Abstract
In the present study, cultured human SHG-44 glioma cells were subjected to a hypoxic environment simulated using the CoCl2 method. Flow cytometry showed increased reactive oxygen species production in these cells. Real-time reverse transcription-PCR showed significantly increased hypoxia-inducible factor-1α mRNA expression in cells exposed to the hypoxic condition. The antioxidant N-acetylcysteine significantly inhibited reactive oxygen species production and reduced hypoxia-inducible factor-1α mRNA expression in normoxic and hypoxic groups, especially in the latter group. These findings indicate that hypoxia induces reactive oxygen species production and hypoxia-inducible factor-1α mRNA expression in human SHG-44 glioma cells, and that the antioxidant N-acetylcysteine can inhibit these changes.
Keywords: neural regeneration, basic research, central nervous system, reactive oxygen species, hypoxia-inducible factor-1α, hypoxia, N-acetylcysteine, glioma, antioxidant, photographs-containing paper, neuroregeneraion
Research Highlights
(1) Hypoxia induced reactive oxygen species production and hypoxia-inducible factor-1α mRNA expression in human SHG-44 glioma cells.
(2) Antioxidant N-acetylcysteine inhibited reactive oxygen species production under both normoxic and hypoxic conditions.
(3) N-acetylcysteine inhibited hypoxia-inducible factor-1α mRNA expression.
INTRODUCTION
Glioma is the most common primary intracranial malignant tumor. It is very important to explore the target during treatment. In the progression of glioma, there is rapid growth and proliferation, which requires substantial oxygen consumption; however, oxygen supply is limited by the lack of sufficient vascularization[1]. In the core of the tumor, cells are in a hypoxic environment, which induces high expression of hypoxia-inducible factor-1α[2].
Glioma cells regulate the expression of hypoxic stress genes to promote survival under hypoxia, enhancing the transcription and expression of numerous factors. This adaptive response allows cells to maintain the growth, metabolism and invasiveness of the tumor[3,4,5]. Thus, the regulation of hypoxia-inducible factor-1α is critical for the ability of the glioma to adapt to hypoxia.
Consequently, the protein is a major therapeutic target. Some studies have shown that hypoxia-inducible factor-1α can activate many target genes during hypoxia and induce downstream effector production to adapt to hypoxic stress[6]. Expression of hypoxia-inducible factor-1α is influenced by many factors[7]. Reactive oxygen species are one of the factors influencing hypoxia-inducible factor-1α expression[8,9].
However, the regulation of hypoxia-inducible factor-1α by reactive oxygen species in gliomas is unclear, and the underlying mechanisms are not well known. In the present study, we investigated hypoxia-inducible factor-1α expression and reactive oxygen species changes under normoxia (regular culture) and hypoxia (CoCl2 -induced chemical hypoxia) using cultured human SHG-44 glioma cells. We also examined the effect of the anti-oxidant N-acetylcysteine. Our results further our understanding of the biological behavior of gliomas and provide insight for the development of novel treatment strategies.
RESULTS
Morphology of SHG-44 glioma cells
After SHG-44 glioma cells were cultured for 72 hours, they were arranged in an orderly manner, with no cell aggregates or lysis. After N-acetylcysteine treatment, cell arrangement became disordered, with no cell aggregates. However, cell aggregates were detected in hypoxia-treated SHG-44 glioma cells, accompanied by some floating lysed cells (Figure 1).
Figure 1.
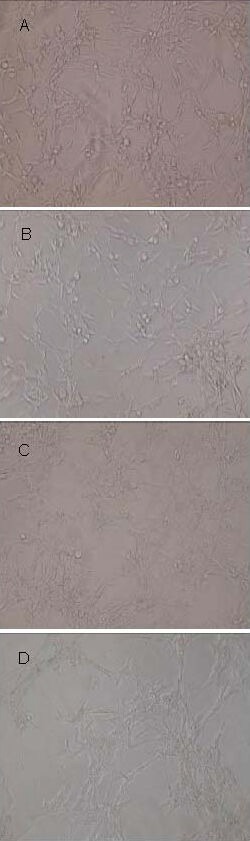
Morphology of human SHG-44 glioma cells after culture for 72 hours (light microscope, × 200).
(A) In the normoxic control group, cells were arranged in an orderly manner.
(B) In the normoxic + NAC group, cells were arranged in an orderly manner.
(C) In the hypoxic control group, cell arrangement was disorderly, with some floating lysed cells.
(D) In the hypoxic + NAC group, cell aggregates formed, with some floating lysed cells.
NAC: N-acetylcysteine.
Reactive oxygen species expression in hypoxic condition
Intracellular reactive oxygen species levels can be assessed indirectly by detecting fluorescent 2’,7’-dichlorofluorescein with flow cytometry[10]. Comparison using variance analysis (the data tested met the assumption of the homogeneity of variance) showed significant differences in the levels of reactive oxygen species among the hypoxic control, normoxic + N-acetylcysteine, hypoxic + N-acetylcysteine and normoxic control groups (P < 0.05). The levels in the normoxic control group were significantly lower than in the hypoxic control group (P < 0.05); those in the normoxic + N-acetylcysteine group were lower than in the normoxic control group (P < 0.05); those in the hypoxic + N-acetylcysteine group were lower than in the hypoxic control group (P < 0.05; Figures 2, 3).
Figure 2.
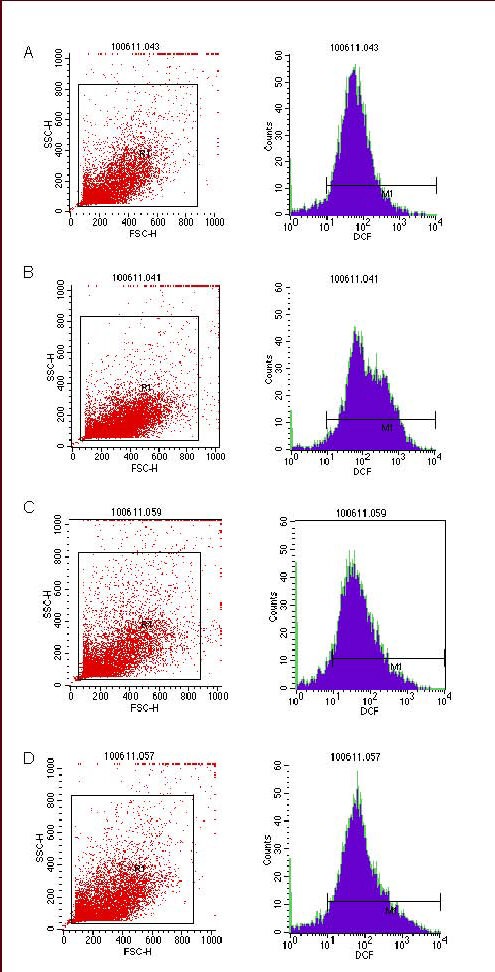
Levels of reactive oxygen species in SHG-44 glioma cells in hypoxic condition detected by flow cytometry (red: cell count detected by flow cytometry; blue: levels of reactive oxygen species).
(A) Normoxic control group; (B) hypoxic control group; (C) normoxic + NAC group; (D) hypoxic + NAC group.
NAC: N-acetylcysteine.
Figure 3.
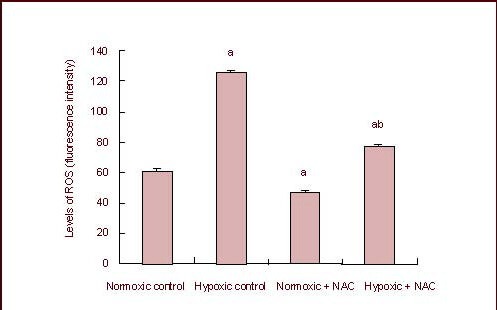
Levels of reactive oxygen species (ROS) in SHG-44 glioma cells in hypoxic condition.
Data are expressed as mean ± SD of six independent experiments. aP < 0.05, vs. normoxic control group; bP < 0.05, vs. hypoxic control group (analysis of variance followed by least significant difference t-test).
NAC: N-acetylcysteine.
Hypoxia-inducible factor-1α mRNA expression in SHG-44 glioma cells
All the procedures for the real-time reverse transcription-PCR experiment were performed in accordance with the instructions provided with the reagents, and the comparative Ct method (2-ΔΔCt) was used for statistical analysis of the raw data from the experiment. 2-ΔΔCt values for each group are shown in Figure 4, and the melting curve and amplification curve of hypoxia-inducible factor-1α and β-actin are shown in Figure 5.
Figure 4.
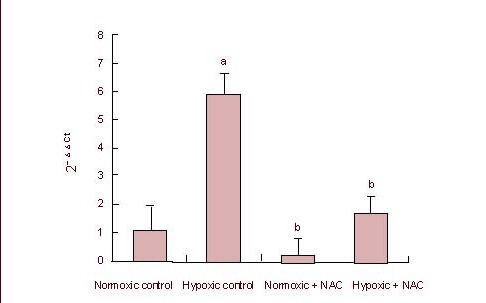
Hypoxia-inducible factor 1α mRNA expression in SHG-44 glioma cells in hypoxic condition.
Data are expressed as mean ± SD of six independent experiments. aP < 0.05, vs. normoxic control group; bP < 0.05, vs. hypoxic control group (analysis of variance followed by least significant difference t-test).
NAC: N-acetylcysteine.
Figure 5.
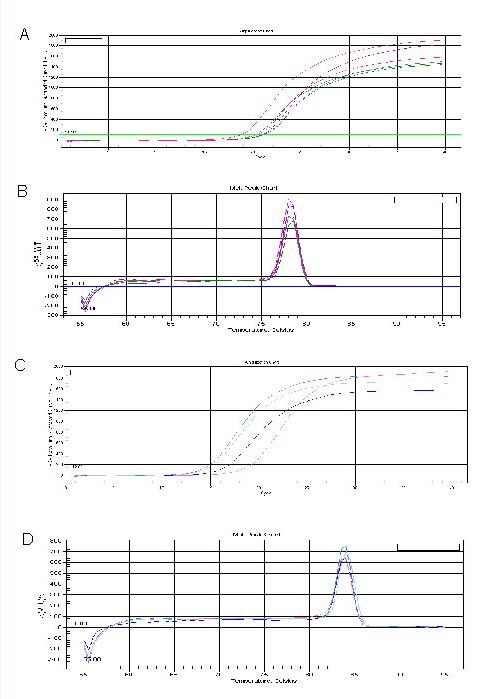
Melting and amplification curves for hypoxia-inducible factor-1α and β-actin.
(A, B) The amplification and melting curves for hypoxia-inducible factor-1α.
(C, D) The amplification and melting curves for β-actin.
The comparison using variance analysis (the data tested met the assumption of the homogeneity of variance) showed that there were statistical differences in the data among the hypoxic control, normoxic + N-acetylcysteine, hypoxic + N-acetylcysteine and normoxic control groups (P < 0.05). Hypoxia-inducible factor-1α mRNA expression in the hypoxic control group was significantly higher than in the normoxic control, hypoxic + N-acetylcysteine and normoxic + N-acetylcysteine groups (P < 0.05); hypoxia-inducible factor-1α mRNA expression in the normoxic control group was higher than in the normoxic + N-acetylcysteine group, but without a significant difference (P > 0.05). Hypoxia-inducible factor-1a mRNA expression was higher in the hypoxic + N-acetylcysteine group compared with the normoxic + N-acetylcysteine group, with no significant difference (P > 0.05).
DISCUSSION
Hypoxia-inducible factor-1 is a hypoxia-inducible transcription factor with DNA binding activity, and it was extracted and isolated from anoxic Hep3 nuclei by Wang and Semenza for the first time in 1992[11]. To respond to the hypoxic microenvironment, hypoxia-inducible factor-1 regulates a variety of target genes involved in cellular adaptation and survival to hypoxic stress, increasing their expression and enhancing cell survival[12].
The expression of hypoxia-inducible factor-1β is persistent and stable in the cytoplasm and is not affected by hypoxia or other factors. In addition, the regulation by hypoxia-inducible factor-1β of hypoxia-inducible factor-1α is divided into regulation of degradation level and regulation of transcriptional level[13]. There are more studies on the former at present, especially studies on the pHDs-pVHL-ubiquitin-proteasome pathway[14]. In addition, other degradation pathways have also been identified, including the pVHL-dependent ubiquitin-proteasome pathway induced by proline hydroxylase and promoted by OS-9[15]. Very few studies have focused on the regulation of hypoxia-inducible factor-1α transcriptional level. It was found by Schnitzer et al[16] that GSK3β can indirectly decrease hypoxia-inducible factor-1α mRNA expression by inhibiting the transcription initiation factor eIF2B; an inhibitor of GSK3β (Indirubin) could significantly improve expression levels.
Galanis et al[17] reported that the increase in reactive oxygen species in tumor cells may be an important factor in hypoxia-inducible factor-1α regulation. Reactive oxygen species may function as a second messenger in signal transduction to impact the transcription of genes, initiating a variety of cell biological effects[18]. Reactive oxygen species are produced by the reaction of electrons (generated by the mitochondrial electron transport chain) with ground-state oxygen passing through the transport chain. Oxygen content is relatively low inside tumor tissues due to uncontrolled growth, and the electron transport chain of mitochondrial aerobic respiration may be blocked to generate reactive oxygen species[19]. The present study confirmed that the levels of reactive oxygen species in SHG-44 glioma cells cultured under hypoxic condition were significantly higher than in those cultured under normoxic condition. However, the levels of reactive oxygen species in the two groups decreased significantly after treatment with the antioxidant N-acetylcysteine.
The study by Koshikawa et al[20] revealed that reactive oxygen species may affect the expression of hypoxia-inducible factor-1α by activating the PI3K-PKB/Akt pathway. The PI3K-PKB/Akt pathway cannot directly regulate the expression or stability of hypoxia-inducible factor-1α, but it can indirectly regulate expression because it has numerous downstream targets. mTOR can initiate the translation of hypoxia-inducible factor-1α by changing the phosphorylation status of the translation regulatory factor (eukaryotic initiation factor 4E-binding protein) and of p70S6 kinase. But a downstream target of hypoxia-inducible factor-1α, REDD1, can inhibit the activity of mTOR to downregulate the expression level of hypoxia-inducible factor-1α, which is also a negative feedback regulatory mechanism of hypoxia-inducible factor-1α itself[21]. Emerling et al[22] reported that the activation of p38MAPK under hypoxic condition may depend on reactive oxygen species generated by mitochondria, which can promote the phosphorylation of the serine and threonine residues of MAPK-upstream kinase to activate the p38MAPK signaling pathway[23]. The expression levels of hypoxia-inducible factor-1α mRNA under hypoxic condition were higher than those under normoxic condition, but the expression levels of hypoxia-inducible factor-1α mRNA declined after treatment with N-acetylcysteine, an antioxidant.
Results from the present study showed that the expression levels of reactive oxygen species in glioma cells cultured under hypoxic condition were significantly higher than those under normoxic condition, while the expression levels of reactive oxygen species under hypoxic and normoxic conditions declined significantly after N-acetylcysteine treatment. Furthermore, the expression levels of hypoxia-inducible factor-1α mRNA under hypoxic condition were higher than those under normoxic condition, but the expression levels of hypoxia-inducible factor-1a mRNA in SHG-44 glioma cells under normoxic and hypoxic conditions both declined after N-acetylcysteine treatment. These findings suggest that reactive oxygen species promote the expression of hypoxia-inducible factor-1α mRNA, and that N-acetylcysteine can block this effect. The present study provides novel insight into glioma biology, and lays the foundation for further research on the application of the antioxidant N-acetylcysteine for the treatment of gliomas.
MATERIALS AND METHODS
Design
In vitro comparative observation of cytology.
Time and setting
The experiment was performed in the Medical School of Xi’an Jiaotong University, China in July 2010.
Materials
Human glioma cell line SHG-44 was provided by Shanghai Institute of Cell Biology, Chinese Academy of Sciences.
Methods
SHG-44 cell culture and passaging
Human glioma cell lines SHG-44 were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA, USA) containing 10% calf serum (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, China) at 37°C in a 5% CO2 incubator for passage after 48 hours (1:2).
CoCl2-simulated hypoxia
CoCl2 solution (the final concentration was 150 μM; Sigma, St. Louis, MO, USA) was added into DMEM in which the human glioma cell line SHG-44 was cultured routinely in the 5% CO2 incubator to block oxygen signal transduction to simulate hypoxic signaling[24].
Grouping and intervention
SHG-44 glioma cells of passage three were collected and seeded at 4 × 104 cells/mL. In the normoxic control group, SHG-44 glioma cells were cultured in normoxia (5% CO2) at 37°C for 72 hours. In the normoxic + N-acetylcysteine group, SHG-44 glioma cells were cultured in normoxia (5% CO2) at 37°C for 24 hours and then treated with N-acetylcysteine (10 mM; Sigma) and cultured for another 48 hours. In the hypoxic control group, SHG-44 glioma cells were cultured in hypoxia (CoCl2 150 μM) at 37°C for 72 hours. In the hypoxic + N-acetylcysteine group, SHG-44 glioma cells were cultured in hypoxia (CoCl2 150 μM) at 37°C for 24 hours and then treated with N-acetylcysteine (10 mM) and cultured for another 48 hours.
CoCl2 and/or N-acetylcysteine were added according to the preset experimental groups. A 6-well plate and a double-well slide were included in each group. SHG-44 glioma cells in exponential growth phase were harvested to prepare a cell suspension of 4 × 104 cells/mL. They were inoculated into 6-well plates (3 mL in each well) for each group. A 1 mL volume of DMEM containing CoCl2 (600 mM) was added to each well of the hypoxic control and hypoxic + N-acetylcysteine groups on the next day so that the final concentration of CoCl2 was 150 μM. The same procedure was performed for the normoxic control and hypoxia + N-acetylcysteine groups, except that DMEM containing CoCl2 was replaced with DMEM. The cell suspensions were diluted to 2 × 104 cells/mL and then inoculated into double-well slides (200 μL in each well) for each group. The supernatant was discarded carefully by suction and 200 μL DMEM containing CoCl2 (150 μM) was added to each well of the hypoxic control and hypoxic + N-acetylcysteine groups on the next day. The same procedure was performed for the normoxic control and normoxic + N-acetylcysteine groups, except that DMEM containing CoCl2 was replaced with DMEM. After the cells in all groups were cultured for 24 hours, N-acetylcysteine (the inhibitor of reactive oxygen species) at a final concentration of 10 mM was added into each well of the normoxic + N-acetylcysteine and hypoxic + N-acetylcysteine groups. The cells in each of these groups were cultured for an additional 48 hours.
Flow cytometry for reactive oxygen species expression
After the fluorescent probe (2′,7′-dichlorofluorescin diacetate [DCFH-DA], 10 mM; Beyotime Institute of Biotechnology, Beijing, China) was prepared, a 5 μL aliquot was added to 5 mL of serum-free DMEM (1:1 000). Cells were digested with 0.25% trypsin (Sigma) and dispersed with a pipette. They were then transferred into a 10 mL centrifuge tube and centrifuged at 1 000 r/min for 5 minutes. The supernatant was discarded, the pellet was centrifuged once more, and the supernatant was discarded again. 500 μL fluorescent probe DCFH-DA diluted with serum-free medium (1:1 000) was added into each well, mixed and transferred to a 1.5 mL EP tube for incubation in a cell incubator at 37°C for 20 minutes so that DCFH-DA which had entered into the cells was oxidized to fluorescent DCF by the reactive oxygen species. After the incubation was complete, the cells were washed three times with serum-free cell culture medium (centrifuged at 1 000 r/min for 5 minutes) to fully remove extracellular DCFH-DA, and then the fluorescence of DCF was detected with flow cytometry (BD FACSCalibur, San Jose, CA, USA) using excitation and emission wavelengths of 488 nm and 525 nm, respectively, to detect intracellular reactive oxygen species levels.
Real-time reverse transcription-PCR detection of hypoxia-inducible factor-1 α mRNA expression
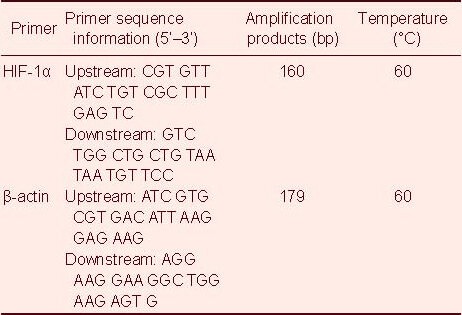
After culture, the cells from each 6-well plate of the four groups were triturated with a pipette and then transferred into 1.5 mL EP tubes (in an ice bath) to rapidly extract RNA using an RNA extraction kit (RNAfast200; Fermentas, Vilnius, Lithuania). 5 μL total RNA was extracted, and mRNA was used as the template to synthesize cDNA by reverse transcription. Then 1 μL reverse transcription reaction mixture (cDNA) was used as the template for amplification reactions. Primer design for hypoxia-inducible factor-1α and β-actin was performed using Primer 5.0 software, and primers were synthesized by Beijing Sunbiotech Co., Ltd., China (Table 1).
Table 1.
Primer sequences for hypoxia-inducible factor-1α (HIF-1α) and β-actin
Total reaction volume was 25 μL for each sample and included the following: ddH2O, 10.5 μL; SYBR® Green Realtime PCR Master Mix (Fermentas), 12.5 μL; upstream primer (10 μM), 0.5 μL; downstream primer (10 μM), 0.5 μL; cDNA by reverse transcription, 1 μL. PCR conditions: 50°C for 2 minutes for 1 cycle and 95°C for 10 minutes for 1 cycle; then 40 cycles of 95°C for 15 seconds, 60°C for 30 seconds and 72°C for 30 seconds. The products were placed into a Bio-Rad iQTM5 Multiple Real-time PCR Instrument (Hercules, CA, USA) after setting the reaction conditions, and the experimental data were analyzed while the reaction proceeded. Ct value was obtained, and results were obtained using 2-ΔΔCt calculation. β-actin was used as reference.
Statistical analysis
The statistical software SPSS 13.0 (SPSS, Chicago, IL, USA) was used for statistical analysis of the experimental data (test level α = 0.05). Intergroup comparison was performed using analysis of variance, and paired comparison was conducted using the least significant difference t-test. A value of P < 0.05 indicated a significant difference.
Footnotes
Conflicts of interest: None declared.
(Edited by Zhou YX, Liu M/Su LL/Wang L)
REFERENCES
- [1].Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- [2].Serganova I, Doubrovin M, Vider J, et al. Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res. 2004;64(17):6101–6108. doi: 10.1158/0008-5472.CAN-04-0842. [DOI] [PubMed] [Google Scholar]
- [3].Qiang L, Wu T, Zhang HW, et al. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2012;19(2):284–294. doi: 10.1038/cdd.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zagzag D, Lukyanov Y, Lan L, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86(12):1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- [5].Eckerich C, Zapf S, Fillbrandt R, et al. Hypoxia can induce c-Met expression in glioma cells and enhance SF/HGF-induced cell migration. Int J Cancer. 2007;121(2):276–283. doi: 10.1002/ijc.22679. [DOI] [PubMed] [Google Scholar]
- [6].Lu H, Li Y, Shu M, et al. HIF-1alpha blocks differentiation of malignant gliomas. FEBS J. 2009;276(24):7291–7304. doi: 10.1111/j.1742-4658.2009.07441.x. [DOI] [PubMed] [Google Scholar]
- [7].Lee JW, Bae SH, Jeong JW, et al. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36(1):1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- [8].Hsieh CH, Lee CH, Liang JA, et al. Cycling hypoxia increases U87 glioma cell radioresistance via ROS induced higher and long-term HIF-1 signal transduction activity. Oncol Rep. 2010;24(6):1629–1636. doi: 10.3892/or_00001027. [DOI] [PubMed] [Google Scholar]
- [9].Hsieh CH, Shyu WC, Chiang CY, et al. NADPH oxidase subunit 4-mediated reactive oxygen species contribute to cycling hypoxia-promoted tumor progression in glioblastoma multiforme. PLoS One. 2011;6(9):e23945. doi: 10.1371/journal.pone.0023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kumar S, Patel S, Jyoti A, et al. Nitric oxide-mediated augmentation of neutrophil reactive oxygen and nitrogen species formation: Critical use of probes. Cytometry A. 2010;77(11):1038–1048. doi: 10.1002/cyto.a.20975. [DOI] [PubMed] [Google Scholar]
- [11].Wang GL, Semenza GL. Characterization of hypoxia inducible factor 1 and regulation of DNA blinding activity by hypoxia. J Biol Chem. 1993;268(29):21513–21518. [PubMed] [Google Scholar]
- [12].Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33(11):526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [13].Kizaka-Kondoh S, Tanaka S, Harada H, et al. The HIF-1-active microenvironment: an environmental target for cancer therapy. Adv Drug Deliv Rev. 2009;61(7-8):623–632. doi: 10.1016/j.addr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [14].Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- [15].Baek JH, Mahon PC, Oh J, et al. OS-9 interacts with hypoxia-inducible factor 1alpha and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1alpha. Mol Cell. 2005;17(4):503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [16].Schnitzer SE, Schmid T, Zhou J, et al. Inhibition of GSK3beta by indirubins restores HIF-1alpha accumulation under prolonged periods of hypoxia/anoxia. FEBS Lett. 2005;579(2):529–533. doi: 10.1016/j.febslet.2004.12.023. [DOI] [PubMed] [Google Scholar]
- [17].Galanis A, Pappa A, Giannakakis A, et al. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008;266(1):12–20. doi: 10.1016/j.canlet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- [18].Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- [19].Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr Opin Cell Biol. 2009;21(6):894–899. doi: 10.1016/j.ceb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Koshikawa N, Hayashi J, Nakagawara A, et al. Reactive oxygen species-generating mitochondrial DNA mutation up-regulates HIF-1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J Biol Chem. 2009;284(48):33185–33194. doi: 10.1074/jbc.M109.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jin HO, An S, Lee HC, et al. Hypoxic condition- and high cell density-induced expression of Redd1 is regulated by activation of HIF-1alpha and Sp1 through the phosphatidylinositol 3-kinase/Akt signaling pathway. Cell Signal. 2007;19(7):1393–1403. doi: 10.1016/j.cellsig.2006.12.014. [DOI] [PubMed] [Google Scholar]
- [22].Emerling BM, Platanias LC, Black E, et al. Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol Cell Biol. 2005;25(12):4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee WY, Liu KW, Yeung JH. Reactive oxygen species-mediated kinase activation by dihydrotanshinone in tanshinones-induced apoptosis in HepG2 cells. Cancer Lett. 2009;285(1):46–57. doi: 10.1016/j.canlet.2009.04.040. [DOI] [PubMed] [Google Scholar]
- [24].Ahn BH, Park MH, Lee YH, et al. Up-regulation of cyclooxygenase-2 by cobalt chloride-induced hypoxia is medi-ated by phospholipase D isozymes in human astroglioma cells. Biochim Biophys Acta. 2007;1773(12):1721–1731. doi: 10.1016/j.bbamcr.2007.06.001. [DOI] [PubMed] [Google Scholar]


