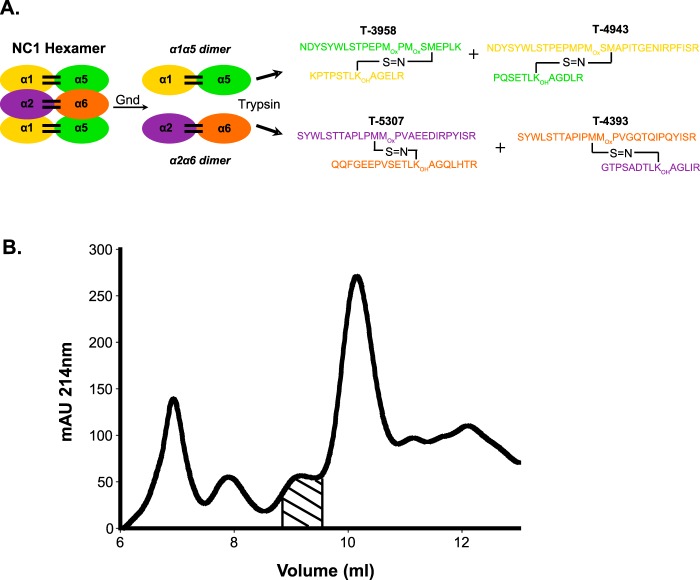
Background: The supramolecular organization of the collagen IV network containing α5 and α6 chains is unknown.
Results: Sulfilimine-cross-linked α1-α5 and α2-α6 NC1 dimers compose the α121–565 hexamer.
Conclusion: The α121-α565 network is formed by a heterotypic interaction between α121 and α565 protomers.
Significance: Sulfilimine bonds cross-link all three collagen IV networks: α121, α345, and α121-α565 networks.
Keywords: Basement Membrane, Collagen, Extracellular Matrix, Extracellular Matrix Protein, Hydroxylysine (Hyl), Mass Spectrometry (MS), Protein Cross-linking, Smooth Muscle, Vascular Smooth Muscle Cells, Sulfilimine Bond
Abstract
Collagen IV is a family of 6 chains (α1-α6), that form triple-helical protomers that assemble into supramolecular networks. Two distinct networks with chain compositions of α121 and α345 have been established. These oligomerize into separate α121 and α345 networks by a homotypic interaction through their trimeric noncollagenous (NC1) domains, forming α121 and α345 NC1 hexamers, respectively. These are stabilized by novel sulfilimine ( S
S N
N ) cross-links, a covalent cross-link that forms between Met93 and Hyl211 at the trimer-trimer interface. A third network with a composition of α1256 has been proposed, but its supramolecular organization has not been established. In this study we investigated the supramolecular organization of this network by determining the chain identity of sulfilimine-cross-linked NC1 domains derived from the α1256 NC1 hexamer. High resolution mass spectrometry analyses of peptides revealed that sulfilimine bonds specifically cross-link α1 to α5 and α2 to α6 NC1 domains, thus providing the spatial orientation between interacting α121 and α565 trimers. Using this information, we constructed a three-dimensional homology model in which the α565 trimer shows a good chemical and structural complementarity to the α121 trimer. Our studies provide the first chemical evidence for an α565 protomer and its heterotypic interaction with the α121 protomer. Moreover, our findings, in conjunction with our previous studies, establish that the six collagen IV chains are organized into three canonical protomers α121, α345, and α565 forming three distinct networks: α121, α345, and α121-α565, each of which is stabilized by sulfilimine bonds between their C-terminal NC1 domains.
) cross-links, a covalent cross-link that forms between Met93 and Hyl211 at the trimer-trimer interface. A third network with a composition of α1256 has been proposed, but its supramolecular organization has not been established. In this study we investigated the supramolecular organization of this network by determining the chain identity of sulfilimine-cross-linked NC1 domains derived from the α1256 NC1 hexamer. High resolution mass spectrometry analyses of peptides revealed that sulfilimine bonds specifically cross-link α1 to α5 and α2 to α6 NC1 domains, thus providing the spatial orientation between interacting α121 and α565 trimers. Using this information, we constructed a three-dimensional homology model in which the α565 trimer shows a good chemical and structural complementarity to the α121 trimer. Our studies provide the first chemical evidence for an α565 protomer and its heterotypic interaction with the α121 protomer. Moreover, our findings, in conjunction with our previous studies, establish that the six collagen IV chains are organized into three canonical protomers α121, α345, and α565 forming three distinct networks: α121, α345, and α121-α565, each of which is stabilized by sulfilimine bonds between their C-terminal NC1 domains.
Introduction
Collagen IV is an evolutionarily conserved protein of basement membranes (BMs),3 a specialized layer of extracellular matrix underlying all epithelia (1, 2). As an important structural component of BMs, one essential function of collagen IV is to maintain tissue architecture during embryonic development, remodeling, and repair (3). Collagen IV also serves as a ligand for integrin cell surface receptors influencing cell behavior including adhesion, migration, and differentiation (4). Importantly, several genetic and acquired diseases affect type IV collagen and thus impair BM function leading to deadly diseases, including Alport syndrome (5, 6), cerebral hemorrhages (7), porencephaly (8), and Goodpasture disease (6).
Collagen IV molecules form triple-helical protomers that further assemble into supramolecular networks stabilized by novel sulfilimine ( S
S N
N ) bonds (9). Sulfilimine bonds cross-link the C-terminal non-collagenous (NC1) domains of interacting protomers, forming a globular structure known as the NC1 hexamer. Catalysis of these cross-links in vivo is promoted by the BM-embedded enzyme peroxidasin via a novel hypobromous acid-mediated mechanism (10). Further studies of sulfilimine bond formation revealed that bromine, a required cofactor of peroxidasin, is an essential trace element for successful embryogenesis and development in Drosophila (11). This unique cross-link is known to be an evolutionarily conserved feature of collagen IV networks, arising at the divergence of Porifera and Cnidaria over 500 Mya (1, 9). Thus, the machinery that assembles sulfilimine bonds is a primordial innovation of collagen IV networks essential for organogenesis and tissue evolution.
) bonds (9). Sulfilimine bonds cross-link the C-terminal non-collagenous (NC1) domains of interacting protomers, forming a globular structure known as the NC1 hexamer. Catalysis of these cross-links in vivo is promoted by the BM-embedded enzyme peroxidasin via a novel hypobromous acid-mediated mechanism (10). Further studies of sulfilimine bond formation revealed that bromine, a required cofactor of peroxidasin, is an essential trace element for successful embryogenesis and development in Drosophila (11). This unique cross-link is known to be an evolutionarily conserved feature of collagen IV networks, arising at the divergence of Porifera and Cnidaria over 500 Mya (1, 9). Thus, the machinery that assembles sulfilimine bonds is a primordial innovation of collagen IV networks essential for organogenesis and tissue evolution.
In mammals, collagen IV is present as a family of six distinct genes encoding the α1-α6 chains. Of all possible combinations, only three collagen IV networks of defined α-chain compositions have been observed: the ubiquitous α121 (12) and the tissue-restricted α345 (13, 14) and α1256 networks (15, 16). The crystal structure of the α121 NC1 hexamer demonstrated that the α121 network results from a homotypic interaction between α121 trimeric protomers (17). Because sulfilimine cross-links bridge the trimer-trimer interface within the NC1 hexamer, forming NC1 dimers, the location of these cross-links also confirmed the relative orientation of interacting protomers. A similar approach using sulfilimine-cross-linked NC1 dimers was used to elucidate the quaternary structure of the α345 hexamer (18), which established that the α345 network is formed by a homotypic interaction between α345 protomers. In contrast, although analyses of BM from X-linked Alport patients, who suffer from an inherited form of kidney disease caused by mutations in the COL4A5 gene, provided clues linking α5 to α6 chain assembly (19), the organization of the α1256 network is not fully understood. Initial characterization of α1256 NC1 hexamers from aorta smooth muscle BM suggested a heterotypic interaction between a putative α565 protomer and a classical α121 protomer. However, inherent limitations of the immunoblotting techniques used to characterize NC1 dimers could not unambiguously establish the organization of the network (16).
The new discovery of sulfilimine bonds, its mechanism of assembly, and the availability of the α121 hexamer crystal structure open new possibilities to gain further insight into the supramolecular organization of the α1256 network. In this study we aimed to determine if sulfilimine cross-linking occurs in aorta smooth muscle BMs and use this chemical information to elucidate the quaternary structure of the α1256 NC1 hexamer. We used high-resolution mass spectrometry (MS) to determine the identity of sulfilimine-cross-linked NC1 dimers resulting from the interaction between protomers. Our results indicate that only α1-α5 and α2-α6 sulfilimine-cross-linked NC1 dimers are present in the α1256 hexamer. These findings unambiguously establish the quaternary organization of the NC1 hexamer revealing a heterotypic interaction between α121 and α565 protomers to form the α1256 network.
EXPERIMENTAL PROCEDURES
Isolation of α1256 NC1 Hexamer from Aorta
Aorta NC1 hexamers were prepared as described previously by Kahsai et al. (20). Briefly, NC1 hexamers were solubilized from bovine aorta tissue by the collagenase digestion method and purified on DE-52 cellulose and Sephacryl S-300 columns.
Separation of the aorta NC1 hexamers of different α-chain composition was performed in an ÄKTA purifier HPLC (GE Healthcare) with a Mono S cation exchange column (GE Healthcare). Aorta NC1 hexamers were loaded into the Mono S column and eluted with a linear gradient of increasing concentrations of NaCl; absorbance was monitored at 280 nm. Each chromatographic peak was analyzed by Western blotting with monoclonal antibodies H11 (anti-α1 NC1 domain), H22 (anti-α2 NC1 domain), H31 (anti-α3 NC1 domain), H44 (anti-α4 NC1 domain), H52 (anti-α5 NC1 domain), and B66 (anti-α6 NC1 domain) (21). Fractions staining for α1, α2, α5, and α6 NC1 domains were pooled and stored at −20 °C.
Mass Spectrometry Analysis of NC1 Dimer Peptides Derived from α1256 NC1 Hexamer
The fraction containing the α1256 hexamer (Mono S peak 2) was denatured and reduced by mixing equal volumes of protein solution with a 0.4 m Tris-HCl, pH 7.5, buffer containing 8 m guanidine-HCl and 50 mm dithiothreitol. After heating samples in a boiling water bath for 10 min, the proteins were alkylated with 50 mm iodoacetamide in the dark for 30 min at room temperature. After ethanol precipitation at −20 °C for 2 h, NC1 subunits were resuspended in 0.1 m ammonium bicarbonate, pH 7.5, and digested with trypsin overnight. Subsequently, tryptic peptides were fractionated on a Superdex Peptide column (GE Healthcare), and polypeptides from 3000 to 6500 Da were collected, lyophilized, and stored at −20 °C before MS analysis.
For mass spectrometric analysis of sulfilimine-cross-linked peptides, dry samples were reconstituted in 0.1% formic acid and loaded onto a capillary reverse phase analytical column (360 μm outer diameter × 100 μm inner diameter) using an Eksigent NanoLC Ultra HPLC and autosampler. The analytical column was packed with 20 cm of C18 reverse phase material (Jupiter, 3 μm beads, 300 Å, Phenomenex) equipped with a laser-pulled emitter tip. Peptides were gradient-eluted at a flow rate of 500 nanoliters/min, and the mobile phase solvents consisted of 0.1% formic acid, 99.9% water (solvent A) and 0.1% formic acid, 99.9% acetonitrile (solvent B). A 90-min gradient was performed and consisted of the following: 0–10 min, 2% B; 10–50 min, 2–35% B; 50–60 min, 35–90% B; 60–65 min, 90% B; 65–70 min, 90–2% B; 70–90 min, 2% B. Upon gradient-elution, peptides were mass analyzed on a LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific, San Jose, CA), equipped with a nanoelectrospray ionization source. The instrument was operated using a data-dependent method where full scan (m/z 400–2000) spectra were acquired with the Orbitrap as the mass analyzer (resolution 60,000), and the three most abundant ions in each MS scan were selected for collision-induced dissociation (CID) fragmentation in the LTQ Velos ion trap, which were followed by data-dependent MS3 analysis of the three most abundant ions in each MS2 scan. An isolation width of 2 m/z, activation time of 30 ms, and 35% normalized collision energy were used to generate MS2 and MS3 spectra. For MS2 scans, a minimum threshold of 500 was used to trigger data-dependent spectra, whereas a threshold of 500 or 100 was used to trigger MS3 spectra. Dynamic exclusion was enabled, with an exclusion duration time of 20 ms.
Sulfilimine Cross-linking Analysis of NC1 Peptide Mass Spectra Data
Mass spectra analyses were performed both manually with Xcalibur 2.1 and computationally with the proteomic analysis software SEQUEST (22, 23) and/or MyriMatch (24). For manual searches, theoretical peptide masses for NC1 peptides containing either Met93 or Lys211 were generated using GPMAW software (Lighthouse Data). Sulfilimine-cross-linked peptide masses were calculated by subtracting 2 hydrogen atoms from the sum of the masses of Met93- and Lys211-containing peptides. Although we hypothesized that the α1256 hexamer results from the interaction of a α121 trimer with a α565 trimer, forming α1-α5 and α2-α6 NC1 dimers, we did not limit our analyses to these combinations and also considered other possible combinatorial arrangements. Sulfilimine-cross-linked peptide complexes were manually searched for using the QualBrowser of Xcalibur 2.1 software.
For sulfilimine-cross-linked peptides not identified manually, we took advantage of the characteristic CID fragmentation of sulfilimine-cross-linked peptides (9), which generates modified Met and Lys residues, to design an alternative unbiased bioinformatics approach to analyze mass spectra. Proteomic analysis software Scaffold 3 (Proteome Software, Portland, OR) was used to compile SEQUEST and/or MyriMatch searches of the bovine subset of the Uniref100 database for sulfilimine cross-link-derived peptides. The MyriMatch algorithm (24) was run on a two six-core Intel Xeon processor HP Proliant DL160 G6 server running Windows server 2008 R2 enterprise using the Bumbershoot suite (25). The search was adapted for identifying peptides with the following amino acid modifications: variable, −48.0034 on Met, +45.9877 or +61.9826 on Lys, and oxidation of methionine (+15.9949) as well as fixed carbamidomethylation of cysteine (+57.021). The proteomic analysis software ScanRanker was used to assess the MS3 spectral quality of peptide sequences containing Met93 or Lys211 (26). Spectra were annotated and validated with the utility program IonMatcher (24).
Homology Modeling of the α121-α565 Hexamer
A homology model of the α121-α565 NC1 hexamer was constructed with SWISS-MODEL server and visualized with UCSF Chimera (27, 28). The crystal structure of bovine α121 hexamer (Protein Data Bank accession code 1T61) was used as the starting template (17). The sequences of bovine α5, α5, and α6 NC1 domains were aligned with bovine α1, α1, and α2 NC1 sequences, respectively, and then threaded onto the α121 trimer template (28). In cases where there were deletions in the primary sequence, the sequence variations were reiteratively modeled and stereochemically refined. The model with the lowest root mean square deviation was retained. The α565 trimer model was superimposed “head-to-head” with the bovine α121 trimer crystal structure to form the α1256 hexamer model. Molecular graphics images were produced using UCSF Chimera 1.6.1.rc and ray-traced with POV-Ray (Version 3.6) (27).
RESULTS
Isolation and Characterization of α1256 Hexamer from Bovine Aorta
The α1256 hexamer was isolated from bovine aorta tissue to provide NC1 peptides amenable to analysis by mass spectrometry. Digestion of the aorta tissue with bacterial collagenase routinely yielded about 1.5 mg of NC1 hexamers per 50 g of wet tissue (Fig. 1). In accord with previous results (16), immunoblot analysis using chain-specific monoclonal antibodies confirmed the presence of the α1, α2, α5, and α6 NC1 domains in the purified aorta NC1 hexamer (Fig. 1B). Notably, α3 and α4 NC1 domains were not detected. Monoclonal antibodies detected NC1 dimeric subunits, suggesting the presence of covalent cross-linking between any combination of α1, α2, α5, and α6 NC1 monomeric subunits.
FIGURE 1.
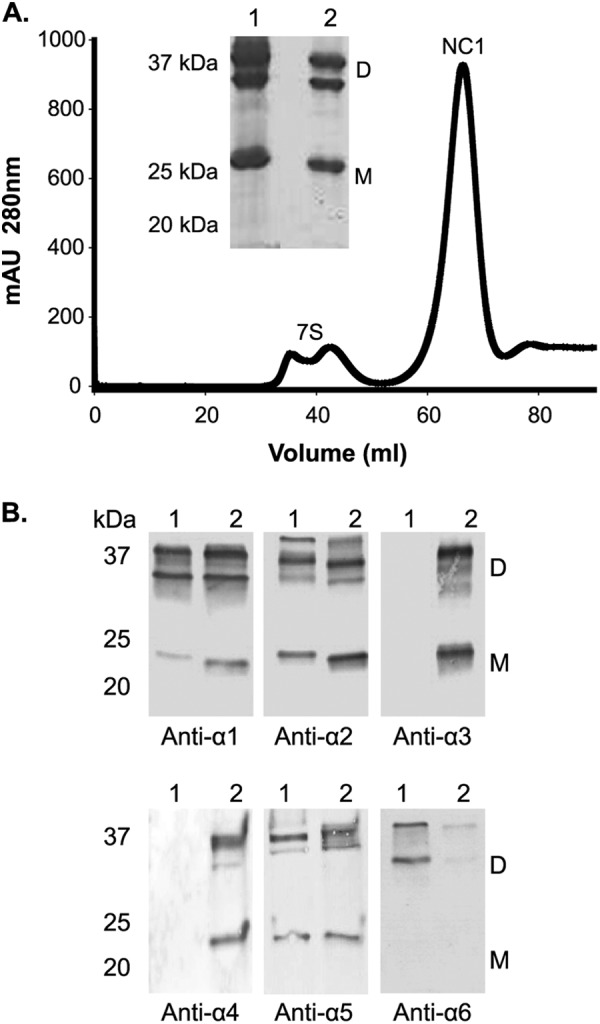
Isolation and characterization of aorta NC1 hexamer with respect to NC1 domain composition. A, isolation of NC1 hexamer from bovine aorta was achieved by size-exclusion chromatography on a S300 column (GE Healthcare). Absorbance was monitored at 280 nm. The inset shows SDS-PAGE analysis in which bovine aorta NC1 hexamer S300 peak (lane 1) is compared with a placenta BM NC1 hexamer (lane 2). mAU, milliabsorbance units. B, composition analysis of bovine aorta NC1 hexamer isolated from S300 column by Western blot using monoclonal antibodies for the α1 (H11), α2 (H22), α3 (H31), α4 (H44), α5 (H53), and α6 (B66) NC1 domains. Lanes 1 and 2 show the staining for bovine aorta NC1 hexamer and bovine testis hexamer (positive control for α1 through α5 NC1 domains), respectively. Dimeric (D) and monomeric (M) NC1 domain subunits are indicated.
In smooth muscle tissues two distinct subpopulations of NC1 hexamers of different α-chain compositions were observed: the α121 and α1256 hexamers (16). To isolate the α1256 hexamer for MS analysis, the preparation of aorta NC1 hexamers was fractionated on a cation-exchange Mono S chromatography column (Fig. 2A). Elution of bound proteins with increasing concentrations of sodium chloride yielded three distinct chromatographic peaks. Immunoblot analysis of each peak using chain-specific monoclonal antibodies showed the α5 and α6 NC1 subunits eluting most abundantly in peaks 1 and 2, with the α1 and α2 NC1 subunits also found in these peaks (Fig. 2B). Peak 3 is predominantly composed of α1 and α2 NC1 subunits, whereas the staining for α5 and α6 NC1 domains was minimal. These data indicate that the α121 hexamer elutes in peak 3, whereas the α1256 hexamer elutes in peaks 1 and 2. Peak 1 and 2 were considered indistinguishable by immunoblot analyses, but because of its greater abundance, peak 2 was used for MS analyses.
FIGURE 2.
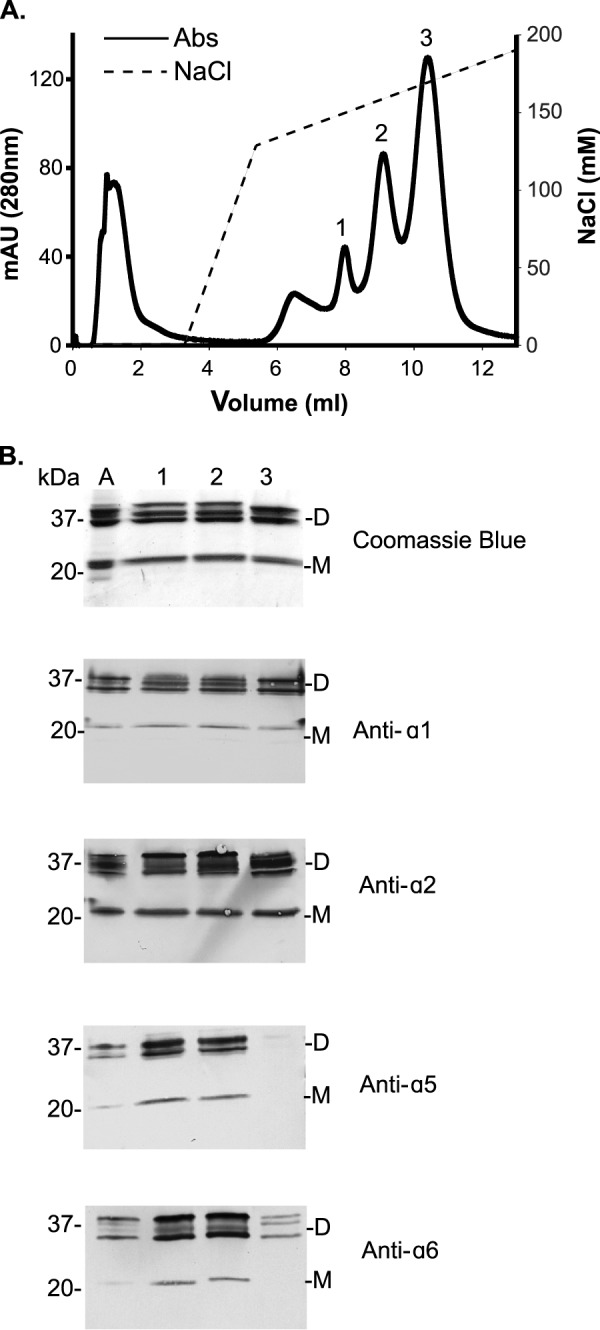
Fractionation of aorta NC1 hexamers. A, Mono S cation-exchange chromatography was used to fractionate bovine aorta NC1 hexamers. Absorbance was monitored at 280 nm (solid line), and the concentration of sodium chloride (NaCl) was monitored by conductivity (dotted line). mAU, milliabsorbance units. B, SDS-PAGE analysis of Mono S chromatographic peaks. Lanes labeled 1–3 correspond to Mono S peaks 1–3, respectively. The same samples were analyzed by immunoblotting using monoclonal NC1-specific antibodies. Unfractionated aorta NC1 hexamer (lane denoted A) was used as a positive control. Dimeric (D) and monomeric (M) NC1 domain subunits are shown. Peak 2 in A, which stained for α1-α5 NC1 and α2-α6 NC1 dimers, was used for further MS analyses.
Mass Spectrometry Analysis of Sulfilimine-Cross-linked NC1 Dimer Peptides
NC1 hexamers are formed by the interaction of the C-terminal NC1 domain trimers of two protomers (17). The large, planar interface formed by the interacting trimers is stabilized by sulfilimine bonds ( S
S N
N ) in which the sulfur atom of Met93 from an NC1 monomer forms a covalent bond with the ϵ-nitrogen from Lys211 (or hydroxylysine) of an adjacent NC1 monomer (9, 29). Because of the spatial alignment of NC1 domains across the trimer-trimer interface, the occurrence of sulfilimine bonds results in the formation of three covalently linked NC1 dimers (Fig. 3). Therefore, the composition of such sulfilimine-cross-linked NC1 dimers reflects the way in which triple-helical protomers interact to form collagen IV networks.
) in which the sulfur atom of Met93 from an NC1 monomer forms a covalent bond with the ϵ-nitrogen from Lys211 (or hydroxylysine) of an adjacent NC1 monomer (9, 29). Because of the spatial alignment of NC1 domains across the trimer-trimer interface, the occurrence of sulfilimine bonds results in the formation of three covalently linked NC1 dimers (Fig. 3). Therefore, the composition of such sulfilimine-cross-linked NC1 dimers reflects the way in which triple-helical protomers interact to form collagen IV networks.
FIGURE 3.
A, experimental strategy for cross-linking analysis of NC1 dimers of the α1256 hexamer. The schematic shows that the α12156 hexamer putatively contains two different types of NC1 dimers, one composed of α1 (yellow) and α5 (green) NC1 domains and another composed of α2 (purple) and α6 (orange) NC1 domains. The NC1 monomers are presumed to be cross-linked by sulfilimine bonds (black bars), which were previously found in NC1 dimers of the α121 hexamer (9). The sulfilimine cross-link is formed between Met93 from an NC1 domain of one trimer and a Hyl211 from an NC1 domain of the opposite trimer (Lys211 is post-translationally modified to Hyl211, noted as KOH). A total of six sulfilimine cross-links (two per NC1 dimer) can be formed at the trimer-trimer interface. Dissociation of α1256 hexamer yields α1-α5 and α2-α6 NC1 dimers. Trypsin digestion of α1-α5 NC1 dimers releases two peptide complexes (T-3958 and T-4943), each containing the sulfilimine cross-link. Likewise, trypsin digestion of α2-α6 NC1 dimers releases two peptide complexes (T-5307 and T-4393). B, fractionation of α1256 hexamer tryptic peptides. Tryptic peptides derived from Mono S peak 2 (Fig. 2A) were fractionated on a Superdex Peptide column (GE Healthcare). The shaded area contained peptides ranging from 3000 to 6500 Da and was collected for mass spectrometry analysis. Absorbance (milliabsorbance units (mAU) was monitored at 214 nm.
In this study we used a MS-based strategy to determine the occurrence of sulfilimine bonds by identifying cross-linked peptides derived from NC1 dimeric subunits. Thus, as previously suggested (16), we hypothesized that this hexamer is formed from a α121 trimer and a α565 trimer. This arrangement may generate up to four distinct sulfilimine cross-linking sites: two sites between α1 and α5 NC1 domains and two sites between α2 and α6 NC1 domains (Fig. 3A). Theoretical masses for α1-α5 and α2-α6 sulfilimine-cross-linked NC1 peptides as well as other possible combinations were calculated as indicated under “Experimental Procedures.” To identify these sulfilimine-cross-linked peptides, Mono S peak 2, containing the α1256 NC1 hexamer (Fig. 2), was digested with trypsin, and the resulting peptides were fractionated by gel filtration (Fig. 3B). Peptides ranging from 3000 to 6500 Da in size were collected and analyzed in a high resolution LTQ Orbitrap Velos in a data-dependent manner as previously described (9, 18). For convenience, sulfilimine-cross-linked peptides identified by manual and bioinformatics analyses of MS data described under “Experimental Procedures” are summarized and presented by the type of NC1 dimer from which they originate.
Mass Spectrometry Analyses of α1-α5 NC1 Sulfilimine-Cross-linked Peptides
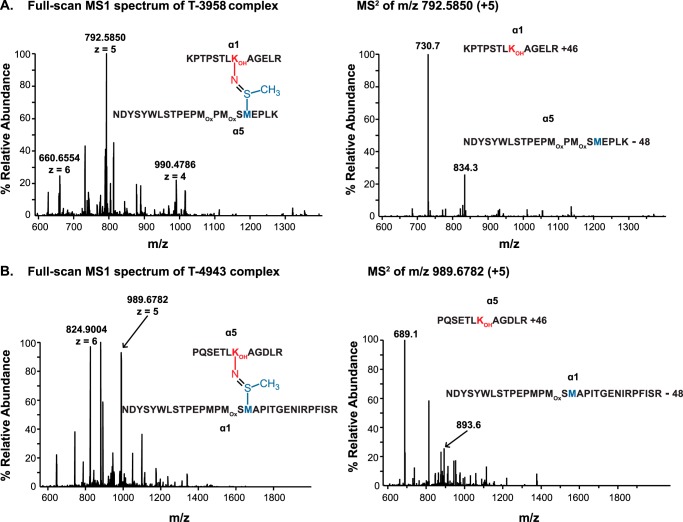
The α1-Hyl211−α5-Met93 complex was found in the full scan mass spectrum containing the multiply protonated ions (+4, +5, +6) that represent the T-3958 complex (Fig. 4A). The difference between observed and calculated masses for this peptide complex reflects the loss of 2 hydrogen atoms upon sulfilimine bond formation (Table 1). To confirm the presence of a sulfilimine bond connecting the two peptides, the m/z 792.5850 (+5) ion was selected for MS2 fragmentation by CID. As observed in the MS2 spectrum (Fig. 4A), CID fragmentation yielded two predominant ions: m/z 834.3 (+3) and m/z 730.7 (+2), which represent the α5-Met93- and α1-Hyl211-containing peptides but deviate from the expected theoretical peptide masses by −48 and +46 atomic mass units, respectively. These peptide modifications correspond to the olefin fragment derived from Met93 and the methylsulfenamide fragment derived from Hyl211 that result from the characteristic gas-phase Cope elimination previously described for sulfilimine-cross-linked peptides (supplemental Figs. S1 and S2) (9). The T-3958 peptide complex demonstrates hydroxylation of α1-Lys211 and establishes the presence of the sulfilimine bond in one of the two cross-linking sites within the α1-α5 NC1 dimer.
FIGURE 4.
Mass spectrometric analysis of sulfilimine-cross-linked α1-α5 NC1 peptides. A, full-scan MS1 and MS2 spectra for tryptic peptide complex T-3958 composed of a α5 NC1 peptide covalently cross-linked to a α1 NC1 peptide by a sulfilimine bond between α5-Met93 and α1-Hyl211. The m/z 990.4786, 792.6860, and 660.6554 correspond to the multiply protonated (+4), (+5), and (+6) ions of the T-3958 complex, respectively. MS2 fragmentation of m/z 792.5850 (+5) by CID yielded two predominant ions; m/z 834.3 (+3) and m/z 730.7 (+2) represent the α5-Met93- and α1-Hyl211-containing peptides, which deviate from the expected theoretical peptide masses by −48 and +46 atomic mass units, respectively. These modifications correspond to the olefin fragment derived from Met93 and a methylsulfenamide fragment derived from Hyl211 that result from the characteristic gas-phase Cope elimination previously described for sulfilimine-cross-linked peptides (9). B, full-scan MS1 and MS2 spectra for tryptic peptide complex T-4943 composed of a α1 NC1 peptide covalently cross-linked to a α5 NC1 peptide by a sulfilimine bond between α1-Met93 and α5-Hyl211. Ions with m/z 989.6782 and 824.9004 correspond to the multiply protonated (+5) and (+6) ions of the T-4943 peptide complex, respectively. CID fragmentation of m/z 824.9004 (+6) yielded a MS2 profile with two predominant peptide fragments: m/z 893.6 (+4) and m/z 689.1 (+2) represent the α1-Met93- and α5-Hyl211-containing peptides, which deviate from the expected theoretical peptide masses by −48 and +46 atomic mass units, respectively, as a result of sulfilimine bond fragmentation (9).
TABLE 1.
Mass spectrometry identified sulfilimine-cross-linked NC1 peptide complexes
Tryptic peptide complexes (T-XXXX) containing Met93 and Hyl211 residues were identified with mass spectrometry and compared to theoretical peptide masses.
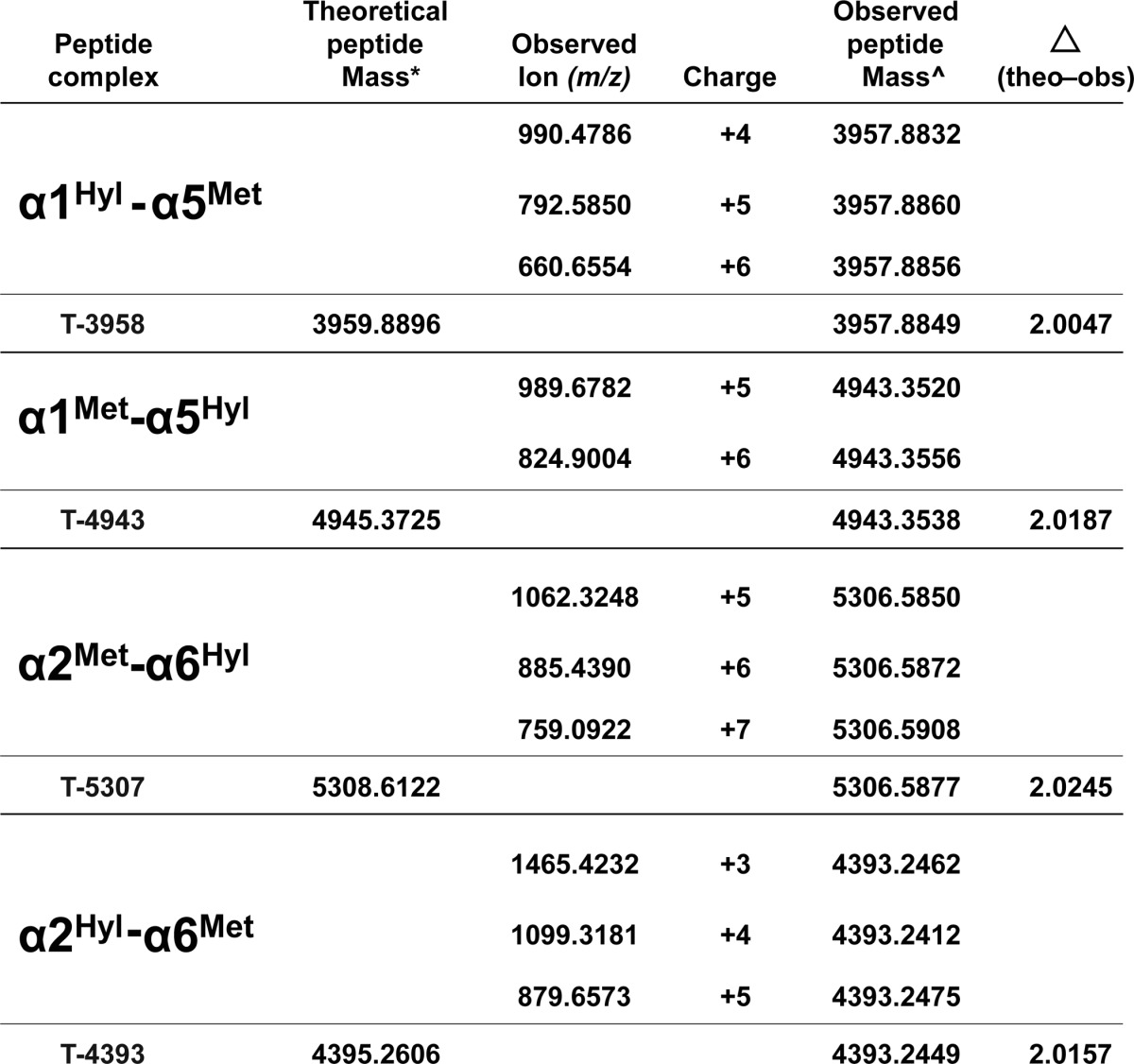
* The theoretical peptide masses were calculated from the sum of the masses of the respective NC1 tryptic peptides containing Met93 and Hyl211.
^ The observed peptide masses were calculated from the monoisotopic ions observed for each multiply protonated precursor ion detected. Calculated peptide masses were averaged and compared to the theoretical mass. A mass difference (theoretical − observed) equivalent to two hydrogen atoms was found in each case.
Similarly for the α1-Met93−α5-Hyl211 NC1 peptide complex, the full scan mass spectrum contained multiply protonated ions (+5 and +6) representing the T-4943 complex (Fig. 4B). The difference between the observed and calculated masses for this peptide complex reflects the loss of 2 hydrogen atoms upon sulfilimine bond formation (Table 1). To confirm the presence of a sulfilimine bond connecting the two peptides, the m/z 824.9004 (+6) ion was selected for MS2 fragmentation by CID. As observed in the MS2 spectrum (Fig. 4B), CID fragmentation yielded two predominant ions; m/z 689.1 (+2) and m/z 893.6 (+4) ions represent the α5-Hyl211- and α1-Met93-containing peptides but deviate from the expected theoretical peptide masses by +46 and −48 atomic mass units and, respectively, which is consistent with a MS2 fragmentation profile characteristic of peptides cross-linked by a sulfilimine bond (9). Furthermore, MS3 fragmentation confirmed the NC1 peptide sequences and localized the peptide modifications to Met93 and Hyl211 (supplemental Figs. S3 and S4). Mass spectrometric analyses of the T-4943 peptide complex demonstrates hydroxylation of α5-Lys211 and establishes the presence of the sulfilimine bond in the second of the two α1-α5 NC1 dimer cross-linking sites.
Mass Spectrometry Analyses of α2-α6 NC1 Sulfilimine-Cross-linked Peptides
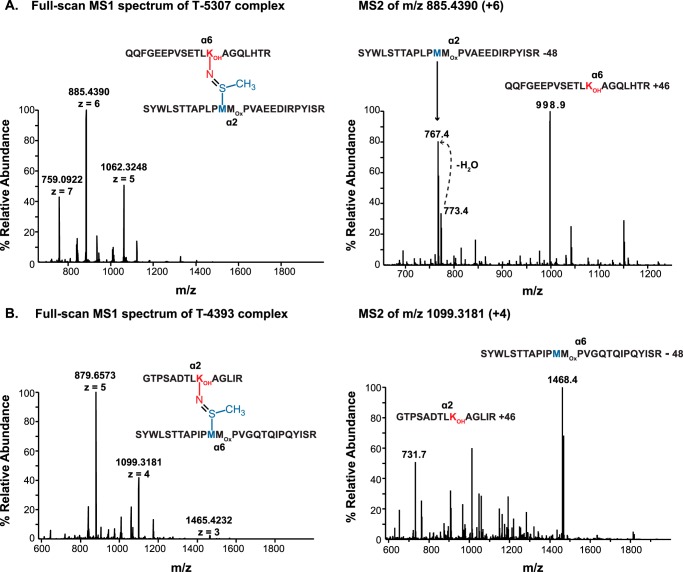
Although the hydroxylation of α6-Lys211 had never been reported, we included this modification in our mass spectra searches because this residue lies embedded within a conserved hydroxylation motif (30). The α2-Met93−α6-Hyl211 NC1 peptide complex was found in the full scan mass spectrum containing the multiply protonated ions (+5, +6, +7) that represent the T-5307 complex (Fig. 5A). The difference between the observed and calculated masses reflects the loss of two hydrogen atoms upon sulfilimine bond formation (Table 1). As shown in Fig. 5A, CID fragmentation of m/z 885.4390 (+6) yielded three predominant ions in the MS2 spectrum: m/z 773.4 (+3) and m/z 767.4 (+3) ions and an m/z 998.9 (+3) ion. These ions represent the α6-Hyl211- and α2-Met93-containing peptides but deviate from the expected theoretical peptide masses by +62 and −48 atomic mass units, respectively, generating a MS2 fragmentation profile characteristic of peptides cross-linked by a sulfilimine bond (9). MS3 fragmentation profiles of the m/z 767.4 (+3) and m/z 998.9 (+3) ions confirmed the NC1 peptide sequences and localized the peptide modifications to Met93 (supplemental Figs. S5 and S6). Furthermore, MS3 validation with IonMatcher localizes the addition of 16 atomic mass units to Lys211 of the α6 NC1 domain (supplemental Fig. S5). These data demonstrate the hydroxylation of α6-Lys211 and establishes the occurrence of a sulfilimine bond in one of the two cross-linking sites within the α2-α6 NC1 dimer.
FIGURE 5.
Mass spectrometric analysis of sulfilimine-cross-linked α2-α6 NC1 peptides. A, full-scan MS1 and MS2 spectra for tryptic peptide complex T-5307 composed of a α2 NC1 peptide covalently cross-linked to a α6 NC1 peptide by a sulfilimine bond between α2-Met93 and α6-Hyl211. The m/z 1062.3248, 885.4390, and 759.0922 correspond to the multiply protonated (+5), (+6), and (+7) ions of the 5306.5930-Da peptide complex, respectively. CID of m/z 885.4390 (+6) yielded a MS2 spectrum with three predominant peptide fragments; m/z 773.4 (+3) and m/z 767.4 (+3) correspond to the Hyl211-containing peptide derived from the α6 NC1 domain, and m/z 998.1 (+3) corresponds to the Met93-containing tryptic peptide derived from the α2 NC1 domain. The α2-Met93-containing and α6-Hyl211-containing peptides deviate from their expected theoretical peptide masses by −48 and +46 atomic mass units, respectively. These modifications correspond to the olefin fragment derived from Met93 and a methylsulfenamide fragment derived from Hyl211 that result from the characteristic gas-phase Cope elimination previously described for sulfilimine-cross-linked peptides (9). B, full-scan MS1 and MS2 spectra for tryptic peptide complex T-4393 composed of a α6 NC1 peptide covalently cross-linked to a α2 NC1 peptide by a sulfilimine bond between α6-Met93 and α2-Hyl211. The m/z 1465.4232, 1099.3181, and 879.6573 correspond to the multiply protonated (+3), (+4), and (+5) ions of the T-4393 peptide complex, respectively. CID of m/z 1099.3181 (+5) yielded a MS2 spectrum with two predominant peptide fragments; m/z 773.4 (+3) and m/z 731.7 (+2) represent the α6-Met93- and α2-Hyl211-containing peptides, which deviate from the expected theoretical peptide masses by −48 and +46 atomic mass units, respectively, as a result of sulfilimine bond fragmentation (9).
Similarly for the α6-Met93−α2-Hyl211 NC1 peptide complex, the full scan MS1 mass spectrum contained multiply protonated ions (+3, +4, +5) representing the T-4393 complex (Fig. 5B). The difference between the observed and calculated mass for this peptide complex reflects the loss of 2 hydrogen atoms upon sulfilimine bond formation (Table 1). To confirm the presence of a sulfilimine cross-link, the m/z 1099.3181 (+4) ion was selected for MS2 fragmentation by CID. As observed in the MS2 spectrum (Fig. 5B), CID fragmentation yielded two predominant ions; m/z 731.7 (+2) and m/z 1468.4 (+2) ions represent the α2-Hyl211- and α6-Met93-containing peptides but deviate from the expected theoretical peptide masses by +46 and −48 atomic mass units, respectively, which is consistent with a MS2 fragmentation profile characteristic of peptides cross-linked by a sulfilimine bond (9). Furthermore, MS3 fragmentation confirmed the NC1 peptide sequences and localized the modifications to Met93 and Hyl211 (supplemental Figs. S7 and S8). Collectively, these results demonstrate hydroxylation of α2-Lys211 and establish the presence of the sulfilimine bond in the second of two cross-linking sites in the α2-α6 NC1 dimer, which along with the four α1-α5 sulfilimine bonds establishes the presence of a total of six sulfilimine bonds per α121-α565 NC1 hexamer. Taken together, our MS studies of NC1 heterodimers support our hypothesis that the α1256 hexamer is formed by the covalent interaction of α121 trimer and the α565 trimer.
Homology Modeling of the α121-α565 Hexamer
Identification of sulfilimine-cross-linked NC1 dimers establishes the spatial arrangement and connectivity of the NC1 domains at the trimer-trimer interface and provides the framework to construct a three-dimensional homology model of the α121-α565 hexamer quaternary structure. The amino acid sequences of α1, α2, α5, and α6 NC1 domains were threaded onto the α121 NC1 hexamer crystal structure template, as described under “Experimental Procedures.” As shown in Fig. 6,4 the overall structure of the homology model for α1256 hexamer is very similar to the already established α121 and α345 hexamer. The model illustrates the interaction of a α121 trimer with a α565 trimer in which the orientation of one with respect to the other is determined by the alignment of α1-α5 and α2-α6 NC1 dimers revealed by the MS analysis of tryptic peptides identified in this study. Henceforth, we propose to change the nomenclature of the α1256 hexamer to α121-α565 hexamer to better represent the heterotypic interaction of α121 and α565 protomers.
FIGURE 6.
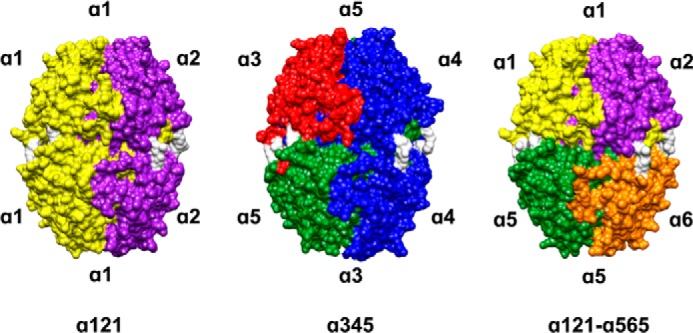
NC1 hexamer structures of the three known mammalian collagen IV networks. The illustration shows that the overall structure of the α121-α565 NC1 hexamer is similar to those of the α121 and α345 hexamer structures determined in previous studies. The sulfilimine bond formed between methionine 93 and lysine (or hydroxylysine) 211 of adjacent NC1 trimers is shown in white for all the α121, the α345, and the α121-α565 NC1 hexamers. Unlike the α121 and α345 NC1 hexamers, the α121-α565 hexameric quaternary structure is formed by a heterotypic interaction between a α121 trimer and a α565 trimer. The alignment of one trimer with respect to the other is defined by the cross-linked α1-α5 and α2-α6 NC1 dimers. Molecular surfaces images were produced using UCSF Chimera (27).
DISCUSSION
Sulfilimine-cross-linked NC1 hexamers provide insightful information regarding the composition and organization of the different collagen IV networks. Previous research described the composition and suggested a quaternary structure for the α121-α565 hexamer, but it could not unequivocally establish covalent linkages and the organization of NC1 domains (16). In this study, we overcame such limitations by using high resolution MS to identify sulfilimine-cross-linked α1-α5 and α2-α6 NC1 dimers. Construction of a three-dimensional model by homology modeling using this chemical information provides the framework for understanding the spatial organization of NC1 domains within the hexamer. Thus, the quaternary structure of the α121-α565 hexamer shown in Fig. 6 defines the supramolecular organization of this collagen IV network.
Initially, the identification of α5 and α6 peptides in the MS data of unfractionated aorta NC1 hexamers was confounded by overcrowding of the spectra by peptides derived from the more prevalent α1 and α2 NC1 domains (supplemental Figs. S9 and S10), as expected based upon previous studies determining the aorta BM abundance of the α121 hexamer as 88% and the α121-α565 hexamer as 12% (16). Therefore, use of the Mono S cation-exchange column to separate the different types of NC1 hexamers was essential for isolating α121-α565 hexamers, from which trypsin digestion produced peptides derived from NC1 dimers identifiable by MS analyses (Fig. 2).
MS analyses identified sulfilimine cross-links at the trimer-trimer interface of the α121-α565 hexamer. Sulfilimine bonds have previously been shown between Met93 and Hyl211of NC1 dimers in the α121 and α345 collagen IV networks, with the exception of the sulfilimine bond between α4-α4 NC1 dimer forming between Met93 and Lys211 (9). In this study MS analyses show that sulfilimine bonds form between Met93 and Hyl211 of α1-α5 and α2-α6 dimers. Notably, the hydroxylation state of the α6-Lys211 was confirmed by MS fragmentation of the T-5307 complex, which revealed an extra 16 atomic mass units localized on the Lys211 of the α6 NC1 peptide (Fig. 5A and supplemental Fig. S5). Hydroxylation of α6-Lys211 is consistent with the α6 NC1 domain containing the XK(A/S/G) sequence motif that triggers hydroxylation of lysyl residues by lysyl hydroxylase (30). These findings together with previous studies in the α121 and α345 hexamers (18, 29) establish that Lys211 of all six NC1 domains form sulfilimine cross-links, and all except α4-Lys211 appear predominantly as Hyl211 in BMs.
Collagen IV plays a role in maintaining the vascular integrity of the aorta, a smooth muscle tissue, which relies on the mechanical properties of the extracellular matrix for proper pulsatile blood flow (31). Indeed, the distinct expression of the α5 and α6 chains has been correlated with BMs of tubular structures that are constantly exposed to mechanical stress or tensile strength (15, 32). Two collagen IV networks (α121 and α121-α565) coexist in the smooth muscle BM of the aorta medial layer. Interestingly, it has been reported that some Alport patients not only endure nephropathy (6) but also have an increased susceptibility to aortic aneurysms (33, 34), possibly due to mutations in the COL4A5 gene leading to loss of the α121-α565 network of the BM surrounding aortic smooth muscle cells. It is conceivable that the absence of this network may weaken the aortic wall by either reducing its mechanical strength and/or by interrupting cell-matrix interactions important for normal smooth muscle cell development. Thus, Alport syndrome, which previously emphasized the important role of the α345 network in molecular ultrafiltration in the kidney, may now also help establish the role of the α121-α565 network in the vascular integrity of the aorta.
The discovery of sulfilimine bonds in the α121-α565 network inspires further exploration to gain a better understanding of the function and regulation of these cross-links in vascular tissues. As we have recently shown in Drosophila (11), the concerted activity of peroxidasin, bromide, and oxidant is an absolute requirement for the formation of both sulfilimine bonds as well as functional collagen IV networks capable of providing mechanical stability to BM and tissues. For instance, the occurrence of sulfilimine bonds demonstrated in this study suggests that all of these elements converge in the aorta BM. In fact, recent reports have shown that peroxidasin, also known as vascular peroxidase (VPO1), is expressed in the vascular wall of arterioles both in the endothelium and medial layer (35). Conditions that may decrease peroxidasin sulfilimine cross-linking activity may result in weakened collagen IV networks, which may lead to abnormalities of the vascular wall such as aortic aneurysm. On the other hand, it is also plausible, for example, that deregulation of peroxidasin could lead to excessive cross-linking of collagen IV network leading to its accumulation in the smooth muscle BM, which may contribute to pathological states such as arteriosclerosis.
In conclusion, the quaternary structures of the three NC1 hexamers representing the three known collagen IV networks are depicted in Fig. 6. Although the α121 and α345 hexamers are formed by a homotypic interaction between protomers, the α121-α565 hexamer is formed by a heterotypic interaction of α121 and α565 protomers. In addition, this study provides chemical evidence supporting the quaternary structure of the α565 protomer illustrated by homology modeling, where the α5 and α6 NC1 domains assemble into a heterotrimer with a structural organization analogous to that of the α121 and α345 heterotrimers. All three triple-helical protomers are the fundamental building blocks of collagen IV networks, and the elucidation of their structures has provided insight into BM function and explained the molecular basis for human diseases including Alport syndrome (6). Taken together, our findings in conjunction with our previous studies establish that the six collagen IV chains are organized into three canonical protomers α121, α345, and α565 forming three distinct networks: α121, α345, and α121-α565, each of which is stabilized by sulfilimine bonds between their C-terminal NC1 domains.
Supplementary Material
Acknowledgments
We thank Mohamed Rafi and Parvin Todd for technical expertise. We are also very grateful to Salisha Hill and Hayes McDonald from the Vanderbilt Mass Spectrometry Center for assistance with MS analysis of some of our samples. We thank Suraj Adhikary for helpful comments with molecular modeling. The LTQ Orbitrap Velos mass spectrometer used in these studies was purchased with funds from National Institutes of Health Grant S10 RR027714 awarded to the Vanderbilt proteomics shared resource.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK18381.

This article contains supplemental Figs. S1–S10.
The homology model of the α121-α565 NC1 hexamer is available at the Vanderbilt Center for Matrix Biology website.
- BM
- basement membrane
- NC1
- noncollagenous
- CID
- collision induced dissociation.
REFERENCES
- 1. Fidler A. L., Vanacore R. M., Chetyrkin S. V., Pedchenko V. K., Bhave G., Yin V. P., Stothers C. L., Rose K. L., McDonald W. H., Clark T. A., Borza D. B., Steele R. E., Ivy M. T., Aspirnauts, Hudson J. K., Hudson B. G. (2014) A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. U.S.A. 111, 331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boute N., Exposito J. Y., Boury-Esnault N., Vacelet J., Noro N., Miyazaki K., Yoshizato K., Garrone R. (1996) Type IV collagen in sponges, the missing link in basement membrane ubiquity. Biol. Cell 88, 37–44 [DOI] [PubMed] [Google Scholar]
- 3. Pöschl E., Schlötzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. (2004) Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131, 1619–1628 [DOI] [PubMed] [Google Scholar]
- 4. Hynes R. O., Lively J. C., McCarty J. H., Taverna D., Francis S. E., Hodivala-Dilke K., Xiao Q. (2002) The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb. Symp. Quant. Biol. 67, 143–153 [DOI] [PubMed] [Google Scholar]
- 5. Kashtan C. E., Kleppel M. M., Butkowski R. J., Michael A. F., Fish A. J. (1990) Alport syndrome, basement membranes, and collagen. Pediatr. Nephrol. 4, 523–532 [DOI] [PubMed] [Google Scholar]
- 6. Hudson B. G., Tryggvason K., Sundaramoorthy M., Neilson E. G. (2003) Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N. Engl. J. Med. 348, 2543–2556 [DOI] [PubMed] [Google Scholar]
- 7. Gould D. B., Phalan F. C., Breedveld G. J., van Mil S. E., Smith R. S., Schimenti J. C., Aguglia U., van der Knaap M. S., Heutink P., John S. W. (2005) Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 308, 1167–1171 [DOI] [PubMed] [Google Scholar]
- 8. Gould D. B., Phalan F. C., van Mil S. E., Sundberg J. P., Vahedi K., Massin P., Bousser M. G., Heutink P., Miner J. H., Tournier-Lasserve E., John S. W. (2006) Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N. Engl. J. Med. 354, 1489–1496 [DOI] [PubMed] [Google Scholar]
- 9. Vanacore R., Ham A. J., Voehler M., Sanders C. R., Conrads T. P., Veenstra T. D., Sharpless K. B., Dawson P. E., Hudson B. G. (2009) A sulfilimine bond identified in collagen IV. Science 325, 1230–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhave G., Cummings C. F., Vanacore R. M., Kumagai-Cresse C., Ero-Tolliver I. A., Rafi M., Kang J. S., Pedchenko V., Fessler L. I., Fessler J. H., Hudson B. G. (2012) Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat. Chem. Biol. 8, 784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCall A. S., Cummings C. F., Bhave G., Vanacore R., Page-McCaw A., Hudson B. G. (2014) Bromine is an essential trace element for assembly of collagen iv scaffolds in tissue development and architecture. Cell 157, 1380–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boutaud A., Borza D. B., Bondar O., Gunwar S., Netzer K. O., Singh N., Ninomiya Y., Sado Y., Noelken M. E., Hudson B. G. (2000) Type IV collagen of the glomerular basement membrane: Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J. Biol. Chem. 275, 30716–30724 [DOI] [PubMed] [Google Scholar]
- 13. Butkowski R. J., Wieslander J., Kleppel M., Michael A. F., Fish A. J. (1989) Basement membrane collagen in the kidney: regional localization of novel chains related to collagen IV. Kidney Int. 35, 1195–1202 [DOI] [PubMed] [Google Scholar]
- 14. Gunwar S., Ballester F., Noelken M. E., Sado Y., Ninomiya Y., Hudson B. G. (1998) Glomerular basement membrane: identification of a novel disulfide-cross-linked network of α3, α4, and α5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J. Biol. Chem. 273, 8767–8775 [DOI] [PubMed] [Google Scholar]
- 15. Seki T., Naito I., Oohashi T., Sado Y., Ninomiya Y. (1998) Differential expression of type IV collagen isoforms, α5(IV) and α6(IV) chains, in basement membranes surrounding smooth muscle cells. Histochem. Cell Biol. 110, 359–366 [DOI] [PubMed] [Google Scholar]
- 16. Borza D. B., Bondar O., Ninomiya Y., Sado Y., Naito I., Todd P., Hudson B. G. (2001) The NC1 domain of collagen IV encodes a novel network composed of the α1, α2, α5, and α6 chains in smooth muscle basement membranes. J. Biol. Chem. 276, 28532–28540 [DOI] [PubMed] [Google Scholar]
- 17. Sundaramoorthy M., Meiyappan M., Todd P., Hudson B. G. (2002) Crystal structure of NC1 domains. Structural basis for type IV collagen assembly in basement membranes. J. Biol. Chem. 277, 31142–31153 [DOI] [PubMed] [Google Scholar]
- 18. Vanacore R. M., Ham A. J., Cartailler J. P., Sundaramoorthy M., Todd P., Pedchenko V., Sado Y., Borza D. B., Hudson B. G. (2008) A role for collagen IV cross-links in conferring immune privilege to the Goodpasture autoantigen: structural basis for the crypticity of B cell epitopes. J. Biol. Chem. 283, 22737–22748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sado Y., Kagawa M., Naito I., Ueki Y., Seki T., Momota R., Oohashi T., Ninomiya Y. (1998) Organization and expression of basement membrane collagen IV genes and their roles in human disorders. J. Biochem. 123, 767–776 [DOI] [PubMed] [Google Scholar]
- 20. Kahsai T. Z., Enders G. C., Gunwar S., Brunmark C., Wieslander J., Kalluri R., Zhou J., Noelken M. E., Hudson B. G. (1997) Seminiferous tubule basement membrane. Composition and organization of type IV collagen chains and the linkage of α3(IV) and α5(IV) chains. J. Biol. Chem. 272, 17023–17032 [DOI] [PubMed] [Google Scholar]
- 21. Ninomiya Y., Kagawa M., Iyama K., Naito I., Kishiro Y., Seyer J. M., Sugimoto M., Oohashi T., Sado Y. (1995) Differential expression of two basement membrane collagen genes, COL4A6 and COL4A5, demonstrated by immunofluorescence staining using peptide-specific monoclonal antibodies. J. Cell Biol. 130, 1219–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eng J. K., McCormack A. L., Yates J. R. (1994) An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 976–989 [DOI] [PubMed] [Google Scholar]
- 23. MacCoss M. J., Wu C. C., Yates J. R., 3rd. (2002) Probability-based validation of protein identifications using a modified SEQUEST algorithm. Anal. Chem. 74, 5593–5599 [DOI] [PubMed] [Google Scholar]
- 24. Tabb D. L., Fernando C. G., Chambers M. C. (2007) MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J. Proteome Res. 6, 654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cociorva D., L Tabb D., Yates J. R. (2007) Validation of tandem mass spectrometry database search results using DTASelect. Curr. Protoc. Bioinformatics 13, 4. [DOI] [PubMed] [Google Scholar]
- 26. Holman J. D., Ma Z. Q., Tabb D. L. (2012) Identifying Proteomic LC-MS/MS Data Sets with Bumbershoot and IDPicker. Curr. Protoc. Bioinformatics 13, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 28. Guex N., Peitsch M. C. (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 29. Vanacore R. M., Friedman D. B., Ham A. J., Sundaramoorthy M., Hudson B. G. (2005) Identification of S-hydroxylysyl-methionine as the covalent cross-link of the noncollagenous (NC1) hexamer of the α1α1α2 collagen IV network: a role for the post-translational modification of lysine 211 to hydroxylysine 211 in hexamer assembly. J. Biol. Chem. 280, 29300–29310 [DOI] [PubMed] [Google Scholar]
- 30. Kivirikko K. I., Pihlajaniemi T. (1998) Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv. Enzymol. Relat. Areas Mol. Biol. 72, 325–398 [DOI] [PubMed] [Google Scholar]
- 31. Wagenseil J. E., Mecham R. P. (2009) Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 89, 957–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hino S., Takemura T., Sado Y., Kagawa M., Oohashi T., Ninomiya Y., Yoshioka K. (1996) Absence of α 6(IV) collagen in kidney and skin of X-linked Alport syndrome patients. Pediatr. Nephrol. 10, 742–744 [DOI] [PubMed] [Google Scholar]
- 33. Kashtan C. E., Segal Y., Flinter F., Makanjuola D., Gan J. S., Watnick T. (2010) Aortic abnormalities in males with Alport syndrome. Nephrol. Dial. Transplant. 25, 3554–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lyons O. T., St John E. R., Morales J. P., Chan Y. C., Taylor P. R. (2007) Ruptured thoracoabdominal aortic aneurysm in a renal transplant patient with Alport's syndrome. Ann. Vasc. Surg. 21, 816–818 [DOI] [PubMed] [Google Scholar]
- 35. Cheng G., Salerno J. C., Cao Z., Pagano P. J., Lambeth J. D. (2008) Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic. Biol. Med. 45, 1682–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.