Abstract
We tested the substrate range of four wild-type Escherichia coli aminoacyl-tRNA synthetases (AARSs) with a library of non-standard amino acids (nsAAs). While these AARSs could discriminate efficiently against the other canonical amino acids, they were able to use many nsAAs as substrates. Our results also showed E. coli tryptophanyl-tRNA synthetase (TrpRS) and tyrosyl-tRNA synthetase to have overlapping substrate ranges. In addition, we found that the nature of the anticodon sequence of tRNATrp altered the nsAA substrate range of TrpRS; this implies that the sequence of the anticodon affects the TrpRS amino acid binding pocket. These results highlight again that inherent AARS polyspecificity will be a major challenge to the goal of incorporating multiple different amino acids site-specifically into proteins.
Keywords: aminoacyl-tRNA synthetase, natural nonsense tRNA suppressor, non-standard amino acid, substrate specificity, synthetic biology
Introduction
Aminoacyl-tRNA synthetases (AARSs) are thought to be highly specific for their cognate amino acid substrates[1]. However, studies over the past fifty years indicated that some non-standard amino acids (nsAAs) are readily incorporated into bacterial proteins[2]. In vitro experiments with a mixture of all Escherichia coli AARSs showed that more than 90 nsAAs can be acylated onto tRNA[3]. Protein engineering directed toward expansion of the genetic code during the past decade[4] led to many variant AARS enzymes with substrate polyspecificity for some of the nsAAs employed[4a, 5]. Some variants of Methanococcus jannaschii tyrosyl-tRNA synthetase (TyrRS)[5i] and of pyrrolysyl-tRNA synthetase can acylate more than ten different compounds[5e, 5f]. Therefore, the high specificity observed by the AARSs against the other canonical amino acids does not extend to a large number of nsAAs.
We decided to examine a number of canonical wild-type E. coli AARSs for their substrate specificity with a large library of nsAAs. As test enzymes we used E. coli leucyl-tRNA synthetase (LeuRS), seryl-tRNA synthetase (SerRS), tryptophanyl-tRNA synthetase (TrpRS) and TyrRS. Each of these enzymes has a natural nonsense suppressor tRNA[6] that, when acylated, can serve to insert its amino acid in response to a stop codon (e.g. the amber (UAG) and opal (UGA) codons) engineered into the second codon of the superfolder green fluorescent protein (sfGFP) mRNA. In order to decrease the competition of the cognate amino acid with the nsAAs to be tested, the experiments were performed in E. coli auxotrophic strains for the cognate amino acid[7]. Our results lend further support to our view of natural AARSs being polyspecific for nsAAs.
Results
Experimental scheme
We used the E. coli wild-type AARS enzymes with their native amber or opal suppressor tRNAs[6]. They were supU (tRNATrpCUA), trpT (tRNATrpTyrUCA), supF (tRNA), supP (tRNALeuCUA CUA) and supD (tRNASerCUA). The TyrRS/supF pair was used to optimize the fluorescence signal arising from read-through of the sfGFP-UAG2 mRNA in a tyrA− auxotrophic stain (Figure S1). The auxotrophic strains (Table S1) transformed with the genes for the AARS/suppressor tRNA pair (Figure S2 and Table S2) were grown at 37°C for 16 h in minimal medium with 1% glycerol supplemented with the 20 canonical amino acids (1 mM). After washing to remove the amino acids and transfer to minimal medium, the strains were cultured in a 384-well plate format where each well contained one member of our nsAA library at 1 mM concentration. The nsAAs library contained 313 members, of which 272 were previously reported [5b] (Tables S3-S5). The fluorescence readout, which was contingent on suppression of the sfGFP-UAG2 mRNA, was used as a measure of the ability of the corresponding endogenous AARS (LeuRS, SerRS, TrpRS and TyrRS) to recognize the nsAA substrates. This allowed a measurement of the range of amino acid substrate specificity for the AARS.
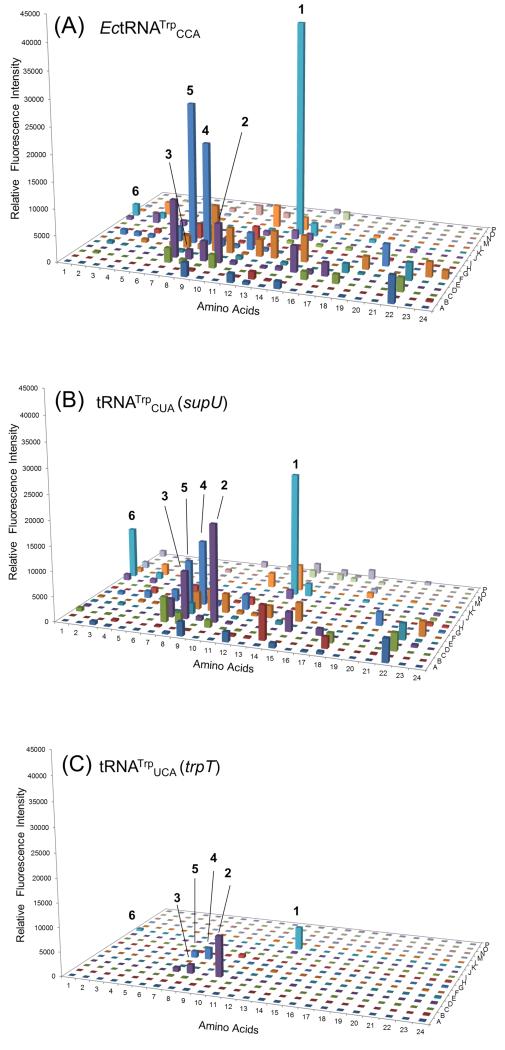
The wild-type protein sequence of sfGFP contains a single Trp (1) residue (W57). Under our incorporation conditions with wild-type tRNATrpCCA this W57 could also be replaced by Trp analogs (2-4), Cys analog 5 and Phe analog 6 (Scheme 1; Figure 1A). The fluorescence intensities generated by nsAAs 4 and 5 were comparable to that of Trp. A similar substrate preference was observed with the TrpRS/supU pair (Figure 1B), although the pattern of suppression efficiencies was different. This change in substrate range stemmed purely from the different anticodon sequence, since the supU tRNA differs only by a single base change from the endogenous tRNATrpCCA isoacceptor.[6] We also tested the TrpRS substrate range with the natural opal suppressor tRNATrpUCA encoded by trpT. As expected, the TrpRS/trpT pair incorporated Trp (1) into sfGFP but at a lower efficiency (Figure 1C). Interestingly, a Trp lactic acid analog (2) (Figure 1C) was a better substrate than Trp for TrpRS/trpT pair. Thus, different tRNAs gave somewhat different suppression profiles suggesting that the nature of the anticodon may affect the amino acid recognition, a property that was also seen with glutaminyl-tRNA synthetase[8]. This suggests that in the strategy to develop orthogonal translation systems for genetic code expansion, modulations in amino acid recognition should be considered when tRNA anticodons are altered.
Scheme 1.
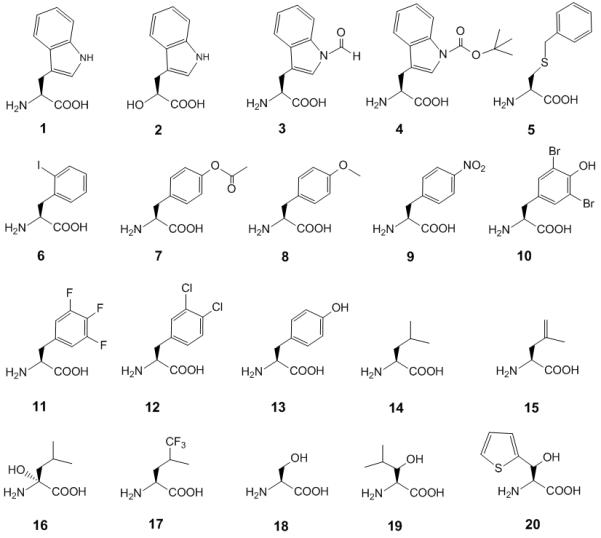
Chemical structures of canonical and non-standard amino acids mentioned in this study.
Figure 1.
Range of substrate specificities of TrpRS. Suppression of the sfGFP-UAG2 and sfGFP-UGA2 genes by the library of nsAA-tRNA was measured by fluorescence intensity. Four wells (A1, A2, I1 and I2) were set as control experiments without adding any nsAA to detect the background signals, which were used to normalize the signals generated by supplied nsAAs. (A) The substrate specificity profile of TrpRS/tRNATrpCCA by production of wild type sfGFP gene. (B) The substrate specificity profile of TrpRS/supU (tRNATrpCUA) by suppression of the sfGFP-UAG2 gene. (C) The substrate specificity profile of TrpRS/trpT (tRNATrpUCA) by suppression of the sfGFP-UGA2 gene. The cell strain JW2580-1 (trpC−) was used in these experiments. The nsAA chemical structures are provided in our reported nsAA library[5b]; Tables S4 and S5 describe the additional nsAAs not previously reported. The labeled nsAA chemical structures are given in Scheme 1.
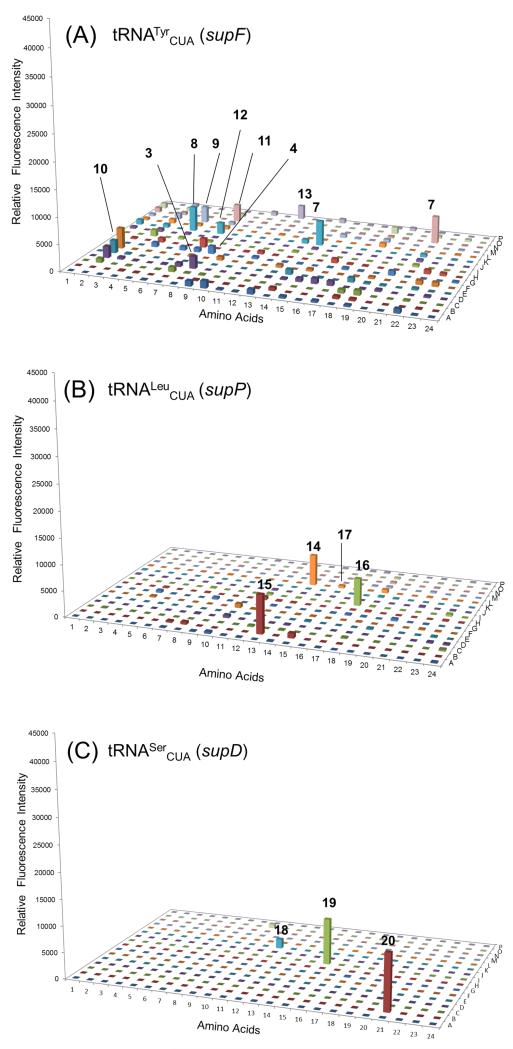
The substrate specificity profile of TyrRS/supF pair indicated that eight aromatic ring-containing nsAAs (3-4, 7-12) were substrates with appreciable signals but had low suppression efficiencies, albeit comparable to that of Tyr (13) (Figure 2A). The LeuRS/supP pair revealed three major fluorescence signals that indicated efficient incorporation of Leu (14) and two related nsAAs (15-16) that contained γ-vinyl and α-hydroxyl functional groups (Figure 2B). Previous studies showed γ-trifluoromethyl-leucine (17) to be a substrate for LeuRS [9]; our nsAA library screening also gave a small positive signal.
Figure 2.
Range of substrate specificities of TyrRS, LeuRS and SerRS. Suppression of the sfGFP-UAG2 gene by the library of nsAA-tRNACUA was measured by fluorescence intensity. Four wells (A1, A2, I1 and I2) were set as control experiments without adding any nsAA to detect the background signals, which were used to normalize the signals generated by supplied nsAAs. (A) The substrate specificity profile of TyrRS/supF (tRNATyrCUA) pair. (B) The substrate specificity profile of LeuRS/supP (tRNALeuCUA) pair. (C) The substrate specificity profile of SerRS/supD (tRNASerCUA) pair. The nsAA chemical structures are provided in our reported nsAA library[5b]; Tables S4 and S5 describe the additional nsAAs not previously reported. The labeled nsAA chemical structures are given in Scheme 1.
Our auxotrophic strain strategy provided sufficient amounts of Trp, Tyr, and Leu, but a much smaller concentration of Ser. Serine is involved in multiple cellular pathways, and is subject to high metabolic flux[10], and the higher conversion rate of serine may have led to a lower amino acid level. Thus, the SerRS/supD pair gave a low fluorescence signal with Ser (Figure 2C; 18), but high signals with two Ser analogs (Figure 2C; 19 and 20). Compared to the wider substrate range of the TyrRS (Figure 2A) and TrpRS (Figure 1B), the smaller substrate range of LeuRS (Figure 2B) and SerRS (Figure 2C) may be explained that the latter two synthetases have an editing function, while the former two do not[1a, 11].
LC-MS/MS analysis of amino acids incorporated at positions 2 and 57 of sfGFP-UAG2
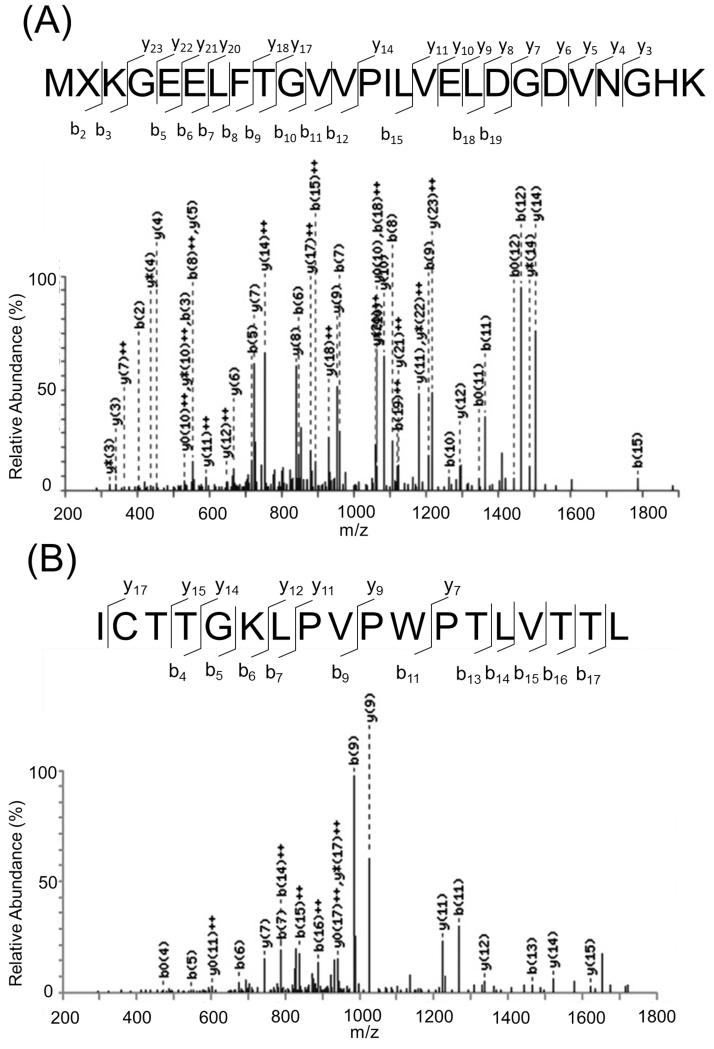
We performed liquid chromatography with tandem mass spectrometry (LC-MS/MS) to confirm the results by fluorescence screening mentioned above. For the TrpRS/supU pair, LC-MS/MS analysis identified nsAA 6 incorporation at position 2 and Trp at position 57 of sfGFP-6 protein produced from adding 1 mM nsAA 6 (Figure 3). Cross-talk events, i.e. Trp incorporation at position 2 and nsAA 6 at position 57, were not detected from the LC-MS/MS analysis. New peaks that appear may be caused by recognition of the introduced tRNA suppressor by AARSs other than the intended AARS (e.g. 6 in Figure 1A and 1B). Such unintended cross-talk may remain undetected; Trp incorporation was not detected at position 2 in the LC-MS/MS analysis of the sfGFP. Therefore, we are not able to rule out the possibility of cross-talk from other AARSs although the amino acid purity had been established.
Figure 3.
Mass spectrometric analyses of sfGFP proteins. The full length sfGFP-6 was produced by using tRNATrpCUA gene in the Trp auxotrophic stain supplied with 1 mM nsAA 6 in the M9 medium. (A) The LC-MS/MS spectrum of the MXKGEELFTGVVPILVELDGDVNGHK (X denotes nsAA 6 incorporated at Ser2TAG position) fragment from sfGFP-6 protein. (B) The LC-MS/MS spectrum of the ICTTGKLPVPWPTLVTTL fragment from sfGFP-6 protein. b0 and y0 denote loss of water from the b or y fragments; y* denotes loss of ammonia (NH3) from y fragments respectively. The full list of m/z values of fragments is provided in Table S6 and S7.
Discussion
The active sites of TrpRS, TyrRS, LeuRS and SerRS
While the nsAA library contained analogs of the cognate substrates of all four tested AARSs, TrpRS and TyrRS displayed a much larger productive substrate range than did LeuRS and SerRS. The four AARSs subjected to the nsAA library screening displayed diverse substrate specificities. TyrRS incorporated two Trp analogs (3 and 4) and Tyr analogs whose phenyl groups were substituted at the para and meta positions (7-13), although the nsAA incorporation efficiency was low (Figure 2A). TyrRS accommodated Tyr analogs as well as larger compounds like Nin-formyl-Trp (3) and Nin-boc-Trp (4). This implies that the TyrRS amino acid binding pocket is wider and deeper than necessary for accommodating Tyr. Sequence and structure based evolutionary analyses show the relationship of TrpRS and TyrRS.[12] There is high backbone conservation between TrpRS and TyrRS suggesting that side chain variations in the amino acid binding pocket are the main source of discrimination between Trp and Tyr.[12a] Thus the use of nsAAs containing aromatic side chains (3 and 4) by both enzymes is plausible. AARSs with narrow amino acid substrate range (SerRS and LeuRS) tend to recognize nsAAs similar to their cognate amino acid. Intrinsic editing mechanism that prevent formation of misacylated tRNA by SerRS and LeuRS may be responsible for the limited substrate response.[1a]
tRNA sequence and amino acid selection
The tRNA anticodon affects the amino acid substrate range of its cognate AARS. Given this differential aminoacylation property, select tRNA anticodon variants may permit specific incorporation of nsAAs at targeted codons. For instance, nsAA 6 was efficiently charged onto tRNATrpCUA (supU) but not tRNATrpCCA (Figure 1A and 1B); this observation was further corroborated by LC-MS/MS analyses that revealed incorporations of nsAA 6 at position 2 and Trp at W57 position, respectively. Since wild-type AARSs differentially aminoacylate their tRNA substrates based on their anticodon sequences, synonymous codons may encode the incorporation of distinct palettes of nsAAs. Superposition of the substrate range profile of tRNA anticodon variants can reveal outliers that may be candidates for specific nsAA incorporation. This approach may allow incorporation of more nsAAs by reducing the degeneracy of the canonical genetic code in vivo.
Conclusion
As suggested by early data[2a] wild-type AARSs can mediate the incorporation of nsAAs into E. coli proteins. This has been borne out in our library screens presented here. While AARSs are exceedingly specific in their substrate responses to the constituents of the normal cell[13], evolution did not provide any defenses against the legions of nsAAs that have been used in the genetic code expansion studies[4a, 14]. This polyspecificity[5f, 5i] by wild-type AARSs and their variants will be the great challenge to the site-specific introduction of multiple nsAAs into proteins during attempts to expand the genetic code[15]. Overcoming these challenges will require more sophisticated genome engineering[16] and inspired, labor-intensive enzyme engineering.
Experimental Section
System optimization and screening of incorporation of canonical or non-standard amino acids by sfGFP production
E. coli strain BW25113 or its auxotrophic strain JW2581-1 (tyrA−) was co-transformed with pBAD-sfGFP-UAG2 and pTech-supF (Figure S1, Table S1 and S2). BW25113 cells were cultured in 10 mL M9 medium (M9 salt supplemented with 1% glycerol, 2 mM MgSO4, 0.1 mM CaCl2) supplemented with 1 mM of the 20 canonical amino acids, 34 μg/mL chloramphenicol (Cm) and 100 μg/mL ampicillin (Amp) at 37 oC. JW2581-1 cells were cultured in 10 mL M9 medium supplemented with 1 mM of the 20 canonical amino acids, 50 μg/mL kanamycin (Kan), 34 μg/mL Cm and 100 μg/mL Amp at 37 °C. When cell A600 reached 0.7 to 1.0, the cells were pelleted and washed thrice with M9 medium, and resuspended in M9 medium (to an A600 of 1.0) supplemented with 1 mM of the appropriate nsAAs without 20 natural amino acids or 1mM Tyr, and arabinose (0.2 %) was added to induce sfGFP protein production for 12 h at 37 °C. Cells were collected by centrifugation and re-suspended in 1× phosphate buffered saline (PBS) with same culture volume. The fluorescence emission intensity of cells was measured at excitation wavelength 485/20 nm and emission wavelength 528/20 nm. The suppression experiments on the five natural tRNA suppressors (pTech-supU, pTech-trpT, pTech-supD and pTech-supP) were performed with the same conditions but in different auxotrophic strains (Figure S2 and Table S1).
For the substrate screening, we used a library that contained 313 nsAAs (containing 41 new entry nsAAs) (Table S3-S5), and its details were previously reported[5b]. E. coli auxotrophic stains were transformed with either pBAD-sfGFP (JW2580 trpC−); pBAD-sfGFP-UGA2 in conjunction with pTech-trpT (JW2580 trpC−); or pBAD-sfGFP-UAG2 in conjunction with pTech-supD (JW2880 serA−), pTech-supP (JW5807 leuB−), pTech-supF (JW2581 tyrA−) or pTech-supU (JW2580 trpC−) plasmids (Table S1 and S2). Cells were cultured in 100 mL M9 medium supplemented with 1 mM of the 20 natural amino acids, 34 μg/mL Cm, 50 μg/mL Kan, and 100 μg/mL Amp at 37 oC. The JW2580 trpC− cell strain containing pBAD-sfGFP was similarly cultured but without Cm in the medium. The cells were pelleted and washed twice with M9 medium, and resuspended in M9 medium (to an A600 of 1.0) supplemented with 0.2% arabinose was added. Aliquots (50 μL) of the cell suspension were transferred into the wells of a 384-well plate containing a different nsAA (1 mM) in each well. Cells were continuous shaken for 12 h at 37 °C, with monitoring of fluorescence intensity (excitation wavelength 485/20 nm; emission wavelength 528/20 nm) and optical density (A600 value) every 10 minutes. Four wells (A1, A2, I1 and I2) without added nsAAs were used as controls to measure the background signals. The nsAA screening experiments were performed in triplicate. For the annotated peaks generated by 1-20 the standard deviations of fluorescence intensities are listed in Table S8.
LC-MS/MS analyses of digested peptides of sfGFP proteins
The sfGFP sample purified from cells expressing supU was analyzed via LC-MS/MS. The full-length protein was trypsin digested by a standard in-gel digestion protocol, and analyzed via LC-MS/MS performed on an LTQ Orbitrap XL equipped with a Waters nanoACQUITY™ UPLC™ system. A Waters Symmetry® C18 180 μm × 20 mm trap column and a 1.7 μm, 100 μm × 250 mm nanoAcquity™ UPLC™ column (35°) were utilized for peptide separation. Trapping was done at 15 μL/min, 99% Buffer A (100% water, 0.1% formic acid) for 1 min. Peptide separation was performed at 300 nL/min with Buffer A and Buffer B (100% CH3CN containing 0.1% formic acid). A linear gradient (51 min) was run with 5% buffer B at initial conditions, 50% B at 50 min, and 85% B at 51 min. MS was acquired in the Orbitrap using 1 microscan, and a maximum inject time of 900 ms followed by data dependent MS/MS acquisitions in the ion trap (via Collision Induced Dissociation, CID). The Mascot search algorithm was used to search for the appropriate non-canonical substitution (Matrix Science).
Supplementary Material
Acknowledgements
This work was supported by the National Institute for General Medical Sciences (GM22854) and the Defense Advanced Research Projects Agency (contract N66001-12-C-4211). We thank Mary LoPresti for preparing the MS samples and Dr. TuKiet Lam and Jean Kanyo for collecting and processing the LC-MS/MS data. We are grateful to the members of the Söll lab (Markus Englert, Li-Tao Guo, Noah Reynolds, Akiyoshi Nakamura, and Carla Polycarpo) for critical discussions and helpful insights.
References
- [1].a) Ling J, Reynolds N, Ibba M. Annu Rev Microbiol. 2009;63:61–78. doi: 10.1146/annurev.micro.091208.073210. [DOI] [PubMed] [Google Scholar]; b) Reynolds NM, Lazazzera BA, Ibba M. Nature Reviews Microbiology. 2010;8:849–856. doi: 10.1038/nrmicro2472. [DOI] [PubMed] [Google Scholar]
- [2].a) Richmond MH. Bacteriol Rev. 1962;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xu ZJ, Love ML, Ma LY, Blum M, Bronskill PM, Bernstein J, Grey AA, Hofmann T, Camerman N, Wong JT. J Biol Chem. 1989;264:4304–4311. [PubMed] [Google Scholar]; c) Li L, Boniecki MT, Jaffe JD, Imai BS, Yau PM, Luthey-Schulten ZA, Martinis SA. Proc Natl Acad Sci USA. 2011;108:9378–9383. doi: 10.1073/pnas.1016460108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hartman MC, Josephson K, Szostak JW. Proc Natl Acad Sci U S A. 2006;103:4356–4361. doi: 10.1073/pnas.0509219103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Liu CC, Schultz PG. Annu Rev Biochem. 2010;79:413–444. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]; b) Johnson JA, Lu YY, Van Deventer JA, Tirrell DA. Curr Opin Chem Biol. 2010;14:774–780. doi: 10.1016/j.cbpa.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lepthien S, Hoesl MG, Merkel L, Budisa N. Proc Natl Acad Sci U S A. 2008;105:16095–16100. doi: 10.1073/pnas.0802804105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Kavran JM, Gundllapalli S, O’Donoghue P, Englert M, Söll D, Steitz TA. Proc Natl Acad Sci U S A. 2007;104:11268–11273. doi: 10.1073/pnas.0704769104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ko JH, Wang YS, Nakamura A, Guo LT, Söll D, Umehara T. FEBS Lett. 2013;587:3243–3248. doi: 10.1016/j.febslet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Nguyen DP, Lusic H, Neumann H, Kapadnis PB, Deiters A, Chin JW. J Am Chem Soc. 2009;131:8720–8721. doi: 10.1021/ja900553w. [DOI] [PubMed] [Google Scholar]; d) Takimoto JK, Dellas N, Noel JP, Wang L. ACS Chem Biol. 2011;6:733–743. doi: 10.1021/cb200057a. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wang YS, Fang X, Chen HY, Wu B, Wang ZU, Hilty C, Liu WR. ACS Chem Biol. 2013;8:405–415. doi: 10.1021/cb300512r. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Wang YS, Fang X, Wallace AL, Wu B, Liu WR. J Am Chem Soc. 2012;134:2950–2953. doi: 10.1021/ja211972x. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Wang YS, Russell WK, Wang Z, Wan W, Dodd LE, Pai PJ, Russell DH, Liu WR. Mol Biosyst. 2011;7:714–717. doi: 10.1039/c0mb00217h. [DOI] [PubMed] [Google Scholar]; h) Yanagisawa T, Ishii R, Fukunaga R, Kobayashi T, Sakamoto K, Yokoyama S. Chem Biol. 2008;15:1187–1197. doi: 10.1016/j.chembiol.2008.10.004. [DOI] [PubMed] [Google Scholar]; i) Young DD, Young TS, Jahnz M, Ahmad I, Spraggon G, Schultz PG. Biochemistry. 2011;50:1894–1900. doi: 10.1021/bi101929e. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Wang ZU, Wang YS, Pai PJ, Russell WK, Russell DH, Liu WR. Biochemistry. 2012;51:5232–5234. doi: 10.1021/bi300535a. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Wang YS, Wu B, Wang Z, Huang Y, Wan W, Russell WK, Pai PJ, Moe YN, Russell DH, Liu WR. Mol Biosyst. 2010;6:1557–1560. doi: 10.1039/c002155e. [DOI] [PubMed] [Google Scholar]; l) Fekner T, Li X, Lee MM, Chan MK. Angew Chem Int Ed Engl. 2009;48:1633–1635. doi: 10.1002/anie.200805420. [DOI] [PubMed] [Google Scholar]
- [6].Eggertsson G, Söll D. Microbiol Rev. 1988;52 doi: 10.1128/mr.52.3.354-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Mol Syst Biol. 2006;2:2006–0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Hong KW, Ibba M, Weygand-Durasevic I, Rogers MJ, Thomann HU, Söll D. EMBO J. 1996;15:1983–1991. [PMC free article] [PubMed] [Google Scholar]; b) Ibba M, Hong KW, Sherman JM, Sever S, Söll D. Proc. Natl. Acad. Sci. USA. 1996;93:6953–6958. doi: 10.1073/pnas.93.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rennert OM, Anker HS. Biochemistry. 1963;2:471–476. doi: 10.1021/bi00903a013. [DOI] [PubMed] [Google Scholar]
- [10].Kanehisa M, Goto S. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Englisch S, Englisch U, von der Haar F, Cramer F. Nucleic Acids Res. 1986;14:7529–7539. doi: 10.1093/nar/14.19.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gruic-Sovulj I, Rokov-Plavec J, Weygand-Durasevic I. FEBS Lett. 2007;581:5110–5114. doi: 10.1016/j.febslet.2007.09.058. [DOI] [PubMed] [Google Scholar]; c) Boniecki MT, Martinis SA. J Biol Chem. 2012;287:11285–11289. doi: 10.1074/jbc.C111.325795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) O’Donoghue P, Luthey-Schulten Z. Microbiol Mol Biol Rev. 2003;67:550–573. doi: 10.1128/MMBR.67.4.550-573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Woese CR, Olsen GJ, Ibba M, Söll D. Microbiol Mol Biol Rev. 2000;64:202–236. doi: 10.1128/mmbr.64.1.202-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ibba M, Söll D. Annu Rev Biochem. 2000;69:617–650. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- [14].Hoesl MG, Budisa N. Curr Opin Biotechnol. 2012;23:751–757. doi: 10.1016/j.copbio.2011.12.027. [DOI] [PubMed] [Google Scholar]
- [15].O’Donoghue P, Ling J, Wang YS, Söll D. Nat Chem Biol. 2013;9:594–598. doi: 10.1038/nchembio.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lajoie MJ, Rovner AJ, Goodman DB, Aerni HR, Haimovich AD, Kuznetsov G, Mercer JA, Wang HH, Carr PA, Mosberg JA, Rohland N, Schultz PG, Jacobson JM, Rinehart J, Church GM, Isaacs FJ. Science. 2013;342:357–360. doi: 10.1126/science.1241459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.