Abstract
Background:
Observational studies suggest that effects of vitamin D may be stronger for cancer mortality than for incidence. Yet, existing randomised controlled trials (RCTs) of vitamin D supplementation have limited power to examine the relationships as their primary end points are not cancer incidence or mortality.
Methods:
Meta-analyses of RCTs of vitamin D supplementation and total cancer incidence and mortality were conducted.
Results:
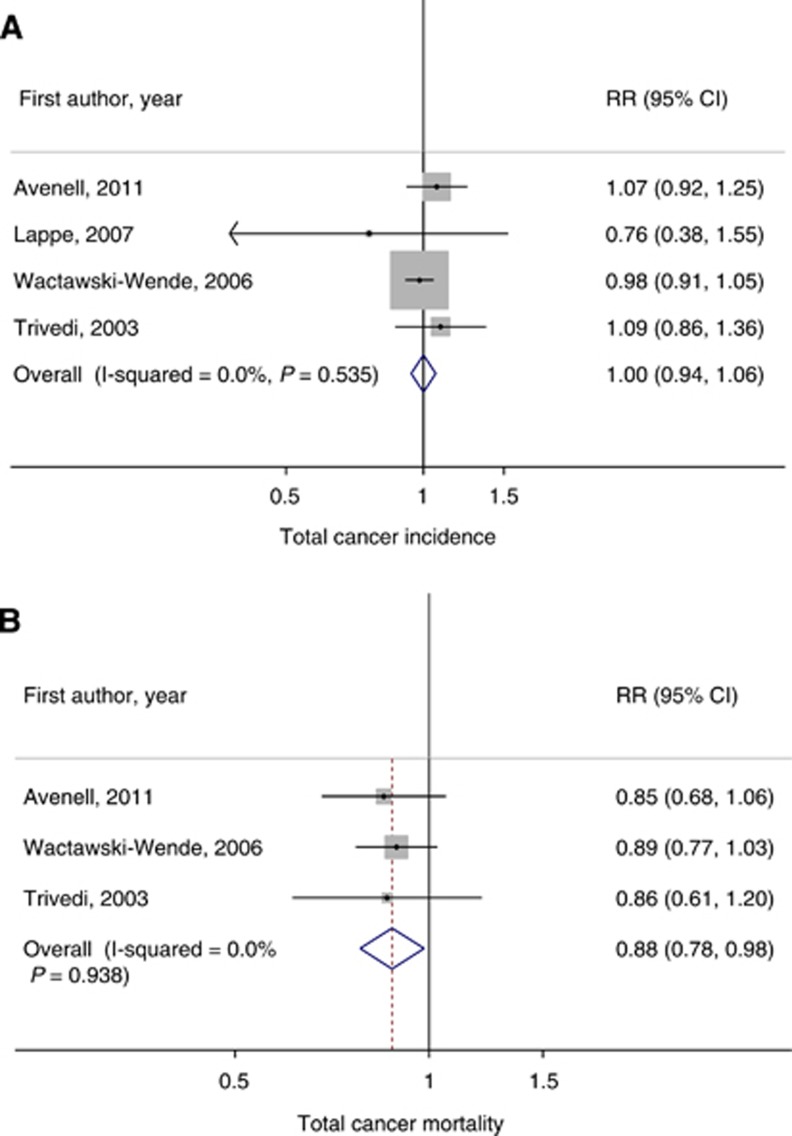
Over 2–7 years of duration, vitamin D supplementations had little effect on total cancer incidence (400–1100 IU per day, summary relative risk (RR)=1.00, 95% confidence interval (CI)=0.94–1.06, I2=0% four RCTs with combined 4333 cases), but significantly reduced total cancer mortality (400–833 IU per day, summary RR=0.88, 95% CI=0.78–0.98, I2=0%, three RCTs with combined 1190 deaths).
Conclusions:
Over 2–7 years of duration, the benefit of vitamin D supplementation may be limited to cancer mortality.
Keywords: vitamin D intake, vitamin D supplements, cancer incidence, cancer mortality, meta-analysis, randomised controlled trial
Based on an inverse association between region ultra-violet-B radiation and colorectal cancer mortality rates, Garland and Garland (1980) first proposed that vitamin D has anti-cancer properties. Subsequently, in numerous animal models, activation of the vitamin D pathway with calcitriol, the active component of vitamin D, or its analogues reduced tumour development and growth (Krishnan et al, 2010; Mehta et al, 2012; Pereira et al, 2012). To date, at least 15 types of cancers, especially colorectal and breast cancers, have been associated with low sun exposure (Grant and Garland, 2006). An inverse association has also been observed between pre-diagnostic circulating 25(OH)D and risk for colorectal cancer (Lee et al, 2011; Ma et al, 2011; Touvier et al, 2011). However, the association has been less consistent for other cancer types (Gallicchio et al, 2010; Gandini et al, 2011).
Several lines of evidence suggest that effects of vitamin D may be stronger for cancer mortality than for incidence. For example, higher ultra-violet-B exposure or other vitamin D surrogates such as predicted 25(OH)D score were more strongly associated with lower cancer mortality than with incidence (Boscoe and Schymura, 2006; Giovannucci et al, 2006; Chen et al, 2010). Also, cancer patients with higher pre-diagnostic 25(OH)D have lower risks of dying from prostate (Fang et al, 2011) and colorectal cancers (Ng et al, 2008; Fedirko et al, 2012). Finally, higher circulating 25(OH)D levels at the time of diagnosis or treatment were related to an improved survival from cancers of the breast (Goodwin et al, 2009), colorectum (Mezawa et al, 2010), prostate (Tretli et al, 2009), lung (Zhou et al, 2007), and melanoma (Newton-Bishop et al, 2009), though the association could be due to confounding by an unknown prognostic factor that predicts a poor prognosis and lowers circulating 25(OH)D levels.
Randomised controlled trials (RCTs) are considered the ‘gold standard' for establishing causality. To date, RCTs have not been conducted to examine the effect of vitamin D on cancer incidence or mortality as primary end points. However, there have been a small number of RCTs of 2–7 years of duration, involving moderate doses of supplemental vitamin D (400–1100 IU per day), and for reasonable numbers for total cancer incidence and mortality. Thus, we conducted meta-analyses of the RCTs of vitamin D supplementation and total cancer incidence and mortality.
Materials and methods
Two authors (EG and NK) participated in the literature search, study selection, and data extraction independently. Inconsistency between researchers was resolved through discussion.
PubMed and Embase were searched for studies published up to April 2014, using the following keywords and corresponding database thesaurus: vitamin D, cholecalciferol, ergocalciferol, cancer, tumour, carcinoma, neoplasm, carcinoma, and words beginning with ‘random'. The search was limited to english articles about humans and no other restrictions were imposed. Abstracts and unpublished results were not included. The reference lists of selected systematic reviews and meta-analyses, and all the articles included in our analysis were reviewed for additional papers.
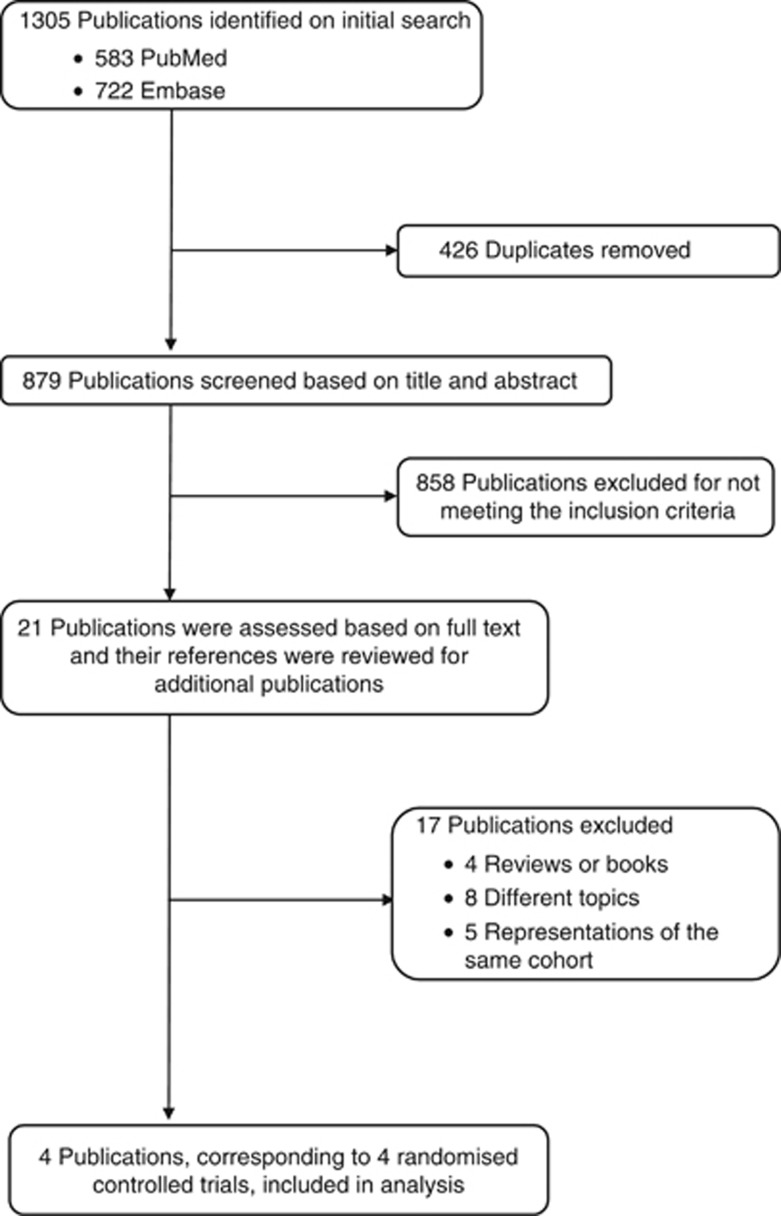
To be included, studies had to be a RCT providing information on the effect of vitamin D supplementation (with or without calcium supplementation) on total cancer incidence or mortality. When there were several publications from the same trial, the publication most fully covering the intervention period was selected. This study selection process is summarised in Figure 1.
Figure 1.
Flowchart of study selection.
The following information was extracted: definitions of interventions and control, most fully adjusted relative risk (RR) (risk ratio or hazard ratio) based on intention-to-treat analysis and 95% confidence interval (CI), level of serum 25(OH)D (at baseline, at follow-up), and relevant study characteristics (Table 1).
Table 1. Main characteristics of the RCTs included in meta-analyses.
| Authors, year, country | Trial name, population (% M), age at baseline | Trial duration | Contrast for RR | Incidence: RR (95% CI), (n case/n total) | Mortality: RR (95% CI), (n case/n total) | 25(OH)D level (nmol l−1): baseline→follow-up | Inclusion/exclusion criteria regarding supplement use |
|---|---|---|---|---|---|---|---|
|
Trivedi et al, 2003, UK |
Pilot community trial, general population (76%) w or w/o history of cancer, 65–85 years |
5 years |
Vit D3 vs placebo Vit D3: 100 000 IU per 4 m (∼833 IU per day) |
1.09 (0.86, 1.36) (188/1345) vs (173/1341) |
0.86 (0.61, 1.20) (63/1345) vs (72/1341) |
Intervention: NA→74.4 at 4 years Control: NA→53.4 at 4 years |
Excluded Vit D supplement users |
|
Wactawski-Wende et al, 2006, USA |
WHI, postmenopausal women (0%) w or w/o history of cancer, 50–79 years |
7 years |
Vit D3+Ca vs placebo Vit D3: 400 IU per day Ca (carbonate): 1000 mg per day |
0.98 (0.91, 1.05) (1634/18 176) vs (1655/18 106) |
0.89 (0.77, 1.03) (344/18 176) vs (382/18 106) |
Intervention: 42 (median)→a54 at 2 years Control: 42 (median)→NA |
Allowed for non-protocol supplement of Vit D up to 600 IU per day; of Ca up to 1000 mg per day |
|
Lappe et al, 2007, USA |
Population-based trial, postmenopausal women (0%) w/o cancer at baseline, 66.7 years (7.3) |
4 years |
Vit D3+Ca vs Ca Vit D3: 1100 IU per day Ca: carbonate 1500 mg per day or citrate 1400 mg per day |
0.76 (0.38, 1.55) (13/446) vs (17/445) |
NA |
Intervention: 71.8→96 at 1 year Control: 71.6→71 at 1 year |
Not specified |
| Avenell et al, 2011, UK | RECORD general population (15%) w/o cancer likely to metastasise to bone within 10 years prior to baseline, 77.2 years (6) | 2–5.2 years | Vit D3 (w, w/o Ca) vs no Vit D3 (w, w/o Ca) Vit D3: 800 IU per day Ca (carbonate): 1000 mg per day | 1.07 (0.92, 1.25) (338/2649) vs (315/2643) | 0.85 (0.68, 1.06) (151/2649) vs (178/2643) | Intervention: 38→62 at 1 year Control: 38→43.6 at 1 year | Excluded supplement users of >200 IU per day of Vit D; >500 mg per day of Ca |
Abbreviations: Ca=calcium; CI=confidence interval; M=male; m=month; n=number; NA=not available; RR=relative risk; Vit=vitamin; w=with; w/o=without.
Estimated based on the statement that serum 25(OH)D level was 28% higher in the intervention group at 2 years after randomisation.
For statistical analyses, the summary RR and 95% CI were calculated using a random effects model. Heterogeneity across studies was assessed by Q test and I2 (Higgins and Thompson, 2002). Potential for small-study effects, such as publication bias, was assessed using Egger's test (Egger et al, 1997) and Begg's test (Begg and Mazumdar, 1994). Sensitivity analysis was performed by excluding the Women's Health Initiative (WHI), as its comparison of combined vitamin D and calcium supplementation against placebo does not allow for testing an independent effect of vitamin D supplementation. For statistical significance, two-sided α was set at P=0.05. All statistical analyses were conducted using STATA 12 (StataCorp, College Station, TX, USA).
Results
Vitamin D supplementation and total cancer incidence
Four RCTs were included in the meta-analysis (4333 cases, 45 151 participants) (Table 1) (Trivedi et al, 2003; Wactawski-Wende et al, 2006; Lappe et al, 2007; Avenell et al, 2012). The summary RR for intervention vs control group was 1.00 (P=0.998, 95% CI=0.94–1.06) with no evidence of heterogeneity (I2=0%, Pheterogeneity=0.54) (Figure 2A). In a sensitivity analysis excluding WHI (Wactawski-Wende et al, 2006), the summary RR was 1.06 (P=0.33, 95% CI=0.94–1.21, I2=0%, Pheterogeneity=0.63). Small-study effects, such as publication bias, were not indicated in both primary (PEgger=0.84, PBegg>0.999) and sensitivity analyses (PEgger=0.32, PBegg=0.60).
Figure 2.
Meta-analyses of RCTs of vitamin D supplementation and total cancer incidence and mortality. (A) Total cancer incidence, (B) total cancer mortality. Abbreviations: CI=confidence interval; RR=relative risk.
Vitamin D supplementation and total cancer mortality
Three RCTs were included in the meta-analysis (1190 deaths, 44 260 participants) (Table 1) (Trivedi et al, 2003; Wactawski-Wende et al, 2006; Avenell et al, 2012). The summary RR for intervention vs control groups was 0.88 (P=0.02, 95% CI=0.78–0.98) with no evidence of heterogeneity (I2=0%, Pheterogeneity=0.94) (Figure 2B). In a sensitivity analysis excluding WHI (Wactawski-Wende et al, 2006), the summary RR was 0.85 (P=0.09, 95% CI=0.71–1.03, I2=0%, Pheterogeneity=0.96). Small-study effects, such as publication bias, were not indicated in both primary (PEgger=0.45, PBegg=0.60) and sensitivity analyses (PEgger=not available, PBegg=0.32).
Discussion
Our results suggest that vitamin D supplementation at doses of up to 800 IU per day and attaining 25(OH)D levels of approximately 54–75 nmol l−1 is unlikely to have an appreciable effect on cancer incidence within 2–7 years. The RCT by Lappe et al (2007) which used 1100 IU per day did indicate a potential short-term effect on incidence, but was based on only 30 cases. Two larger UK studies (Trivedi et al, 2003; Avenell et al, 2012) (with 1104 combined cases) were of comparable duration, used slightly lower doses (800–833 IU per day) and achieved comparable increments of 25(OH)D within the intervention groups (24 nmol l−1) (Avenell et al, 2012) or contrasts between intervention and control group (21 nmol l−1) (Trivedi et al, 2003) as did the Lappe study (1100 IU per day; 24.2 nmol l−1 increment; 25 nmol l−1 contrast). Yet these studies indicated no comparable effect on total cancer incidence. Because the Lappe study population had higher baseline 25(OH)D levels and the dose was slightly higher, the attained 25(OH)D level of 96 nmol l−1 was higher than that in the two null studies (74.4 and 62 nmol l−1) (Trivedi et al, 2003; Avenell et al, 2012). The WHI, the largest study, showed a negligible effect on incidence, but the increment was smaller (12 nmol l−1) and the estimated attained median level of 25(OH)D was only 54 nmol l−1. A recent article that reviewed 20 meta-analyses of observational studies on cancer outcomes also concluded that circulating 25(OH)D or 1,25(OH)2D concentrations are unlikely to be related to cancer incidence (Theodoratou et al, 2014).
Unlike cancer incidence, vitamin D supplementation was related to a statistically significant 12% reduction in cancer mortality. Publication bias was unlikely as we did not identify comparably large studies that had data on cancer mortality. Furthermore, unlike for cancer incidence, an inverse relationship was consistently observed in all the studies included in the meta-analysis on cancer mortality. A recent meta-analysis also found a statistically significant inverse association between circulating 25(OH)D levels and cancer mortality, based on 12 primary prevention cohort studies in which reverse causation is less likely than secondary prevention cohort studies (Chowdhury et al, 2014). Animal models support mechanisms whereby vitamin D status may influence processes such as metastasis (Krishnan et al, 2010; Mehta et al, 2012; Pereira et al, 2012), which could affect mortality.
Our analyses inherit the limitations of the available RCTs to examine many facets of the vitamin D-cancer hypothesis, in terms of doses, attained 25(OH)D levels, duration, and effects on specific cancer types. Further, only one of the four RCTs included in the meta-analysis examined vitamin D-only supplementation. The remaining three RCTs added calcium in their intervention regimes; while WHI could not distinguish an independent effect of vitamin D, calcium was balanced between the vitamin D and non-vitamin D groups in the two other trials. Yet, since the effect of vitamin D was tested in calcium-replete populations, our findings might not be generalisable to populations with a low calcium intake as calcium may be a modifier. Lastly, despite that meta-analysis generally enhances statistical power, considering that total cancer incidence and mortality were not the primary end points in the RCTs included, our meta-analysis might have had inadequate power to detect a meaningful association. Nevertheless combining RCTs allowed us to examine an interesting range of attained 25(OH)D levels for total cancer incidence and mortality over a period ranging from 2–7 years. The results should not be generalised beyond these limits.
Our findings may have implications for future research. Increasing 25(OH)D levels to a range of 75 nmol l−1 is unlikely to influence total cancer incidence within 5 years. Whether attaining higher levels in the range of 90–100 nmol l−1 would reduce incidence remains unclear. Ongoing RCTs of relatively high doses will be able to test this hypothesis. Additionally, some interventions may require more than 5 years to elicit a substantial reduction in cancer incidence, as demonstrated for aspirin and colorectal cancer (Rothwell et al, 2010). Because very long-term RCTs may be unfeasible, further observational studies addressing long-term effects may provide useful information on the required duration.
It is unclear whether the potential benefit for vitamin D status on cancer mortality operates in the pre-diagnostic stages by influencing tumour aggressive behaviour and metastatic seeding, during treatment through interactions with therapies, in post-diagnostic stages by improving survival, or during multiple stages. Indeed, the RCTs did not exclude people with a prior history of cancer and the benefit was observed in such mixed populations. Given the high prevalence of vitamin D deficiency at the time of diagnosis, RCTs could feasibly be conducted in which high doses of vitamin D are provided to rapidly increase vitamin D stores at the time shortly before treatment to test the hypothesis that vitamin D status may favourably interact with treatment or be protective for survival. RCTs over a period of 5 years or so, such as VITAL, will be able to test whether vitamin D intervention begun in the pre-diagnostic period can reduce cancer mortality.
In the UK, approximately 159 000 people die of cancer annually, so a 15% reduction would result in a substantial number of potentially preventable deaths from cancer. Although not definitive, these data offer some support of attaining 25(OH)D levels of at least 75 nmol l−1, as has been recommended by the Endocrine Society (Holick et al, 2011). There is no credible evidence of harmful effects of vitamin D in this range of exposure (Bischoff-Ferrari et al, 2010). Whether attaining considerably higher levels would provide further benefits on cancer currently cannot be addressed adequately based on the available RCT data.
The authors declare no conflict of interest.
References
- Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA. Long-term follow-up for mortality and cancer in a randomised placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial) J Clin Endocrinol Metab. 2012;97:614–622. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2010;21:1121–1132. doi: 10.1007/s00198-009-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Clements M, Rahman B, Zhang S, Qiao Y, Armstrong BK. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control. 2010;21:1701–1709. doi: 10.1007/s10552-010-9599-1. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, Feldman BS, Pan A, Johnson L, Crowe F, Hu FB, Franco OH. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. doi: 10.1136/bmj.g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Kasperzyk JL, Shui I, Hendrickson W, Hollis BW, Fall K, Ma J, Gaziano JM, Stampfer MJ, Mucci LA, Giovannucci E. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PLoS One. 2011;6:e18625. doi: 10.1371/journal.pone.0018625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirko V, Riboli E, Tjonneland A, Ferrari P, Olsen A, Bueno-de-Mesquita HB, van Duijnhoven FJ, Norat T, Jansen EH, Dahm CC, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Racine A, Lukanova A, Teucher B, Boeing H, Aleksandrova K, Trichopoulou A, Benetou V, Trichopoulos D, Grioni S, Vineis P, Panico S, Palli D, Tumino R, Siersema PD, Peeters PH, Skeie G, Brustad M, Chirlaque MD, Barricarte A, Quiros JR, Sanchez MJ, Dorronsoro M, Bonet C, Palmqvist R, Hallmans G, Key TJ, Crowe F, Khaw KT, Wareham N, Romieu I, McKay J, Wark PA, Romaguera D, Jenab M. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in Western European populations. Cancer Epidemiol Biomarkers Prev. 2012;21:582–593. doi: 10.1158/1055-9965.EPI-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicchio L, Helzlsouer KJ, Chow WH, Freedman DM, Hankinson SE, Hartge P, Hartmuller V, Harvey C, Hayes RB, Horst RL, Koenig KL, Kolonel LN, Laden F, McCullough ML, Parisi D, Purdue MP, Shu XO, Snyder K, Stolzenberg-Solomon RZ, Tworoger SS, Varanasi A, Virtamo J, Wilkens LR, Xiang YB, Yu K, Zeleniuch-Jacquotte A, Zheng W, Abnet CC, Albanes D, Bertrand K, Weinstein SJ. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:10–20. doi: 10.1093/aje/kwq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer. Int J Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26:2687–2699. [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Trump DL, Johnson CS, Feldman D. The role of vitamin D in cancer prevention and treatment. Endocrinol Metab Clin North Am. 2010;39:401–418. doi: 10.1016/j.ecl.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomised trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- Lee JE, Li H, Chan AT, Hollis BW, Lee IM, Stampfer MJ, Wu K, Giovannucci E, Ma J. Circulating levels of vitamin D and colon and rectal cancer: the Physicians' Health Study and a meta-analysis of prospective studies. Cancer Prev Res. 2011;4:735–743. doi: 10.1158/1940-6207.CAPR-10-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–3782. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- Mehta RG, Peng X, Alimirah F, Murillo G, Mehta R. Vitamin D and breast cancer: Emerging concepts. Cancer Lett. 2012;334 (1:95–100. doi: 10.1016/j.canlet.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Mezawa H, Sugiura T, Watanabe M, Norizoe C, Takahashi D, Shimojima A, Tamez S, Tsutsumi Y, Yanaga K, Urashima M. Serum vitamin D levels and survival of patients with colorectal cancer: Post-hoc analysis of a prospective cohort study. BMC Cancer. 2010;10:347. doi: 10.1186/1471-2407-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Bishop JA, Beswick S, Randerson-Moor J, Chang YM, Affleck P, Elliott F, Chan M, Leake S, Karpavicius B, Haynes S, Kukalizch K, Whitaker L, Jackson S, Gerry E, Nolan C, Bertram C, Marsden J, Elder DE, Barrett JH, Bishop DT. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, Fuchs CS. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26:2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- Pereira F, Larriba MJ, Munoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012;19:R51–R71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Riboli E, Hercberg S, Norat T. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- Tretli S, Hernes E, Berg JP, Hestvik UE, Robsahm TE. Association between serum 25(OH)D and death from prostate cancer. Br J Cancer. 2009;100:450–454. doi: 10.1038/sj.bjc.6604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. Br Med J. 2003;326:469–475. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- Zhou W, Heist RS, Liu G, Asomaning K, Neuberg DS, Hollis BW, Wain JC, Lynch TJ, Giovannucci E, Su L, Christiani DC. Circulating 25-hydroxyvitamin d levels predict survival in early-stage non-small-cell lung cancer patients. J Clin Oncol. 2007;25:479–485. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]