Summary
Background
Use of antiretroviral treatment for HIV-1 infection has decreased AIDS-related morbidity and mortality and prevents sexual transmission of HIV-1. However, the best time to initiate antiretroviral treatment to reduce progression of HIV-1 infection or non-AIDS clinical events is unknown. We reported previously that early antiretroviral treatment reduced HIV-1 transmission by 96%. We aimed to compare the effects of early and delayed initiation of antiretroviral treatment on clinical outcomes.
Methods
The HPTN 052 trial is a randomised controlled trial done at 13 sites in nine countries. We enrolled HIV-1-serodiscordant couples to the study and randomly allocated them to either early or delayed antiretroviral treatment by use of permuted block randomisation, stratified by site. Random assignment was unblinded. The HIV-1-infected member of every couple initiated antiretroviral treatment either on entry into the study (early treatment group) or after a decline in CD4 count or with onset of an AIDS-related illness (delayed treatment group). Primary events were AIDS clinical events (WHO stage 4 HIV-1 disease, tuberculosis, and severe bacterial infections) and the following serious medical conditions unrelated to AIDS: serious cardiovascular or vascular disease, serious liver disease, end-stage renal disease, new-onset diabetes mellitus, and non-AIDS malignant disease. Analysis was by intention-to-treat. This trial is registered with ClinicalTrials.gov, number NCT00074581.
Findings
1763 people with HIV-1 infection and a serodiscordant partner were enrolled in the study; 886 were assigned early antiretroviral treatment and 877 to the delayed treatment group (two individuals were excluded from this group after randomisation). Median CD4 counts at randomisation were 442 (IQR 373–522) cells per μL in patients assigned to the early treatment group and 428 (357–522) cells per μL in those allocated delayed antiretroviral treatment. In the delayed group, antiretroviral treatment was initiated at a median CD4 count of 230 (IQR 197–249) cells per μL. Primary clinical events were reported in 57 individuals assigned to early treatment initiation versus 77 people allocated to delayed antiretroviral treatment (hazard ratio 0·73, 95% CI 0·52–1·03; p=0·074). New-onset AIDS events were recorded in 40 participants assigned to early antiretroviral treatment versus 61 allocated delayed initiation (0·64, 0·43–0·96; p=0·031), tuberculosis developed in 17 versus 34 patients, respectively (0·49, 0·28–0·89, p=0·018), and primary non-AIDS events were rare (12 in the early group vs nine with delayed treatment). In total, 498 primary and secondary outcomes occurred in the early treatment group (incidence 24·9 per 100 person-years, 95% CI 22·5–27·5) versus 585 in the delayed treatment group (29·2 per 100 person-years, 26·5–32·1; p=0·025). 26 people died, 11 who were allocated to early antiretroviral treatment and 15 who were assigned to the delayed treatment group.
Interpretation
Early initiation of antiretroviral treatment delayed the time to AIDS events and decreased the incidence of primary and secondary outcomes. The clinical benefits recorded, combined with the striking reduction in HIV-1 transmission risk previously reported, provides strong support for earlier initiation of antiretroviral treatment.
Funding
US National Institute of Allergy and Infectious Diseases.
Introduction
Combination antiretroviral treatment has reduced morbidity and mortality associated with HIV-1 infection over the past two decades.1,2 However, the best timing of treatment initiation in people with high CD4 cell counts remains unknown. Findings of observational studies of treatment for HIV-1 infection lend support to early initiation of antiretroviral treatment,3–6 but data from randomised studies are scarce. In a randomised trial from Haiti,7 HIV-1 disease progression was delayed and survival extended when antiretroviral treatment was started at CD4 counts of 200–350 cells per μL, compared with initiation at CD4 counts of less than 200 cells per μL.
The HIV Prevention Trials Network (HPTN) 052 study is a worldwide, multicentre, randomised controlled trial designed to compare early versus delayed antiretroviral treatment for HIV-1-infected adults with CD4 counts of 350–550 cells per μL. Interim results after 1·7 years of follow-up showed a 96% reduction in HIV-1 transmission to a sexual partner and delayed time to AIDS events with early treatment.8 Here, we present a planned analysis of HPTN 052, focusing on clinical outcomes that reflect overall recorded morbidity and mortality related to HIV-1 infection. These outcomes might be a result of HIV-1 disease progression, the effect of HIV-1 infection on serious non-AIDS clinical events, adverse events resulting from treatment, or a combination of these.
Methods
Study population
We enrolled HIV-1-serodiscordant couples (one with HIV-1 infection and the other without) from nine countries: Botswana (one site), Brazil (two sites), India (two sites), Kenya (one site), Malawi (two sites), South Africa (two sites), Thailand (one site), the USA (one site), and Zimbabwe (one site).8 Since the US site enrolled only two couples during the pilot phase of the study and did not participate in the full study, the two HIV-1-infected participants enrolled at that site were excluded from the current analyses. Inclusion criteria were a CD4 count of 350–550 cells per μL in the HIV-1-infected member of every couple (index case) and no previous long-term use of antiretroviral treatment. The study protocol was approved by the institutional review board or ethics committee at every site, in addition to other local regulatory bodies as appropriate. All study participants provided written informed consent.
Randomisation and masking
We randomly assigned HIV-1-infected participants to either early or delayed antiretroviral treatment. Random-isation was done centrally by use of permuted blocks, and we stratified randomisation by study site.8 Participants and clinicians were aware of their treatment allocation.
Outcomes
Primary clinical outcomes were death, new-onset WHO stage 4 HIV-1 disease, tuberculosis, severe bacterial infections, serious cardiovascular or vascular events, serious liver disease, end-stage renal disease, new-onset diabetes mellitus, and non-AIDS defining malignant diseases (appendix pp 26–27). Secondary clinical outcomes included WHO stage 2 and 3 HIV-1 events and other medical disorders such as malaria, chronic renal insufficiency, hepatic transaminitis, lipodystrophy, dyslipidaemia, peripheral neuropathy, hypertension, lactic acidosis, and thrombocytopenia (appendix p 28).
Procedures
Early treatment started at enrolment; delayed anti retroviral treatment started either after two consec u tive CD4 counts of 250 cells per μL or less were recorded or after development of an AIDS-related illness. Prophylaxis with isoniazid, co-trimoxazole (trimethoprimsulfamethoxazole), or both, was available, according to local guidelines. After enrolment, we did periodic clinical and laboratory assessment, measured drug adherence, and provided counselling. Full details of study implementation and other study results have been described elsewhere.8 We did all current data analyses in accordance with a prespecified plan (appendix pp 1–25).
We captured incident cases of primary and secondary outcomes prospectively during the study with a targeted case report form (appendix pp 34–41). All primary outcomes and prespecified selected secondary outcomes underwent masked case review. We either designated outcomes as confirmed or probable or rejected them, according to AIDS Clinical Trials Group Criteria for Clinical and Other Events (appendix pp 42–145)9 and WHO case definitions of HIV-1 for surveillance and revised clinical staging and immunological classification of HIV-1-related disease in adults and children.10 We reviewed the study database further for laboratory events, including persistent elevation of hepatic aminotransferase enzymes or creatinine and thrombo cytopenia (appendix p 28). In all analyses, we pooled outcomes across disposition (confirmed or probable). With the exception of recurrent tuberculosis, bacterial infections, malaria, anaemia, and transaminitis after resolution, we did not include repeat events.
Statistical analysis
We estimated time to first primary outcome with the Kaplan-Meier method and compared the dist ri butions by treatment group with the log-rank test. We summarised distributions with cumulative event probabilities over 2 years (median follow-up). The incidence of primary outcomes, and combined primary and secondary outcomes, were estimated by treatment group with Poisson regression. We used robust standard errors to accommodate multiple and repeated events. In secondary analyses, using methods described previously,8 we examined the cumulative incidence of prespecified subcategories of primary outcomes: AIDS events (including WHO stage 4 events, tuberculosis, and severe bacterial infections), non-AIDS events, and deaths. We calculated the relative hazard of primary outcomes for delayed versus early treatment by prespecified sub groups (geographical region, sex, and baseline CD4 count [<450 cells per μL vs ≥450 cells per μL]) using Cox proportional-hazards regression. We used Cox regression stratified by geographical region to examine factors associated with the hazard of primary outcome. One analysis only included pretreatment risk factors and a covariate for treatment group; a second analysis included a time-updated covariate for proximal CD4 cell count (the CD4 cell count measured closest to each observed event time) and, thus, did not include a treatment group covariate.11 All estimated effect sizes are given with 95% CIs.
For every participant randomly allocated to the delayed treatment group, the rate of change in CD4 count from study entry to antiretroviral treatment initiation was estimated with least-squares regression, using all available CD4 counts during that period. We presented the distribution of these estimated rates of CD4 count change and all continuous outcomes as medians and IQR.
We analysed data by intention-to-treat, irrespective of antiretroviral treatment status. We included all data to May 11, 2011 (the day before public release of HPTN 052 primary prevention study results), for HIV-1-infected participants enrolled at sites outside the USA. This date was chosen because, after that time, providers and participants would make decisions about their care based on the results of the trial, which would affect patients’ outcomes and induce bias. This Article includes 3 months’ additional follow-up, compared with the previous analysis,8 and excludes two individuals enrolled at the US site. We censored follow-up for all participants at May 11, 2011, or when the patient came off study, or 6 months after their last visit, whichever occurred first.
This trial is registered with ClinicalTrials.gov, number NCT00074581.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
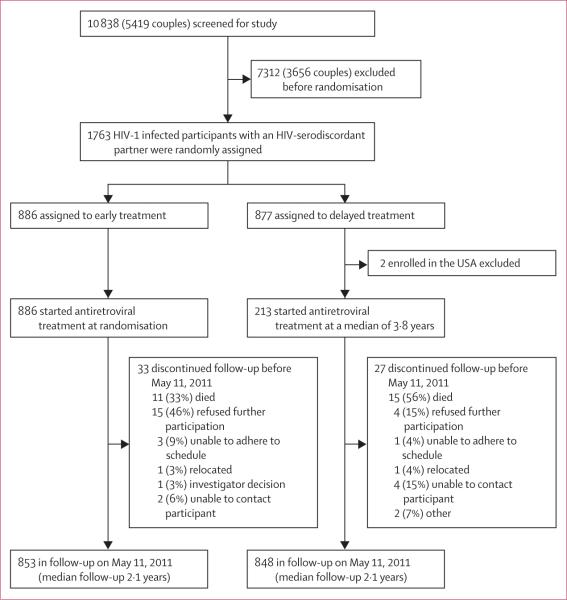
1763 HIV-1-infected individuals with a serodiscordant partner were enrolled in the trial and underwent randomisation; 886 were assigned to the early treatment group and 877 were allocated to delayed antiretroviral treatment (figure 1). Two patients from the USA were excluded from the delayed treatment group after randomisation. Table 1 presents baseline characteristics. Median age of participants was 33 years (IQR 27–39), and half the study population were men. The median baseline CD4 count was 436 cells per μL (IQR 364–522), and the median plasma viral load was 4·4 log10 copies per mL (IQR 3·8–4·9); 368 (21%) participants had plasma HIV-1 RNA greater than 100 000 copies per mL. At enrolment, 199 (11%) participants in total were taking co-trimoxazole; at 12 months, this number had declined to 32 (4%) in the early treatment group and 65 (8%) in the delayed treatment group. Only 14 participants were receiving isoniazid at study entry; an additional 38 patients (25 allocated to early treatment and 13 assigned to the delayed treatment group) initiated prophylaxis with isoniazid during the course of the study. Median follow-up was 2·1 years (IQR 1·5–2·9).
Figure 1.
Trial profile
Table 1.
Baseline characteristics
| Early treatment (n=886) | Delayed treatment (n=875) | |
|---|---|---|
| Age | ||
| <25 years | 112 (13%) | 117 (13%) |
| 25-39 years | 563 (64%) | 559 (64%) |
| ≥40 years | 211 (24%) | 199 (23%) |
| Women | 432 (49%) | 441 (50%) |
| Region | ||
| South America | 142 (16%) | 134 (15%) |
| Africa | 267 (30%) | 264 (30%) |
| Asia | 477 (54%) | 477 (55%) |
| HIV1 RNA (log10 copies per mL) | 4·4 (3·8-4·9) | 4·4 (3·9-4·9) |
| Missing data | 3 | 4 |
| CD4 count (cells per μL) | 442 (373-522) | 428 (357-522) |
| Missing data | 6 | 5 |
| Hepatitis B positive | 46 (5%) | 45 (5%) |
| Haemoglobin (≥85 g/L) | 36 (4%) | 46 (6%) |
| History of tuberculosis | 21 (2%) | 16 (2%) |
| Ongoing tuberculosis | 23 (3%) | 18 (2%) |
| Active treatment* | 10 | 5 |
| Maintenance treatment | 13 | 13 |
| Isoniazid prophylaxis | 5 (1%) | 9 (1%) |
| Co-trimoxazole prophylaxis | 98 (11%) | 101 (12%) |
Data are number of participants (%) or median (IQR).
HIV-1-infected participants were ineligible for the study if they were in the intensive phase of tuberculosis treatment; these 15 individuals enrolled during the intensive phase of tuberculosis treatment constitute eligibility violations but were included in all analyses.
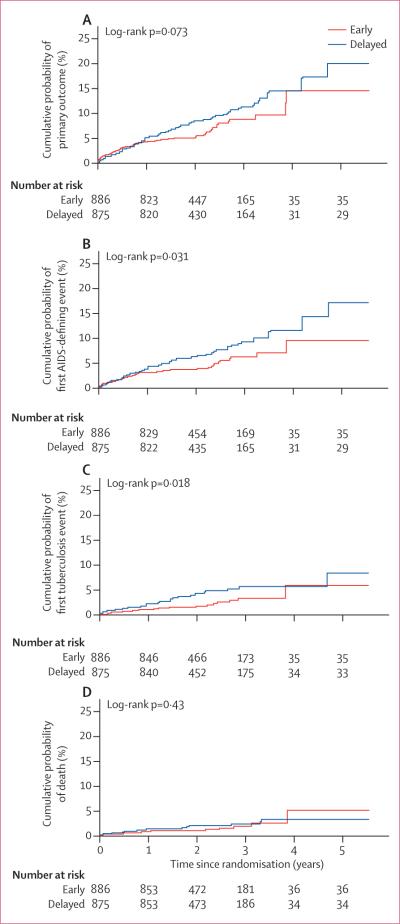
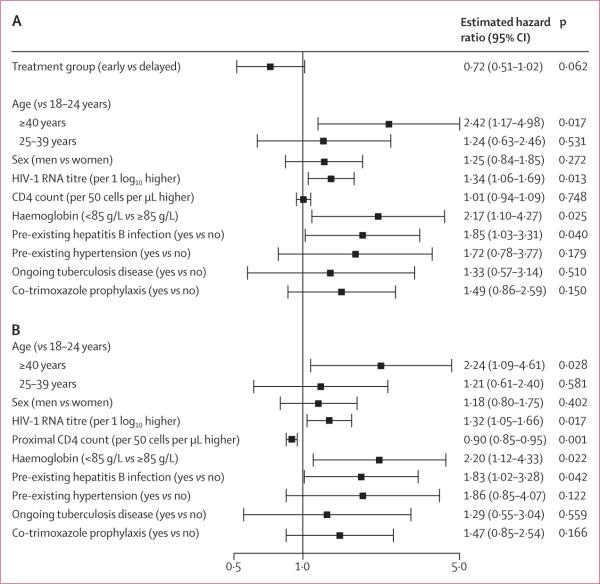
57 (6%) participants assigned to the early treatment group and 77 (9%) individuals allocated to delayed antiretroviral treatment had at least one primary outcome event (table 2). The cumulative probability of a primary outcome event over 2 years was 4·8% (95% CI 3·6–6·5) in the early treatment group and 7·9% (6·2–10·1) in the delayed treatment group (figure 2A) The estimated relative hazard (early vs delayed treatment) of primary events was 0·73 (95% CI 0·52–1·03; p=0·074); no differences in the effect of early versus delayed treatment were apparent by geographical region, sex, and baseline CD4 count (p>0·81; figure 3).
Table 2.
Summary of primary and secondary outcomes
| Early treatment (n=886) | Delayed treatment (n=875) | Hazard ratio (95% CI) | p value | |
|---|---|---|---|---|
| Primary events | ||||
| Any serious clinical event | 57 (6%) | 77 (9%) | 0.73 (0·52-1·03) | 0·074 |
| Any AIDS event | 40 (5%) | 61 (7%) | 0-64 (0·43-0·96) | 0·031 |
| Tuberculosis | 17 (2%) | 34 (4%) | 0·49 (0·28-0·89) | 0·018 |
| Severe bacterial infection* | 20 (2%) | 13 (1%) | · · | · · |
| Any WHO stage 4 event (excluding tuberculosis) | 9 (1%) | 19 (2%) | · · | · · |
| Chronic herpes simplex† | 2 | 8 | · · | · · |
| Oesophageal candidosis | 2 | 2 | · · | · · |
| Recurrent severe bacterial pneumonia* | 2 | 1 | · · | · · |
| Invasive cervical carcinoma | 0 | 2 | · · | · · |
| Wasting syndrome due to HIV-1 infection | 0 | 2 | · · | · · |
| Kaposi's sarcoma | 1 | 1 | · · | · · |
| Recurrent septicaemia | 2 | 0 | · · | · · |
| Extrapulmonary cryptococcosis | 1 | 0 | · · | · · |
| HIV-1-related encephalopathy | 0 | 1 | · · | · · |
| Pneumocystis pneumonia | 0 | 1 | · · | · · |
| Primary CNS lymphoma | 0 | 1 | · · | · · |
| Secondary events | ||||
| Non-AIDS event | 12 (1%) | 9 (1%) | 1·35 (0·57-3·19)† | 0·50 |
| Diabetes mellitus | 4 | 5 | · · | · · |
| Non-AIDS malignant disease | 3 | 3 | · · | · · |
| Serious cardiovascular or vascular disease | 3 | 1 | · · | · · |
| Serious liver disease | 2 | 0 | · · | · · |
| End-stage renal disease | 0 | 0 | · · | · · |
| Mortality | ||||
| All deaths | 11 (1%) | 15 (2%) | 0·73 (0·34-1·59)† | 0·43 |
| Deaths associated with a primary event | 1 | 4 | · · | · · |
| Deaths from other causes | 10 | 11 | · · | · · |
Data are the number (%) of participants who had at least one such type of outcome. All primary events underwent masked review to ensure they met prespecified diagnostic criteria.
One recurrent severe bacterial pneumonia event reported in the primary paper8 was changed to severe bacterial infection by the site clinician after the data lock for the primary paper.
One chronic herpes simplex event reported previously in the primary paper8 was rejected by masked review and, thus, was excluded from this table.
Post-hoc analyses that did not form part of the original study analysis plan.
Figure 2. Kaplan-Meier curves showing time to primary and secondary outcomes.
(A) Primary outcome. (B) First AIDS event. (C) First tuberculosis event. (D) Death.
Figure 3. Forest plot showing relative hazard of primary outcome for early versus delayed treatment.
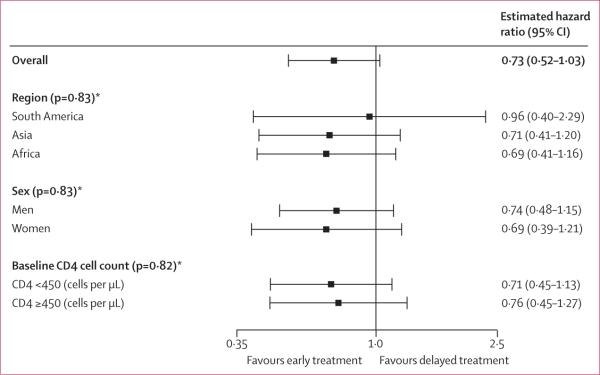
Errors bars show 95% CI. *Test against the null hypothesis of no treatment modification by given subgroup (interaction).
40 (5%) participants assigned to the early treatment group had AIDS events compared with 61 (7%) people allocated delayed antiretroviral treatment. The cumulative probability of having an AIDS event over 2 years was 3·3% (2·4–4·9) in the early treatment group compared with 6·0% (4·5–7·9) in the delayed treatment group (figure 2B). The estimated relative hazard was 0·64 (95% CI 0·43–0·96; p=0·031).
17 (2%) people assigned early antiretroviral treatment reported a tuberculosis event versus 34 (4%) individuals who were allocated to the delayed treatment group. The cumulative probability of tuberculosis events over 2 years was 1·2% (0·6–2·2) in patients assigned to the early treatment group and 3·7% (2·5–5·3) in those allocated delayed antiretroviral treatment (figure 2C). The estimated relative hazard was 0·49 (95% CI 0·28–0·89; p=0·018). With respect to secondary outcomes, serious non-AIDS events were rare and were reported in 12 patients assigned to early antiretroviral treatment and in nine participants who were allocated to the delayed treatment group (table 2).
26 people died, 11 who were assigned early antiretroviral treatment and 15 allocated delayed treatment (table 2). The distribution of time to death did not differ between treatment groups (p=0·43; figure 2D). All but three deaths have been described previously.8 The three additional deaths were identified during the additional follow-up period: one individual assigned to the early treatment group died from sepsis; and two people who were allocated delayed antiretroviral treatment died from indeter minate causes. In total, five deaths were associated with primary outcomes, and the remainder were attributable to causes that either did not meet predefined primary outcome diagnosis criteria or were suicides, accidental deaths, or of indeterminate cause.
162 primary outcome events were recorded in total, over 4007 person-years of follow-up, which includes repeated and multiple outcomes in the same individual. 71 primary events were noted in people assigned to the early treatment group (incidence 3·5 events per 100 person-years, 95% CI 2·7–4·7) versus 91 in individuals allocated delayed antiretroviral treatment (4·5 events per 100 person-years, 3·6–5·7; p=0·19).
AIDS events were the most frequently reported outcomes, with 71 events noted in 61 patients assigned delayed antiretroviral treatment and 49 events in 40 people allocated early treatment (p=0·089). Severe bacterial infections happened more frequently in patients assigned early treatment (20 events in 20 people) than in those allocated delayed antiretroviral treatment (15 events in 13 patients). 54 cases of tuberculosis were recorded in 51 participants: 17 were noted in people allocated early treatment (incidence 0·8 events per 100 person-years, 95% CI 0·5–1·4) and 37 were reported in individuals allocated delayed antiretroviral treatment (1·8 events per 100 person-years, 95% CI 1·3–2·6; p=0·009). Tuberculosis cases are presented in the appendix (pp 29–33).
On multivariable analysis of baseline factors associated with time to first primary outcome (figure 4A), a higher risk was noted with older age (p=0·017), higher baseline HIV-1 RNA titre (p=0·013), an amount of haemoglobin less than 85 g/L (p=0·025), and hepatitis B virus co-infection (p=0·040). The estimated effect of early versus delayed antiretroviral treatment was unchanged after adjustment for these baseline factors. A proximal (time-updated) increase in CD4 cell count of 50 cells per μL was associated with a 10% lower hazard of a primary outcome (hazard ratio 0·90, 95% CI 0·85–0·95; p=0·001; figure 4B).
Figure 4. Forest plots showing risk factors for primary outcome.
Errors bars show 95% CI. (A) Treatment group and risk factors at study entry. (B) Risk factors at study entry with time-updated CD4 count.
To provide a more comprehensive measure of the clinical effect of early versus delayed antiretroviral treatment in individuals with HIV-1 infection, secondary outcomes in our study included WHO stage 2 and 3 events and other medical disorders reflecting HIV-1 disease, potential toxic effects of drugs, or both. 615 (35%) participants had at least one such secondary outcome (table 3), 298 (34%) in patients assigned to the early treatment group and 317 (36%) in those allocated delayed antiretroviral treatment. The most frequently reported secondary outcomes were recurrent upper-respiratory-tract infections, moderate and severe unexplained weight loss, smear-positive malaria, papular pruritic eruptions, herpes zoster, and persistent candidosis (table 3). Of note, secondary events potentially associated with antiretroviral treatment included dyslipidaemia and lipodystrophy, and these disorders were most frequently reported in patients allocated early antiretroviral treatment, whereas other events linked to antiretroviral treatment—eg, peripheral neuro pathy, hepatic transaminitis and chronic renal failure—were not (table 3).
Table 3.
Summary of targeted events
| Early treatment (n=886) | Delayed treatment (n=875) | |
|---|---|---|
| Any targeted event (including primary events) | 326 (37%) | 347 (40%) |
| Non-primary targeted event | 298 (34%) | 317 (36%) |
| WHO stage 2 or 3 event | 210 (24%) | 250 (29%) |
| Upper-respiratory-tract infection | 72 | 87 |
| Moderate unexplained weight loss | 76 | 61 |
| Papular puritic eruptions | 33 | 52 |
| Herpes zoster (shingles) | 17 | 53 |
| Persistent oral candidosis | 22 | 47 |
| Unexplained severe weight loss | 37 | 21 |
| Seborrhoeic dermatitis | 7 | 18 |
| Oral ulcerations | 10 | 9 |
| Fungal nail infections | 5 | 10 |
| Oral hairy leukoplakia | 3 | 10 |
| Angular cheilitis | 7 | 6 |
| Unexplained anaemia | 0 | 6 |
| Acute necrotising ulcerative stomatitis, gingivitis, or periodontitis | 0 | 4 |
| Unexplained chronic diarrhoea | 0 | 1 |
| Other targeted medical disorder | ||
| Smear-positive malaria | 49 | 49 |
| Peripheral neuropathy | 15 | 14 |
| Dyslipidaemia | 23 | 7 |
| Hypertension | 12 | 8 |
| Lipodystrophy | 10 | 0 |
| Hepatic transaminitis | 9 | 8 |
| Thrombocytopenia | 3 | 6 |
| Lactic acidosis (symptomatic) | 1 | 1 |
| Chronic renal insufficiency | 0 | 0 |
Data are the number (%) of participants who had at least one such type of outcome.
Primary and secondary outcomes combined were reported in 326 (37%) patients assigned early anti-retroviral treatment and in 347 (40%) participants allocated to the delayed treatment group (table 3). The incidence of all primary and secondary outcomes was lower in participants randomly allocated to early antiretroviral treatment (24·9 events per 100 person-years, 95% CI 22·5–27·5) than in individuals assigned to the delayed treatment group (29·2 events per 100 person-years, 95% CI 26·5–32·1; p=0·025). This difference was driven by HIV-1-related outcomes.
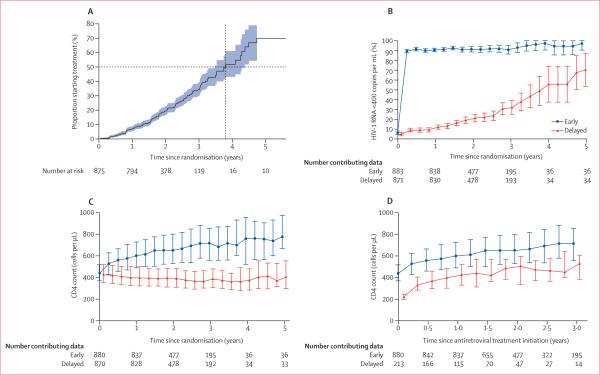
213 (24%) participants assigned to the delayed treatment group ultimately began antiretroviral treatment. Median time to treatment initiation was 3·8 years from enrolment (95% CI 3·5–4·4; figure 5A), with a median CD4 count of 230 cells per μL (IQR 197–249) and median HIV-1 RNA titre of 5·0 log10 copies per mL (IQR 4·5–5·5) at treatment initiation. Median duration of antiretroviral treatment was 2·1 years (IQR 1·5–2·9) for patients assigned early treatment and 1·0 years (0·5–1·7) for individuals allocated to the delayed group. Choice of antiretroviral drug has been described previously and was predominantly a combination of zidovudine, lamivudine, and efavirenz.8 In the early treatment group, the proportion of individuals with an HIV-1 RNA titre less than 400 copies per mL remained greater than 89% for the entire follow-up. A gradual increase in the proportion of people with an HIV-1 RNA titre less than 400 copies per mL was noted in the delayed treatment group, which grew as HIV-1-infected study participants began antiretroviral treatment (figure 5B). Overall, the median change in CD4 count over 2 years was a 225 cells per μL increase (IQR 96 to 373 increase) in patients allocated to early antiretroviral treatment, compared with a median change in CD4 count in the delayed treatment group of a 37 cells per μL decline (IQR 123 decline to 51 increase; figure 5C). Before starting antiretroviral treatment, the median change in CD4 count was a 39 cells per μL decline (IQR 0 to 80 decline) in patients allocated to the delayed treatment group per year, but after treatment initiation, the CD4 count changed by a similar extent in both groups (median change of 246 cells per μL increase [IQR 144 to 384 increase] over 2 years). However, the CD4 count in people allocated to early antiretroviral treatment remained higher than in those assigned to the delayed treatment group over the course of the study (figure 5D).
Figure 5. Plots showing time to initiation of treatment, HIV-1 RNA amounts, and CD4 counts.
(A) Initiation of antiretroviral treatment in patients assigned to the delayed treatment group. Dotted lines represent median time to treatment initiation. Shaded blue region represents 95% CIs. (B) Proportion of participants with HIV-1 RNA <400 copies per mL. Error bars represent 95% CIs. (C) Median CD4 count in the intention-to-treat population. Error bars represent IQR. (D) Median CD4 count after initiation of antiretroviral treatment. Error bars represent IQR. For readability, outcomes at each timepoint for the delayed group are shifted slightly off-centre.
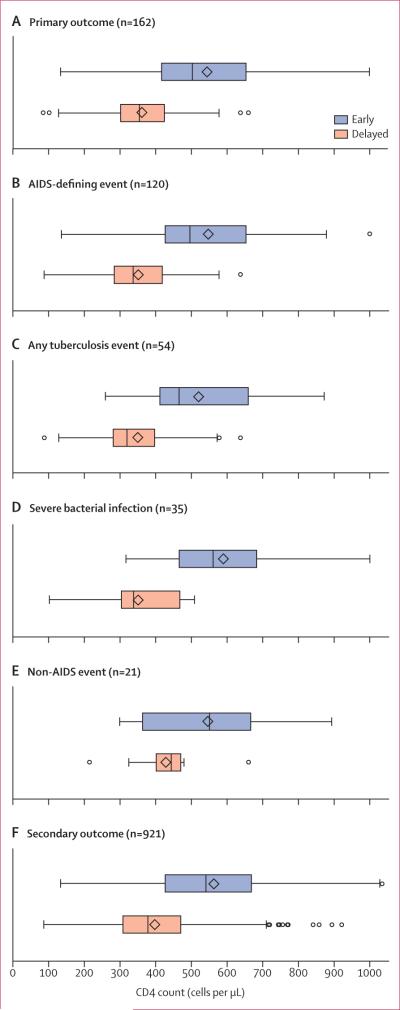
Primary and secondary endpoints were not concentrated among participants with low CD4 counts; in fact, most outcome events were recorded when the most recent CD4 count was higher than 350 cells per μL (figure 6). The median CD4 count for primary clinical events was 353 cells per μL (IQR 301–425) in patients assigned to delayed antiretroviral treatment compared with 502 cells per μL (417–653) in participants allocated to the early treatment group.
Figure 6.
Distribution of CD4 counts at study visit before outcome
Discussion
Our findings from the HPTN 052 study cohort show the broader clinical effect of early initiation of antiretroviral treatment, including effects in relation to serious non-AIDS medical disorders and HIV-1-associated events. Time to first serious clinical event was longer in patients assigned early antiretroviral treatment, despite the fact that serious non-AIDS events were uncommon. However, a substantial number of AIDS-related and HIV-1-related events were recorded among study participants despite fairly high baseline CD4 counts. Time to an AIDS event was longer in patients allocated to the early treatment group than in those assigned delayed antiretroviral treatment (p=0·031); similarly, the overall incidence of primary or secondary outcomes was higher in the delayed group, predominantly tuberculosis and WHO stage 2 and 3 events (p=0·025).
The results of our current multicentre randomised study extend previous study findings (panel).12 We have reported8 that antiretroviral treatment prevents 96% of HIV-1 transmission events from an infected individual to their sexual partner when initiated at CD4 counts of 350–550 cells per μL, compared with starting treatment at a CD4 count lower than 250 cells per μL; further, antiretroviral treatment was associated with a reduction in AIDS events. Findings of the Comprehensive International Program of Research on AIDS (CIPRA) study, done in Haiti,7 showed a benefit when antiretroviral treatment was initiated at CD4 counts between 250 and 350 cells per μL compared with counts lower than 200 cells per μL. Post-hoc analysis of a subset of antiretroviral treatment-naive people, and patients off-therapy who had been treated previously, who were enrolled in the Strategies for Management of Antiretroviral Therapy (SMART) randomised controlled trial, showed a benefit of earlier antiretroviral treatment,13 with most events occurring in previously treated individuals. Detailed analyses of several large cohorts show a benefit of treatment initiation at higher CD4 cell counts.3–6 The strengths and limitations of these earlier studies have been addressed.6,14,15
The burden of AIDS and mortality seen in our study, and in other cohorts in Africa,16 is higher than in European populations with similar CD4 counts.17 Time-to-first AIDS event was significantly longer in patients allocated to the early treatment group than in those assigned to delayed antiretroviral treatment. This difference mainly reflects prevention of tuberculosis, which is a leading cause of death in people living with HIV-1 infection.18 The noted reduction in risk is similar to that seen in the CIPRA study7 and accords with the 65% reduction in tuberculosis incidence associated with use of antiretroviral treat ment across all CD4 counts that was reported in a meta-analysis.19 We recorded both pulmonary and extrapulmonary tuberculosis in our study participants (appendix pp 29–33). The frequency of extrapulmonary tuberculosis in people with HIV-1 infection,20,21 and difficulties in diagnosis of extrapulmonary tuberculosis,22,23 have been noted. Prevention of tuberculosis ascribed to antiretroviral treatment provides an important rationale for early treatment of HIV-1 infection. Addition of isoniazid to antiretroviral treatment could potentially decrease tuberculosis incidence further.24
In this report, we included clinical outcomes that might result not only from HIV-1 infection but also from antiretroviral treatment, to provide a more complete picture of the health of individuals living with HIV-1 infection. Early antiretroviral treatment reduced the incidence of WHO stage 2 and 3 events; although such events are not life-threatening, they can lead to substantial morbidity, even among patients with high CD4 counts.16 Not unexpectedly, dyslipidaemia and lipodystrophy were recorded more frequently in patients assigned to the early treatment group versus those allocated to delayed antiretroviral treatment. We reported previously that serious (grade 3 and 4) laboratory toxic effects were uncommon in both treatment groups.8 In this study, we focused on serious clinical diagnoses that might be a result of adverse effects of treatment or HIV-1, including dyslipidaemia, end-stage renal disease, and serious liver disease. Our analyses did not include a focus on anaemia, neutropenia, and psychiatric events,8 which were numerically more common with early antiretroviral treatment possibly because of use of zidovudine or efavirenz.25
Complications unrelated to AIDS are an emerging concern in resource-limited settings.26,27 In this current analysis, the reported incidence of non-AIDS events was low, with no difference recorded between study groups. Serious adverse events possibly attributable to anti-retroviral treatment were also infrequent.8 The short duration of follow-up restricts our ability to note cardiovascular, renal, hepatic, or malignant events that might be associated with untreated HIV-1 infection. Long-term complications of antiretroviral treatment might also not be apparent in view of the duration of follow-up. After unmasking of the results of HPTN 052, all HIV-1-infected study participants were offered antiretroviral treatment,8,28 most of whom have chosen to initiate treatment.28 We continue to follow up couples enrolled in HPTN 052 for complications arising from the delay in antiretroviral treatment initiation, and for additional HIV-1 transmission events.
Although prevention of most clinical events was favoured by early initiation of antiretroviral treatment, we did not record an effect on all-cause mortality. This finding is possibly accounted for by the lower risk of death within the range of CD4 cell counts (350–550 cells per μL) assessed in our study and the high-quality clinical care provided at the study sites, which guaranteed that patients presenting with disease progression would receive antiretroviral treatment.
Initiation of antiretroviral treatment had the anticipated therapeutic benefits on HIV-1 RNA titres and CD4 cell counts. HIV-1 RNA was suppressed for most participants, and CD4 cell counts rose after treatment was started. In patients assigned to delayed antiretroviral treatment, CD4 counts fell without any treatment and rose once treatment was initiated, at a similar rate to that noted in people allocated to the early treatment group. However, the absolute CD4 cell count did not reach the level recorded in individuals assigned to early antiretroviral treatment, suggesting that delayed treatment might diminish or defer full restoration of immunity.29
The decision about when to start antiretroviral treatment has led to policy recommendations that differ across organisations and countries—differences that, in part, are based on resources.12,30–32 We believe the decision to initiate antiretroviral treatment must take into consideration the complex mix of the public health benefits of reduced HIV-1 transmission ascribed to antiretroviral treatment8 and the potential costs and benefits to every individual who is infected with HIV-1. On the basis of our study results, early antiretroviral treatment—defined as initiation of antiretroviral treatment at a CD4 count of 350–550 cells per μL— can be expected to delay the time to AIDS-defining events, tuberculosis, and WHO stage 2 and 3 events and significantly reduce the incidence of these events, compared with patients in whom antiretroviral treatment is delayed until CD4 counts reach less than 250 cells per μL. The absolute differences recorded between groups in clinical events were modest. However, they offer the potential for reduced morbidity among the millions of people with HIV-1 infection. Coupling clinical benefit with a striking effect on transmission risk gives additional strength to the argument for early initiation of antiretroviral treatment.
Supplementary Material
Panel: Research in context.
Systematic review
In 2013, WHO did a systematic review12 to address when to start antiretroviral treatment for individuals living with HIV-1 infection with CD4 cell counts higher than 350 cells per μL. The review considered results from three clinical trials and 21 observational studies. The effects of early antiretroviral treatment on morbidity, mortality, and immunological and virological outcomes were studied. The findings showed that initiation of antiretroviral treatment at a CD4 count greater than 350 cells per μL, compared with treatment at a CD4 count of 350 cells per μL or lower, reduced the risk of progression to AIDS or death, cut the risk of tuberculosis, diminished the risk of developing a non-AIDS-defining illness, and increased the likelihood of immune recovery. Further analysis showed a decreased risk of death with early initiation of antiretroviral treatment in 13 studies and a diminished risk of progression to AIDS or death in nine studies.
Interpretation
HIV Prevention Trials Network (HPTN) 052 is the first randomised controlled trial, with study sites from nine countries in four continents, to directly compare early versus delayed antiretroviral treatment for HIV-1-infected adults with CD4 counts of 350–550 cells per μL. We assessed a broad range of clinical outcomes that reflect overall HIV-1-related morbidity and mortality that might be related to disease progression, and we looked at the effect of HIV-1 infection on serious clinical events unrelated to AIDS and adverse events resulting from antiretroviral treatment. Our findings show that early antiretroviral treatment delayed the time to AIDS events, tuberculosis, and WHO stage 2 and 3 events and significantly reduced the incidence of these events, compared with patients for whom antiretroviral treatment was delayed until CD4 cell counts reached less than 250 cells per μL. Early antiretroviral treatment led to a rapid rise in CD4 cell counts to near normal levels. Our results, combined with the striking reduction in risk of HIV-1 transmission resulting from suppression of HIV-1 replication, provide strong support for early initiation of antiretroviral treatment.
Acknowledgments
This study was supported by the HIV Prevention Trials Network (HPTN) and by grants from the National Institute of Allergy and Infectious Diseases to the HPTN Network Laboratory (UM1-AI068619, U01-AI068619, UM1-AI068613, and U01-AI068613), to the HPTN Statistical and Data Management Center (UM1-AI068617 and U01-AI068617), and to DH, HJR, and SS (U01-AI068636). The National Institute of Allergy and Infectious Diseases assumes all sponsor responsibilities through an investigational new drug application with the US Food and Drug Administration. The antiretroviral drugs used in this study were donated by Abbott Laboratories, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, and Merck.
Footnotes
Contributors
BG was a site investigator and led writing of the report. MCH was a site investigator and was a member of the writing committee. HJR, YQC, and LW were study statisticians and members of the writing committee. SS, JE, and DH were members of the study oversight committee and the writing committee. S-SO was a study statistician. MA was a study clinical affairs safety associate. MM and TG led protocol development and helped manage the study. NK, JGH, JK, JHSP, SVG, SC, MGdM, KHM, JM, LAM, RP, IS, TET, KN-S, and ME were site investigators. SHE and EP-M worked at the HPTN Network Laboratory. JG was a member of the study oversight committee. IH led protocol development and was a site investigator. DC contributed to protocol development and was a site investigator. MSC was the principal investigator.
Declaration of interests
JE is a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, Janssen, Merck, and ViiV HealthCare, and has received research funding to the University of North Carolina (UNC) from GlaxoSmithKline, Abbvie, and Merck. JG has served on scientific advisory boards or has received consulting income from Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, and Merck; has served on a Data Safety Monitoring Board for Takara Bio; and has received research funding to his institution from Bristol-Myers Squibb, Merck, Sangama Bio Sciences, Vertex Pharmaceuticals, and ViiV Healthcare (to Southwest CARE Center) and Gilead Sciences (to Johns Hopkins University and Southwest CARE Center). JGH is on the board of Mylan Pharmaceuticals. DH is principal investigator on an NIH-funded study that receives drugs from Gilead for its participants. KHM has received unrestricted research grants from Merck, Gilead, and Bristol-Myers Squibb. All other authors declare that they have no competing interests.
References
- 1.Ray M, Logan R, Sterne JA, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–37. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braitstein P, Brinkhof MW, Dabis F, et al. for the Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration, and ART Cohort Collaboration (ART-CC) groups Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 3.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–15. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Committee for the CASCADE Collaboration Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–69. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell TB, Smeaton LM, Kumarasamy N, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9:e1001290. doi: 10.1371/journal.pmed.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO [Feb 26, 2013];WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2006 Aug 7; http://www.who.int/hiv/pub/guidelines/hivstaging/en/index.html.
- 11.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–95. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO [Jan 20, 2014];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013 Jun; http://www.who.int/hiv/pub/guidelines/arv2013/en. [PubMed]
- 13.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 14.Hughes MD, Ribaudo HR. The search for data on when to start treatment for HIV infection. J Infect Dis. 2008;197:1084–86. doi: 10.1086/586712. [DOI] [PubMed] [Google Scholar]
- 15.Siegfried N, Uthman UA, Rutherford G. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev. 2010;3:CD008272. doi: 10.1002/14651858.CD008272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anglaret X, Minga A, Gabillard D, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d'Ivoire. Clin Infect Dis. 2012;54:714–23. doi: 10.1093/cid/cir898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips AN, Gazzard B, Gilson R, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21:1717–21. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- 18.WHO [Jan 14, 2014];Global tuberculosis control. 2011 http://whqlibdoc.who.int/publications/2011/9789241564380_eng.pdf.
- 19.Suthar AB, Lawn SD, Del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naing C, Mak JW, Maung M, Wong SF, Kassim AI. Meta-analysis: the association between HIV infection and extrapulmonary tuberculosis. Lung. 2012;191:27–34. doi: 10.1007/s00408-012-9440-6. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Kong Y, Wilson F, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004;38:199–205. doi: 10.1086/380644. [DOI] [PubMed] [Google Scholar]
- 22.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 23.Chakravorty S, Sen MK, Tyagi JS. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J Clin Microbiol. 2005;43:4357–62. doi: 10.1128/JCM.43.9.4357-4362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifford DB, Evans S, Yang Y, et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143:714–21. doi: 10.7326/0003-4819-143-10-200511150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Belloso WH, Orellana LC, Grinsztejn B, et al. Analysis of serious non-AIDS events among HIV-infected adults at Latin American sites. HIV Med. 2010;11:554–64. doi: 10.1111/j.1468-1293.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- 27.Wester CW, Koethe JR, Shepherd BE, et al. Non-AIDS-defining events among HIV-1-infected adults receiving combination antiretroviral therapy in resource-replete versus resource-limited urban setting. AIDS. 2011;25:1471–79. doi: 10.1097/QAD.0b013e328347f9d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batani J, Brum T, Calvet G, et al. Acceptance of ART in the delay arm after notification of interim study results: data from HPTN 052.. 20th Conference on Retrovirus and Opportunistic Infections; Atlanta, GA, USA. March 3–6, 2013; p. 550. (poster) [Google Scholar]
- 29.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–30. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society—USA panel. JAMA 2012. 308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 31.Williams I, Churchill D, Anderson J, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012. HIV Med 2012. 13(suppl 2):1–85. doi: 10.1111/j.1468-1293.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 32.Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. [Aug 3, 2013];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012 http://aidsinfo.nih.gov/contentfiles/lvguidelines/ Adult and Adolescent GL.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.