Significance
Mechanisms controlling immune reactivity prevent excessive inflammation and autoimmunity, but generally dampen antitumor activity. The tumor necrosis factor alpha-induced protein 3 gene encoding the A20 protein, a key molecule controlling NF-κB activation, has been linked to the development of multiple inflammatory pathologies in humans, some of which are recapitulated in mice with selective deletion of A20 in myeloid, dendritic, or B cells. Here, mice with selective deletion of A20 in mature conventional T cells presented no detectable pathology. CD8 T cells from these mice showed increased antigen sensitivity with enhanced production of IL-2 and IFNγ. Importantly, A20-deleted CD8 T cells possessed heightened antitumor activity in vivo. Targeting this gene in adoptively transferred CD8 T cells could represent a promising mechanism to achieve tumor rejection.
Keywords: T-cell activation, tumor immunity, inflammation
Abstract
The transcription factor NF-κB is central to inflammatory signaling and activation of innate and adaptive immune responses. Activation of the NF-κB pathway is tightly controlled by several negative feedback mechanisms, including A20, an ubiquitin-modifying enzyme encoded by the tnfaip3 gene. Mice with selective deletion of A20 in myeloid, dendritic, or B cells recapitulate some human inflammatory pathology. As we observed high expression of A20 transcripts in dysfunctional CD8 T cells in an autochthonous melanoma, we analyzed the role of A20 in regulation of CD8 T-cell functions, using mice in which A20 was selectively deleted in mature conventional T cells. These mice developed lymphadenopathy and some organ infiltration by T cells but no splenomegaly and no detectable pathology. A20-deleted CD8 T cells had increased sensitivity to antigen stimulation with production of large amounts of IL-2 and IFNγ, correlated with sustained nuclear expression of NF-κB components reticuloendotheliosis oncogene c-Rel and p65. Overexpression of A20 by retroviral transduction of CD8 T cells dampened their intratumor accumulation and antitumor activity. In contrast, relief from the A20 brake in NF-κB activation in adoptively transferred antitumor CD8 T cells led to improved control of melanoma growth. Tumor-infiltrating A20-deleted CD8 T cells had enhanced production of IFNγ and TNFα and reduced expression of the inhibitory receptor programmed cell death 1. As manipulation of A20 expression in CD8 T cells did not result in pathologic manifestations in the mice, we propose it as a candidate to be targeted to increase antitumor efficiency of adoptive T-cell immunotherapy.
Mechanisms controlling immune reactivity prevent excessive inflammation and autoimmunity, but generally dampen antitumor activity (1, 2). It is thus important to understand the consequences of release from immune control mechanisms in terms of increase in antitumor efficacy on the one hand and with respect to the possibility of development of autoimmune pathologies on the other hand. The transcription factor NF-κB is central to inflammatory signaling, as well as to activation of innate and adaptive immune functions. Activation of the NF-κB pathway is regulated by ubiquitination and is tightly controlled by several feedback mechanisms (3). A20, an ubiquitin-modifying enzyme encoded by the tnfaip3 gene, is one of the major inhibitors of the canonical NF-κB signaling pathway (4). Genome-wide association studies (GWAS) have linked germ-line single nucleotide polymorphisms of the TNFAIP3 gene with susceptibility to multiple human pathologies, including systemic lupus erythematosus (SLE) and psoriasis (5). For the latter autoimmune diseases, causal mutations have been characterized that control either the level of expression or the function of A20.
When A20 is ubiquitously knocked out, mice are viable but develop severe multiorgan inflammation leading to premature death (6). Using mouse models expressing the recombinase Cre in specific cell types crossed to A20 flox/flox (A20fl/fl) mice, A20 deficiency has been well studied in B cells, myeloid cells, and dendritic cells (DCs) (7–12). With each cell type, specific deletion of A20 led to the development of various degrees of autoimmune signs. Specific A20 deletion in B cells led to the progressive development of a SLE-type pathology (7, 9, 12), whereas mice with A20 deletion in cells of myeloid origin developed spontaneous polyarthritis with the production of type II collagen autoantibodies. Mice with DC-specific A20 deletion developed either features of SLE (10) or features of human inflammatory bowel disease (IBD) in independent studies (8). In both cases the lack of A20 in DCs induced aberrant activation and proliferation of T cells. To our knowledge, no study of A20 deficiency in primary T cells has been conducted, although the involvement of A20 in T-cell receptor (TCR)-mediated signaling in cultured cells has been reported (13, 14).
We observed a sustained high level expression of A20 transcripts in dysfunctional CD8 T cells isolated from a progressing autochthonous melanoma in mice. This provided a strong incentive to analyze the consequences of A20 deletion in mature CD8 T cells on their differentiation in basal conditions and their capacity to develop antitumor activity.
Results
Characteristics of Mice with Specific Deletion of A20 in Peripheral T Cells.
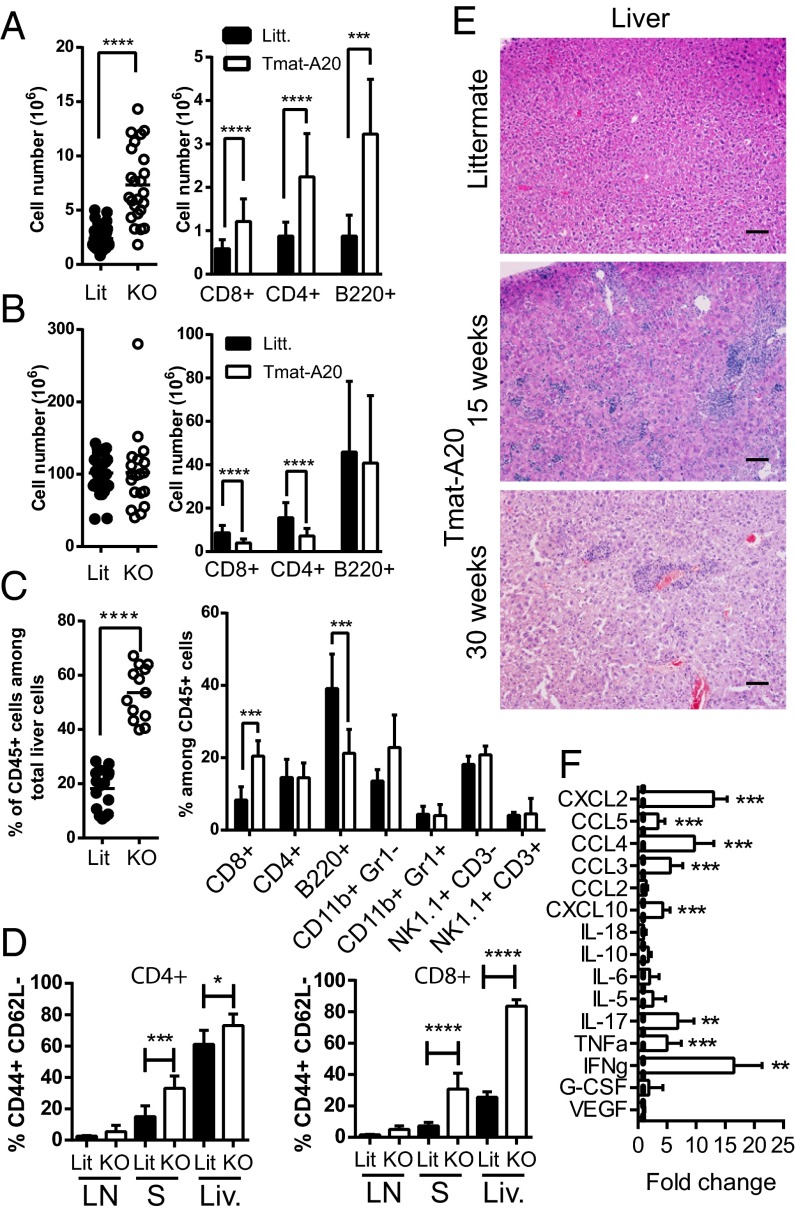
To achieve A20 deletion in primary T cells we crossed A20fl/fl mice (15) with mature T-Cre (maT-Cre) mice that specifically express the Cre recombinase in 80% of the peripheral CD8 T cells, around 50% of peripheral CD4 T cells, and 25% of regulatory T cells (maT-A20, Fig. S1). Recombination was limited in thymocytes (Fig. S1 A and C) as well as in B cells (<1%), natural killer (NK) cells (<6%), CD11b+ myeloid cells (<3%), and CD11b+ CD11c+ DCs (<4%) (Fig. S1 B and D). These mice developed lymphadenopathy as early as 6 wk after birth with an increase in CD4 and CD8 T cells as well as B220+ cells in lymph nodes (LNs) (Fig. 1A). LN CD8 and CD4 T cells retained a naïve phenotype (Fig. 1D). Total splenocyte numbers were similar but splenic CD4 and CD8 T-cell numbers were decreased in maT-A20 mice compared with littermates (Fig. 1B). Interestingly 35% of the remaining splenic T cells showed an effector-like phenotype (Fig. 1D). In the liver, we observed an increased infiltration of CD45+ cells, with no significant variation of major immune populations except an increase in the proportion of CD8 T cells (Fig. 1C). Most of these CD8 T cells had an effector-like phenotype (Fig. 1D). Increased infiltration in the liver or the lung was detectable at 6, 15, or 30 wk of age, with a phenotype that did not evolve after 15 wk of age (Fig. 1E and Fig. S1E). Given the very low recombination in myeloid populations, it seems unlikely that the activated T-cell phenotype observed in the maT-A20 mice resulted from the presence of A20-deleted non-T cells as previously shown in mice with A20-deleted macrophages (16) or DCs (8, 10). A multiplex analysis of the serum of 15-wk-old mice showed augmented levels of proinflammatory cytokines including IFNγ, TNFα, or IL-17. We also detected increased levels of several chemokines in the serum from maT-A20 mice (CXCL2, CXCL10, CCL3, CCL4, and CCL5/Rantes, Fig. 1F). The latter three chemokines may be produced directly by T cells (17), whereas the former two may be produced as the result of the indirect activation of myeloid cells by T-cell–produced IFNγ or TNFα (18). In conclusion, in a model with a specific deletion of A20 in peripheral T cells, we observed the development of some lymphadenopathy and infiltration of T cells in peripheral organs. Nevertheless, we did not detect any weight loss or any difference in survival when comparing maT-A20 mice to control littermates (Fig. S1 F and G).
Fig. 1.
T-cell–specific deletion of A20 in mice induces lymphadenopathy and infiltration of T cells in peripheral organs. (A–C) Total cell number (Left) or number of the indicated population (Right) per LN (A), spleen (B), or percentage of CD45+ cells among total cells in the liver (Left) and percentage of the indicated population among CD45+ cells (Right) in the liver (C) of maT-A20 mice (KO, open circles) or WT littermates (Litt., black circles). Each circle represents the number of cells from 1 mouse. Results from 6-, 9-, or 15-wk-old mice were pooled as we did not observe any significant variation between the various ages. Histograms represent pooled data from at least 10 mice per condition. (D) Percentage of CD62L− CD44+ T cells was determined by flow cytometry in LN, spleen (S), or liver (Liv.) from Littermate (Litt.) or maT-A20 mice (KO). (E) Hematoxilin/eosin labeling was performed on livers from 15-wk-old maT-A20 mice or littermate controls. Representative pictures from 6 individual mice are shown. (Scale bars, 100 µm.) (F) Fold increase of the indicated cytokine/chemokine in serum of maT-A20 mice (n = 12) versus littermate controls (n = 8) as measured by Luminex analysis.
To further evaluate the in vivo consequence of A20 deletion in CD8 T cells, we reconstituted Rag-2−/− mice with either CD8 T cells alone or CD8 T cells plus CD4 T cells purified from maT-A20 mice LNs (Fig. S2). Results showed that transfer of A20-deleted CD8 T cells alone did not lead to organ infiltration (Fig. S2 B and C), whereas transfer of A20-deleted CD8 and CD4 T cells resulted in a preferential expansion of splenic CD4 over CD8 T cells (Fig. S2A) and strong liver infiltration by T cells (Fig. S2 B and C). Relative to their representation in the spleen, CD8 T cells were highly enriched within the liver (Fig. S2 A and B). Altogether, these results suggested that the phenotype observed in the maT-A20 mice resulted from A20 deletion in both conventional CD4 and CD8 T cells. A20-deleted CD8 T cells alone did not cause autoimmune pathology.
A20 Deletion in CD8 T Cells Increases Their Cytokine Production Through an Increased NF-κB Activation.
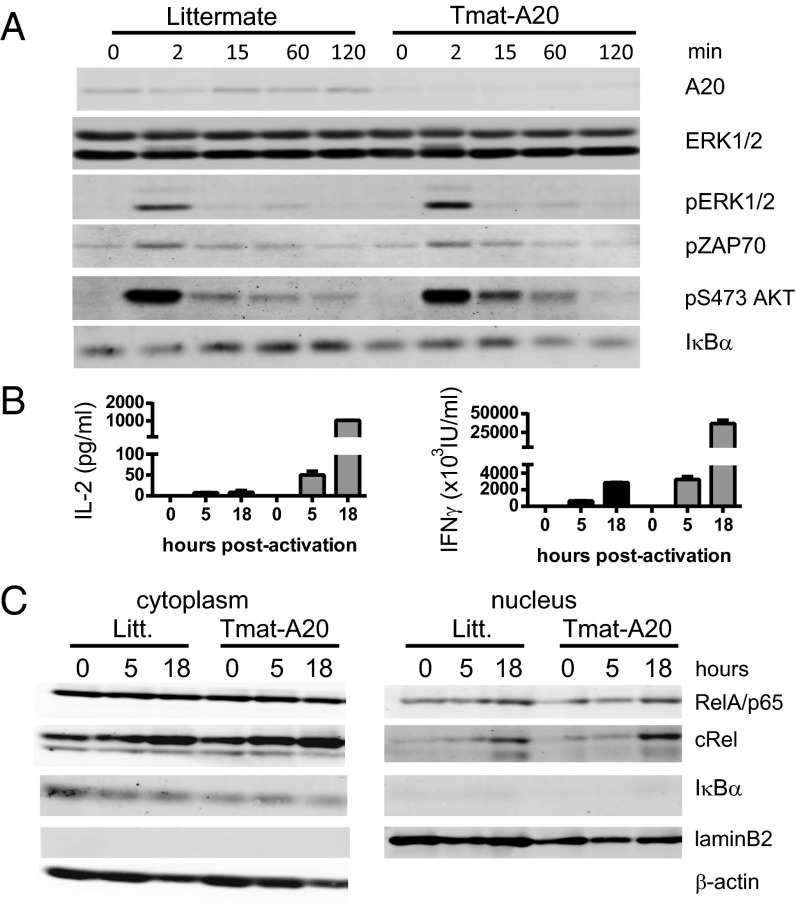
To measure the effect of A20 deletion on early T-cell activation, naïve CD8 T cells from LNs of maT-A20 mice or littermates were stimulated for 2–120 min with cross-linked anti-CD3 and anti-CD28 mAb. No difference was detected in ZAP70, ERK, or protein kinase AKT phosphorylation between the two samples (Fig. 2A). Antigen receptor activation of the canonical NF-κB pathway requires phosphorylation followed by degradation of the Inhibitor of NF-κB alpha (IκBα) to allow the translocation of active NF-κB components to the nucleus. We observed a sustained decrease in the level of the IκBα protein in CD8 T cells from maT-A20 mice after stimulation with anti-CD3/CD28 (at 60 and 120 min) compared with CD8 T cells from WT mice (Fig. 2A and Fig. S3A).
Fig. 2.
CD8 T cells from maT-A20 mice have increased NF-κB activation upon stimulation. Purified CD8 T cells from maT-A20 mice (maT-A20) or littermates (Litt.) were stimulated with cross-linked anti-CD3 and anti-CD28 (A) or with coated anti-CD3 and soluble anti-CD28 (B and C) for the indicated time period. Cytoplasmic protein extracts (A) or cytoplasmic and nuclear protein extracts (C) were analyzed by Western blot for the indicated proteins. (B) Levels of IFNγ and IL-2 were measured by ELISA in the in vitro cultures used in C.
When stimulated with coated anti-CD3 and soluble anti-CD28, CD8 T cells from maT-A20 mice produced a much higher amount of IL-2 and IFNγ compared with CD8 T cells from WT mice as early as 5 h poststimulation, with up to 100-fold more IL-2 and 10-fold more IFNγ produced 18 h poststimulation (Fig. 2B). At the latter time point, we detected a slightly increased level of RelA/p65 and a clearly increased level of c-Rel protein in the nucleus of CD8 T cells from maT-A20 mice that correlated with a marked decrease in IκBα in the cytoplasm of the cells (Fig. 2C and Fig. S3B). Altogether, CD8 T cells from maT-A20 mice have an increased capacity to produce IL-2 and IFNγ upon stimulation. These characteristics correlate with an increased cytoplasmic degradation of IκBα, the inhibitor of NF-κB, and a higher level of c-Rel in the nucleus of these cells.
A20 Deletion in TCR Transgenic CD8 T Cells Increases Their Capacity to Produce Cytokines in Response to Low Doses of Peptide.
To further study how A20 deletion affects CD8 T-cell activation and differentiation, we crossed maT-A20 mice with mice transgenic for the P14 TCR specific for lymphocytic choriomeningitis virus (LCMV) GP33 associated with H-2Db (GP33/H-2Db) (P14-maT-A20) (19). In these mice, we did not detect increased infiltration in the liver (Fig. S4A). CD8 T cells detected in the LNs, spleen, or liver kept a naïve phenotype (Fig. S4B) with a comparable expression of the Vα and the Vβ chains of the P14 TCR as in WT P14 mice (Fig. S4C).
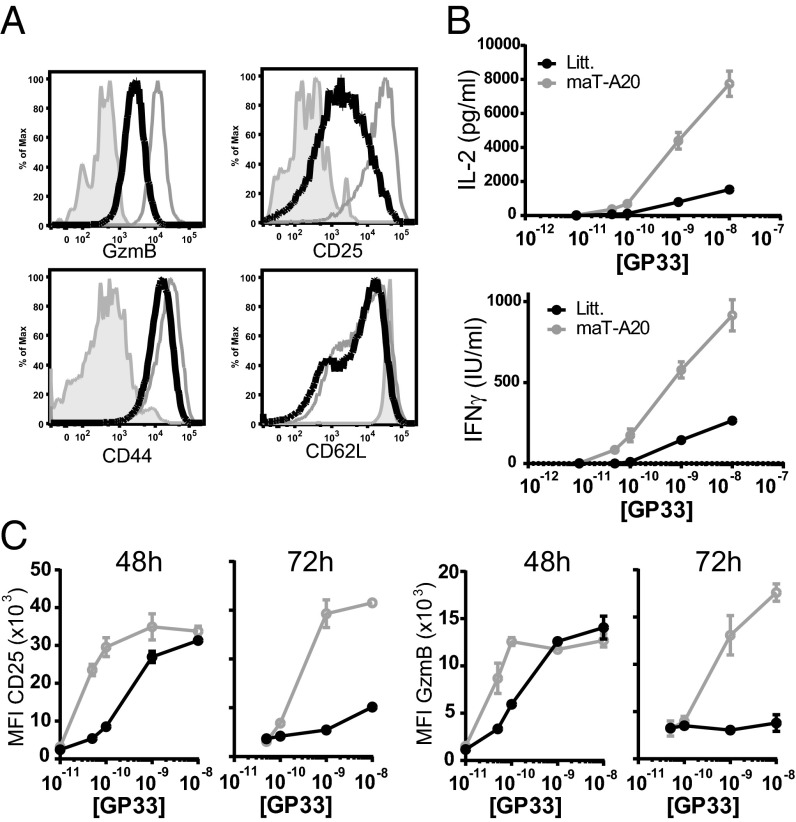
After 24 h of in vitro stimulation with various amounts of antigenic peptide (from 10−11 M to 10−8 M), the T cells from WT or maT-A20 mice expressed similar levels of CD69 (Fig. S4D). At 48/72 h they both down-regulated CD62L expression at their surface to the same extent and expressed a high level of CD44 similarly (Fig. 3A and Fig. S4E). The major differences between the two cell types were the expression of CD25 and granzyme B (GzmB), both present at a higher level at 48 h and more strikingly at 72 h poststimulation in P14-maT-A20 CD8 T cells (Fig. 3 A and C). Furthermore, P14-maT-A20 CD8 T cells produced five times more IL-2 compared with WT P14 T cells and three to four times more IFNγ, depending on the concentration of peptide (Fig. 3B). These results showed that A20 deletion in CD8 T cells increased their capacity to produce large amounts of cytokines after stimulation with low amounts of peptide.
Fig. 3.
Increased cytokine production by CD8 T cells from maT-A20 mice. (A–C) CD8 T cells from LNs of P14-maT-A20 (gray line) or WT P14 littermate (black line) mice were left unstimulated (solid gray, A) or stimulated with GP33 peptide at a concentration ranging from 10−8 to 10−11 M and analyzed by flow cytometry for the indicated molecule. (A) Histograms represent the level of expression of the indicated molecules at 72 h postactivation (10−9 M GP33 peptide). (B) Supernatants from the P14-maT-A20 or WT P14 T cells stimulation with the indicated concentration of GP33 peptide were tested in triplicates by ELISA for content of IL-2 (24 h) or IFNγ (48 h). (A and B) Results are representative of three independent experiments. (C) Mean fluorescence intensity (MFI) of the indicated molecules was measured 48 or 72 h after the initial stimulation of WT P14 (black line) or P14-maT-A20 (gray line) CD8 T cells with the indicated concentration of peptide.
High Levels of A20 in Tumor-Specific CD8 T Cells Correlate with Poor Intratumor Accumulation.
We previously developed a mouse model of induced melanoma based on conditional deletion of tumor suppressor genes with concomitant expression of a natural mouse tumor antigen (Tyr-iRas-P1A-transgenic Ink4a/Arf flox/flox or TiRP mice) (20). In this model, tumor-intrinsic factors control the development of aggressive tumors and their expression of an inflammatory/ immunosuppressive program (21). Intratumor T cells expressed inhibitory receptors such as PD-1 and showed a low level of GzmB and poor capacity to produce IFN-γ upon restimulation (22, 23). We showed by quantitative RT-PCR that A20/tnfaip3 transcripts are overexpressed in CD8+ tumor infiltrating lymphocytes (TILs) sorted from TiRP melanomas compared with naïve or activated T cells (Fig. 4A).
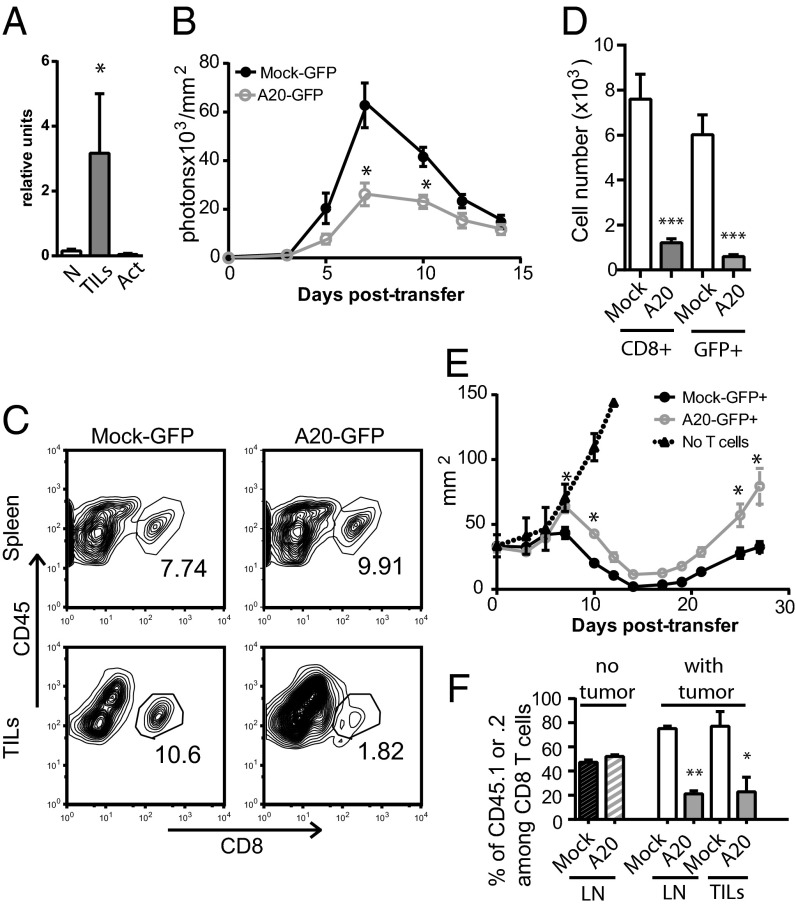
Fig. 4.
Inefficient tumor infiltration/elimination by CD8 T cells expressing high level A20. (A) CD8 T cells were sorted from melanomas that developed in TiRP mice (three independent samples). RNA levels of A20 from these cells (TILs) were compared with those from naïve CD8 T cells (N) and from in vitro activated CD8 T cells (Act) by quantitative RT-PCR. (B–F) The 5 × 104 sorted GFP+ TCRP1A-Luc CD8 T cells transduced with mock or A20 encoding retroviral constructs were injected in Rag−/−B10.D2 mice, injected with 106 P511 mastocytoma 7 d earlier. (B) P511 infiltration by TCRP1A-Luc T cells was monitored by bioluminescence. (C) At day 7, splenocytes and TILs were analyzed by flow cytometry for the indicated markers. Percentages of CD45+ CD8+ T cells are indicated on the dot plots. (D) TCRP1A CD8 T-cell numbers in TILs from three pooled experiments are shown. (E) Tumor growth was monitored every 2–3 d and pooled results from two independent experiments (10 mice per condition) are shown. (F) After sorting, CD45.1 mock-transduced and CD45.2 A20-transduced TCRP1A T cells were mixed at a 1:1 ratio and transferred in Rag−/−B10.D2 mice (hatched bars) or Rag−/−B10.D2 mice injected with 106 P511 mastocytoma 7 d earlier (empty white or gray bars). Mean percentage CD45.1+ or CD45.2+ among CD8 T cells at day 6 posttransfer in the LNs or in TILs (two experiments with 6 mice per condition) are shown.
To assess the consequences of a high level expression of A20 in T cells on their capacity to accumulate in a tumor and to affect tumor progression, we used a system of adoptive transfer of TCR transgenic CD8 T cells specific for the P1A35–43 epitope (TCRP1A) previously shown to induce regression of the P1A-expressing P511 mastocytoma (17). We cloned the cDNA encoding A20 into a retroviral vector also coding for GFP. We next activated and transduced tumor-specific TCRP1A CD8 T cells that express luciferase as a transgene (TCRP1A-Luc) with a control retrovirus or with the retrovirus encoding A20 (Fig. S5A). Transduced CD8 T cells expressed a higher level of the relevant transcripts and of the A20 protein compared with mock-transduced T cells (Fig. S5 B and C). They were sorted on the basis of their GFP expression (Fig. S5A) and transferred into Rag−/−B10.D2 mice that had developed a solid tumor after injection of P511 mastocytoma 7 d before, as described (17). We measured by bioluminescence the infiltration of the tumor by T cells. We detected a threefold lower luminescence intensity when using A20-transduced compared with mock-transduced TCRP1A-Luc T cells at day 7, the peak of infiltration of the tumor (Fig. 4B). This was associated with a similar decrease in CD8 T-cell number recovered from the tumors at that time (Fig. 4 C and D), despite a similar proportion of TCRP1A T cells found in the spleens of these animals (Fig. 4C). This dampened intratumor accumulation correlated with a delay in P511 regression upon adoptive transfer of A20-transduced compared with mock-transduced TCRP1A T cells (Fig. 4E). In this transplanted tumor model, tumor antigen-loss variants escape adoptive therapy by TCRP1A T cells leading to tumor regrowth after 20 d (17, 24). Regression of the P511 tumor, which remained incomplete upon adoptive transfer of A20-transduced TCRP1A T cells, was also followed by a more robust tumor regrowth (Fig. 4E). The GFP level in mock- versus A20-transduced TCRP1A T cells was similar before adoptive transfer into the mice (Fig. S5A). As early as 4 or 7 d after transfer, we observed a dramatic decrease in GFP expression in A20-transduced TCRP1A T cells (Fig. S5 D and E), suggesting a negative effect of high level A20 expression on T-cell fitness and a selective expansion/survival of A20-low T cells. To determine whether this negative effect is linked to an early defect in survival after transfer or to a defect occurring after tumor-induced reactivation, we mixed mock-transduced GFP+ CD45.1 with A20-transduced GFP+ CD45.2 TCRP1A T cells at a 1:1 ratio. We then transferred these cells into Rag−/−B10.D2 mice that were injected or not with P511 mastocytomas 7 d earlier. In tumor-free mice, the ratio of CD45.1 to CD45.2 T cells remained identical 7 d posttransfer of the mixed TCRP1A T cells (Fig. 4F). This observation ruled out any differential survival capacity during homeostasis after adoptive transfer. However, in tumor-bearing mice, a 3.5 ratio in favor of the mock-transduced TCRP1A T cells was observed both in the LNs and inside the tumor (Fig. 4F). This result suggests that A20-expressing cells developed a defective tumor-induced secondary response compared with control T cells and failed to accumulate in tumor-bearing hosts. Maintenance of a same ratio of CD45.1 to CD45.2 cells between LNs and TILs also ruled out a potential defect in migration of A20-transduced T cells. In vitro, 48 h posttransduction, we did not detect any increase in annexin V labeling of either A20- or mock-transduced GFP+ CD8 T cells (Fig. S5F). We detected only a slight delay in the proliferation of GFP+ A20-transduced CD8 T cells (Fig. S5G). Altogether, we show that high level expression of A20 in CD8 T cells is detrimental for their antitumor efficiency.
A20 Deletion in CD8 T Cells Increases Their Capacity to Eliminate Tumor Burden.
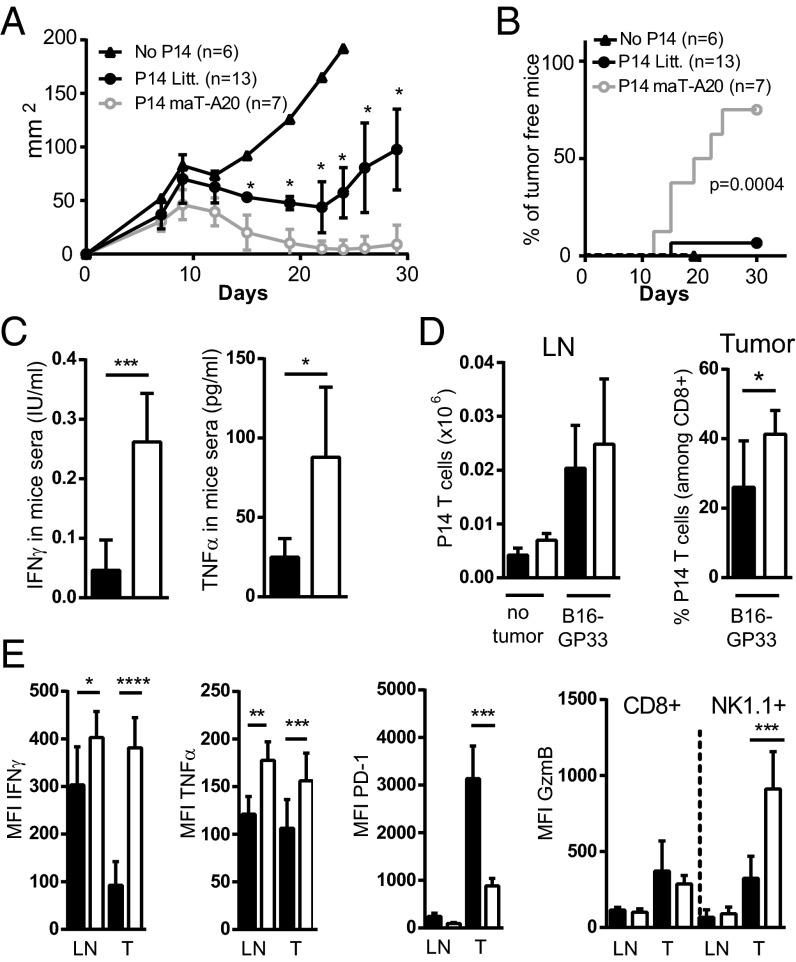
Given the high level of A20 in nonresponsive TILs and the increased capacity for CD8 T cells lacking A20 to develop effector functions, we further tested the capacity to eliminate tumor cells by naïve tumor-specific CD8 T cells from maT-A20 compared with WT mice. For this, we used GP33/H-2Db-specific CD8 T cells from P14 mice, which are inefficient at preventing the growth of B16F10 melanoma cells expressing the LCMV GP33 epitope (B16-GP33) (25). We injected WT C57BL/6 mice with B16-GP33 melanoma cells and the following day we transferred purified naïve P14 T cells from WT or maT-A20 mice. Tumor growth was significantly decreased in the presence of P14-maT-A20 CD8 T cells compared with WT P14 CD8 T cells (Fig. S6A). In another protocol, we first injected 106 B16-GP33 cells in C57BL/6 CD45.1 mice and waited for the formation of a solid mass (at day 7) before transferring 6–8 106 in vitro preactivated CD45.2 P14-maT-A20 or WT P14 CD8 T cells. We observed a reduction of the tumor mass with both cell types (Fig. 5A) but only P14-maT-A20 CD8 T cells cleared the tumor burden (in 75% of the mice, Fig. 5B). At day 7 after T-cell transfer, we measured a threefold increase of IFNγ and TNFα in the serum of the mice that received P14-maT-A20 compared with WT P14 CD8 T cells (Fig. 5C). The analysis of the transferred CD45.2 CD8 T cells revealed similar numbers of cells in the LNs and slightly increased P14-maT-A20 compared with WT P14 CD8 TILs (Fig. 5D). When tested ex vivo for their capacity to produce IFNγ in response to stimulation by their cognate peptide GP33, P14-maT-A20 CD8 T cells from LNs responded slightly better than the WT P14 counterparts. The difference was much increased in favor of the P14-maT-A20 cells when TILs were compared (Fig. 5E for MFI; Fig. S6B for percentage of positive cells). A similar trend was observed when TNFα producing CD8 T cells were analyzed (Fig. 5E and Fig. S6B). The expression of GzmB was higher in TILs than in LNs for both WT P14 and P14-maT-A20 CD8 T cells (Fig. 5E and Fig. S6B) without a significant difference between the two groups. Because high expression of the inhibitory receptor PD-1 has been associated with dysfunction of intratumor T cells, we compared its level of expression on the two types of transferred T cells. The expression of PD-1 was about threefold lower on P14-maT-A20 than on WT P14 TILs (Fig. 5E). Interestingly, this PD-1 level of the adoptively transferred P14-maT-A20 CD8 T cells was also lower than that of endogenous CD8 T cells (CD45.1) present in the same tumor (Fig. S6C), indicating that low level expression of PD-1 is a property intrinsic to the P14-maT-A20 CD8 T cells and is not related to differences in the tumor microenvironment. We also observed that endogenous NK cells (CD45.1) present within the tumors of mice that received P14-maT-A20 CD8 T cells had more than twofold higher expression of GzmB than those from mice that received WT P14 CD8 T cells (Fig. S6D). This property of “bystander activation” may further contribute to antitumor activity.
Fig. 5.
P14 CD8 T cells from maT-A20 mice have increased antitumor potential. B16-GP33 cells (106) were injected s.c. in CD45.1 C57BL/6 mice and in vitro preactivated WT CD45.2 P14 or CD45.2 P14-maT-A20 CD8 T cells (6–8 × 106 cells) were transferred 7 d later. (A and B) Tumor growth was monitored with a caliper every 2–3 d. (C) At day 7 after transfer, levels of IFNγ and TNFα in mice sera were measured by ELISA. (D) On the same day, the number of CD45.2+ P14 T cells present in LNs and their proportion in TILs among total CD8 T cells was determined; number of CD45.2+ P14 T cells present in LNs of mice that received no tumor (no tumor) is also shown. (E) MFI of the indicated molecules was determined by flow cytometry on CD45.2+ P14 T cells and on endogenous CD45.1+ NK1.1+ cells (for GzmB only) present in the LNs or in TILs (T) directly ex vivo (PD-1 and GzmB) or after 4 h restimulation with GP33 peptide (IFNγ and TNFα). (A–E) Results are representative of two independent experiments. Numbers of mice are indicated in A and B and six mice per condition were used for C–E.
In conclusion P14 T cells deleted for A20 expression showed an improved capacity to eliminate tumor cells. This improvement was associated with their enhanced capacity to produce IFNγ and TNFα and their moderate expression of the inhibitory receptor PD-1. In addition, presence of these T cells led to bystander activation of NK cells within the tumor microenvironment. The latter property may further contribute to contain the escape from T-cell therapy of tumor antigen- or MHC-loss variants (17).
Discussion
We show that deletion of A20 in peripheral CD8 and CD4 conventional T cells, under conditions that did not affect the majority of regulatory T cells, induced early enlargement of lymph nodes and lymphocytic infiltration of primary organs. Surprisingly, this phenotype observed in 6-wk-old mice did not worsen with age. In maT-A20 mice crossed with P14 TCR transgenic mice, infiltration of the lung or liver with CD8 T cells was abrogated compared with the phenotype we observed in maT-A20 mice with a full TCR repertoire. In addition to the restricted TCR repertoire expressed by the P14 CD8 T cells, the development of CD4 T cells is greatly diminished in P14 transgenic mice (19). The paucity of A20-deleted CD4 T cells in these mice may be the reason for the absence of lymphocytic infiltration in peripheral organs as our data on reconstitution of Rag-2−/− mice indicated that A20-deleted CD8 T cells alone failed to reproduce the organ infiltration that was observed upon coinjection of A20-deleted CD4 T cells. The control of the infiltration over time in WT A20 mice could depend on the establishment of mechanisms of peripheral tolerance exerted extrinsically, such as by T regulatory cells or intrinsically by induction of deletion or anergy in the CD8 T cells (26). In vitro A20-deficient CD8 T cells had an increased capacity to produce IFNγ and IL-2 in response to limited amounts of antigen. These results are consistent with previous observations using Jurkat cells transfected with siRNA targeting A20 (14).
NF-κB signaling is tightly controlled by ubiquitination, and A20, through its deubiquinating activity, is one of the proteins that affects this process. The CARMA1, BCL10, and MALT1 signalosome bridges TCR signaling to the IκB kinase (IKK) complex activating the canonical NF-κB pathway, which involves mainly NF-κB heterodimers consisting of RelA, c-Rel, and p50 (27). Activation of this pathway involves the ubiquitin-dependent proteasomal degradation of the IκB inhibitory proteins upon their phosphorylation at the IKK complex. Release from the IκB proteins leads to nuclear translocation of the NF-κB dimers and their DNA binding. In studies performed in the Jurkat line, A20 has been shown to control the ubiquitination of MALT1, thereby preventing the interaction between the ubiquitinated MALT1 protein and the IKK complex, acting as a negative regulator of the NF-κB pathway (14). In our study, we observed an increase in nuclear translocation of c-Rel compared with RelA NF-κB components in the response to CD3/CD28 engagement of A20-deleted versus WT CD8 T cells. Whether the relief from A20 control of MALT1 activity favors the activation of c-Rel over RelA remains to be determined. This is suggested to occur in B cells, where MALT1 directs BCR-induced canonical NF-κB signaling selectively to the c-Rel subunit (28). Interestingly, for CD8 T-cell activation, c-Rel deficiency has been reported to phenocopy deficiency of PKCθ thought to activate the CARMA1, BCL10, and MALT1 signalosome, but not BCL10 deficiency (29). Irrespective of the detailed mechanism of increase in NF-κB activity, we show that A20 deletion in CD8 T cells greatly enhances their capacity to produce IL-2 and IFNγ. This is coherent with the described defects in IL-2 production by T cells lacking c-Rel (30) and c-Rel requirement for chromatin remodeling across the IL-2 gene promoter (31). It is also consistent with the observed strict dependency of IFNγ gene transcription upon binding of NF-κB to cis-regulatory elements of the gene for both TCR- and cytokine (IL-12/IL-18)-induced signaling in CD8 T cells (32).
We have observed that dysfunctional CD8 T cells isolated from a progressing autochthonous melanoma in mice overexpressed A20 transcripts compared with naïve or acutely activated CD8 T cells. We demonstrated that overexpression of A20 by retroviral transduction of tumor-specific CD8 T cells led to a drastic inhibition of their intratumor accumulation. We show that this effect is not related to the homing capacity of the T cells and that it requires restimulation induced by the tumor. Because of a rapid loss of the T cells expressing high levels of A20, we were not able to determine whether it was related to a defect in activation, proliferation, or survival.
Importantly, relief from the A20 brake in NF-κB activation in adoptively transferred antitumor CD8 T cells led to increased efficiency in induction of regression of the B16 melanoma with concomitant detection of high levels of IFNγ and TNFα in the serum of the mice. In addition to a small increase (1.6-fold) in the number of A20-deleted compared with WT CD8 T cells infiltrating the tumors, the A20-deleted CD8 TILs produced higher amounts of IFNγ and TNFα ex vivo. Intratumor IFNγ has been shown to have beneficial antitumor effects, including the activation of tumor-associated macrophages toward a tumoricidal rather than tumor-promoting program (33) as well as angiostatic effects (34). In synergy with TNFα, IFNγ further mediates the destruction of the tumor stroma (35). Whereas injection of these cytokines may produce unwanted toxicity, tumor antigen-specific T cells localize to the tumors and release cytokines without evidence for systemic toxicity. In contrast to the beneficial antitumor effects of IFNγ, this cytokine has also been shown to contribute to the adaptive immune resistance of tumor cells (1). This is thought to occur by its capacity to increase the expression on tumor cells of the ligand PDL-1 of the inhibitory receptor PD-1. PD-1 is transiently expressed upon T-cell activation, but its sustained expression on CD8 T cells contributes to the generation of dysfunctional CD8 T cells in numerous settings involving persistent antigen, such as chronic viral infections and cancer. Engagement of T-cell–expressed PD-1 by tumor-expressed PDL-1 has been shown to inhibit CD8 T-cell effector functions (36). The intrinsic characteristic of moderate levels of expression of PD-1 on A20-deleted CD8 TILs observed in our study may thus contribute to the enhanced antitumor efficacy of these cells upon adoptive immunotherapy.
This work contributes to the identification of a major pathway affected in dysfunctional CD8 T cells within tumors, which, in the case studied here, could be reversed without apparent pathologic consequences. Thus, A20 may be an interesting candidate to be targeted in the context of adoptive T-cell immunotherapy.
Materials and Methods
Mice and Cell Lines.
TiRP-10B; Ink4a/Arf flox/flox mice, “TiRP mice,” were kept on a B10.D2 background and treated with 4OH-tamoxifen as described (20, 23). A20flox/flox mice (15) were crossed on maT-Cre mice (37) (Fig. S1). Mice heterozygous for the H-2Ld/P1A35–43-specific TCR-transgene (TCRP1A) (17) and luciferase expressing TCRP1A mice (TCRP1A-Luc) (24) were kept on the Rag-1−/−B10.D2 background. P14 TCR mice (19), Rag-1−/−B10.D2, and C57BL/6 (CD45.2) as well as congenic C57BL/6 (CD45.1) mice were also used. All mice were bred in the Centre d'Immunologie de Marseille–Luminy animal facility. Animal experiments were approved by the regional Provence-Alpes-Côte d'Azur committee on ethics for animal experimentation and respected French and European directives. B16F10 melanoma cells expressing the glycoprotein epitope amino acids 33–41 (B16GP33) were a kind gift from H. Pircher (University of Freiburg, Freiburg, Germany). P511 mastocytoma cells expressing the P1A gene were used as described (17).
CD8 T-Cell Activation and Retroviral Infections.
FL cDNA vector coding for A20 (clone ID 6838303 from Thermo Scientific ABgene) was cloned into the retroviral vector pMX-IRES-GFP after PCR amplification using PFUultra HF (Agilent) using the following primers GGAAGATCTCCCCAAGAGGCCTTGT/TTGCTGGACCTGTCAAT (A20) and TCGGAAATATACCA AAGC/TTTTCCTTTTGCGGCCGCTTCTTTGACGTGCTTGG (NR4A2). Retroviral particles were produced as described (38). TCRP1A T cells were activated with 10−7 M of the P1A35–43 (LPYLGWLVF) peptide, retrovirally transduced after 20 h as described (38) and further cultured for another 48 h.
Additional materials and methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Y. Hamon for technical advice, L. Leserman for suggestions on the manuscript, T. Lawrence for his support, and the Centre d'Immunologie de Marseille–Luminy imaging and animal facilities for assistance. This work was supported by funding from Institut National de la Santé et de la Recherche and Centre National de la Recherche Scientifique and by grants from the Agence Nationale de la Recherche (ANR Retour Post-Doctorants to G.V.) and the Association pour la Recherche sur le Cancer (to A.-M.S.-V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406259111/-/DCSupplemental.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: From tumor tolerance to eradication. Immunol Rev. 2011;241(1):104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 4.Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol. 2014;35(1):22–31. doi: 10.1016/j.it.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Ma A, Malynn BA. A20: Linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12(11):774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee EG, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu Y, et al. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood. 2011;117(7):2227–2236. doi: 10.1182/blood-2010-09-306019. [DOI] [PubMed] [Google Scholar]
- 8.Hammer GE, et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol. 2011;12(12):1184–1193. doi: 10.1038/ni.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hövelmeyer N, et al. A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies. Eur J Immunol. 2011;41(3):595–601. doi: 10.1002/eji.201041313. [DOI] [PubMed] [Google Scholar]
- 10.Kool M, et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35(1):82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Matmati M, et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet. 2011;43(9):908–912. doi: 10.1038/ng.874. [DOI] [PubMed] [Google Scholar]
- 12.Tavares RM, et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity. 2010;33(2):181–191. doi: 10.1016/j.immuni.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coornaert B, et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol. 2008;9(3):263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- 14.Düwel M, et al. A20 negatively regulates T cell receptor signaling to NF-kappaB by cleaving Malt1 ubiquitin chains. J Immunol. 2009;182(12):7718–7728. doi: 10.4049/jimmunol.0803313. [DOI] [PubMed] [Google Scholar]
- 15.Vereecke L, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207(7):1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, et al. A20 controls macrophage to elicit potent cytotoxic CD4(+) T cell response. PLoS ONE. 2012;7(11):e48930. doi: 10.1371/journal.pone.0048930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanker A, et al. CD8 T cell help for innate antitumor immunity. J Immunol. 2007;179(10):6651–6662. doi: 10.4049/jimmunol.179.10.6651. [DOI] [PubMed] [Google Scholar]
- 18.Griffin GK, et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188(12):6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pircher H, Bürki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 20.Huijbers IJ, et al. An inducible mouse model of melanoma expressing a defined tumor antigen. Cancer Res. 2006;66(6):3278–3286. doi: 10.1158/0008-5472.CAN-05-3216. [DOI] [PubMed] [Google Scholar]
- 21.Wehbe M, et al. Epithelial-mesenchymal-transition-like and TGFβ pathways associated with autochthonous inflammatory melanoma development in mice. PLoS ONE. 2012;7(11):e49419. doi: 10.1371/journal.pone.0049419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auphan-Anezin N, et al. Immunosuppression in inflammatory melanoma: Can it be resisted by adoptively transferred T cells? Pigment Cell Melanoma Res. 2013;26(2):167–175. doi: 10.1111/pcmr.12056. [DOI] [PubMed] [Google Scholar]
- 23.Soudja SM, et al. Tumor-initiated inflammation overrides protective adaptive immunity in an induced melanoma model in mice. Cancer Res. 2010;70(9):3515–3525. doi: 10.1158/0008-5472.CAN-09-4354. [DOI] [PubMed] [Google Scholar]
- 24.Grange M, et al. Activated STAT5 promotes long-lived cytotoxic CD8+ T cells that induce regression of autochthonous melanoma. Cancer Res. 2012;72(1):76–87. doi: 10.1158/0008-5472.CAN-11-2187. [DOI] [PubMed] [Google Scholar]
- 25.Prévost-Blondel A, et al. Tumor-infiltrating lymphocytes exhibiting high ex vivo cytolytic activity fail to prevent murine melanoma tumor growth in vivo. J Immunol. 1998;161(5):2187–2194. [PubMed] [Google Scholar]
- 26.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22(3):275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Paul S, Schaefer BC. A new look at T cell receptor signaling to nuclear factor-κB. Trends Immunol. 2013;34(6):269–281. doi: 10.1016/j.it.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferch U, et al. MALT1 directs B cell receptor-induced canonical nuclear factor-kappaB signaling selectively to the c-Rel subunit. Nat Immunol. 2007;8(9):984–991. doi: 10.1038/ni1493. [DOI] [PubMed] [Google Scholar]
- 29.Deenick EK, et al. c-Rel phenocopies PKCtheta but not Bcl-10 in regulating CD8+ T-cell activation versus tolerance. Eur J Immunol. 2010;40(3):867–877. doi: 10.1002/eji.200939445. [DOI] [PubMed] [Google Scholar]
- 30.Köntgen F, et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9(16):1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 31.Rao S, Gerondakis S, Woltring D, Shannon MF. c-Rel is required for chromatin remodeling across the IL-2 gene promoter. J Immunol. 2003;170(7):3724–3731. doi: 10.4049/jimmunol.170.7.3724. [DOI] [PubMed] [Google Scholar]
- 32.Balasubramani A, et al. Modular utilization of distal cis-regulatory elements controls Ifng gene expression in T cells activated by distinct stimuli. Immunity. 2010;33(1):35–47. doi: 10.1016/j.immuni.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 34.Qin Z, et al. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63(14):4095–4100. [PubMed] [Google Scholar]
- 35.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118(4):1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roncagalli R, et al. Quantitative proteomics analysis of signalosome dynamics in primary T cells identifies the surface receptor CD6 as a Lat adaptor-independent TCR signaling hub. Nat Immunol. 2014;15(4):384–392. doi: 10.1038/ni.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdeil G, Puthier D, Nguyen C, Schmitt-Verhulst AM, Auphan-Anezin N. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. J Immunol. 2006;176(8):4834–4842. doi: 10.4049/jimmunol.176.8.4834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.