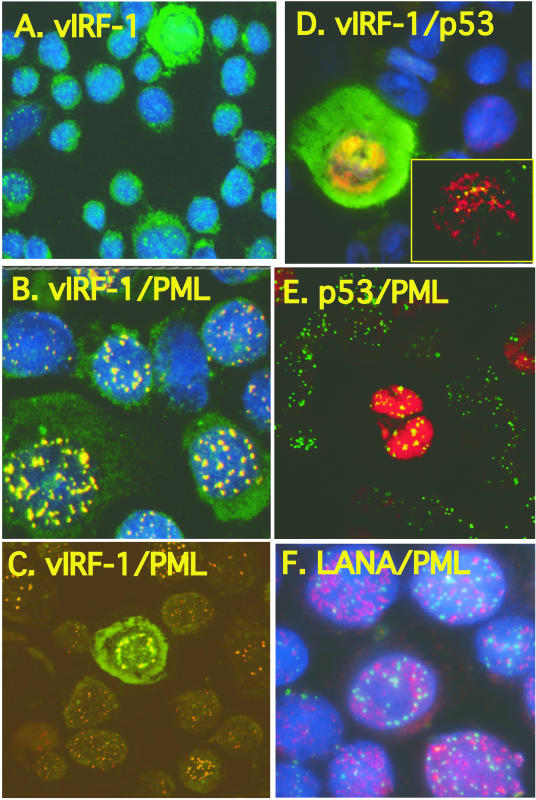
In addition to the strong immunoreactivity for vIRF-1 that was seen in a subset of cells, punctate immunoreactivity for vIRF-1 in the nucleus was observed in nearly all BCBL-1 cells (Fig. 3A). The punctate vIRF-1 protein found in all cells was distinct from the strong immunofluorescence for vIRF-1 that was associated with lytic replication (e.g., Fig. 3A has a single strongly positive cell surrounded by cells with the punctate nuclear vIRF-1 immunofluorescence). We hypothesized that vIRF-1 might be present in PML bodies, because vIRF-1 specifically binds to p53, p300, and CREB (37, 45, 60, 61), proteins that can be found in PML bodies (33, 70), and the punctate nature and nuclear localization of the vIRF-1 immunoreactivity suggested that it was specific. Dual IFA with antibodies to vIRF-1 and PML demonstrated complete colocalization of PML and vIRF-1 in latently infected cells producing yellow immunofluorescence (Fig. 3B), whereas confocal microscopy demonstrated that BCBL-1 cells that expressed vIRF-1 at a high level during lytic replication had vIRF-1 both within and outside the PML bodies (Fig. 3C, green cell). When cells were simultaneously examined for expression of vIRF-1 and p53, strong expression of p53 primarily occurred when HHV-8 was within the lytic phase of viral replication, with cells expressing high levels of vIRF-1 also expressing elevated levels of p53 (Fig. 3D). Confocal microscopy revealed that both proteins colocalized to some punctate areas in the nucleus (Fig. 3D, yellow dots in inset), but the levels of both proteins were so high that the majorities of p53 and vIRF-1 were not colocalized (Fig. 3D). Confocal microscopy revealed that the majority of BCBL-1 cells expressed PML without expressing any detectable p53 (Fig. 3E). Colocalization of p53 and PML was detected in cells that expressed p53, and the amount of p53 present far exceeded the amount of PML. Not all viral proteins examined colocalized with PML. For example, LANA and PML protein did not colocalize in any cell (Fig. 3F). Thus, vIRF-1, but not p53, was found associated with PML of all BCBL-1 cells, whereas p53 was colocalized with PML in BCBL-1 cells supporting lytic replication of HHV-8. The levels of vIRF-1 that were in PML bodes were low compared to the levels in some cells undergoing lytic replication of HHV-8, and cells expressing elevated levels of vIRF-1 also expressed elevated levels of p53. All subsequent studies exploring induction of vIRF-1 and the consequences of its expression during lytic replication focused on cells that had vIRF-1 levels that far exceeded the levels restricted to the PML bodies.
FIG. 3.
Colocalization of vIRF-1 and PML in latently infected BCBL-1 cells. BCBL-1 cells were incubated for 48 h in standard medium. Single and double immunofluorescent staining was done with vIRF-1 polyclonal antibody (A to D), p53 monoclonal antibody (D and E), LANA monoclonal antibody (F), and PML monoclonal antibody (B, C, E, and F), with secondary antibodies conjugated to Alexa Fluor 488 (green) and Alexa Fluor 568 (red). For all images, vIRF-1 was secondarily labeled with Alexa Fluor 488 (green), and p53 and LANA were secondarily labeled with Alexa Fluor 568 (red). PML was labeled with Alexa Fluor 568 (red) in panels B, C, and F and with Alexa Fluor 488 (green) in panel E. Cellular nuclei were counterstained with DAPI (blue). Imaging was performed with a Nikon Eclipse E-800 microscope equipped with an Optronics MagnaFire S99800 digital camera (A, B, D, and F) or with a Zeiss axioplasm laser-scanning confocal microscope with a Zeiss X100 1.3 oil emersion objective (C and E). Magnifications, ×400 (A) and ×1,000 (B to F).
Centrosome patterns in multinucleated BCBL-1 cells.
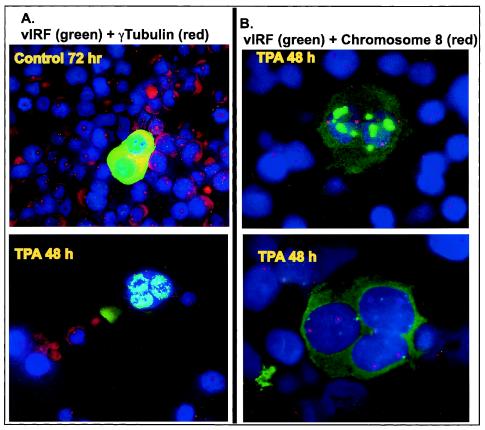
Many, but not all, cells that expressed high levels of vIRF-1 protein had a multinucleated appearance. There are three potential explanations for the multinucleated appearance. (i) vIRF-1 has been reported to alter chromatin structure, preventing binding of propidium iodide to condensed DNA (37). Thus, vIRF-1 might be preventing access of DAPI to the DNA in some regions of the nucleus, thereby generating multiple nuclear lobes within a single nucleus. (ii) Some cells within the lytic phase might have undergone cellular DNA replication and nuclear division without cytokinesis, leading to multinucleated cells. (iii) Alternatively, abnormal chromosomal segregation of incompletely duplicated chromosomes could generate micronuclei. To explore these possibilities, we used γ-tubulin to identify centrosomes within the cells, since γ-tubulin is an essential component of centrosomes and centrosomes are required for appropriate chromosomal segregation during nuclear division (30-32, 44, 46). None of the cells that expressed high levels of vIRF-1 had more than one centrosome (red dots close to the nucleus), and many of the cells that expressed high levels of vIRF-1 lacked any detectable centrosome, even when they appeared to be multinucleated (Fig. 4A and data not shown). This is in contrast to the case for non-TPA-stimulated cells, which contained either one or two centrosomes in all cells except in spontaneously lytic cells that strongly expressed vIRF-1 and were deficient in centrosomes. The presence of multiple nuclear elements without multiple centrosomes in some vIRF-1-expressing cells suggested that there might not be multiple nuclei present. To assess this possibility, FISH with a probe for sequences in the centromere region of chromosome 8 was performed. Multinucleated cells should have one or two copies of chromosome 8 in each nucleus, whereas a hyperlobated nucleus or a micronucleus would not contain chromosome 8 in each element. IFA followed by FISH demonstrated that each nuclear element that expressed high levels of vIRF-1 contained genetic material from chromosome 8 (Fig. 4B), providing evidence that each nuclear element contained replicated chromosomes and hence had undergone nuclear division with appropriate chromosomal segregation. This indicates that there was a defect in cytokinesis that led to the formation of multinucleated cells during lytic replication of HHV-8. It has not yet been determined which viral gene(s) is responsible for these changes.
FIG. 4.
Multinucleated BCBL-1 cells and deficient centrosomes in cells expressing elevated levels of vIRF-1. (A) Double immunofluorescent staining was done with vIRF-1 polyclonal antibody and γ-tubulin monoclonal antibody to detect centrosomes. Secondary antibodies were conjugated to Alexa Fluor 488 (green) and Alexa Fluor 568 (red) for vIRF-1 and γ-tubulin, respectively. Cellular nuclei were counterstained with DAPI. Magnification, ×400. (B) Immunofluorescent staining with vIRF-1 antibody detected by Alexa Fluor 488 (green) followed by FISH with a probe for the centromere region of chromosome 8 conjugated to SpectrumOrange (red). Cellular nuclei were counterstained with DAPI. Magnification, ×1,000.
Short duration of vIRF-1 expression.
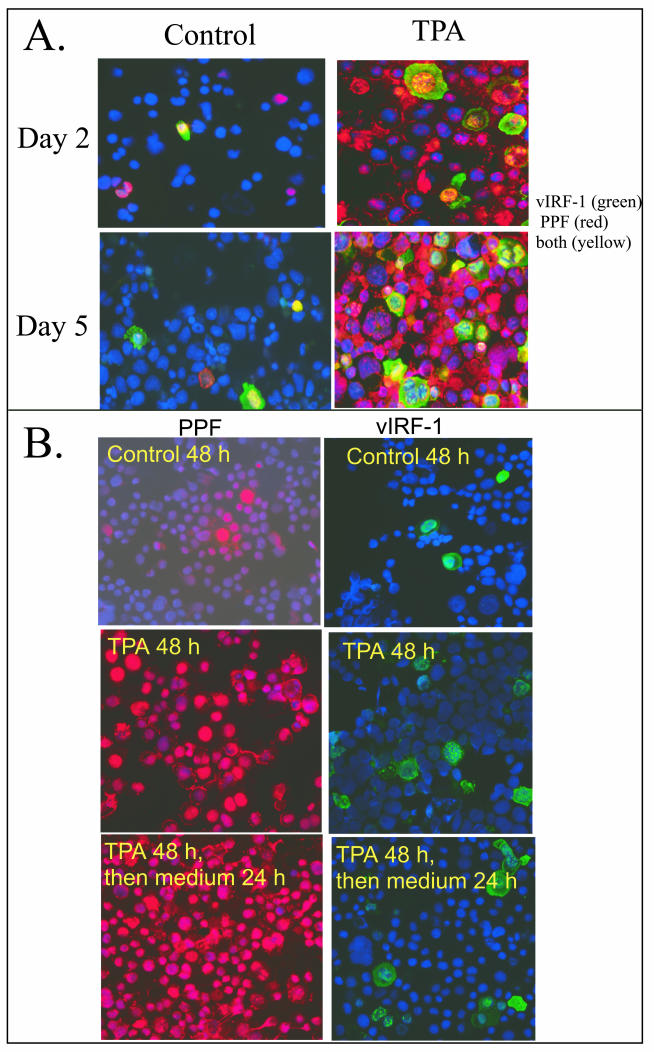
We were surprised by the relatively low percentage of cells that expressed high levels of vIRF-1 during prolonged incubation with TPA. This could occur if only a low percentage of cells were entering the lytic cascade. Alternatively, it could occur if high levels of vIRF-1 were expressed for only a short time during lytic replication. We explored these possibilities by comparing expression of vIRF-1 with that of PPF (encoded by ORF 59), a delayed-early viral protein expressed exclusively during lytic replication (9, 10). Cells that were not incubated with TPA included very few cells that expressed elevated levels of vIRF-1 or PPF (Fig. 5A), but low levels of vIRF-1 were present in the PML bodies as seen under higher magnification (data not shown). The percentage of cells that expressed high levels of vIRF-1 increased in response to incubation with TPA and stabilized after 2 days of TPA, whereas the percentage that expressed PPF continued to increase, with the majority of cells expressing PPF after 5 days of incubation with TPA (Fig. 5A). The majority of cells that expressed high levels of vIRF-1 also expressed PPF, whereas many cells expressed PPF without having elevated levels of vIRF-1. The high percentage of cells that expressed PPF indicated that most BCBL-1 cells entered the lytic cascade in response to prolonged incubation with TPA, yet not all cells within the lytic cascade expressed vIRF-1. This suggested that the expression of vIRF-1 might be of shorter duration than the expression of PPF.
FIG.5.
Expression of vIRF-1 and PPF. (A) BCBL-1 cells were incubated in standard medium (control) or in medium supplemented with TPA (20 ng/ml) for 2 and 5 days, and expression of vIRF-1 (green) and PPF (red) was analyzed by IFA. Colocalization of vIRF-1 and PPF appears yellow. The nuclei of all cells were counterstained with DAPI (blue). Magnification, ×200. (B) BCBL-1 cells were incubated in standard medium (control) or in medium supplemented with TPA (20 ng/ml) for 2 days. The medium was then changed, cells were maintained in fresh medium for 24 h, and expression of vIRF-1 (green) and PPF (red) was individually determined by IFA. The nuclei of all cells were counterstained with DAPI (blue). Magnification, ×200.
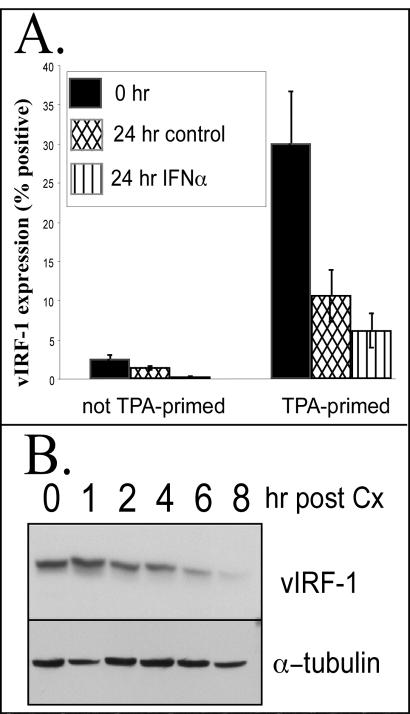
We then assessed the consequences of the removal of TPA after 48 h of stimulation on the expression of vIRF-1 and PPF in the population, looking at expression of each protein individually by IFA. Most cells in the population expressed high levels of PPF in response to incubation with TPA for 48 h, and this percentage did not decline when TPA was withdrawn and incubation was continued for 24 h in medium lacking TPA (Fig. 5B). In contrast, there was a decline in the percentage of cells that expressed high levels of vIRF-1 when TPA was withdrawn (Fig. 5B and 6A). This suggested that cells continued to be recruited into the lytic phase of viral replication when incubation with TPA was continued, whereas withdrawal of TPA prevented additional cells from entering the lytic cascade yet allowed cells within the cascade to progress. The decline that occurred in the percentage of cells that expressed vIRF-1 when TPA was withdrawn suggested that high-level vIRF-1 was expressed relatively early in the lytic cascade and did not persist as cells progressed to later stages of the cascade, whereas PPF expression was of longer duration. The percentage of cells that expressed high levels of vIRF-1 declined from approximately 30 to 10 to 12% over a 24-h period when TPA was removed from cells that had been primed for 48 h (Fig. 6A). When IFN-α was added to cells after the TPA was removed, the percentage of cells that expressed high levels of vIRF-1 declined more rapidly.
FIG. 6.
Duration of vIRF-1 expression. (A) BCBL-1 cells were primed with TPA (20 ng/ml) for 48 h or were not primed. The medium was then changed, and cells were maintained in fresh medium or medium supplemented with IFN-α (1,000 U/ml) for 24 h. The percentage of cells that expressed high levels of vIRF-1 was determined by IFA with anti-vIRF-1 antibody. BCBL-1 cells from nine random fields were photographed at a magnification of ×200 for each of duplicate samples prepared per experimental condition. The number of vIRF-1-positive cells within each field was determined with Image Pro Plus software. Error bars indicate standard deviations. (B) BCBL-1 cells were incubated with TPA for 48 h. Protein synthesis was inhibited by incubation with cycloheximide (Cx) (10 μg/ml) for the indicated durations. Total cellular protein (50 μg) was size fractionated by SDS-polyacrylamide gel electrophoresis and analyzed by Western blot analysis for expression of vIRF-1 and α-tubulin.
The rapid decline in the percentage of cells that expressed high levels of vIRF-1 suggested that the protein was not stable. This hypothesis was confirmed when TPA-stimulated BCBL-1 cells were incubated with cycloheximide to inhibit protein synthesis. This led to a rapid decline in vIRF-1 expression in TPA-stimulated BCBL-1 cells and revealed that the protein half-life was less than 3 h (Fig. 6B). In contrast, levels of α-tubulin were more stable during incubation with cycloheximide. Thus, vIRF-1 protein levels would rapidly decline unless de novo synthesis of vIRF-1 was occurring.
Inhibitory effect of vIRF-1 on responses to IFN-α in BCBL-1 cells.
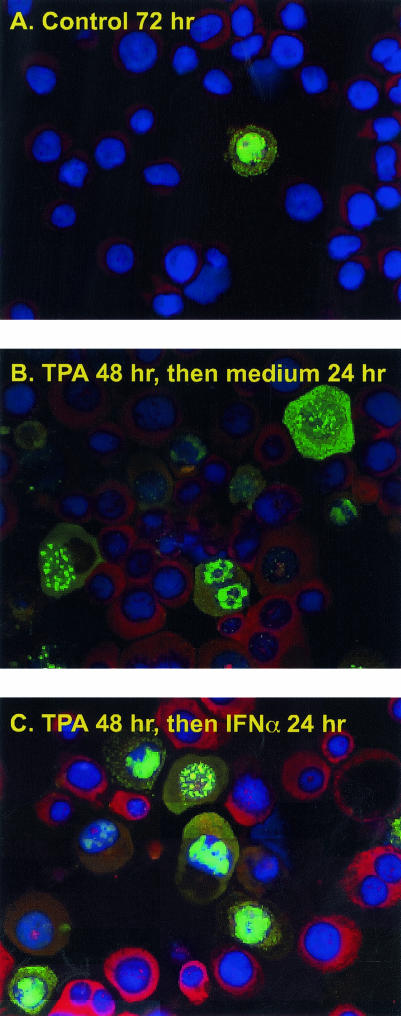
Based on the short half-life of vIRF-1 and the expression of high levels of vIRF-1 for only a short portion of the lytic cascade, we considered that vIRF-1 might not be expressed at high levels for a duration sufficient to protect cells from IFN-α-induced changes. To explore this possibility, we used IFA to determine whether cells that expressed high levels of vIRF-1 in response to TPA were resistant to induction of PKR protein by IFN-α. The percentage of cells that expressed high levels of vIRF-1 increased in response to incubation with TPA for 48 h (Fig. 7A and B). Incubation with IFN-α induced high levels of PKR protein expression in cells that lacked elevated levels of vIRF-1 but not in cells that expressed elevated levels of vIRF-1 (Fig. 7C). Examination using filters that exclusively measured PKR expression confirmed that PKR protein was not expressed when levels of vIRF-1 were elevated (data not shown), providing evidence that vIRF-1 offered some protection from IFN-α-induced changes.
FIG. 7.
Effect of vIRF-1 on IFN-α-induced PKR expression. BCBL-1 cells were incubated in the absence (A) or presence (B and C) of TPA for 48 h. After the medium was changed, cells were incubated for 24 h in standard medium (A and B) or in the presence of IFN-α (1,000 U/ml) for 24 h (C). Expression of vIRF-1 was detected by using vIRF-1 polyclonal antibody followed by Alexa Fluor 488-conjugated antibody (green). Expression of PKR was determined by using PKR monoclonal antibody followed by Alexa Fluor 568-cojugated antibody (red). Cellular DNA was counterstained with DAPI. Magnification, ×400.
Consequences of TPA priming for gene induction by IFN-α.
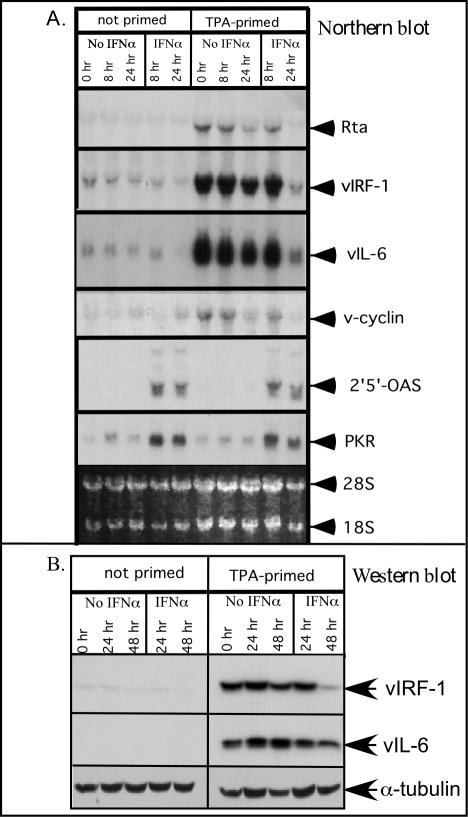
Cells that expressed high levels of vIRF-1 showed some protection from IFN-α-induced changes, yet the percentage of TPA-stimulated cells that expressed high levels of vIRF-1 was never more than 35%, even though most cells entered the lytic cascade. This suggested that many of the cells within the lytic cascade were likely to respond to IFN-α with increased expression of antiviral genes. This issue was explored by using Northern blot analysis to compare changes in gene expression resulting from incubation with IFN-α in nonprimed and TPA-primed cells. As expected, TPA-primed cells expressed much higher levels of the lytic cycle mRNAs (Rta, vIRF-1, and vIL-6) than did cells that were not primed with TPA (Fig. 8A). In addition, there was an increase in the level of the latency-associated transcript v-cyclin, but this increase was minor compared to the increase of the lytic-associated genes. Importantly, both TPA-primed and nonprimed cells had similar increases in PKR and 2′,5′-OAS mRNAs when incubated with IFN-α for 8 h, demonstrating that cells harboring HHV-8 in the lytic and latent phases responded comparably to IFN-α as a population, even if individual cells expressing high levels of vIRF-1 or other vIRFs were more resistant. The product of the viral gene Rta is both necessary and sufficient for inducing HHV-8 to enter the lytic cascade (40, 63). Removal of TPA for 24 h reduced the amount of Rta expression by more than 80%, whereas it was undetectable if cells were incubated with IFN-α for 24 h after TPA was removed. Incubation of TPA-primed cells with IFN-α for 24 h also decreased the expression of vIRF-1 and vIL-6 by at least 90% compared to the same time point in the absence of IFN-α, but the levels of PKR and 2′,5′-OAS did not decline to the same extent. Incubation of non-TPA-primed cells with IFN-α did not induce increased expression of vIL-6 mRNA, in contrast to the induction of vIL-6 by IFN-α that was reported to occur in BCP-1 cells (12). Western blot analysis confirmed that TPA induced high levels of both vIRF-1 and vIL-6 protein expression, and IFN-α was insufficient to induce vIL-6 in nonprimed BCBL-1 cells (Fig. 8B). Furthermore, incubation of TPA-primed cells with IFN-α accelerated the decline in vIRF-1 protein levels. From these studies, we can conclude that (i) prolonged incubation with TPA led to the recruitment of additional cells into the lytic phase of viral replication, whereas removal of the TPA decreased the recruitment, leading to a decline in vIRF-1 and Rta expression; (ii) despite the high-level vIRF-1 expression, the population of TPA-stimulated cells remained as responsive to IFN-α as nonprimed cells; and (iii) incubation of TPA-primed cells with IFN-α reduced the expression of vIRF-1 mRNA and protein and the expression of other lytic-associated genes, indicating that cells within the lytic cascade were not fully protected from IFN-α.
FIG. 8.
Effect of TPA priming on gene induction by IFN-α. BCBL-1 cells at a density of 5 × 105/ml were incubated in the absence or presence of TPA (20 ng/ml) for 48 h and then switched at time zero to standard medium or medium supplemented with IFN-α (1,000 U/ml). Cells were harvested at the indicated time points. (A) Total cellular RNA (15 μg) was analyzed by Northern blotting with the indicated probes. Ethidium bromide staining was carried out to assess RNA loading (bottom panel). (B) Whole-cell extracts (50 μg) were analyzed by Western blotting with antibodies to vIRF-1, vIL-6, and α-tubulin.
Decreased proliferation and apoptosis in response to TPA priming.
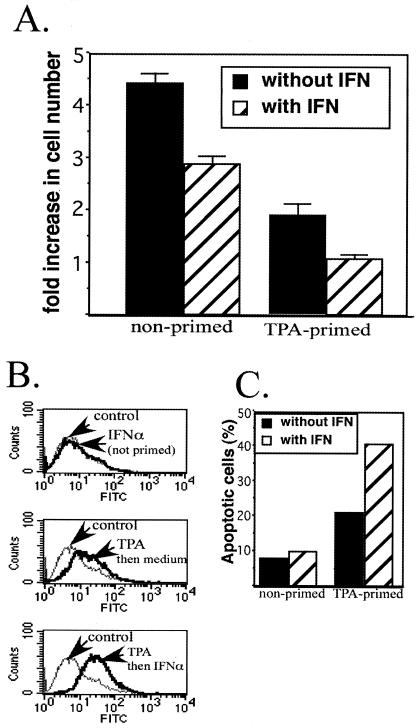
The loss of centrosomes in cells undergoing lytic replication of HHV-8 combined with the expression of replication-associated protein, a viral protein that blocks cellular DNA replication (68, 71), should prevent cellular replication as HHV-8 progresses through the lytic phase. We explored this issue by examining changes in cell number over a 48-h period for TPA-primed and unprimed BCBL-1 cells. Cells that were not induced into the lytic cycle increased in number by over fourfold over 48 h, whereas there was a twofold increase in the number of TPA-primed cells (Fig. 9A). When TPA-primed cells were incubated with IFN-α, there was no increase in cell number during the 48-h incubation. Incubation of nonprimed cells with IFN-α had less of an impact on cell number than priming with TPA, with cells increasing threefold in number over the 48-h incubation. Thus, IFN-α reduced the number of TPA-primed cells more effectively than the number of nonprimed cells.
FIG. 9.
Effect of TPA priming and incubation with IFN-α on cell number and apoptosis. Cells were incubated in control medium (nonprimed) or medium supplemented with 20 ng of TPA per ml (primed) for 48 h. At time zero, the cell number was adjusted to 5 × 105/ml, and the medium was then replaced with fresh medium with no supplements (control) or supplemented with IFN-α (1,000 U/ml) for 24 and 48 h. (A) BCBL-1 cell number and viability were measured by direct counting of cells that excluded trypan blue by using a hemacytometer as described in the text. The fold increase was determined by dividing the number of cells after a 48-h incubation by the number at time zero. Error bars indicate standard deviations for triplicate experiments. (B) Apoptosis in nonprimed and TPA-primed cells was assessed at 24 h following stimulation by using the TUNEL assay. For each sample, 10,000 events were acquired. The percentage of apoptotic cells was determined by using a gate that contained 90% of the peak from the nonprimed control but lacked the shoulder to the right of the peak. A shift to the right is indicative of increased TUNEL labeling, indicating increased apoptosis. FITC, fluorescein isothiocyanate. (C) The percentage of cells to the right of the gate for each of the experimental conditions was determined and plotted as percent apoptotic cells.
We used the TUNEL assay to assess the role of apoptosis in the changes in cell number that occurred in response to TPA and IFN-α. We found a greater-than-twofold increase in the percentage of cells undergoing apoptosis at 24 h after the completion of TPA priming (Fig. 9B and C). When TPA-primed cells were incubated with IFN-α for 24 h, the percentage of TUNEL-positive cells increased to more than 40%, whereas incubation with IFN-α had very little effect on amount of apoptosis that occurred in nonprimed cells in this time frame (approximately 10% in the absence and presence of IFN-α). These results demonstrate that many BCBL-1 cells that entered the lytic cascade in response to TPA priming withdrew from the cell cycle and underwent apoptosis, and the level of apoptosis was doubled if TPA-primed cells were incubated with IFN-α. In contrast, incubation with IFN-α for 24 h marginally increased the amount of apoptosis in latently infected cells.
Effect of IFN-α on production of infectious HHV-8 in TPA-stimulated BCBL-1 cells.
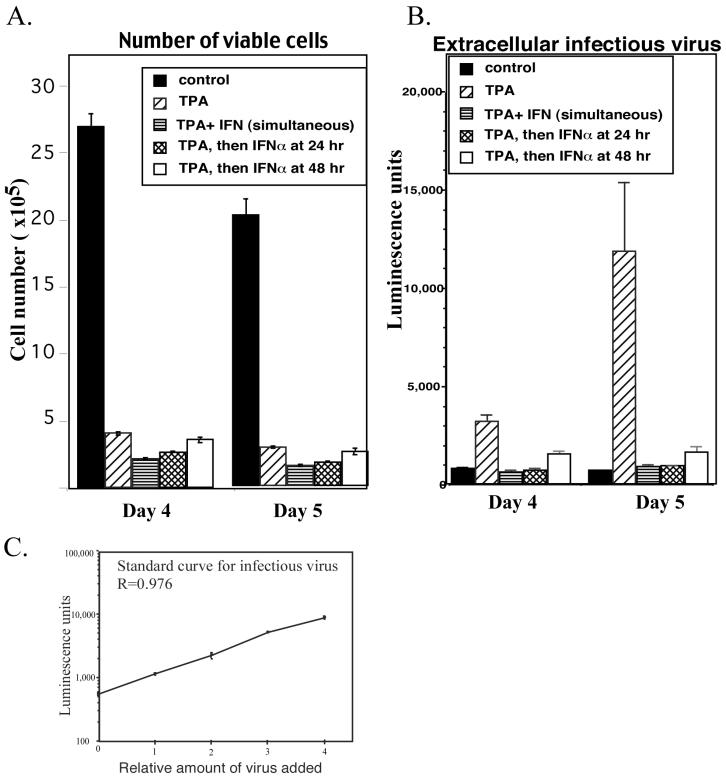
Many cells within the lytic cascade responded to IFN-α with enhanced expression of antiviral genes and enhanced apoptosis, suggesting that neither vIRF-1 nor other viral proteins blocked responses to IFN-α in many BCBL-1 cells that entered the lytic cascade. This suggested that IFN-α might have a profound effect on production of infectious virus by preventing successful completion of the process. Alternatively, the disruption of responses to IFN-α that occurred when high levels of vIRF-1 were transiently expressed during the lytic cascade might have been sufficient to ensure production of infectious virus. To explore these possibilities, we quantitated the amount of infectious virus produced by TPA-stimulated BCBL-1 cells and examined the impact of IFN-α on the process. Production of infectious virus was measured by using an Rta-dependent reporter cell line whose transcriptional activation was directly proportional to the amount of infectious HHV-8, as previously reported (27) and as shown in Fig. 10C. In the absence of IFN-α, infectious virus began to appear in conditioned medium 3 days following incubation with TPA and climbed to peak levels by day 5 (data not shown). We thus examined changes in cell number and the amount of infectious virus present in conditioned medium at 4 and 5 days following TPA stimulation. When BCBL-1 cells were incubated with TPA for 4 and 5 days, the number of viable cells was reduced by approximately 80% compared to control cells grown in the absence of TPA (Fig. 10A). In contrast, incubation with IFN-α in the absence of TPA reduced the cell number by less than 20% (data not shown). The reduction in cell number that occurred in response to incubation with TPA was expected, since entry into the lytic cascade blocked cellular proliferation and induced apoptosis, as shown in Fig. 9. Despite the low number of viable cells, infectious virus was readily detectable in the conditioned medium from TPA-stimulated cells and continued to increase between days 4 and 5, whereas there was minimal infectious virus in conditioned medium from nonstimulated cells on either day even though the number of viable cells was much higher (Fig. 10B). When IFN-α was present throughout the incubation with TPA, all TPA-induced accumulation of extracellular virus was eliminated. There was also a reduction in the number of viable cells, but the impact of IFN-α on the TPA-induced changes in cell number were minor compared to the impact on TPA-induced changes in infectious virus production (compare TPA to TPA+ IFN-α in Fig. 10A and B). When the addition of IFN-α was delayed until 24 or 48 h after the addition of TPA, the number of surviving cells approached the number that resulted from incubation with TPA alone (Fig. 10A), yet the production of infectious virus was still severely compromised (Fig. 10B). When the addition of IFN-α was delayed until 48 h after TPA stimulation, then extracellular infectious virus appeared, but the amount was small and levels did not increase between days 4 and 5, unlike the case for TPA-stimulated cells in the absence of IFN-α. Thus, IFN-α was effective at preventing production of infectious virus, especially when present early in the lytic cascade.
FIG. 10.
Impact of IFN-α on cell number and production of infectious HHV-8 in TPA-stimulated cells. BCBL-1 cells were incubated in the absence of TPA (control) or with TPA (20 ng/ml), and IFN-α (1,000 U/ml) was added at the same time as TPA (simultaneous) or 24 or 48 h after TPA stimulation. The amount of infectious virus in the cultured medium and the number of surviving cells were examined 4 and 5 days after TPA stimulation. (A) The number of cells was determined by direct counting of cells that excluded trypan blue by using a hemacytometer. The data represent means and standard deviations from triplicate determinations. (B) The amount of infectious virus in 200 μl of the medium was determined by using the T1H6 reporter cell line and is shown as relative luminescence units. (C) A standard curve was generated by using aliquots of the infectious virus from BCBL-1 cells.
DISCUSSION
This study demonstrates that the expression of vIRF-1 is more complex than previously reported, with vIRF-1 expressed at low levels in PML bodies of all virally infected cells and at high levels transiently during lytic replication. When vIRF-1 was found exclusively in PML bodies, the levels were insufficient to block responses to IFN-α, whereas the high levels that were expressed transiently during lytic replication of HHV-8 blocked some responses to IFN-α, including induction of PKR expression. Maximum expression of vIRF-1 within the population required at least 48 h of incubation with TPA, but not all BCBL-1 cells entered the lytic cascade simultaneously in response to TPA. This contributed to no more than 35% of the population expressing high levels of vIRF-1 at any point in time after incubation with TPA. Withdrawal of TPA led to a decline in the expression of both Rta and vIRF-1 as recruitment into the lytic cascade terminated, whereas expression of PPF was maintained as viral replication progressed to later stages of the lytic cascade. Both non-TPA-stimulated and TPA-stimulated BCBL-1 cells responded to IFN-α with enhanced expression of antiviral genes, yet the consequences of IFN-α were more pronounced with the TPA-stimulated cells. During lytic replication, IFN-α enhanced apoptosis and blocked production of infectious extracellular virus, especially when present early in the lytic cascade.
The amount of vIRF-1 that was associated with PML bodies was considerably smaller than the amount that blocked IFN-α-induced expression of PKR. Colocalization of vIRF-1 and PML occurred in all HHV-8-infected cells, irrespective of whether HHV-8 was latent or undergoing lytic replication, but the vIRF-1 in the PML bodies could be easily overlooked since levels were much lower than the high levels expressed during lytic replication. While all cells contained vIRF-1 in PML bodies, most latently infected cells did not express detectable p53, so that p53 was found in PML bodies mainly in cells supporting lytic replication of HHV-8. PML bodies have been implicated in various cellular processes, and viruses often disrupt PML bodies to reduce cellular antiviral defenses (23, 51). Both herpes simplex virus and human cytomegalovirus disseminate PML bodies, whereas multiple HHV-8 encoded proteins bind without triggering dissemination of the PML bodies (70, 71). The presence of vIRF-1 in all latently infected cells is consistent with a recent report indicating that vIRF-1 transcript is coexpressed with latency mRNAs in KS biopsies (18). Distinct transcriptional start sites have been identified for vIRF-1 expressed during latent and lytic replication, and promoter elements responsible for lytic expression of vIRF-1 lead to levels of transcription that are at least 10-fold higher than those for elements that regulate latent vIRF-1 expression (14). Thus, it is not surprising that mRNA for vIRF-1 in latently infected cells was below the level of detection by the ISH method that we used.
While vIRF-1 has been reported to deacetylate histones and reduce the ability of DNA to bind to intercalating dyes (37), the multinucleated appearance of cells that expressed high vIRF-1 was not a manifestation of abnormal binding of DAPI to DNA. Centrosomes are required for successful chromosomal segregation (21, 32), yet the multinucleated cells had inadequate numbers of centrosomes without any apparent abnormalities in chromosomal segregation. This suggests that the centrosomes were lost after nuclear division was complete. The lack of cytokinesis and the loss of centrosomes might reflect a viral strategy to shift resources away from cellular replication towards viral replication. Many viral genes are expressed when HHV-8 enters the lytic cascade, and the genes responsible for the accumulation of multinucleated cells and the loss of centrosomes have not been identified. Viruses that disrupt the formation or function of centrosomes include vaccinia virus and simian virus 40. Vaccinia virus infection results in a severe reduction of proteins at the centrosome and loss of centrosomal microtubule nucleation efficiency (49), and this has been implicated in enhancing viral spread to the cell periphery. Simian virus 40 blocks mitosis yet allows cells to enter a second S phase, with the majority of infectious virus produced during the G2 and second S phases (36). In contrast to the decrease in centrosome number that we observed when HHV-8 was undergoing lytic replication, an increase in centrosome number and cellular DNA replication can occur when viral replication is linked to cellular DNA replication, as occurs during latency. The E6 and E7 proteins of human papillomavirus uncouple centrosome duplication from cell division, leading to multiple centrosomes, mitotic defects, and genomic instability (20). This can also occur when v-cyclin from HHV-8 is transfected into p53-deficient cells (66). A block in cytokinesis, leading to multinucleated cells that display polyploidy and aneuploidy, accompanies the increase in centrosome number, but this occurs only when p53 is absent. We did not see any increase in centrosome number in untreated BCBL-1 cells, but it is possible that the changes are rare events in latently infected cells and hence were not observed with the limited numbers of cells examined. Not all cells that expressed high levels of vIRF-1 were multinucleated, but this was a common occurrence. The lack of centrosomes occurred relatively early in the lytic cascade when high levels of vIRF-1 were expressed. Not surprisingly, some cells were multinucleated at later time points when high levels of vIRF-1 were no longer expressed.
Lytic replication induced by TPA was accompanied by a reduction in cell number due to reduced proliferation and enhanced apoptosis, but this did not prevent the release of large amounts of infectious virus into the conditioned medium. TPA induces entry into the lytic cascade by inducing expression of Rta, and TPA also affects cellular gene expression. Thus, it is possible that there are some differences in the consequences of lytic replication induced by TPA compared to lytic replication initiated exclusively by Rta.
At the population level, IFN-α induced comparable increases in mRNAs for both PKR and 2′,5′-OAS in cells that were latently and lytically infected with HHV-8, yet the biologic consequences of incubation with IFN-α differed considerably when HHV-8 was in the different replicative states. IFN-α did not cause much of a reduction in cell number or induce much apoptosis in nonprimed cells, whereas IFN-α added to TPA-primed cells enhanced apoptosis and reduced the expression of viral mRNAs and proteins that are normally expressed during the lytic cascade. Both PKR and 2′,5′-OAS generally require an activation event for enzymatic activity, an event that is more likely to occur during lytic replication when multiple viral genes are expressed (1, 13, 65). Activation of 2′,5′-OAS can lead to a reduction in mRNA levels through the activation of a latent RNase, and activation of PKR can reduce protein synthesis by phosphorylating eIF2α, an initiation factor for mRNA translation. The activation of these proteins can also contribute to apoptosis. Thus, the greater reduction in expression of lytic viral genes and the enhanced apoptosis that occurred when TPA-primed cells were incubated with IFN-α might have resulted from the enhanced activation of these and other antiviral proteins by viral gene products expressed during lytic replication. IFN-α can also induce higher levels of expression of the tumor suppressor gene product p53 (1, 13, 64, 65), a transcription factor that contributes to antiviral responses by enhancing expression of proapoptotic genes (47). Viral proteins that alter the function of antiviral proteins can modulate the efficacy of these antiviral pathways. For example, vIRF-1 blocks responses to IFN-α, overexpression of vIRF-2 blocks activation of PKR but not activation of 2′,5′-OAS (3, 22), and overexpression of LANA-1, vIRF-1, or Rta can disrupt the function of p53 (25, 45, 53, 61, 67). Our studies demonstrated that viral proteins expressed during the lytic cascade were insufficient to prevent IFN-α from functioning as an effective antiviral cytokine. IFN-α had the most profound effect on the production of infectious HHV-8 if IFN-α was present early in the lytic cascade prior to peak expression of vIRF-1. When incubation with IFN-α was delayed until after 48 h of incubation with TPA, extracellular infectious virus was produced, but the levels were much lower than those that occurred in TPA-primed cells in the absence of IFN-α.
These studies demonstrate that IFN-α functions as an antiviral cytokine during lytic replication of HHV-8 despite the expression of vIRF-1 and other viral proteins that block responsiveness to IFN-α. The susceptibility of BCBL-1 cells to the antiviral effects of IFN-α were more pronounced early in the lytic cascade, a time when most cells were not yet expressing high levels of vIRF-1. The expression of high levels of vIRF-1 was of short duration, and the decline in vIRF-1 that occurred as cells progressed to later stages of the lytic cascade also may have contributed to responsiveness to IFN-α. Incubation of latently infected cells with IFN-α altered gene expression, but there was very little inhibition of proliferation or apoptosis until cells were induced into the lytic phase, a process that induced apoptosis prior to production of infectious virus.
Acknowledgments
This study was supported by NIH grant RO-1 CA79402.
We thank Patrick Moore and Yuan Chang for antibodies to vIL-6, Todd Kroll for advice on FISH, and Reneé Shaw and William Kaiser for technical assistance and useful discussions.
REFERENCES
- 1.Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. [DOI] [PubMed] [Google Scholar]
- 2.Benech, P., Y. Mory, M. Revel, and J. Chebath. 1985. Structure of two forms of the interferon-induced (2′5′)-oligo A synthetase of human cells based on cDNAs and gene sequences. EMBO J. 4:2249-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burysek, L., and P. M. Pitha. 2001. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J. Virol. 75:2345-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burysek, L., W. S. Yeow, B. Lubyova, M. Kellum, S. L. Schafer, Y. Q. Huang, and P. M. Pitha. 1999. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J. Virol. 73:7334-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burysek, L., W. S. Yeow, and P. M. Pitha. 1999. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2). J. Hum. Virol. 2:19-32. [PubMed] [Google Scholar]
- 6.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 7.Cesarman, E., and D. M. Knowles. 1999. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin. Cancer Biol. 9:165-174. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman, E., R. G. Nador, K. Aozasa, G. Delsol, J. W. Said, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am. J. Pathol. 149:53-57. [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, S. R., C. Bloomer, and B. Chandran. 1998. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology 240:118-126. [DOI] [PubMed] [Google Scholar]
- 10.Chan, S. R., and B. Chandran. 2000. Characterization of human herpesvirus 8 ORF59 protein (PF-8) and mapping of the processivity and viral DNA polymerase-interacting domains. J. Virol. 74:10920-10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee, M., J. Osborne, G. Bestetti, Y. Chang, and P. S. Moore. 2002. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science 298:1432-1435. [DOI] [PubMed] [Google Scholar]
- 13.Chawla-Sarkar, M., D. J. Lindner, Y. F. Liu, B. R. Williams, G. C. Sen, R. H. Silverman, and E. C. Borden. 2003. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 8:237-249. [DOI] [PubMed] [Google Scholar]
- 14.Chen, J., K. Ueda, S. Sakakibara, T. Okuno, and K. Yamanishi. 2000. Transcriptional regulation of the Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor gene. J. Virol. 74:8623-8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotter, M. A., II, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 16.D'Agostino, G., E. Arico, L. Santodonato, M. Venditti, P. Sestili, L. Masuelli, A. Coletti, A. Modesti, G. Picchio, D. E. Mosier, M. Ferrantini, and F. Belardelli. 1999. Type I consensus IFN (IFN-con1) gene transfer into KSHV/HHV-8-infected BCBL-1 cells causes inhibition of viral lytic cycle activation via induction of apoptosis and abrogates tumorigenicity in sCID mice. J. Interferon Cytokine Res. 19:1305-1316. [DOI] [PubMed] [Google Scholar]
- 17.Decker, T., S. Stockinger, M. Karaghiosoff, M. Muller, and P. Kovarik. 2002. IFNs and STATs in innate immunity to microorganisms. J. Clin. Invest. 109:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dittmer, D. P. 2003. Transcription profile of Kaposi's sarcoma-associated herpesvirus in primary Kaposi's sarcoma lesions as determined by real-time PCR arrays. Cancer Res. 63:2010-2015. [PubMed] [Google Scholar]
- 19.Du, M. Q., T. C. Diss, H. Liu, H. Ye, R. A. Hamoudi, J. Cabecadas, H. Y. Dong, N. L. Harris, J. K. Chan, J. W. Rees, A. Dogan, and P. G. Isaacson. 2002. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood 100:3415-3418. [DOI] [PubMed] [Google Scholar]
- 20.Duensing, S., L. Y. Lee, A. Duensing, J. Basile, S. Piboonniyom, S. Gonzalez, C. P. Crum, and K. Munger. 2000. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. USA 97:10002-10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duensing, S., and K. Munger. 2001. Centrosome abnormalities, genomic instability and carcinogenic progression. Biochim. Biophys. Acta 1471:M81-M88. [DOI] [PubMed] [Google Scholar]
- 22.Esteban, M., M. A. Garcia, E. Domingo-Gil, J. Arroyo, C. Nombela, and C. Rivas. 2003. The latency protein LANA2 from Kaposi's sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J. Gen. Virol. 84:1463-1470. [DOI] [PubMed] [Google Scholar]
- 23.Everett, R. D. 2001. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene 20:7266-7273. [DOI] [PubMed] [Google Scholar]
- 24.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 25.Gwack, Y., S. Hwang, H. Byun, C. Lim, J. W. Kim, E. J. Choi, and J. Choe. 2001. Kaposi's sarcoma-associated herpesvirus open reading frame 50 represses p53-induced transcriptional activity and apoptosis. J. Virol. 75:6245-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harcourt, J. L., and M. K. Offermann. 2000. Interferon-alpha synergistically enhances induction of interleukin-6 by double stranded RNA in HeLa cells. Eur. J. Biochem. 267:2768-2777. [DOI] [PubMed] [Google Scholar]
- 27.Inoue, N., J. Winter, R. B. Lal, M. K. Offermann, and S. Koyano. 2003. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J. Virol. 77:8147-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 29.Jenner, R. G., M. M. Alba, C. Boshoff, and P. Kellam. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi, H. C. 1993. Gamma-tubulin: the hub of cellular microtubule assemblies. Bioessays 15:637-643. [DOI] [PubMed] [Google Scholar]
- 31.Kapoor, T. M., and D. A. Compton. 2002. Searching for the middle ground: mechanisms of chromosome alignment during mitosis. J. Cell Biol. 157:551-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karsenti, E., J. Newport, and M. Kirschner. 1984. Respective roles of centrosomes and chromatin in the conversion of microtubule arrays from interphase to metaphase. J. Cell Biol. 99:47s-54s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katano, H., K. Ogawa-Goto, H. Hasegawa, T. Kurata, and T. Sata. 2001. Human-herpesvirus-8-encoded K8 protein colocalizes with the promyelocytic leukemia protein (PML) bodies and recruits p53 to the PML bodies. Virology 286:446-455. [DOI] [PubMed] [Google Scholar]
- 34.Katze, M. G. 1995. Regulation of the interferon-induced PKR: can viruses cope? Trends Microbiol. 3:75-78. [DOI] [PubMed] [Google Scholar]
- 35.Kedes, D. H., M. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehman, J. M., J. Laffin, and T. D. Friedrich. 2000. Simian virus 40 induces multiple S phases with the majority of viral DNA replication in the G2 and second S phase in CV-1 cells. Exp. Cell Res. 258:215-222. [DOI] [PubMed] [Google Scholar]
- 37.Li, M., B. Damania, X. Alvarez, V. Ogryzko, K. Ozato, and J. U. Jung. 2000. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor. Mol. Cell. Biol. 20:8254-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin, R., P. Genin, Y. Mamane, M. Sgarbanti, A. Battistini, W. J. Harrington, Jr., G. N. Barber, and J. Hiscott. 2001. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20:800-811. [DOI] [PubMed] [Google Scholar]
- 39.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monini, P., F. Carlini, M. Sturzl, P. Rimessi, F. Superti, M. Franco, G. Melucci-Vigo, A. Cafaro, D. Goletti, C. Sgadari, S. Butto, P. Leone, C. Chiozzini, C. Barresi, A. Tinari, A. Bonaccorsi, M. R. Capobianchi, M. Giuliani, A. di Carlo, M. Andreoni, G. Rezza, and B. Ensoli. 1999. Alpha interferon inhibits human herpesvirus 8 (HHV-8) reactivation in primary effusion lymphoma cells and reduces HHV-8 load in cultured peripheral blood mononuclear cells. J. Virol. 73:4029-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore, P. S., and Y. Chang. 1998. Kaposi's sarcoma (KS), KS-associated herpesvirus, and the criteria for causality in the age of molecular biology. Am. J. Epidemiol. 147:217-221. [DOI] [PubMed] [Google Scholar]
- 43.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In B. Fields, D. Knipe, and P. Howley (ed.), Field's virology, 4th ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 44.Musacchio, A., and K. G. Hardwick. 2002. The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell. Biol. 3:731-741. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura, H., M. Li, J. Zarycki, and J. U. Jung. 2001. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J. Virol. 75:7572-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasmyth, K. 2002. Segregating sister genomes: the molecular biology of chromosome separation. Science 297:559-565. [DOI] [PubMed] [Google Scholar]
- 47.Oren, M. 2003. Decision making by p53: life, death and cancer. Cell Death Differ. 10:431-442. [DOI] [PubMed] [Google Scholar]
- 48.Paulose-Murphy, M., N. K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ploubidou, A., V. Moreau, K. Ashman, I. Reckmann, C. Gonzalez, and M. Way. 2000. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 19:3932-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S. J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regad, T., and M. K. Chelbi-Alix. 2001. Role and fate of PML nuclear bodies in response to interferon and viral infections. Oncogene 20:7274-7286. [DOI] [PubMed] [Google Scholar]
- 52.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 53.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roan, F., N. Inoue, and M. K. Offermann. 2002. Activation of cellular and heterologous promoters by the human herpesvirus 8 replication and transcription activator. Virology 301:293-304. [DOI] [PubMed] [Google Scholar]
- 55.Roan, F., J. C. Zimring, S. Goodbourn, and M. K. Offermann. 1999. Transcriptional activation by the human herpesvirus-8-encoded interferon regulatory factor. J. Gen. Virol. 80:2205-2209. [DOI] [PubMed] [Google Scholar]
- 56.Sarid, R., O. Flore, R. Bohenzky, Y. Chang, and P. Moore. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body-cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schindler, C. 1999. Cytokines and JAK-STAT signaling. Exp. Cell Res. 253:7-14. [DOI] [PubMed] [Google Scholar]
- 58.Schindler, C., and S. Brutsaert. 1999. Interferons as a paradigm for cytokine signal transduction. Cell. Mol. Life Sci. 55:1509-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schindler, C. W. 2002. JAK-STAT signaling in human disease. J. Clin. Investig. 109:1133-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seo, T., D. Lee, B. Lee, J. H. Chung, and J. Choe. 2000. Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) binds to, and inhibits transactivation of, CREB-binding protein. Biochem. Biophys. Res. Commun. 270:23-27. [DOI] [PubMed] [Google Scholar]
- 61.Seo, T., J. Park, D. Lee, S. G. Hwang, and J. Choe. 2001. Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J. Virol. 75:6193-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 63.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takaoka, A., S. Hayakawa, H. Yanai, D. Stoiber, H. Negishi, H. Kikuchi, S. Sasaki, K. Imai, T. Shibue, K. Honda, and T. Taniguchi. 2003. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence Nature 424:516-523. [DOI] [PubMed] [Google Scholar]
- 65.Tan, S. L., and M. G. Katze. 1999. The emerging role of the interferon-induced PKR protein kinase as an apoptotic effector: a new face of death? J. Interferon Cytokine Res. 19:543-554. [DOI] [PubMed] [Google Scholar]
- 66.Verschuren, E. W., J. Klefstrom, G. I. Evan, and N. Jones. 2002. The oncogenic potential of Kaposi's sarcoma-associated herpesvirus cyclin is exposed by p53 loss in vitro and in vivo. Cancer Cell 2:229-241. [DOI] [PubMed] [Google Scholar]
- 67.Viejo-Borbolla, A., E. Kati, J. A. Sheldon, K. Nathan, K. Mattsson, L. Szekely, and T. F. Schulz. 2003. A domain in the C-terminal region of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated herpesvirus affects transcriptional activation and binding to nuclear heterochromatin. J. Virol. 77:7093-7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang, S. E., F. Y. Wu, M. Fujimuro, J. Zong, S. D. Hayward, and G. S. Hayward. 2003. Role of CCAAT/enhancer-binding protein alpha (C/EBPalpha) in activation of the Kaposi's sarcoma-associated herpesvirus (KSHV) lytic-cycle replication-associated protein (RAP) promoter in cooperation with the KSHV replication and transcription activator (RTA) and RAP. J. Virol. 77:600-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang, X. P., Y. J. Zhang, J. H. Deng, H. Y. Pan, F. C. Zhou, E. A. Montalvo, and S. J. Gao. 2001. Characterization of the promoter region of the viral interferon regulatory factor encoded by Kaposi's sarcoma-associated herpesvirus. Oncogene 20:523-530. [DOI] [PubMed] [Google Scholar]
- 70.Wu, F. Y., J. H. Ahn, D. J. Alcendor, W. J. Jang, J. Xiao, S. D. Hayward, and G. S. Hayward. 2001. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol. 75:1487-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu, F. Y., Q. Q. Tang, H. Chen, C. ApRhys, C. Farrell, J. Chen, M. Fujimuro, M. D. Lane, and G. S. Hayward. 2002. Lytic replication-associated protein (RAP) encoded by Kaposi sarcoma-associated herpesvirus causes p21CIP-1-mediated G1 cell cycle arrest through CCAAT/enhancer-binding protein-alpha. Proc. Natl. Acad. Sci. USA 99:10683-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu, Y., J. B. Black, C. S. Goldsmith, P. J. Browning, K. Bhalla, and M. K. Offermann. 1999. Induction of human herpesvirus-8 DNA replication and transcription by butyrate and TPA in BCBL-1 cells. J. Gen. Virol. 80:83-90. [DOI] [PubMed] [Google Scholar]
- 73.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimring, J. C., S. Goodbourn, and M. K. Offermann. 1998. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1 mediated transcription. J. Virol. 72:701-707. [DOI] [PMC free article] [PubMed] [Google Scholar]