Abstract
Background
Short-course antiretroviral therapy (ART) in primary human immunodeficiency virus (HIV) infection may delay disease progression but has not been adequately evaluated.
Methods
We randomly assigned adults with primary HIV infection to ART for 48 weeks, ART for 12 weeks, or no ART (standard of care), with treatment initiated within 6 months after seroconversion. The primary end point was a CD4+ count of less than 350 cells per cubic millimeter or long-term ART initiation.
Results
A total of 366 participants (60% men) underwent randomization to 48-week ART (123 participants), 12-week ART (120), or standard care (123), with an average follow-up of 4.2 years. The primary end point was reached in 50% of the 48-week ART group, as compared with 61% in each of the 12-week ART and standard-care groups. The average hazard ratio was 0.63 (95% confidence interval [CI], 0.45 to 0.90; P = 0.01) for 48-week ART as compared with standard care and was 0.93 (95% CI, 0.67 to 1.29; P = 0.67) for 12-week ART as compared with standard care. The proportion of participants who had a CD4+ count of less than 350 cells per cubic millimeter was 28% in the 48-week ART group, 40% in the 12-week group, and 40% in the standard-care group. Corresponding values for long-term ART initiation were 22%, 21%, and 22%. The median time to the primary end point was 65 weeks (95% CI, 17 to 114) longer with 48-week ART than with standard care. Post hoc analysis identified a trend toward a greater interval between ART initiation and the primary end point the closer that ART was initiated to estimated seroconversion (P = 0.09), and 48-week ART conferred a reduction in the HIV RNA level of 0.44 log10 copies per milliliter (95% CI, 0.25 to 0.64) 36 weeks after the completion of short-course therapy. There were no significant between-group differences in the incidence of the acquired immunodeficiency syndrome, death, or serious adverse events.
Conclusions
A 48-week course of ART in patients with primary HIV infection delayed disease progression, although not significantly longer than the duration of the treatment. There was no evidence of adverse effects of ART interruption on the clinical outcome. (Funded by the Wellcome Trust; SPARTAC Controlled-Trials.com number, ISRCTN76742797, and EudraCT number, 2004-000446-20.)
Although the use of highly active antiretroviral therapy (ART) in human immunodeficiency virus (HIV) disease reduces morbidity and mortality,1-3 the role of ART in the management of primary HIV infection remains controversial.4-6 Immunologic damage after HIV acquisition occurs rapidly and is not wholly reversible by later ART.7-9 Observational studies have suggested that a short course of ART during primary HIV infection may preserve immune function,10,11 decrease viral evolution,12 and limit the viral reservoir.13-15
Two randomized, controlled trials — the AIDS Clinical Trials Group Setpoint Study and the Primo-SHM trial, involving 130 and 115 participants, respectively — were designed to evaluate the effect of short-course ART versus no therapy on HIV RNA levels and showed modest delays in the initiation of long-term ART and a transient lowering of the viral set point.16,17 However, there is currently no evidence from randomized trials on whether initiating ART during primary HIV infection delays disease progression.
The Short Pulse Anti-Retroviral Therapy at Sero-conversion (SPARTAC) trial was designed to determine whether short-term ART during primary HIV infection can lengthen the time until patients reach a CD4+ count of less than 350 cells per cubic millimeter or require long-term ART.
Methods
Study Design and Participants
The SPARTAC trial was an open-label, randomized, controlled trial that enrolled adults with primary HIV infection from August 2003 through July 2007 in eight countries. Primary HIV infection was defined as infection meeting one or more of the following criteria: a positive HIV-antibody test within 6 months after a negative test (criterion 1), a negative HIV-antibody test with a positive reverse-transcription–polymerase-chain-reaction assay for HIV RNA (criterion 2), a low level of HIV antibodies (optical density units [OD], <0.6) according to a serologic testing algorithm for recent infection (subtype B strain only)18 (criterion 3), an equivocal HIV-antibody test with a repeat test within 2 weeks showing an increase in the level of HIV antibodies (criterion 4), or clinical manifestations of symptomatic HIV seroconversion illness supported by antigen positivity and less than 4 positive bands on Western blot analysis (criterion 5).
Eligible participants underwent randomization within 6 months after the antibody test showing negative results, equivocal results, or a low antibody level. Persons in whom ART was indicated were ineligible. All participants gave written informed consent; the trial was approved by research ethics committees in each country.
The time of seroconversion was estimated as the midpoint between the most recent negative or equivocal test and the first positive test for patients who met criterion 1 or 4; as the date of the test for patients who met criterion 2, 3 (if OD ≤0.01), or 5; and as the date of the test − [(OD × 150) ÷ 2] days for patients who met criterion 3 if the antibody level was greater than 0.01 OD.
Study Oversight
An independent data and safety monitoring committee reviewed interim data annually (five times in total) with the use of Haybittle–Peto criteria to guide recommendations for stopping the study or modifying the protocol. The study was funded by the Wellcome Trust, and Imperial College, London, was the sponsor; Abbott Laboratories provided lopinavir–ritonavir (Kaletra and Aluvia) for the African sites. None of those bodies had any role in the trial design; the collection, analysis, or interpretation of the data; or the writing of the manuscript. The trial writing group vouches for the accuracy and completeness of the data presented and for the fidelity of this report to the study protocol, which is available with the full text of this article at NEJM.org.
Randomization and Follow-up
Participants were randomly assigned, in a 1:1:1 ratio, to receive ART for 48 weeks, ART for 12 weeks, or no therapy (standard of care), in blocks of randomly varying size and with stratification according to country and evidence of HIV seroconversion (categorized as definitive [criterion 1 or 2] or presumptive [any other criterion]).
The intended treatment regimen was chosen by the treating clinician before randomization; two nucleoside reverse-transcriptase inhibitors (NRTIs) plus a ritonavir-boosted protease inhibitor were recommended, to avoid the risk of resistance to nonnucleoside reverse-transcriptase inhibitors (NNRTIs) on stopping owing to their differential clearance19,20 and to avoid the increased risk of rash with NNRTIs21 (rash is already common in primary HIV infection). Long-term ART was administered according to local HIV treatment guidelines or at the discretion of the treating clinician.
Clinic visits were scheduled at weeks 0, 4, 12, 16, and 24, and every 12 weeks thereafter. Follow-up was planned until the 360th enrolled participant had completed 3.5 years, which occurred on November 7, 2010; participants not seen on or after this date were considered lost to follow-up.
End Points
The primary end point was a composite of a CD4+ count of less than 350 cells per cubic millimeter (documented >3 months after randomization and confirmed within 4 weeks) or initiation of long-term ART. Secondary end points included the separate components of the primary end point, the acquired immunodeficiency syndrome (AIDS) or death, HIV-specific CD4+ and CD8+ responses at week 60, the slope of decline in the CD4+ count from 24 weeks after ART interruption in the treatment groups as compared with the slope of decline from randomization in the standard-care group, the development of drug resistance not present at baseline by week 120, changes in blood pressure from randomization to weeks 12 and 48, and virologic failure of the first long-term ART regimen (HIV RNA level, >400 copies per milliliter). We also examined changes in the CD4+ count and HIV RNA level over time. For full details of the study design, see the protocol and the statistical analysis plan, available at NEJM.org.
Procedures
The lower limit of detection for the plasma HIV RNA level was 50 copies per milliliter, except in South Africa and Uganda (400 copies per milliliter). Clinical AIDS events excluded a CD4+ count of less than 200 cells per cubic millimeter. Serious adverse events, deaths, and AIDS events were reviewed by two clinicians (one independent) who were not aware of the study-group assignments.
To determine whether there was new drug resistance by week 120, viral sequencing was performed at baseline, 4 weeks after the interruption of ART in the treatment groups, and at the last time point, up to 120 weeks, at which the virus was detectable (≥50 copies per milliliter) in participants receiving long-term ART. Resistance mutations were classified according to the algorithm used by the Stanford University HIV Drug Resistance Database, and HIV subtype was determined according to the Rega Institute system22 in March and April 2011.
An interferon-γ enzyme-linked immunosorbent spot (ELISpot) assay23 was used to measure HIV type 1–specific CD8+ responses across Gag overlapping peptides in all participants with the sub-type B strain of HIV; CD4+ responses were also measured in U.K. participants with subtype B. All assays were performed in duplicate.
Statistical Analysis
On the basis of data from the CASCADE (Concerted Action on Seroconversion to AIDS and Death in Europe) study,24 we calculated that we would need to enroll 360 patients in order to provide 90% power to detect a relative reduction in the risk of progression to the original primary end point (a CD4+ count of <350 cells per cubic millimeter) of 50% with 48-week ART and 25% with 12-week ART, as compared with standard care, at a significance level of 5%. We assumed a recruitment period of 18 months, a minimum of 3.5 years of follow-up, and a 10% rate of loss to follow-up.
The time to the primary end point and other time-to-event end points was estimated with the use of Kaplan–Meier plots, and differences between groups were summarized by means of average hazard ratios that were estimated with the use of proportional-hazards models (adjusted for randomization stratification factors), with the two components of the primary end point also considered as competing risks. Categorical outcomes were compared between groups with the use of chi-square tests and logistic-regression models, and continuous outcomes were compared with the use of linear regression, with adjustment for baseline values. Slopes of decline in the CD4+ count were modeled with the use of linear mixed models. Average CD4+ counts and HIV RNA levels were estimated with the use of a time-averaged area-under-the-curve analysis. Analyses were performed on an intention-to-treat basis.
Results
Baseline Characteristics of the Participants
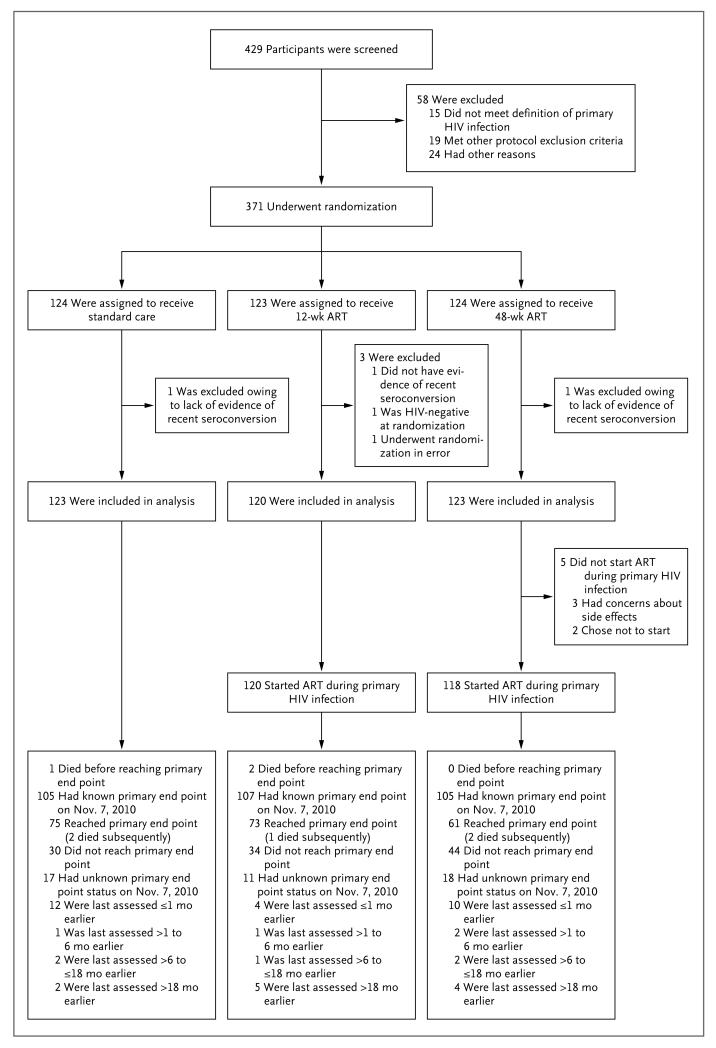
Of 371 participants who underwent randomization, 5 met major ineligibility criteria and were excluded from all analyses, as specified by the statistical analysis plan (Fig. 1). Of the remaining 366 participants (123 in the 48-week ART group, 120 in the 12-week ART group, and 123 in the standard-care group), 246 (67%) and 9 (2%) were eligible under definitive criteria 1 and 2, respectively, and 72 (20%), 1 (0.3%), and 38 (10%) were eligible under presumptive criteria 3, 4, and 5, respectively. Nine participants had Fiebig stage I infection (acquired during the past several days), and the remainder had stage V or VI infection (positive HIV-antibody test).25
Figure 1. Study Enrollment, Randomization, and Outcomes.
The primary end point was a confirmed CD4+ count of less than 350 cells per cubic millimeter or the initiation of long-term antiretroviral therapy (ART). One additional patient in the standard-care group is known to have died after November 7, 2010 (a patient in Johannesburg, who died in January 2011). HIV denotes human immunodeficiency virus.
The characteristics of the participants were balanced across the study groups (Table 1). The majority of participants were men infected through sex between men (205 participants [56%]) and women infected through sex with men (146 participants [40%]). The median interval between seroconversion and randomization was 12 weeks (interquartile range, 9 to 15), the median baseline CD4+ count was 559 cells per cubic millimeter (interquartile range, 435 to 700), and the median plasma HIV RNA level was 4.53 log10 copies per milliliter (interquartile range, 3.67 to 5.18). Of the 364 participants for whom information about the subtype strain was available, 208 (57%) and 120 (33%) were infected with HIV subtypes B and C, respectively, and 21 (6%) had evidence of transmitted drug-resistant virus.
Table 1.
Baseline Characteristics of the 366 Eligible Participants Enrolled in the Trial.*
| Characteristic | Standard Care (N = 123) |
12-Week ART (N = 120) |
48-Week ART (N = 123) |
Total (N = 366) |
|---|---|---|---|---|
| Sex — no. (%) | ||||
| Male | 74 (60) | 71 (59) | 74 (60) | 219 (60) |
| Female | 49 (40) | 49 (41) | 49 (40) | 147 (40) |
| Age — yr | ||||
| Median (interquartile range) | 31 (25–39) | 32 (24–39) | 33 (26–41) | 32 (25–40) |
| Range | 19–63 | 19–61 | 20–61 | 19–63 |
| Evidence of seroconversion — no. (%)† | ||||
| Definitive: criterion 1 or 2 | 86 (70) | 85 (71) | 84 (68) | 255 (70) |
| Presumptive: criterion 3, 4, or 5 | 37 (30) | 35 (29) | 39 (32) | 111 (30) |
| Estimated time since seroconversion at randomization — wk‡ | ||||
| Median (interquartile range) | 11 (8–15) | 12 (9–15) | 12 (9–15) | 12 (9–15) |
| Range | 2–24 | 1–23 | 1–22 | 1–24 |
| CD4+ count — cells/mm3 | ||||
| Median (interquartile range)§ | 543 (404–715) | 519 (433–638) | 605 (463–750) | 559 (435–700) |
| Range | 130–1226 | 95–1243 | 195–1399 | 95–1399 |
| HIV RNA level — copies/ml | ||||
| Median (interquartile range) | 4.70 (3.68–5.24) | 4.39 (3.59–5.18) | 4.43 (3.81–5.13) | 4.53 (3.67–5.18) |
| Range | 1.70–6.22 | 1.40–6.22 | 2.22–6.47 | 1.40–6.47 |
| Any drug resistance at Stanford level 4 oar 5 — no. (%)¶ | 8 (7) | 5 (4) | 8 (7) | 21 (6) |
| Reported HIV seroconversion–type illness — no. (%) | 81 (66) | 64 (53) | 70 (57) | 215 (59) |
Characteristics were compared across randomized groups with the use of the Kruskal–Wallis test for medians and the chi-square test for categorical variables. None of these characteristics differed significantly among the three groups (P>0.05 for all comparisons), unless otherwise noted. ART denotes antiretroviral therapy, and HIV human immunodeficiency virus.
Primary HIV infection was defined as infection meeting one or more of the following criteria: a positive HIV-antibody test within 6 months after a negative test (criterion 1), a negative HIV-antibody test with a positive reverse-transcription–polymerase-chain-reaction assay for HIV RNA (criterion 2), a low level of HIV antibodies (optical density units [OD], <0.6) according to a serologic testing algorithm for recent infection (subtype B strain only)18 (criterion 3), an equivocal HIV-antibody test with a repeat test within 2 weeks showing an increase in the level of HIV antibodies (criterion 4), or clinical manifestations of symptomatic HIV seroconversion illness supported by antigen positivity and less than 4 positive bands on Western blot analysis (criterion 5).
The time of seroconversion was estimated as the midpoint between the most recent negative or equivocal test and the first positive test for patients who met criterion 1 or 4; as the date of the test for patients who met criterion 2, 3 (if OD ≤0.01), or 5; and as the date of the test − [(OD × 150) ÷ 2] days for patients who met criterion 3 if the antibody level was greater than 0.01 OD.
P<0.05 (but P>0.01) for the overall comparison among the three groups.
Levels range from 1 to 5, with a higher level indicating a greater degree of resistance. Data were not available for 1 participant in the 12-week ART group (the HIV RNA value was too low) and 1 participant in the 48-week ART group (no sample was available).
Follow-up
The median follow-up was 4.2 years (interquar-tile range, 3.7 to 4.7), and an average of 89% of scheduled visits were attended, a rate that was balanced across the groups. A total of 46 participants (13%) were lost to follow-up for the primary end-point analysis (15% of the 48-week ART group, 9% of the 12-week ART group, and 14% of the standard-care group), although 26 of these participants (57%) had been assessed for the primary end point in the previous month.
All but 5 participants who were randomly assigned to ART started therapy (all 5 were in the 48-week ART group), with more than 99% initiating therapy in 14 days or less and 93% in 3 days or less after randomization; 91% received an initial regimen of two NRTIs and a ritonavir-boosted protease inhibitor.
Primary End Point
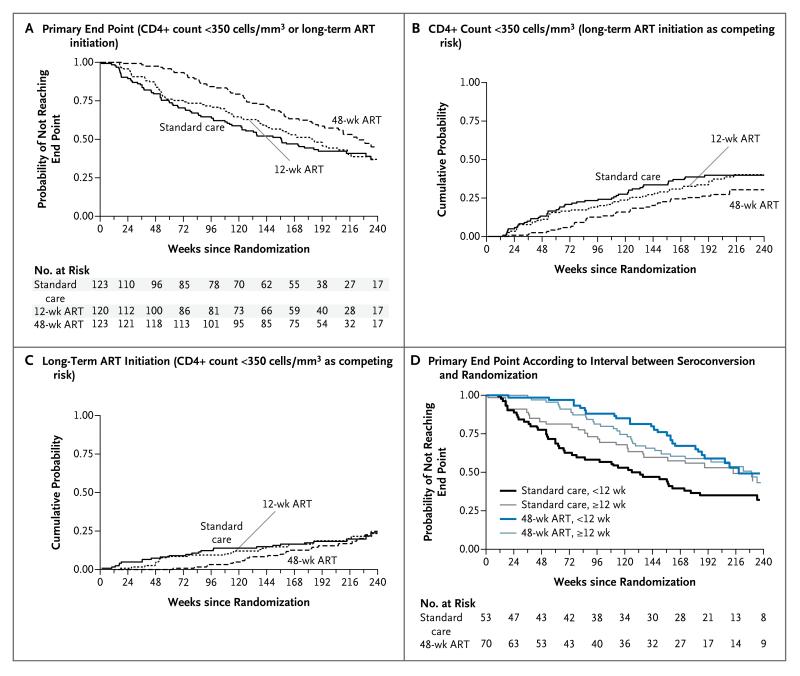
A total of 209 participants reached the primary end point (61 in the 48-week ART group [50%], 73 in the 12-week ART group [61%], and 75 in the standard-care group [61%]) (Fig. 2A). Of these participants, 130 had a confirmed CD4+ count of less than 350 cells per cubic millimeter (34 in the 48-week ART group, 48 in the 12-week ART group, and 48 in the standard-care group), and 79 started long-term ART without a confirmed CD4+ count of less than 350 cells per cubic millimeter (27 in the 48-week ART group, 25 in the 12-week ART group, and 27 in the standard-care group). The hazard ratio for the primary end point with 48-week ART as compared with standard care was 0.63 (95% confidence interval [CI], 0.45 to 0.90; P = 0.01), and the hazard ratio with 12-week ART as compared with standard care was 0.93 (95% CI, 0.67 to 1.29; P = 0.67).
Figure 2. Time from Randomization to End Points According to Study Group.
Panels A and D show data for the primary end point, and Panels B and C show data for the individual components of the primary end point. Primary HIV infection was defined as infection meeting one or more of the following criteria: a positive HIV-antibody test within 6 months after a negative test (criterion 1), a negative HIV-antibody test with a positive reverse-transcription–polymerase-chain-reaction assay for HIV RNA (criterion 2), a low level of HIV antibodies (optical density units [OD], <0.6) according to a serologic testing algorithm for recent infection (subtype B strain only)18 (criterion 3), an equivocal HIV-antibody test with a repeat test within 2 weeks showing an increase in the level of HIV antibodies (criterion 4), or clinical manifestations of symptomatic HIV seroconversion illness supported by antigen positivity and less than 4 positive bands on Western blot analysis (criterion 5). The time of seroconversion was estimated as the midpoint between the most recent negative or equivocal test and the first positive test for patients who met criterion 1 or 4; as the date of the test for patients who met criterion 2, 3 (if OD ≤0.01), or 5; and as the date of the test − [(OD × 150) ÷ 2] days for patients who met criterion 3 if the antibody level was greater than 0.01 OD.
Median times to the primary end point were 222 weeks in the 48-week ART group (95% CI, 189 to 270), 184 weeks in the 12-week ART group (95% CI, 140 to 214), and 157 weeks in the standard-care group (95% CI, 114 to 213). The difference between the 48-week ART group and the standard-care group was 65 weeks (95% CI, 17 to 114), which was not significantly longer than the duration of the treatment (48 weeks). The difference between the 12-week ART group and the standard-care group was 27 weeks (95% CI, −25 to 79).
With the two components of the primary end point analyzed as competing risks, the cumulative probability of a confirmed CD4+ count of less than 350 cells per cubic millimeter by 4 years was 0.27 in the 48-week ART group (95% CI, 0.19 to 0.35), 0.38 in the 12-week ART group (95% CI, 0.29 to 0.47), and 0.39 in the standard-care group (95% CI, 0.31 to 0.48) (Fig. 2B); the corresponding values for long-term ART initiation were 0.17 (95% CI, 0.11 to 0.24), 0.19 (95% CI, 0.12 to 0.26), and 0.18 (95% CI, 0.12 to 0.26) (Fig. 2C). We found no evidence of a different treatment effect on the time to the primary end point according to viral subtype (P = 0.56).
In a post hoc analysis, there was a trend toward a greater effect of 48-week ART, as compared with standard care, the sooner that randomization occurred after seroconversion (P = 0.09). For example, the hazard ratio for the primary end point was 0.41 (95% CI, 0.22 to 0.77) for an interval of 4 weeks between seroconversion and randomization, 0.65 (95% CI, 0.46 to 0.92) for an interval of 12 weeks, and 1.28 (95% CI, 0.54 to 3.05) for an interval of 24 weeks. Median times to the primary end point among participants who had seroconversion 12 weeks or less before randomization were 218 weeks in the 48-week ART group (95% CI, 183 to ∞), 181 weeks in the 12-week ART group (95% CI, 115 to 275), and 126 weeks in the standard-care group (95% CI, 74 to 171), as compared with 229 weeks (95% CI, 151 to ∞), 185 weeks (95% CI, 138 to 241), and 213 weeks (95% CI, 123 to ∞), respectively, among those with seroconversion more than 12 weeks before randomization (Fig. 2D).
In participants who started long-term ART, CD4+ counts at initiation tended to be higher in the 48-week ART group than in the 12-week ART and standard-care groups (median, 335 cells per cubic millimeter [interquartile range, 260 to 440] vs. 314 cells per cubic millimeter [interquar-tile range, 236 to 378] and 270 cells per cubic millimeter [interquartile range, 220 to 349], respectively; P = 0.33 for the comparison with 12-week ART and P=0.02 for the comparison with standard care, by the Wilcoxon rank-sum test). This finding may be related to the calendar times of initiation (median, January 2009 in the 48-week ART group, July 2008 in the 12-week ART group, and May 2008 in the standard-care group), because a change in the treatment guidelines during the period of follow-up favored the initiation of long-term ART at a higher CD4+ count.26-28 Of the 172 participants who started long-term ART, 21 (12%) switched to second-line ART during follow-up.
Secondary End Points
Nineteen participants received a diagnosis of AIDS (6 in the 48-week ART group, 7 in the 12-week ART group, and 6 in the standard-care group), and 8 participants died (2 in the 48-week ART group, 3 in the 12-week ART group, and 3 in the standard-care group) (see the Supplementary Appendix, available at NEJM.org). For the composite of the primary end point, AIDS, or death, hazard ratios were very similar to the hazard ratio for the primary end point.
The proportions of participants with a positive CD4+-specific response at week 60, among the 108 participants for whom this information was available, were 75% in the 48-week ART group, 78% in the 12-week ART group, and 51% in the standard-care group (P = 0.04 for 48-week ART vs. standard care and P = 0.02 for 12-week ART vs. standard care). Corresponding values for CD8+-specific responses among 142 participants were 80%, 89%, and 87% (P = 0.32 for 48-week ART vs. standard care and P = 0.78 for 12-week ART vs. standard care).
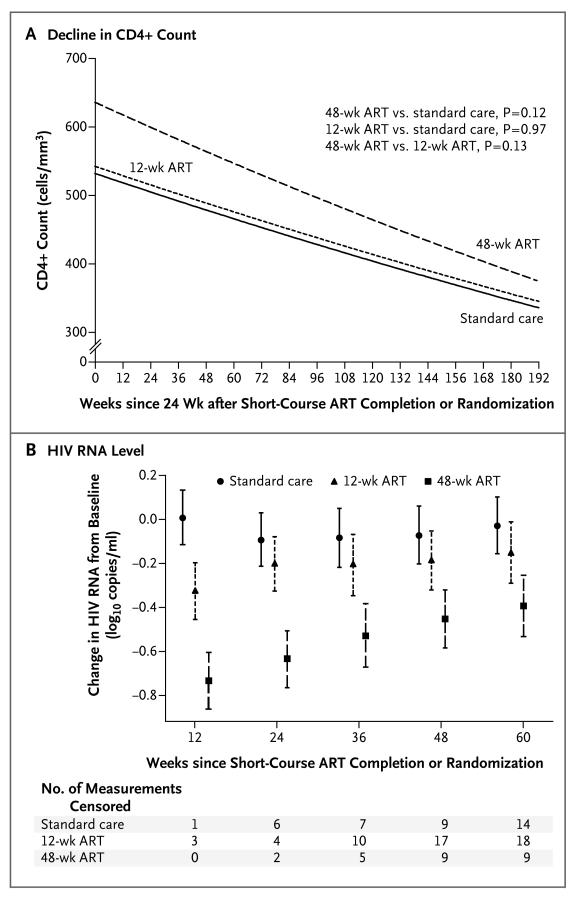
There was no significant difference in the decline in the CD4+ count from 24 weeks after completion of the short-course regimen in the treatment groups as compared with the decline from randomization in the standard-care group (on a square-root scale per 12 weeks: −0.37 cells per cubic millimeter in the 48-week ART group [95% CI, −0.43 to −0.30], −0.29 cells per cubic millimeter in the 12-week ART group [95% CI, −0.36 to −0.23], and −0.30 cells per cubic millimeter in the standard-care group [95% CI, −0.35 to −0.24]; P = 0.12 for 48-week ART vs. standard care and P = 0.97 for 12-week ART vs. standard care) (Fig. 3A).
Figure 3. Changes in the CD4+ Count and HIV RNA Level.
Panel A shows the output from the statistical model for the decline in the CD4+ count from 24 weeks after completion of the study regimen in the 48-week and 12-week ART groups and from randomization in the standard-care group. Panel B shows the differences from baseline in HIV RNA levels at 12, 24, 36, 48, and 60 weeks after completion of the study regimen in the 48-week and 12-week ART groups and from randomization in the standard-care group, with adjustment for the baseline value. Some measurements were censored owing to initiation of long-term ART. I bars indicate 95% confidence intervals.
The mean change from baseline in the HIV RNA level at 36 weeks after completion of the 12-week course of ART was similar to that at the same time point from randomization in the standard-care group (difference, −0.12 log10 copies per milliliter [95% CI, −0.32 to 0.07]). However, the mean change was significantly greater in the 48-week ART group than in the standard-care group (difference, −0.44 log10 copies per milliliter [95% CI, −0.64 to −0.25]), and the difference was sustained at 60 weeks (Fig. 3B).
Of the 339 participants for whom an evaluation of new drug-resistance mutations was available, 6 had resistance at Stanford level 4 or 5 (range, 1 to 5, with a higher level indicating a greater degree of resistance) by week 120 (3 in the 48-week ART group, 1 in the 12-week ART group, and 2 in the standard-care group). Of these 6 participants, 5 had NNRTI-associated mutations (2 in the 48-week ART group, 2 in the 12-week ART group, and 1 in the standard-care group) and 1 (in the 48-week ART group) had a protease inhibitor–associated mutation (46I mutation).
Adverse Events
A total of 139 serious adverse events were reported in 93 participants (109 events required hospitalization or resulted in prolonged hospitalization, 12 were life-threatening, 8 were fatal, 3 were non-AIDS cancers, and 7 were other medical conditions) (see the Supplementary Appendix), and 345 grade 3 or 4 adverse events were reported in 165 participants, with similar rates across the groups.
CD4+ Counts and HIV RNA after Initiation of Long-Term ART
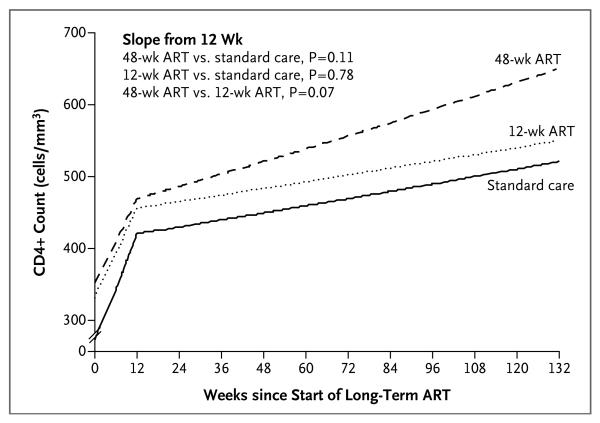
Of the 172 participants who started long-term ART, 36 (21%) had virologic failure, with no evidence of a significant difference between groups (hazard ratio, 0.73 for 48-week ART vs. standard care [95% CI, 0.31 to 1.72] and 0.91 for 12-week ART vs. standard care [95% CI, 0.42 to 1.99]). Increases in CD4+ counts after initiation of long-term ART were similar across the three groups, although slightly steeper in the 48-week ART group (after 12 weeks, on a square-root scale per 12 weeks: 0.39 cells per cubic millimeter in the 48-week ART group [95% CI, 0.23 to 0.54], 0.21 cells per cubic millimeter in the 12-week ART group [95% CI, 0.09 to 0.33], and 0.23 cells per cubic millimeter in the standard-care group [95% CI, 0.13 to 0.34]) (Fig. 4).
Figure 4.
Statistical Model of the CD4+ Response to the Initiation of Long-Term ART
Overall Experience
Over the period of the trial, the average CD4+ count was higher by 138 cells per cubic millimeter (95% CI, 90 to 185) in the 48-week ART group than in the standard-care group, and the average HIV RNA level was lower by 0.40 log10 copies per milliliter (95% CI, 0.20 to 0.61). The average CD4+ count was 649 cells per cubic millimeter in the 48-week ART group (95% CI, 616 to 683), 534 cells per cubic millimeter in the 12-week ART group (95% CI, 500 to 568), and 512 cells per cubic millimeter in the standard-care group (95% CI, 479 to 545); the HIV RNA level was 3.23 log10 copies per milliliter (95% CI, 3.08 to 3.37), 3.53 log10 copies per milliliter (95% CI, 3.38 to 3.68), and 3.63 log10 copies per milliliter (95% CI, 3.49 to 3.78), respectively. The average duration of ART during the median 4.2 years of follow-up was 1.5 years, 1.2 years, and 1.1 years, respectively.
Discussion
The SPARTAC trial, which involved men and women from disparate settings in eight countries, assessed the efficacy of short-course ART during primary HIV infection. Our choice of a clinically relevant primary end point with several years of follow-up (median, >4 years) allowed an evaluation of the effect of ART given during primary infection on disease progression, as compared with the current standard of care of no treatment. A 48-week course of ART during primary HIV infection delayed disease progression and the consequent need for long-term ART, although the delay was not significantly longer than the duration of initial treatment. We found no benefit of 12-week ART given during primary HIV infection.
The trial was designed pragmatically to include persons in whom seroconversion had occurred within 6 months before randomization, although we found that in the group receiving standard care, participants who underwent randomization within 12 weeks after seroconversion had more rapid disease progression than those with a longer interval between seroconversion and randomization, a finding consistent with findings from a large observational study.24 Post hoc analyses identified a greater benefit of 48-week ART for participants who received therapy closer to seroconversion, supporting findings from other studies.15,29 Future research focusing on patients with acute infection (seroconversion within the previous 12 weeks), a challenging group to identify, would be required to confirm this observation.
Observational studies have suggested that ART given during primary HIV infection could confer a durable beneficial effect by limiting HIV-mediated immunologic damage,10,30-33 which was the main hypothesis underpinning our trial. Although there was a significant increase in HIV-specific responses with 48-week ART, as compared with standard care, there also appeared to be a similar increase with 12-week ART. This finding was at odds with the main trial results, as were the results of the CD8+-specific responses. It would therefore appear that the observed effects of short-course ART during primary HIV infection are not mediated through HIV-specific immunity, as determined by the frequency of positive ELISpot tests.
The higher overall average CD4+ count with 48-week ART than with standard care is encouraging and may confer a clinical benefit. The sustained CD4+ benefit after completion of a short course of ART in patients with primary HIV infection contrasts with the findings in studies involving patients with chronic infection, which have shown a blunted CD4+ response when long-term ART is interrupted.34 Whether the lower HIV RNA levels after cessation of 48-week ART or the improved CD4+ recovery after initiation of long-term ART can be maintained and will translate into a clinical benefit in the years ahead requires longer follow-up, which is planned.
Drug-resistant mutations developed in six participants by week 120. In five of these participants, the mutations were most likely induced by long-term ART (NNRTI mutations). One case of drug resistance was probably induced by the short-course ART (46I mutation).
In summary, we found that a 48-week course of ART during primary HIV infection delayed a decline in the CD4+ count to less than 350 cells per cubic millimeter or the initiation of long-term ART, but this delay was not significantly longer than the 48-week treatment period. However, our finding that, with the use of a realistic, clinically relevant definition of primary HIV infection, 48 weeks of ART altered the course of the two main markers of HIV disease progression — namely, the CD4+ count and the HIV RNA level — beyond the treatment period is an intriguing observation that requires further evaluation. For maximal individual benefit, future studies that examine longer courses of therapy, therapy initiated earlier in primary HIV infection, or both may be required.
Supplementary Material
Acknowledgments
Funded by a grant from the Wellcome Trust (069598/Z/02/Z). Dr. Fidler reports receiving lecture fees from Bristol-Myers Squibb Pharmaceuticals through her institution. Dr. Fisher reports receiving lecture fees from Abbott Laboratories, Bristol-Myers Squibb Pharmaceuticals, Gilead Sciences, Janssen-Cilag, Merck, and ViiV Healthcare. Dr. Kelleher reports receiving consulting fees, lecture fees, and payment for development of educational material from ViiV Healthcare through his institution; receiving grant support, lecture fees, and payment for development of educational material (all through his institution) and payment for travel and accommodations from Merck; receiving lecture fees from Boehringer Ingelheim through his institution; and being a named inventor in a patent with Becton Dickinson. Dr. Kinloch reports receiving payment for travel and accommodations from Bristol-Myers Squibb Pharmaceuticals. Dr. Miro reports serving on the board and receiving lecture fees (and grant support through his institution) from Cubist Pharmaceuticals, serving on the board and receiving lecture fees (and grant support through his institution) from Novartis, receiving consulting fees and lecture fees from Abbott Laboratories, Bristol-Myers Squibb Pharmaceuticals, Gilead Sciences, Merck, Pfizer, and Theravance, and receiving lecture fees from Boehringer Ingelheim, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Roche, Schering-Plough, and ViiV Healthcare. Dr. Porter reports receiving lecture fees from Janssen Therapeutics (formerly Tibotec Therapeutics). Dr. Tambussi reports serving on the board and receiving consulting fees and payment for development of educational material (and grant support through his institution) from ViiV Healthcare, receiving grant support from Abbott Laboratories through his institution, and receiving payment for travel and accommodations from Gilead Sciences and Bristol-Myers Squibb Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank all the participants and staff at all the sites participating in the trial; the Wellcome Trust for long-term support; and the National Institute for Health Research Comprehensive Biomedical Research Centre for infrastructure support in the United Kingdom.
appendix
The members of the writing group — Sarah Fidler, M.B., B.S., Ph.D., Faculty of Medicine, Imperial College, London; Kholoud Porter, Ph.D., Medical Research Council Clinical Trials Unit, London; Fiona Ewings, Ph.D., Medical Research Council Clinical Trials Unit, London; John Frater, M.B., B.S., Ph.D., Peter Medawar Building for Pathogen Research, University of Oxford, Oxford, United Kingdom; Gita Ramjee, Ph.D., HIV Prevention Unit, Medical Research Council, Durban, South Africa; David Cooper, M.D., D.Sc., Kirby Institute, University of New South Wales, Sydney; Helen Rees, M.D., Wits Reproductive Health and HIV Institute, University of Witswatersrand, Hillbrow Health Precinct, Johannesburg; Martin Fisher, F.R.C.P., Royal Sussex County Hospital, Brighton, United Kingdom; Mauro Schechter, M.D., Ph.D., Hospital Escola Sao Francisco de Assis, Universidade Federal do Rio de Janeiro, Rio de Janeiro; Pontiano Kaleebu, M.D., Ph.D., Medical Research Council/Uganda Virus Research Institute, Entebbe, Uganda; Giuseppe Tambussi, M.D., Ospedale San Raffaele, Milan; Sabine Kinloch, M.D., Royal Free Hospital, London; Jose M. Miro, M.D., Ph.D., Hospital Clinic–Institut d’in vestigacions Biomèdiques August Pi I Sun yer, University of Barcelona, Barcelona; Anthony Kelleher, M.D., Ph.D., St. Vincent’s Hospital, Sydney; Myra McClure, Ph.D., F.R.C.Path., Faculty of Medicine, Imperial College, London; Steve Kaye, Ph.D., Faculty of Medicine, Imperial College, London; Michelle Gabriel, B.Sc., Medical Research Council Clinical Trials Unit, London; Rodney Phillips, M.D., M.B., B.S., Peter Medawar Building for Pathogen Research, University of Oxford, Oxford, United Kingdom; Jonathan Weber, F.R.C.P., Faculty of Medicine, Imperial College, London; and Abdel Babiker, Ph.D., Medical Research Council Clinical Trials Unit, London — assume responsibility for the content and integrity of the article. Drs. Weber and Babiker contributed equally to this article.
References
- 1.Detels R, Muñoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280:1497–503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Ray M, Logan R, Sterne JA, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–37. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell SK, Little SJ, Rosenberg ES. Clinical management of acute HIV infection: best practice remains unknown. J Infect Dis. 2010;202(Suppl 2):S278–S288. doi: 10.1086/655655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidler S, Fox J, Porter K, Weber J. Primary HIV infection: to treat or not to treat? Curr Opin Infect Dis. 2008;21:4–10. doi: 10.1097/QCO.0b013e3282f428bf. [DOI] [PubMed] [Google Scholar]
- 6.D’Souza MP, Axten KL, Hecht FM, Altfeld M. Acute HIV-1 infection: what’s new? Where are we going? J Infect Dis. 2010;202(Suppl 2):S267–S269. doi: 10.1086/655650. [DOI] [PubMed] [Google Scholar]
- 7.Centlivre M, Sala M, Wain-Hobson S, Berkhout B. In HIV-1 pathogenesis the die is cast during primary infection. AIDS. 2007;21:1–11. doi: 10.1097/QAD.0b013e3280117f7f. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011;364:1943–54. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–94. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg ES, LaRosa L, Flynn T, Robbins G, Walker BD. Characterization of HIV-1-specific T-helper cells in acute and chronic infection. Immunol Lett. 1999;66:89–93. doi: 10.1016/s0165-2478(98)00165-5. [DOI] [PubMed] [Google Scholar]
- 11.Hecht FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–33. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 12.Chamberland A, Sylla M, Boulassel MR, et al. Effect of antiretroviral therapy on HIV-1 genetic evolution during acute infection. Int J STD AIDS. 2011;22:146–50. doi: 10.1258/ijsa.2010.010292. [DOI] [PubMed] [Google Scholar]
- 13.Pires A, Hardy G, Gazzard B, Gotch F, Imami N. Initiation of antiretroviral therapy during recent HIV-1 infection results in lower residual viral reservoirs. J Acquir Immune Defic Syndr. 2004;36:783–90. doi: 10.1097/00126334-200407010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Schmid A, Gianella S, von Wyl V, et al. Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection. PLoS One. 2010;5(10):e13310. doi: 10.1371/journal.pone.0013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianella S, von Wyl V, Fischer M, et al. Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. Antivir Ther. 2011;16:535–45. doi: 10.3851/IMP1776. [DOI] [PubMed] [Google Scholar]
- 16.Hogan CM, Degruttola V, Sun X, et al. The Setpoint Study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012;205:87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grijsen ML, Steingrover R, Wit FW, et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: the randomized Primo-SHM trial. PLoS Med. 2012;9(3):e1001196. doi: 10.1371/journal.pmed.1001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy G, Parry JV. Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill. 2008;13:ii, 1896613. [PubMed] [Google Scholar]
- 19.Cressey TR, Green H, Khoo S, et al. Plasma drug concentrations and virologic evaluations after stopping treatment with nonnucleoside reverse-transcriptase inhibitors in HIV type 1-infected children. Clin Infect Dis. 2008;46:1601–8. doi: 10.1086/587657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kikaire B, Khoo S, Walker AS, et al. Nevirapine clearance from plasma in African adults stopping therapy: a pharmacokinetic substudy. AIDS. 2007;21:733–7. doi: 10.1097/QAD.0b013e3280121801. [DOI] [PubMed] [Google Scholar]
- 21.Ananworanich J, Moor Z, Siangphoe U, et al. Incidence and risk factors for rash in Thai patients randomized to regimens with nevirapine, efavirenz or both drugs. AIDS. 2005;19:185–92. doi: 10.1097/00002030-200501280-00011. [DOI] [PubMed] [Google Scholar]
- 22.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42:1608–18. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox J, Scriba TJ, Robinson N, Weber JN, Phillips RE, Fidler S. Human immunodeficiency virus (HIV)-specific T helper responses fail to predict CD4+ T cell decline following short-course treatment at primary HIV-1 infection. Clin Exp Immunol. 2008;152:532–7. doi: 10.1111/j.1365-2249.2008.03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Walker AS. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–74. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 25.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 26.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Office of AIDS Research Advisory Council; Bethesda, MD: Dec 1, 2007. [Google Scholar]
- 27.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Office of AIDS Research Advisory Council; Bethesda, MD: Dec 1, 2009. [Google Scholar]
- 28.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society–USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 29.Steingrover R, Garcia EF, van Valkengoed IG, et al. Transient lowering of the viral set point after temporary antiretroviral therapy of primary HIV type 1 infection. AIDS Res Hum Retroviruses. 2010;26:379–87. doi: 10.1089/aid.2009.0041. [DOI] [PubMed] [Google Scholar]
- 30.Al-Harthi L, MaWhinney S, Connick E, et al. Immunophenotypic alterations in acute and early HIV infection. Clin Immunol. 2007;125:299–308. doi: 10.1016/j.clim.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malhotra U, Berrey MM, Huang Y, et al. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;181:121–31. doi: 10.1086/315202. [DOI] [PubMed] [Google Scholar]
- 32.Oxenius A, Price DA, Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2000;97:3382–7. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streeck H, Jolin JS, Qi Y, et al. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol. 2009;83:7641–8. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Sadr WM, Grund B, Neuhaus J, et al. Risk for opportunistic disease and death after reinitiating continuous anti-retroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008;149:289–99. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.