Abstract
Chronic liver injury promotes hepatic inflammation, representing a prerequisite for organ fibrosis. We hypothesized a contribution of chemokine receptor CCR6 and its ligand, CCL20, which may regulate migration of T-helper (Th)17, regulatory, and gamma-delta (γδ) T cells. CCR6 and CCL20 expression was intrahepatically up-regulated in patients with chronic liver diseases (n = 50), compared to control liver (n = 5). Immunohistochemistry revealed the periportal accumulation of CCR6+ mononuclear cells and CCL20 induction by hepatic parenchymal cells in liver disease patients. Similarly, in murine livers, CCR6 was expressed by macrophages, CD4 and γδ T-cells, and up-regulated in fibrosis, whereas primary hepatocytes induced CCL20 upon experimental injury. In two murine models of chronic liver injury (CCl4 and methionine-choline-deficient diet), Ccr6−/− mice developed more severe fibrosis with strongly enhanced hepatic immune cell infiltration, compared to wild-type (WT) mice. Although CCR6 did not affect hepatic Th-cell subtype composition, CCR6 was explicitly required by the subset of interleukin (IL)-17- and IL-22-expressing γδ T cells for accumulation in injured liver. The adoptive transfer of WT γδ, but not CD4 T cells, into Ccr6−/− mice reduced hepatic inflammation and fibrosis in chronic injury to WT level. The anti-inflammatory function of hepatic γδ T cells was independent of IL-17, as evidenced by transfer of Il-17−/− cells. Instead, hepatic γδ T cells colocalized with hepatic stellate cells (HSCs) in vivo and promoted apoptosis of primary murine HSCs in a cell-cell contact-dependent manner, involving Fas-ligand (CD95L). Consistent with γδ T-cell-induced HSC apoptosis, activated myofibroblasts were more frequent in fibrotic livers of Ccr6−/− than in WT mice. Conclusion: γδ T cells are recruited to the liver by CCR6 upon chronic injury and protect the liver from excessive inflammation and fibrosis by inhibiting HSCs.
Chronic inflammation is the key factor promoting hepatic fibrogenesis and subsequently leading to cirrhosis and liver failure.1 Hepatic inflammation is tightly regulated by chemokines and their receptors that control recruitment of immune cells to the liver. Comprehensive analyses in experimental models of chronic liver injury revealed crucial functions for infiltrating monocytes and macrophages, but much less is known about T-cell attraction to injured liver.2 The chemokine receptors, CCR5 and CXCR3, have been linked to CD4 T-cell recruitment to the liver3,4 and CCR7 to infiltration of CD8 T cells.5 Almost nothing is known about mechanisms recruiting unconventional or innate T-cell subsets, such as gamma-delta (γδ) T cells, although the liver is one of the richest sources of γδ T cells in the body.6 Hepatic γδ T cells had been suggested as a critical early modulator of liver inflammation in acute acetaminophen- or Concanavalin A-induced hepatitis in mice,7,8 but their contribution to chronic inflammation and fibrosis is currently unclear.
The lymphocyte-associated chemokine receptor, CCR6, has important functions in mucosal immunity.9 Its CC-type chemokine ligand is CCL20, also termed macrophage inflammatory protein-3alpha (MIP-3α). Among CD4 T cells, CCR6 is specifically expressed on T-helper (Th)17 and regulatory (Treg), but not on Th1 or Th2 T cells.4 CCR6-mediated chemotaxis of T cells has been functionally linked to distinct immune-mediated diseases, because CCR6-deficiency aggravated experimental glomerulonephritis and autoimmune encephalomyelitis (EAE) in mice.10,11 More recently, CCR6 has also been identified on subsets of γδ T cells, where CCR6 expression is clearly associated with interleukin (IL)-17 production of these cells.12 Similar to CD4 T cells, these IL-17-producing γδ T cells have been associated with immune-mediated diseases, such as EAE.13
The role of CCR6 in liver diseases is largely obscure. A preliminary study investigating 34 patients found elevated levels of CCR6-expressing hepatic T cells and enhanced intrahepatic levels of CCL20 in fibrotic livers.14 More recently, CCR6-CCL20 has been described in patients with cholestatic diseases for positioning of Th17 cells around inflamed portal tracts in human liver.4 In this study, we investigated the functional relevance of CCR6 in hepatic inflammation and fibrosis. We demonstrate the activation of the CCR6-CCL20 pathway in patients with chronic liver diseases (CLDs) and murine hepatic fibrosis and provide experimental evidence that the CCR6-dependent recruitment of IL-17-producing γδ T cells into the injured liver critically limits hepatic inflammation and fibrosis.
Materials and Methods
Human Liver Samples
Human liver tissue was acquired either from biopsies for routine clinical purposes or explants of cirrhotic livers obtained during liver transplantation.15
Mice
C57bl/6 wild-type (WT), congenic CD45.1, Actin-eGFP, Il-17−/−,16 and Ccr6−/− mice9 were housed under specific pathogen-free conditions. Experiments were performed with age- and sex-matched animals at 6–12 weeks of age under ethical conditions approved according to German legal requirements.
Induction of Liver Injury and Fibrosis
Mice were injected intraperitoneally (IP) with 0.6 mL/kg body weight of CCl4 (Merck, Darmstadt, Germany) mixed with corn oil. For induction of liver fibrosis, CCl4 was injected thrice-weekly for 4 weeks. Mice were sacrificed 48 hours after the last injection. For induction of steatohepatitis, mice were fed with a methionine-choline-deficient (MCD) diet for 3–8 weeks (catalog no. 390439; MP Biomedicals, Solon, OH).
For details on methodology, please see the Supporting Materials.
Results
The Chemokine Receptor, CCR6, and Its Ligand, CCL20, Are Significantly Up-Regulated in Chronic Liver Injury in Mice and Men
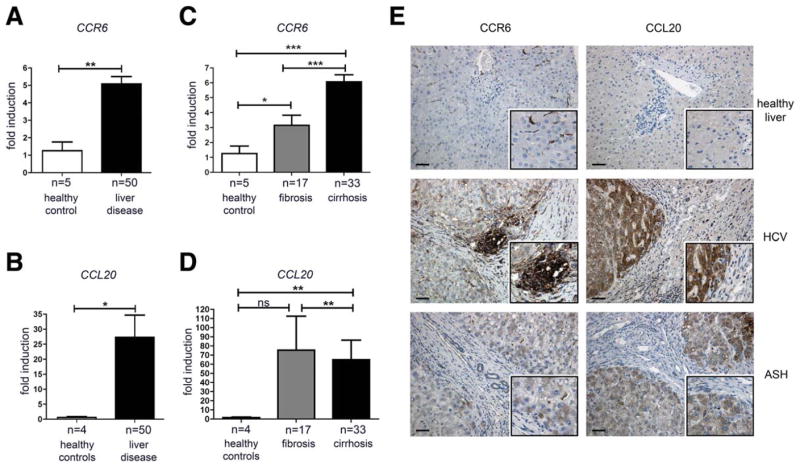
To investigate the potential role of CCR6 in CLD, liver samples of patients with different stages of chronic hepatic injury were assessed for expression of CCR6 and its cognate ligand, CCL20. In comparison to healthy controls (n = 5), hepatic messenger RNA (mRNA) expression of both markers was significantly up-regulated in patients with CLDs (n = 50; Fig. 1A,B). CCR6 expression was even higher in cirrhosis than fibrosis (Fig. 1C), whereas highest CCL20 expression was observed in fibrotic livers (Fig. 1D). Of note, CCR6 expression was elevated in patients with viral hepatitis, compared to other disease etiologies, whereas highest CCL20 was observed in primary biliary cirrhosis (Supporting Fig. 1A,B). Immunohistochemistry (IHC) confirmed enhanced CCR6 expression on protein level in liver disease patients and specifically detected CCR6 on lymphocytes in periportal infiltrates (Fig. 1E). In contrast, CCL20 protein expression was remarkably up-regulated by hepatocytes and, to a lesser extent, by biliary epithelial cells (BECs) in diseased versus control livers (Fig. 1E). In patients with cholestatic diseases, larger bile ducts strongly expressed CCL20, especially in regions with large clusters of inflammatory cells and damaged biliary epithelium (Supporting Fig. 1C).
Fig. 1.
CCR6 and CCL20 are up-regulated in human CLD. (A–D) Liver samples of patients with CLD and control tissue were analyzed for CCR6 and CCL20 expression levels by quantitative real-time polymerase chain reaction, normalized to β-actin. (E) Human liver sections were stained for CCR6 and CCL20; representative pictures from n = 3 individual patients per group are shown. Inserts display higher magnifications of positively stained areas. Scale bar: 100 μm. HCV, chronic hepatitis C virus infection, ASH, alcoholic steatohepatitis. *P < 0.05; **P < 0.01; ***P < 0.001. Data are shown as mean ± standard error of the mean.
To assess whether CCR6 and CCL20 are also up-regulated in experimental murine fibrosis, c57bl/6 mice were IP injected with CCl4 thrice-weekly for 4 weeks. CCl4-challenged mice showed significantly higher hepatic gene expression levels of Ccr6 and Ccl20, compared to controls (Supporting Fig. 2A,B), resembling the results obtained in human samples. Furthermore, murine primary hepatocytes, but not freshly isolated primary hepatic stellate cells (HSCs) and Kupffer cells (KCs)/macrophages, clearly up-regulated Ccl20 upon CCl4 treatment, compared to cells from untreated livers (Supporting Fig. 2C,D). Together, these results demonstrated that the CCR6/CCL20 pathway is activated in CLD in men and mice, and that injured hepatocytes strongly induce Ccl20 expression, thus likely regulating CCL20-mediated chemotaxis on CCR6-expressing leukocytes upon liver injury.
Ccr6−/− Mice Develop More Severe Hepatic Fibrosis Upon Chronic Liver Injury
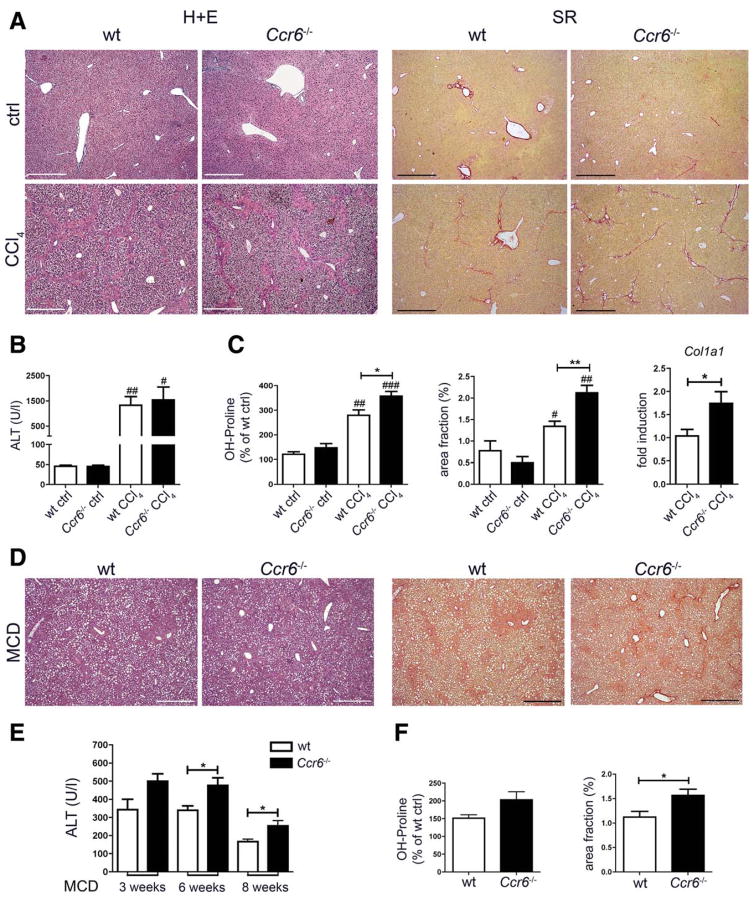
To elucidate the functional relevance of CCR6/CCL20 for liver disease progression, liver fibrosis was induced by repetitive CCl4 injections in WT and Ccr6−/− mice. Conventional histology stainings showed severely damaged liver architecture in WT and Ccr6−/− mice with large areas of necrotic hepatocytes and dense periportal inflammatory infiltrates, alongside strongly elevated aminotransferase activities in serum (Fig. 2A,B). Strikingly, extracellular collagen deposition, as revealed by Sirius Red stainings, was significantly enhanced in Ccr6−/− mice, compared to WT mice (Fig. 2A). Accelerated fibrosis development in Ccr6−/− mice was confirmed by increased hepatic concentrations of hydroxyproline, an amino acid abundantly present in collagen matrices, higher area fraction by Sirius Red staining, and increased expression of Collagen1A1 in fibrotic livers of Ccr6−/−, compared to WT, mice (Fig. 2C).
Fig. 2.
CCR6 deficiency promotes hepatic fibrosis in mice. (A–C) WT and Ccr6−/− mice were challenged with CCl4 thrice-weekly for 4 weeks or left untreated. (A) Representative hematoxylin and eosin (H+E) and Sirius Red (SR) stainings of WT and Ccr6−/− mice. (B) Alanine aminotransferase (ALT) serum activities in WT and Ccr6−/− mice. (C) Hydroxyproline (OH-Proline) measurement, quantification of matrix deposition from Sirius Red stainings (derived from stainings as shown in A) and expression of Collagen1a1 (Col1a1) in livers of WT and Ccr6−/− mice. (D–F) WT and Ccr6−/− mice were fed with MCD diet for 8 weeks to induce steatohepatitis. (D) Representative H+E and Sirius Red stainings. (E) Serum ALT levels at indicated time points. (F) Hydroxyproline measurement and quantification of matrix deposition from livers of WT and Ccr6−/− mice. */#P < 0.05; **/##P < 0.01; ***/###P < 0.001. #Compared to control conditions. Scale bars: 500 μm. Data are derived from n = 7–10 mice per group and shown as mean ± standard error of the mean. To accurately compare results for fibrosis from different experiments, data were normalized to the respective WT control liver (=100%) from the same experiment.
To confirm the general relevance of the CCR6/CCL20 pathway for hepatic fibrogenesis, mice were also subjected to MCD diet as an independent model of chronic metabolic liver injury, resulting in severe steatohepatitis.17 Liver injury was enhanced in Ccr6−/−, compared to WT, mice during MCD diet, as evidenced by histology and liver enzymes (Fig. 2D,E and data not shown). Moreover, Ccr6−/− mice displayed more severe hepatic fibrosis after 8 weeks of MCD diet, compared to WT animals (Fig. 2D,F). Collectively, these results indicated that CCR6/CCL20 represents a protective chemokine pathway during chronic liver injury and hepatofibrogenesis.
Infiltration of Leukocytes Into Damaged Liver is Enhanced in Ccr6−/− Mice
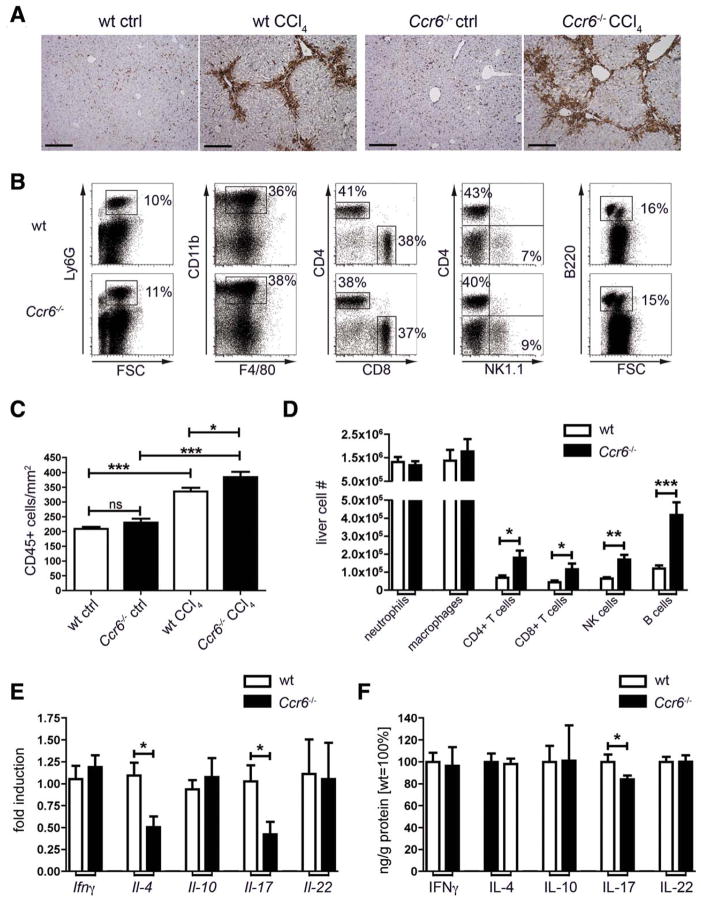
Livers of Ccr6−/− mice showed significantly higher intrahepatic leukocyte accumulation upon injury, compared to WT mice, as evidenced by IHC for CD45 (Fig. 3A). By fluorescence-activated cell sorting (FACS) analysis of intrahepatic immune cell subsets (Fig. 3B), we could not observe differences in neutrophils or macrophages, whereas all major lymphocyte subsets (T, B, and natural killer [NK] cells) were significantly increased in livers of Ccr6−/− CCl4-treated mice, compared to WT animals (Fig. 3C,D). Importantly, the relative composition of the different major immune cell populations in injured livers did not significantly differ between WT and Ccr6−/− mice (Fig. 3B and Supporting Fig. 3).
Fig. 3.
Leukocyte infiltration and Th subset composition in injured livers of WT and CCR6-deficient mice. Wt and Ccr6−/− mice were treated thrice-weekly with CCl4 for 4 weeks or left untreated. (A) IHC for the pan-leukocyte marker CD45 in livers of WT and Ccr6−/− mice. Scale bar: 200 μm. (B) Liver leukocytes (CD45+, Hoechst-33258 negative) were subdivided into neutrophils (Ly6G+), macrophages (CD11b+F4/80+), T cells (CD3+CD4+, CD3+CD8+), NK cells (CD3−NK1.1+), and B cells (B220+). Representative FACS plots from livers of CCl4-treated mice. (C) Quantification of (A). (D) Absolute numbers of intrahepatic leukocyte subpopulations shown in (B). (E) Expression of signature cytokines for CD4 Th cell subtypes was measured by quantitative polymerase chain reaction from total liver, namely, Ifnγ (Th1), Il-4 (Th2), Il-10 (Treg), and Il-17 and Il-22 (Th17). (F) Concentrations of cytokines were measured by enzyme-linked immunosorbent assay from liver and normalized to total protein content in liver extracts. Because of different absolute cytokine concentrations, values are displayed as % of fibrotic WT liver. *P < 0.05; **P < 0.01; ***P < 0.001. Data are derived from n = 6–10 mice per group and shown as mean ± standard error of the mean.
CCR6 expression had been reported on distinct CD4 T-cell subsets, in particular, Th17 and Treg cells.18 Thus, we analyzed the expression of the signature cytokines for Th1, Th2, Th17, and Treg cells in the liver, which are interferon (IFN)-γ, IL-4, IL-17, and IL-10, respectively. There was no difference in IFN-γ or IL-10 gene expression and protein concentrations between WT and Ccr6−/− mice (Fig. 3E,F). Il-4 gene expression was significantly reduced in livers of CCl4-treated Ccr6−/− mice, compared to WT mice (Fig. 3E), but not on protein level (Fig. 3F).
More striking, IL-17 expression, but not IL-22, was significantly reduced in livers of Ccr6−/−, compared to WT, mice, both on mRNA and protein level (Fig. 3E,F). Nonetheless, we also checked for gene and protein expression of Th-lineage-specific transcription factors T-bet (Th1), Gata3 (Th2), FoxP3 (Treg), or Rorγt (Th17; Supporting Fig. 4A–C), indicating that differentiation of intrahepatic Th cells did not change upon the absence of CCR6. Hence, whereas our results demonstrated that Ccr6−/− mice develop more severe hepatic inflammation after CCl4 treatment with higher immune cell infiltration, compared to WT mice, this effect appeared to not be related to alterations in the CD4 Th cell compartment.
Infiltration of IL-17-Producing γδ T Cells Is Reduced in the Absence of CCR6
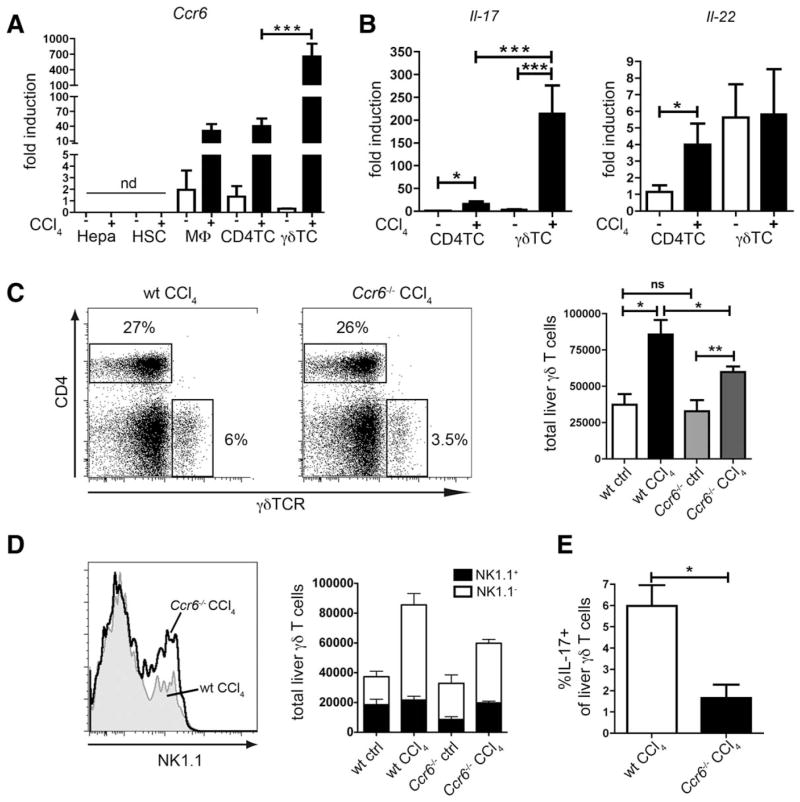
To identify the CCR6-dependent cellular compartment responsible for limiting the inflammatory response in injured liver, we isolated different primary cell types by FACS sorting from murine livers to analyze Ccr6 expression. We could not detect Ccr6 expression in liver-resident cell populations, such as hepatocytes and HSCs, but noted a clear Ccr6 induction in macrophages and CD4 T cells upon injury (Fig. 4A). However, the most remarkable Ccr6 up-regulation was detected on γδ T cells after CCl4 treatment (Fig. 4A). Moreover, both cell types up-regulated Il-17 after CCl4 treatment, but, again, expression in γδ T cells was much higher than in CD4 T cells (Fig. 4B). Il-22 was also expressed by both CD4 T cells and γδ T cells, with a tendency toward higher expression in γδ T cells (Fig. 4B).
Fig. 4.
Intrahepatic accumulation of IL-17 producing γδ T cells is impaired in Ccr6−/− mice upon chronic injury. (A) Hepatocytes (Hepa), HSC, macrophages (MΦ), CD4+ T cells (CD4TC), and γδ T cells (γδ TC) were isolated by FACS sorting from livers of WT mice treated with CCl4 or control mice. Ccr6 expression was determined by quantitative polymerase chain reaction. (B) CD4+ T cells and γδ T cells were FACS sorted from WT livers. Il-17 and Il-22 expression by quantitative polymerase chain reaction. (C) Absolute numbers of γδ T cells (CD3+CD4−γδTCR+) in livers of WT or Ccr6−/− mice treated with CCl4 or control mice. Representative FACS plots from livers of CCl4-treated mice. (D and E) Same as (C) with additional staining for NK1.1 and IL-17 after restimulation with phorbol 12-myristate 13-acetate PMA/ionomycin to discriminate γδ T-cell subtypes. (D) Representative histograms of NK1.1 expression by hepatic γδ T cells (CCl4-treated mice, gated on CD3+CD4−γδTCR+) and quantification of NK1.1+ and NK1.1− γδ T cells. (E) Quantification of IL-17+ γδ T cells in the liver (CCl4-treated mice, IL-17 expression determined by intracellular FACS staining). *P < 0.05; **P < 0.01; ***P < 0.001. Data are derived from n = 6–10 mice per group and shown as mean ± standard error of the mean.
These analyses hinted at a CCR6-dependent hepatic recruitment of γδ T cells in chronic liver injury. In fact, γδTCR+ T cells increased upon CCl4 treatment in livers of WT and Ccr6−/− mice (Fig. 4C). However, Ccr6−/− mice showed significantly reduced intrahepatic γδ T cells, compared to WT mice, after CCl4 treatment (Fig. 4C).
γδ T cells can be classified into two major subsets according to their surface expression of NK1.1 or CCR6. NK1.1+ γδ T cells produce mainly IFN-γ, whereas CCR6+ γδ T cells primarily produce IL-17 and share some features with Th17 cells.12 In healthy livers, approximately half of the γδ T cells were NK1.1+ in WT, but only 25% in Ccr6−/− mice (Fig. 4D). After CCl4 treatment, the relative amount of NK1.1+ γδ T cells decreased significantly in WT mice as a result of an influx of CCR6+ γδ T cells (Fig. 4D). In contrast, the NK1.1+ subset did not change considerably in Ccr6−/− after CCl4 treatment, shifting the balance of both subtypes toward NK1.1+ γδ T cells in the injured liver of Ccr6−/− mice (Fig. 4D). Importantly, in Ccr6−/− mice, the numbers of IL-17+ γδ T cells were significantly lower than in WT mice upon CCl4 treatment (Fig. 4E). Taken together, these results strongly indicated that the infiltration of IL-17-producing γδ T cells into injured liver depended on the chemokine receptor, CCR6.
IL-17-Producing γδ T Cells Require CCR6 to Traffic to the Liver
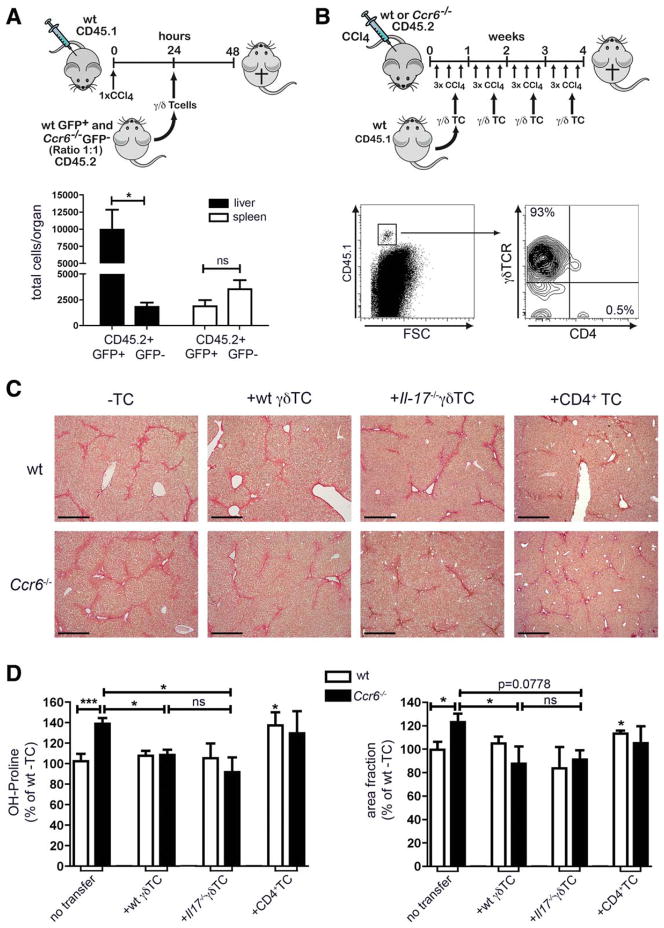
Thus, we tested the hypothesis that the IL-17-producing subset of γδ T cells employs CCR6 to migrate to injured liver. Therefore, splenic γδ T cells were isolated from enhanced green fluorescent protein (eGFP) transgenic WT mice and GFP− Ccr6−/− mice (both from the CD45.2 background), mixed in a ratio of 1:1 and injected intravenously (IV) into congenic WT mice (carrying the CD45.1 allele) that had been treated once with CCl4 24 hours before γδ T-cell transfer (Fig. 5A). Twenty-four hours after transfer, γδ T cells were analyzed in liver and spleen. In livers, we found overwhelming numbers of GFP+ (=WT) γδ T cells, but only very few GFP− (=Ccr6−/−) γδ T cells. Very little transferred cells could be found in the spleen with no difference in numbers between WT and Ccr6−/− T-cells, corroborating that CCl4 administration induced a strong recruitment signal for transferred γδ T cells to the liver (Fig. 5A). These results indicated that γδ T cells indeed require CCR6 to traffic to liver upon injury.
Fig. 5.
Reconstitution of Ccr6−/− mice with γδ T cells, but not with CD4+ T cells, reduces hepatic fibrosis. (A) Splenic γδ T cells were isolated from actin-eGFP or Ccr6−/− mice, mixed in a 1:1 ratio and injected into CD45.1+ B6-mice that had been treated with CCl4 once (24 hours before T-cell injection). Twenty-four hours after T-cell injection absolute numbers of CD45.2+GFP+ WT and CD45.2+GFP− Ccr6−/− γδ T cells in liver and spleen were determined. (B–D) WT and Ccr6−/− mice were treated with CCl4 thrice-weekly over 4 weeks. Simultaneously, splenic CD4+ or γδ T cells were isolated from CD45.1+ mice and injected IV once per week. Mice received either wt γδ T cells (100,000–150,000 cells), Il-17−/− γδ T cells (100,000–150,000 cells), or WT CD4+ T cells (1,000,000–1,500,000 cells) during CCl4 treatment. (B) Leukocytes were isolated from liver and stained for CD45.1, CD4, and γδ TCR to identify transferred cells (representative plot shown). (C) Sirius Red stainings of liver paraffin sections. Scale bar: 500 μm. (D) Measurement of hepatic hydroxyproline and quantification of ECM deposition. *P < 0.05. Data are derived from n = 6 mice per group and shown as mean ± standard error of the mean. To accurately compare results for fibrosis from different experiments, data were normalized to the respective WT liver without cell transfer (=100%) from the same experiment.
Adoptive Transfer of WT γδ T Cells, but Not of CD4 T Cells, Limits Hepatic Fibrogenesis in Ccr6−/− Mice Independent of IL-17
To provide functional evidence that the recruitment of CCR6-expressing γδ T cells is responsible for limiting CCl4-mediated hepatic injury and fibrogenesis, we reconstituted Ccr6−/− mice with WT γδ T cells. Therefore, WT and Ccr6−/− mice were treated with repetitive CCl4 injections, and mice additionally received WT γδ T cells (100,000–150,000 cells) isolated from spleens of congenic mice (CD45.1) IV once per week (Fig. 5B). After 4 weeks of CCl4 treatment, a clear population of (transferred) CD45.1+ cells could be found in the liver that expressed the γδ T-cell receptor (TCR), but not CD4 (Fig. 5B). Ccr6−/− mice that received wt γδ T cells showed significantly less hepatic fibrosis than without transfer (Fig. 5C,D). Moreover, the weekly adoptive transfer of WT γδ T cells to Ccr6−/− mice was capable of fully restoring the fibrosis phenotype to the level of WT mice (Fig. 5C,D). Notably, the adoptive transfer of γδ T cells into WT mice did not significantly affect fibrosis progression, which might be related to the overall low numbers of transferred γδ T cells, compared to the endogenous hepatic γδ T-cell pool already present in fibrotic WT mice.
Next, we investigated the mechanisms by which CCR6-expressing γδ T cells restrict hepatic inflammation and fibrosis. IL-17 was the primary cytokine produced by hepatic CCR6+ γδ T cells in injured liver, but IL-17 signaling in macrophages and HSCs is considered profibrogenic in murine liver fibrosis.19 To test whether CCR6-expressing γδ T cells limit hepatofibrogenesis by IL-17, we reconstituted Ccr6−/− mice with IL-17-deficient γδ T cells. The fibrosis phenotype of Ccr6−/− mice injected with Il-17−/− γδ T cells was fully restored to WT level, similar to adoptive transfer of Il-17+ γδ T cells (Fig. 5C,D).
To ensure that accelerated fibrosis in Ccr6−/− mice was a consequence of reduced γδ T cells, but not of alterations in the CD4 T-cell compartment, we also transferred WT CD4+ T cells into CCl4-challengend WT and Ccr6−/− mice. As anticipated,2 the adoptive transfer of CD4 T cells enhanced collagen deposition in WT mice, but did not further increase the strong fibrosis in Ccr6−/− mice (Fig. 5C,D), corroborating that CCR6 was specifically important for γδ T cells in vivo. Taken together, our results provided experimental evidence that γδ T cells modulate hepatic inflammation and restrict fibrosis progression, but these effects were unrelated to IL-17 secretion by these cells.
Hepatic γδ T Cells Directly Promote HSC Apoptosis
We hypothesized that γδ T cells interact with HSCs, the major collagen-producing cell type in the liver.1 We isolated γδ T cells, CD4+ T cells, and NK cells from livers of WT mice that had received a single CCl4 injection and cocultured these cells with GRX cells, a murine HSC cell line, for 48 hours. As a positive control, GRX cells were stimulated with transforming growth factor (TGF)-β1, whereas untreated cells served as negative controls. Hepatic NK cells were included in this experiment because they have been shown to exert antifibrotic functions through killing of activated HSCs.20 GRX cells that were left untreated, incubated with TGF-β, or cocultured with CD4+ T cells showed a typical appearance of proliferating, myofibroblast-like cells after 48 hours (Supporting Fig. 5A). In contrast, upon coculture with hepatic γδ T cells, GRX cells grew less dense and had more round-shaped apoptotic myofibroblasts in culture (Supporting Fig. 5A). As expected, stimulation of GRX cells with TGF-β1 resulted in up-regulated expression of Collagen1 and the HSC activation marker PDGF-receptor-β, whereas coculture with NK cells reduced expression of these markers (Supporting Fig. 5B). Coculture of GRX cells with γδ T cells also significantly reduced Collagen1 expression, suggesting that γδ T cells impair HSC-mediated extracellular matrix (ECM) production (Supporting Fig. 5B). This function appeared cell-contact dependent, because coculture with γδ T-cell supernatant did not affect HSC activation markers (Supporting Fig. 5B). Notably, coculture of γδ T cells appeared to moderately increase the fraction of apoptotic cells detected by propidium iodide (PI)-based FACS analysis in GRX culture, similar to effects observed with NK cells (Supporting Fig. 5C).
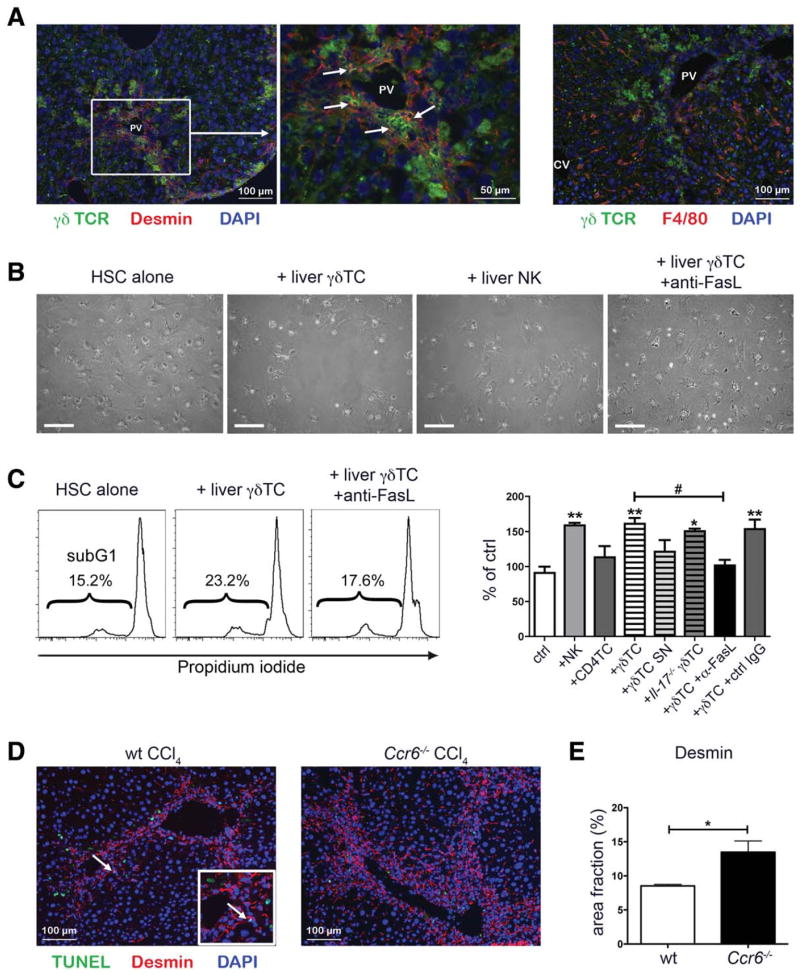
Next, we performed immunofluorescent double staining for γδ T cells (γδ TCR) and HSC (desmin) in fibrotic mouse livers to assess cell-cell interactions in vivo. In fact, HSCs frequently colocalized with γδ T cells in periportal areas of fibrotic livers (Fig. 6A), whereas hepatic macrophages (identified by F4/80) did not commonly show proximity to γδ T cells. Based on these observations in vitro (GRX cell line) and in vivo (CCl4 model), we hypothesized that γδ T cells might promote apoptosis of HSCs, similar to the antifibrotic effects of NK cells. In fact, hepatic γδ T cells isolated from fibrotic, compared to control, livers showed increased expression of the apoptosis-inducing protein Fas ligand (FasL; CD95L; Supporting Fig. 5D). Coculture of primary HSCs, freshly isolated from murine livers, with either isolated hepatic γδ T or NK cells, but not with hepatic CD4 T cells, significantly increased the fraction of apoptotic primary HSCs, as evidenced by the sub-G1 fraction upon PI staining, in culture (Fig. 6B,C). Coculture of HSCs with Il-17−/− γδ T cells also significantly increased the fraction of apoptotic HSCs (Fig. 6C), demonstrating that this mechanism is IL-17 independent. It had been reported that IL-22, which is expressed by hepatic γδ T cells (Fig. 4B), might be a paracrine factor to induce senescence of HSCs.21 However, the effects of hepatic γδ T cells on HSCs were cell-cell contact dependent, because γδ T-cell supernatant (SN) did not increase HSC apoptosis (Fig. 6C). Moreover, specific inhibition by an anti-IL-22 antibody (Ab) did not abrogate γδ T-cell-induced HSC apoptosis (Supporting Fig. 5E). When a monoclonal FasL-blocking Ab was added to the coculture with hepatic γδ T cells, the rate of apoptotic primary HSCs was significantly reduced to baseline levels (Fig. 6B,C).
Fig. 6.
γδ T cells induce HSC apoptosis. (A) WT mice were treated thrice-weekly with CCl4 for 4 weeks. Liver sections were stained for γδ TCR to identify γδ T cells (green) and desmin to identify HSC (red, left and middle panel) or F4/80 to identify macrophages (red, right panel). Nuclei were counterstained with DAPI (blue). PV, portal vein; CV, central vein. (B and C) Hepatic CD4+ T cells, γδ T cells, and NK cells were isolated by FACS sorting from WT mice 48 hours after a single CCl4 injection. Either isolated cells or supernatant from overnight cultures were incubated with primary HSCs, isolated from WT mice, for 48 hours. (B) Representative pictures of cocultures. Scale bar: 200 μm. (C) HSCs were fixed, permeabilized, and stained with PI for FACS analysis. Representative histograms with sub-G1 cells are shown. Amounts of apoptotic cells are shown as percent of control. (D) WT and Ccr6−/− mice were treated thrice-weekly with CCl4 for 4 weeks. Liver sections were stained for desmin to identify HSCs (red) and TUNEL to identify apoptotic cells (green). Nuclei were counterstained with DAPI (blue). Arrow indicates a representative apoptotic HSC in liver of CCl4-treated WT mice. (E) Quantification of desmin-positive areas in pictures shown in (D). */#P < 0.05; **P < 0.01; ***P < 0.001. *Compared to control. Data are expressed as mean ± standard error of the mean from three independent experiments. DAPI, 4′,6-diamidino-2-phenylindole.
To assess whether γδ T-cell-induced HSC apoptosis is involved in limiting hepatic fibrosis of WT, compared to Ccr6−/−, mice, liver sections of CCl4-treated WT and Ccr6−/− mice were analyzed for the presence of apoptotic HSCs by costaining for desmin and TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling, which marks DNA fragmentation of apoptotic cells). Although the rate of apoptotic HSCs was low, even in WT livers, Ccr6−/− mice showed tremendously higher numbers of desmin+ HSCs in portal areas, consistent with reduced HSC apoptosis during the course of chronic liver injury (Fig. 6D,E). These data strongly support that hepatic γδ T cells promote apoptosis of HSCs, in a FasL-dependent manner, thereby limiting profibrogenic functions of stellate cells.
Discussion
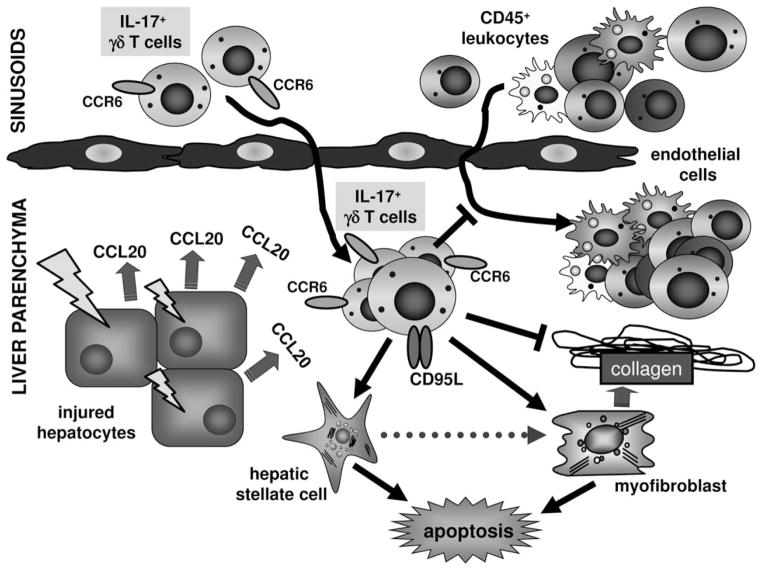
Chronic liver injury characteristically provokes a complex inflammatory reaction with tightly regulated interactions between damaged parenchymal cells, as well as resident and infiltrating immune cells. During recent years, increased expression of CC and CXC chemokines and their receptors has been revealed in hepatic fibrosis.2 However, very little is known about chemokine-mediated pathways that down-regulate inflammation or exert antifibrotic actions. As such, CX3CR1-CX3CL1 represents an anti-inflammatory and prosurvival signal for intrahepatic monocyte-derived macrophages in liver fibrosis,22 or CXCR3 and its ligand, CXCL9, modulate intrahepatic T-cell responses toward a net antifibrotic effect.23 In our study, we demonstrate that CCR6-CCL20 is functionally involved in chronic liver injury and fibrogenesis. Our data revealed that the CCR6-dependent recruitment of γδ T cells into injured liver critically limits hepatic inflammation and fibrosis in vivo (summarized schematically in Fig. 7).
Fig. 7.
Functional role of CCL20/CCR6 and γδ T cells in liver inflammation and fibrosis. Schematic depiction summarizing results from in vitro and in vivo experiments. Upon liver injury, hepatocytes and (especially in cholestatic diseases) injured large bile ducts induce expression of the chemokine CCL20 thereby attracting CCR6+ IL-17+ γδ T cells into injured liver. These γδ T cells reduce hepatic inflammation and fibrosis in chronic injury, independent of its signature cytokine IL-17. One important mechanism appears to be that hepatic γδ T cells promote apoptosis of HSCs and myofibroblasts in a cell-cell contact-dependent manner, involving FasL (CD95L).
Our functional studies in murine liver fibrosis were prompted by our observation that patients with different stages of hepatic fibrosis showed increased intrahepatic expression of both CCR6 and CCL20, in line with an earlier study.14 In human cholestatic liver diseases, CCR6 expression was linked to Th17 and CCL20 to BECs.4 Our data now revealed that also hepatocytes, in conditions of hepatic fibrosis, strongly up-regulate CCL20 in patients with CLDs. Concordantly, activation of the CCR6-CCL20 pathway was resembled in murine liver fibrosis, in which CCL20 expression was strongly induced by hepatocytes upon injury.
In agreement with our observation of an accumulation of CCR6-expressing mononuclear cells in periportal regions of fibrotic human livers, we detected CCR6 expression on macrophages, CD4 T cells, and γδ T cells in experimental murine fibrosis. Interestingly, we did not observe a migratory defect in CD4 T cells or macrophages in Ccr6-deficient mice. In fact, CD4 T cells were significantly elevated in the absence of CCR6. This contrasts studies with Ccr6−/− mice in autoimmune-mediated inflammatory diseases, such as EAE or glomerulonephritis, 10,11 in which lack of CCR6 significantly impaired recruitment of distinct CD4 T-cell subsets, namely, Treg and Th17 cells, to the inflamed organ. In both cases, Ccr6−/− mice developed aggravated disease, most probably because of reduced numbers of regulatory T cells. Our model of CCl4-induced liver fibrosis markedly differs from EAE or glomerulonephritis, because liver injury by CCl4 is not directly immune mediated, but inflammation evolves as a consequence of tissue damage. Moreover, the liver is anatomically distinct from other organs, such as kidney or brain, because of its vast blood supply from portal veins and hepatic arteries, its rather leaky endothelium, and also its abundance of resident innate immune cells, including macrophages, NKT, or other unconventional T cells.6 Our results clearly showed that neither migration of hepatic CD4 T cells was impaired in Ccr6−/−, compared to WT, mice in CCl4-induced fibrogenesis nor was the differentiation of the main Th cell subsets affected in fibrotic Ccr6−/− animals. In addition, we could show that reconstitution of Ccr6−/− mice with WT CD4 T cells did not affect fibrosis progression in these mice. However, WT mice that received CD4 T cells showed enhanced fibrogenesis, compared to WT mice that did not receive T cells, most likely as a result of the transfer of profibrogenic (e.g., Th17 cells)19 Th cell subsets.
Our experiments revealed that the main CCR6-expressing and -responsive cell type in injured liver are γδ T cells. By isolating primary immune cells from fibrotic murine livers, γδ T cells were found to express CCR6 much higher than CD4 T cells or macrophages, alongside higher IL-17 and IL-22 expression, compared to CD4 T cells. This is consistent with recent work describing CCR6 and NK1.1 as surface markers of γδ T cells that allow distinguishing between IL-17- and IFN-γ-producing γδ T cells, respectively.12 Although it has been noted before that the liver is highly enriched for γδ T cells in general,6 their functional role in liver fibrosis had remained elusive. In a model of mild chronic injury, mice deficient for the γδ TCR showed similar early-stage fibrosis as WT mice.24 In our models of advanced experimental fibrosis, we found significantly less hepatic γδ T cells in Ccr6−/−, compared to WT, mice. Furthermore, this reduction of hepatic γδ T cells in Ccr6−/− mice was specifically caused by a lack of recruitment of IL-17-producing γδ T cells. Adoptive transfer experiments revealed that (IL-17-producing) γδ T cells utilize the CCR6-CCL20 axis to migrate to the injured liver and that this subset of γδ T cells was functionally responsible for restricting inflammation and fibrosis in the chronic liver injury model.
Very little is known about the functional role of CCR6+ γδ T cells in inflammatory conditions in vivo. CCR6+ γδ T cells were described to express TLRs TLR1 and TLR2, possibly enabling them to directly interact with certain pathogens. Thus, γδ T cells may act as an efficient first line of defense that could orchestrate an inflammatory response to pathogen-derived, as well as environmental, signals long before adaptive immunity (e.g., Th17 cells) has sensed bacterial invasion. 25 Similar observations were reported in a model of crescentic glomerulonephritis, in which renal γδ T cells produced IL-17 and IL-23, thus providing proinflammatory signals very early in the course of disease.26 However, in CCl4-induced chronic liver damage, CCR6-expressing γδ T cells accumulated in injured liver and restricted the overall inflammatory response as well as subsequent fibrosis development. Interestingly, an anti-inflammatory function of γδ T cells has been recently observed in an experimental model of colitis as well.27 Further studies are needed to define different subsets of γδ T cells in humans, especially in specific compartments, such as healthy or diseased livers. Translational research from other disease models, such as skin inflammation, support that functionally distinct subsets of human γδ T cells exist as well, as exemplarily revealed in patients with psoriasis.28
Importantly, the anti-inflammatory and -fibrotic effects of hepatic γδ T cells were not mediated by its signature cytokine, IL-17. This is in full agreement with earlier studies that assigned profibrogenic functions to IL-17, derived by Th17 and myeloid cells, in inflamed liver.19,29 In fact, our experiments unraveled direct interactions of hepatic γδ T cells with stellate cells in the liver, because γδ T cells were found to facilitate HSC apoptosis in vitro. Concordantly, HSC numbers and activation were strongly enhanced in vivo in CCl4-treated Ccr6−/−, compared to WT, livers. These processes appeared cell-cell contact dependent, because HSC apoptosis could neither be induced by supernatants of γδ T cells nor blocked by anti-IL-22 Ab, but could be inhibited by FasL-blocking Ab. However, this does not exclude other protective mechanisms apart from Fas-mediated HSC apoptosis, for example, induction of HSC senescence by IL-2221 or alterations of the hepatic microenvironment by other soluble factors.
Taken together, our study not only unraveled a yet-unrecognized anti-inflammatory and -fibrotic function for CCR6/CCL20 in liver fibrosis in vivo, but also illuminates, for the first time, functional aspects of the pathogenic involvement of hepatic γδ T cells in chronic liver injury. The subset of IL-17/IL-22-producing γδ T cells is recruited by CCR6 to injured liver and restricts inflammation and fibrogenesis, likely by inhibiting HSC functionality. Future studies are needed to address the therapeutic potential of augmenting the CCR6/CCL20 pathway and/or γδ T-cell functionality in liver fibrosis.
Supplementary Material
Acknowledgments
The authors sincerely thank Aline Roggenkamp, Carmen Tag, Sibille Sauer-Lehnen, and Christiane Esch for their excellent technical assistance.
This work was supported by the German Research Foundation (DFG; Ta434/2-1, SFB/TRR 57) and Interdisciplinary Center for Clinical Research (IZKF), Aachen. The authors thank Prof. Ralf Weiskirchen for providing GRX cells.
Abbreviations
- Ab
antibody
- BECs
biliary epithelial cells
- CCL
C-C motif chemokine ligand
- CCR
C-C motif chemokine receptor
- CLDs
chronic liver diseases
- EAE
experimental autoimmune encephalomyelitis
- ECM
extracellular matrix
- eGFP
enhanced green fluorescent protein
- FACS
fluorescence-activated cell sorting
- FasL
Fas ligand
- HSC
hepatic stellate cell(s)
- IHC
immunohistochemistry
- IV
intravenously
- KC
Kupffer cells
- IFN
interferon
- IL
interleukin
- IP
intraperitoneally
- MIP-3α
macrophage inflammatory protein 3alpha
- MCD
methionine-choline deficient
- mRNA
messenger RNA
- NK
natural killer
- PI
propidium iodide
- TCR
T cell receptor
- TGF
transforming growth factor
- Th
T helper
- Treg
regulatory T-cells
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
wild type
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets. 2011;10:509–536. doi: 10.2174/187152811798104890. [DOI] [PubMed] [Google Scholar]
- 3.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 4.Oo YH, Banz V. CXCR3 dependent recruitment and CCR6 mediated positioning of Th-17 cells in the inflamed liver. J Hepatol. 2012;57:1044–1051. doi: 10.1016/j.jhep.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060–1076. doi: 10.1016/s0016-5085(03)01194-6. [DOI] [PubMed] [Google Scholar]
- 6.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Sun R, Wei H, Tian Z. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: interaction of gammadelta T cells with macrophages. Hepatology. 2013;57:373–384. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 8.Zhao N, Hao J, Ni Y, Luo W, Liang R, Cao G, et al. Vgamma4 gammadelta T cell-derived IL-17A negatively regulates NKT cell function in Con A-induced fulminant hepatitis. J Immunol. 2011;187:5007–5014. doi: 10.4049/jimmunol.1101315. [DOI] [PubMed] [Google Scholar]
- 9.Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 10.Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martinez AC, Varona R. CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol. 2009;39:1671–1681. doi: 10.1002/eji.200839123. [DOI] [PubMed] [Google Scholar]
- 11.Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel JH, Erhardt A, et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol. 2010;21:974–985. doi: 10.1681/ASN.2009070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, et al. CCR6 and NK1. 1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 13.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu Y, Murata H, Kashii Y, Hirano K, Kunitani H, Higuchi K, Watanabe A. CC-chemokine receptor 6 and its ligand macrophage inflammatory protein 3alpha might be involved in the amplification of local necroinflammatory response in the liver. Hepatology. 2001;34:311–319. doi: 10.1053/jhep.2001.26631. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, et al. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–426. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 21.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, et al. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology. 2010;52:1769–1782. doi: 10.1002/hep.23894. [DOI] [PubMed] [Google Scholar]
- 23.Wasmuth HE, Lammert F, Zaldivar MM, Weiskirchen R, Hellerbrand C, Scholten D, et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology. 2009;137:309–319. 319.e1–3. doi: 10.1053/j.gastro.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Turner JE, Krebs C, Tittel AP, Paust HJ, Meyer-Schwesinger C, Bennstein SB, et al. IL-17A production by renal gammadelta T cells promotes kidney injury in crescentic GN. J Am Soc Nephrol. 2012;23:1486–1495. doi: 10.1681/ASN.2012010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, et al. Retinoic acid expression associates with enhanced IL-22 production by gammadelta T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187:2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. doi: 10.1155/2011/345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.