Abstract
The diterpenoids are classically defined by their composition, four isoprenyl units (20 carbons), and are generally derived from [E,E,E]-geranylgeranyl diphosphate (GGPP). Such metabolism seems to be ancient and has been extensively diversified, with ~12,000 diterpenoid natural products known. Particularly notable are the gibberellin phytohormones, whose requisite biosynthesis has provided a genetic reservoir giving rise to not only a large super-family of ~7,000 diterpenoids, but to some degree all plant terpenoid natural products. This review focuses on the diterpenoids, particularly the defining biosynthetic characteristics of the major superfamilies defined by the cyclization and/or rearrangement of GGPP catalyzed by diterpene synthases/cyclases, although some discussion also is provided of the important subsequent elaboration in those few cases where molecular genetic information is available. In addition, the array of biological activity providing the selective pressure driving the observed gene family expansion and diversification, along with biosynthetic gene clustering, will be discussed as well.
Keywords: biosynthesis, terpene synthases, cytochromes P450, phytoalexins, allelochemicals
INTRODUCTION
The ancient role of GGPP in plant metabolism is perhaps best highlighted by its incorporation into the central photosynthetic pigment chlorophyll – i.e., as the derived/reduced phytyl side chain, which is also found in the accessory tocopherols. Indeed, two GGPP also are condensed together en route to the carotenoids. Accordingly, photosynthetic pigment production is the primary metabolic fate for GGPP in plants. However, this review is focused on diterpenoid natural products, which serve less central, although still important, roles. While not required for normal growth and development, leading to their designation as secondary, or more specialized, metabolites, these compounds serve in often critical ecological roles, mediating interactions between the producing plant and other organisms, which can range from other plants to herbivores and microbes (Figure 1). In addition, a number of diterpenoids have been important in human industry, such as the historicals role of conifer resin acids in the naval stores industry, and the blockbuster anti-cancer drug taxol (paclitaxel).
Figure 1.
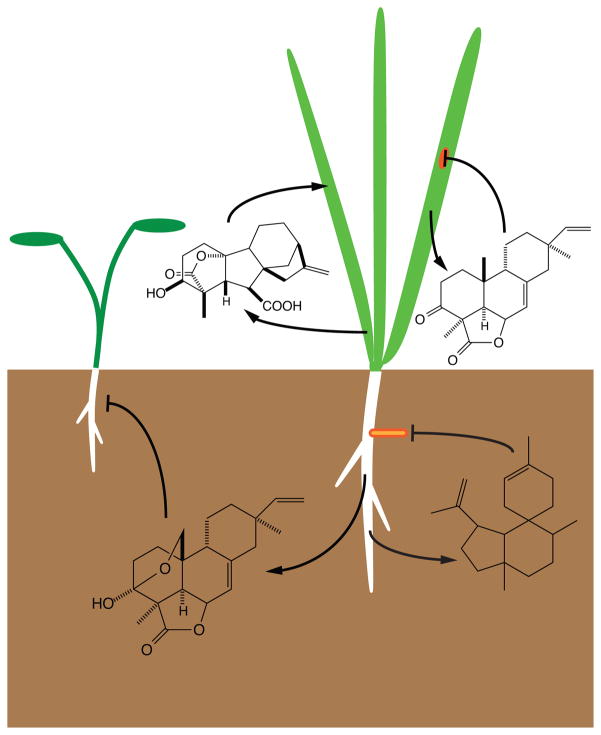
Schematic of the biological roles played by diterpenoid natural products, ranging from the growth promoting effects of the GA phytohormones to roles in ecological interactions – e.g., illustrated are the activities of momilactone B as a rice allelochemical, rhizathalene A as an herbivore (insect) antifeedant, and momilactone A as an antibiotic/phytoalexin against the rice fungal blast pathogen.
Notably, the larger size of the diterpenoids reduces their volatility relative to smaller terpenoids. As a result, they function in more localized roles. Given that there are ~12,000 known diterpenoid natural products, the vast majority of which are from plants, it is perhaps not surprising that the physiological roles of only a very small fraction are understood in any detail. Yet their biological activity must underlie the observed diversification, specifically to drive expansion of the underlying biosynthetic enzyme gene families, and this critical point will be discussed here. Included in this point will be discussion of the intriguing observation that, in certain cases, some of the biosynthetic enzyme genes from a common pathway are clustered together in the relevant plant genome.
Diterpenoid natural products originate in the plastids, although most likely leucoplasts rather than chloroplasts. Regardless, the isoprenyl precursors are then derived from the 2-C-methyl-D-erythritol 4-phosphate (MEP) rather than mevalonate (MVA) dependent pathway (Figure 2). These isoprenoid precursor pathways have been recently reviewed (104), and will not be further discussed here. The resulting dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) units are utilized (DMAPP + 3×IPP) to construct a linear four-isoprenyl unit (20-carbon) diterpenoid precursor. This series of condensation/elongation reactions is most often catalyzed by a trans-isoprenyl diphosphate synthase to produce the usual transoid precursor GGPP, using a straightforward reaction mechanism that has been recently reviewed and also will not be further described here (17; 102). Notably, there is a recent report indicating that an analogous set of elongation reactions can be catalyzed by a cis-isoprenyl diphosphate synthase family member, which then produces the corresponding cisoid four isoprenyl unit (20C) precursor nerylneryl diphosphate (1). However, this has not yet been well-characterized, with relatively little known about any derived diterpenoids, and will not be further discussed here, leaving the focus on natural products originating from GGPP.
Figure 2.
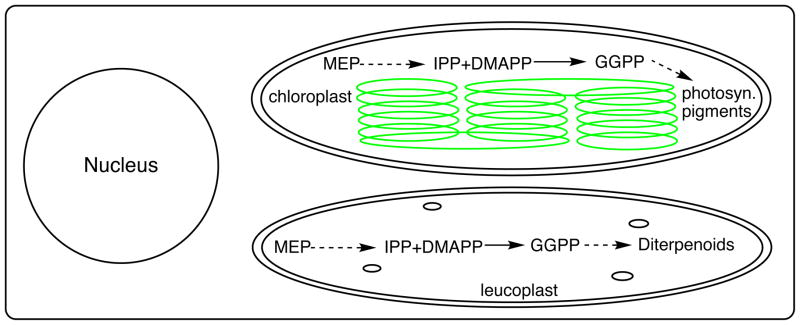
Sub-cellular localization of the initial stages of diterpenoid biosynthesis to plastids. Schematic of a plant cell with relevant chloroplast and leucoplast organelles shown, along with upstream MEP-dependent isoprenoid precursor pathway described elsewhere (104), as well as production of the general diterpenoid precursor GGPP catalyzed by isoprenyl diphosphate synthases also described elsewhere (17; 102).
BIOSYNTHETIC ORIGINS
While GGPP can be incorporated into other compounds, such as the photosynthetic pigments mentioned above, such (mero)diterpenoids are not otherwise covered here. Although biosynthesis of the acyclic plaunotol is initiated by the production of geranylgeraniol from GGPP that seems to be catalyzed by a membrane-associated phosphatase in Croton stellatopilosus (59; 60), it seems worth mentioning that terpene synthases also can produce such primary alcohols, as demonstrated for the production of geraniol from geranyl diphosphate in herbal monoterpenoid biosynthesis (35). Regardless, diterpenoid natural products biosynthesis is, nevertheless, almost invariably initiated by diterpene synthases. The resulting hydrocarbon skeletal structures, which are generally cyclized and/or rearranged, provide the first differentiation of GGPP into diterpenes that, upon further transformation(s), lead into derived, structurally related families. This section of the review then follows a similar pattern, discussing first the early steps mediated by diterpene synthases/cyclases, followed by a necessarily brief discussion of the few further transformations understood at the molecular level (i.e., where the relevant enzymatic genes have been cloned), here largely focused on the mono-oxygenase cytochromes P450 (CYPs), whose introduction of oxygen is critical for increasing solubility and introducing hydrogen bonding potential into the olefins that most often result from diterpene synthase activity.
To bicycle or not to bicycle
Notably, diterpenoid biosynthesis can be initiated by either of two distinct classes of reactions. While both involve carbocationic cascades, these are triggered in very different ways. The reactive allylic diphosphate ester bond present in GGPP invariably undergoes lysis/ionization to trigger one such carbocationic cascade, in reactions catalyzed by class I diterpene synthases (EC 4.2.3.x). However, this can be preceded by a protonation-initiated (bi)cyclization reaction (Figure 3), catalyzed by class II diterpene cyclases (EC 5.5.1.x), which leaves the allylic diphosphate ester bond intact for ionization by a subsequently acting class I diterpene synthase. From the trivial labdane name assigned to the most commonly observed hydrocarbon skeletal structure resulting from such class II bicyclization, the derived polycyclic natural products have been termed the labdane-related diterpenoids (65). Such metabolism is universally found in vascular plants due to the requisite production of GAs, whose biosynthesis proceeds through such a sequential pair of class II and class I cyclization reactions.
Figure 3.
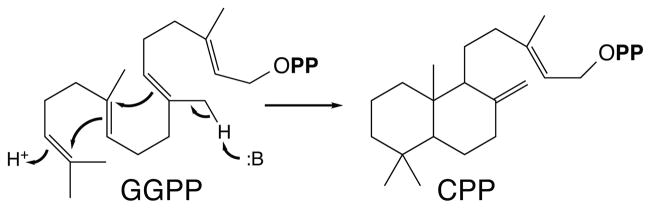
Bicyclization of GGPP to labdane hydrocarbon backbone containing CPP that can proceed more prototypical allylic diphosphate ester ionization initiated reaction catalyzed by (class I) terpene synthases in diterpenoid biosynthesis.
To bicycle first: The labdane-related diterpenoids
Of the more than 12,000 diterpenoids ~7,000 fall into the labdane-related super-family, highlighting the widespread diversification of such biosynthesis. The vast majority of these natural products are found in plants, where the requisite production of gibberellins (GAs) seems to have provided a genetic reservoir for derivation of more specialized labdane-related diterpenoids, particularly stemming from duplication of the genes encoding the two diterpene synthases/ cyclases, as will become evident in the following sections. Notably, these also are ancestral to all the plant (class I) terpene synthases involved in hemi-, mono-, sesqui-, as well as di- terpenoid biosynthesis (7), which form a moderately sized gene family (20+ members) in vascular plants (10). These enzymes produce the hydrocarbon backbones that define structurally related families, and represent the initial step in such terpenoid biosynthesis. Accordingly, GAs provided not only the origins of the diterpenoid metabolism discussed here, but to some extent that of the smaller terpenoid natural products as well. This broader evolutionary scenario has been recently reviewed (17), and almost certainly only applies to plants, not to microbes as suggested elsewhere (61), as will become evident in the discussions below.
Gibberellins: The ancestral labdane-related diterpenoids
GA metabolism has been comprehensively reviewed in several recent publications (5; 28; 66; 121). Here only the early steps in GA biosynthesis are described (Figure 4). In particular, those leading up to formation of the characteristic 6-5-6-5 ring structure, as it is duplication of these upstream enzymes that has led to the observed diversity of labdane-related diterpenoid natural products. GA biosynthesis is initiated by bicyclization of GGPP to copalyl/labdadienyl diphosphate (CPP), with enantiomeric stereochemistry (as defined relative to that found in cholesterol), in reactions catalyzed by class II diterpene cyclases then termed CPP synthase (CPS). The resulting ent-CPP is then further cyclized to the ent-kaurene, with this class I diterpene synthase then termed kaurene synthase (KS), which catalyzes a complex bicyclization and ring rearrangement reaction (Supplemental Movie). This tetracyclic 6-6-6-5 olefin is further transformed by a multiply reactive CYP, kaurene oxidase (KO), that converts C19 from a methyl group to carboxylic acid, via series of hydroxylation reactions, with intervening dehydration (54). Notably, while CYPs are typically found associated with the outer membrane of the endoplasmic reticulum (ER), at least the KO from Arabidopsis thaliana is found on the outer membrane of the plastid instead (31). This alternative sub-cellular localization presumably provides preferential access to the plastid-derived ent-kaurene. The resulting ent-kaurenoic acid is then finally converted to the gibberellane skeletal structure by another CYP, kaurenoic acid oxidase (KAO).
Figure 4.
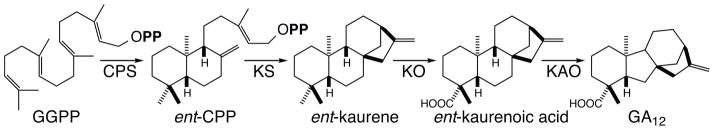
Early steps in GA biosynthesis, from GGPP to first gibberellane intermediate, along with relevant enzymes (as defined in the text).
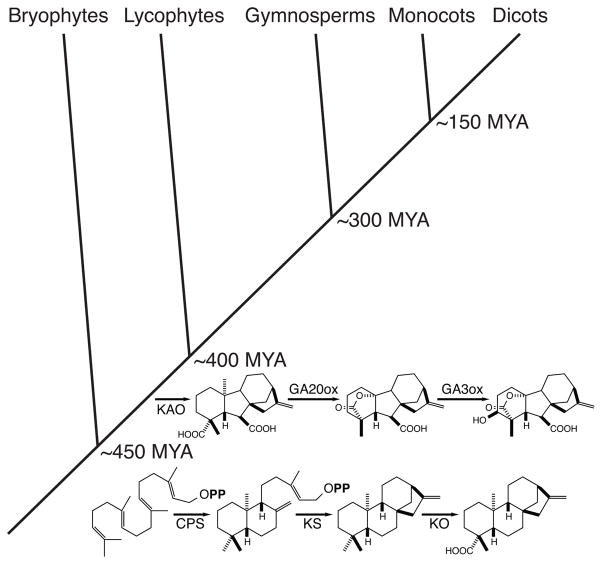
Not surprisingly, the amount of bioactive GAs is tightly regulated in plants. There clearly is tight transcriptional regulation of the genes for both anabolic and catabolic enzymes (28; 121). Notably, this includes transcription of CPS, whose activity can be considered to initiate GA biosynthesis (91). In addition, it has been suggested that the CPS involved in GA biosynthesis are subject to inhibition exerted by physiologically relevant levels of the divalent magnesium ion (which varies in plant plastids in response to light), and that also serves as an enzymatic cofactor for such class II diterpene cyclases, while those dedicated to more specialized metabolism are exempt from such inhibition (72). Susceptibility to this co-factor inhibition effect has been associated with the identity of a single amino acid residue, with a histidine found at this position in all CPS unambiguously associated with GA biosynthesis, while this is an arginine (or glutamine) in other class II diterpene cyclases. Strikingly, exchange of these residues is sufficient to ‘switch’ enzymatic susceptibility to such inhibition (49). Thus, it seems likely that this cofactor inhibition is a physiologically relevant regulatory mechanism. Recent work lends itself to speculation about the evolution of plant GA biosynthesis. This is based in part on the availability of genome sequences for the moss/bryophyte Physcomitrella patens and the lycophyte Selaginella moellendorffii, whose divergence from the angiosperm lineage is nearly as ancient (3; 79). It has been shown that S. moellendorffii produces and responds to GA, using genes homologous to those identified in angiosperms, while P. patens does not produce or respond to GA (34; 124). On the other hand, P. patens does produce ent-kaurene and ent-kaurenoic acid using enzymes homologous to those found in vascular plants (27; 52), and these diterpenoids further seem to have some physiological role in the moss (2; 26). Thus, it seems likely that the production of ent-kaurenoic acid arose early in plant evolution, before the divergence of bryophytes and the vascular plants some 450 million years ago (MYA), with the more specific evolution of GA biosynthesis arising in the next 50 million years – i.e., before separation of the lycophyte lineage from that of the angiosperms that occurred some 400 MYA (Figure 5).
Figure 5.
Staggered evolution of GA biosynthesis. While the production of ent-kaurenoic acid seems to have evolved early in land plants, as suggested by the presence of CPS/KS and KO homologs in the bryophyte P. patens, while GA biosynthesis more specifically seems to have arisen later, during the evolution of vascular plants, as suggested by the presence of homologs to the remaining genes in the early diverging, yet vascular, lycophyte S. moellendorffii (GA20ox, GA C20-oxidase; GA3ox, GA C3-oxidase). Shown is simplified plant phylogeny with dates of divergence (MYA, millions of years ago), along with components of the GA biosynthetic pathway alongside the approximate period in which they evolved.
Regardless of evolutionary history, it is clear that GAs are required for normal plant growth and development. Notably, this includes not only stem elongation and flowering (as shown in Figure 6), but also seed germination, such that plants with severe GA deficiency are sterile. Hence, the enzymatic genes underlying GA biosynthesis must be present in all plant genomes, providing the aforementioned genetic reservoir that can be drawn upon by either individual gene or whole genome duplication to derive more specialized labdane-related diterpenoid metabolism, as well as the more distantly descended class I terpene synthases, as will be discussed in the following sections.
Figure 6.
Effect of GA phytohormone deficiency on stem elongation and flowering of A. thaliana.
Conifer resin acids: Early labdane-related diterpenoids
An abietadiene synthase involved in resin acid biosynthesis from grand fir, Abies grandis was the first diterpene synthase from more specialized labdane-related diterpenoid metabolism to be cloned (94). This bifunctional enzyme, AgAS, catalyzes both class II and I cyclization reactions (Figure 7), and was clearly related to both the CPS from A. thaliana (AtCPS) and maize (Zea mays), and the KS from pumpkin (Cucurbita maxima), which also had been cloned by that time (4; 96; 122). As noted in this original report, AgAS contained motifs in common with both. Specifically, a DxDD motif in common with the CPSs, and a DDxxD motif in common with the KS, as well as other known class I (di)terpene synthases. However, it also was noted that AgAS was similar in length to both the CPSs and KS, which are similar in length to each other, and all these shared at least some regions of significant similarity throughout their sequences (94). While the evolutionary relationship between all of these terpene synthases/cyclases was somewhat enigmatic at that time, later work demonstrated that at least some early diverging (non-vascular) plants contain a similarly bifunctional CPS/KS (27; 40). This led to the suggestion that GA biosynthesis may have originally relied upon such a bifunctional CPS/KS, arising from fusion of separate CPS and KS from a bacterial origin, which then underwent an early gene duplication, allowing sub-functionalization to the separate CPS and KS observed in vascular plants (53). Intriguingly, the genome of the lycophyte S. moellendorffii contains bifunctional diterpene synthases also involved in more specialized metabolism (47; 95), but these appear to have a separate evolutionary origin from those involved in conifer resin acid biosynthesis such as AgAS (10). While a number of bifunctional diterpene synthases have since been identified from gymnosperms (43; 50; 80; 86; 125), it should be pointed out that conifers seem to have monofunctional CPS and KS for GA biosynthesis (42), and also recently have been shown to have monofunctional class I diterpene synthases involved in more specialized metabolism as well (21). Thus, work on resin acid biosynthesis has both provided key insights into the evolutionary origins of labdane-related diterpenoids, particularly of the key diterpene synthases/cyclases, and the manifold ways in which such evolution unfolds.
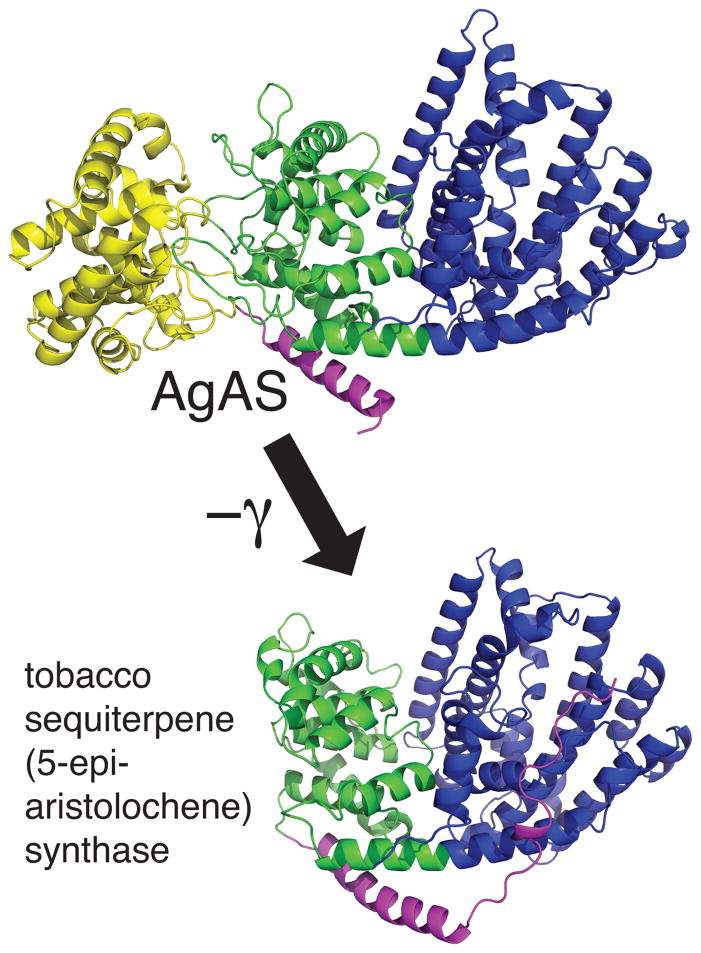
Figure 7.
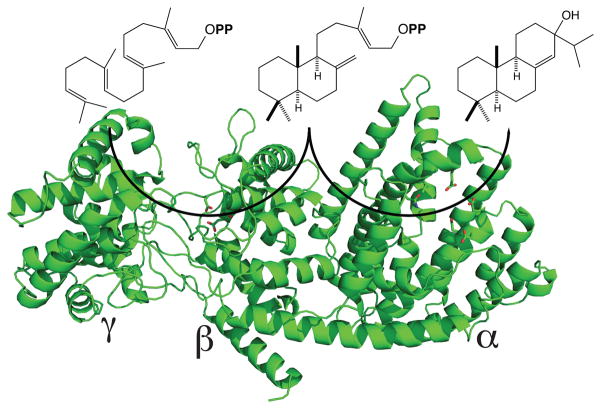
Structure of the abietadiene synthase from A. grandis (AgAS), along with catalyzed reactions. AgAS exhibits a tri-domain structure (γβα, as indicated), wherein the class II active site sits between the N-terminal γβ domains, and the class I active site is within the C-terminal α domain. The structure is shown as a ribbon diagram, with the side-chains of the catalytic class II associated DxDD and class I associated DDxxD motifs shown in stick format.
In addition, AgAS has served as a model diterpene synthase/cyclase for investigation of terpene synthase structure-function relationships. This includes work with labeled substrates and analogs of high energy intermediates (70; 71; 73; 75–78), as well as extensive mutagenesis (49; 68; 69; 73). One of the key findings from this work was demonstration that the class I and II active sites were separate, and catalytically independent (albeit structurally interdependent), opening up the possibility that these were located in distinct domains (67; 71). Another was the demonstration that broadly conserved residues in both the class I and II associated domains (active sites) played important roles in catalysis (68; 69). More recently, a crystal structure has been determined for AgAS (127), revealing a tri-domain (γβα) protein structure wherein the class II active site sits at the interface between the N-terminal γ and β domains, while the class I active site falls within the C-terminal α-domain (Figure 7). These observations not only verified the modular nature predicted for the diterpene synthases/cyclases, but also the existence of an extensive interface between the domains associated with each activity (i.e., consistent with the previously demonstrated structural interdependence), particularly the β and α domains. Moreover, this atomic level structure has already led to additional insights into the enzymatic mechanism of at least the class II cyclization reaction as well (13).
Cereals: a model system for investigation of labdane-related diterpenoids
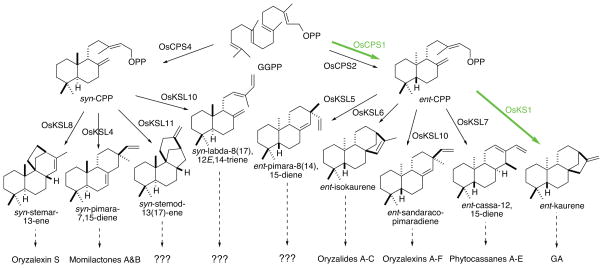
Expanded and diversified diterpene synthase gene families have been observed in angiosperms as well, most notably in the cereal crop plant family. Rice (Oryza sativa) has been known to produce more specialized labdane-related diterpenoids for over 40 years (38), and the relatively early availability of its genome sequence enabled application of a functional genomics approach to investigation of the underlying biosynthetic enzymes (64; 100; 123). This led to biochemical characterization of its full complement of both CPS and KS-like (KSL) enzymes, which significantly expanded the range of reactions for which the relevant enzymes were molecularly identified, as previously only those for the production of ent-kaurene and abietadiene were known (Figure 8). Moreover, despite extensive phytochemical investigations, the rice KSL (OsKSL) actually exhibited greater diversity than was expected from the known natural products, with the resulting diterpenes easily detectable in planta (55; 56). This demonstrated the ability of such a functional genomics approach to elucidate metabolism, as well as highlighting the extensive elaboration of labdane-related diterpenoid metabolism in rice.
Figure 8.
Metabolic map of rice diterpenoid biosynthesis. Shown are the characterized diterpene synthases/cyclases along with the catalyzed reactions, and downstream natural products where known. Indicated in green, and by thicker arrows, are those enzymes and reactions required for GA biosynthesis in rice (83). Adapted from ref. 108, with permission.
Characterization of the OsKSL family led to detailed insight into the plasticity of class I terpene synthases. The alleles for OsKSL5 from ssp. indica and japonica were found to catalyze distinct cyclization reactions with ent-CPP, with that from japonica producing tricyclic ent-pimaradiene (37), while that from indica produced tetracyclized and rearranged ent-isokaurene (119). Comparison of their sequences led to identification of single residue responsible for this dramatic change in product outcome (Figure 9), which was found to be applicable to not only these alleles and the closely related OsKSL6, but also to even distantly related KS (120). It further was possible to apply this single residue ‘switch’ to increase the complexity of the reaction catalyzed by OsKSL4, from production of a tricyclic syn-pimaradiene to a rearranged tetracycle (57), as well as apply a similar ‘switch’ in AgAS (112). Moreover, these results, coupled with additional mechanistic investigation (82), highlighted role for electrostatic interactions, particularly with the diphosphate anion co-product, in such class I terpene synthase catalysis (128).
Figure 9.
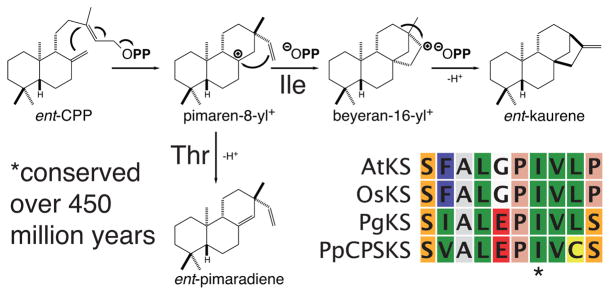
Effect of single residue ‘switch’ on reaction catalyzed by ent-(iso)kaurene synthases. Shown is a scheme of the reaction catalyzed in the usual production of ent-kaurene from ent-CPP, which requires the presence of the conserved Ile residue indicated in the alignment of KSs from A. thaliana (AtKS), rice (OsKS), spruce (PgKS), and P. patens (PpCPSKS), which spans ~450 million years of evolution. If this residue is changed to Thr, the enzymes largely catalyze the abortive production of ent-pimaradiene instead. The potential role of the ionized pyrophosphate anion in driving carbocation migration also is indicated by its positioning within the scheme.
Molecular evidence of a role for more specialized labdane-related diterpenoids in maize (i.e., cloning and characterization of a second, inducible CPS), actually preceded identification of such natural products in this cereal crop plant (24; 87). Subsequent release of the maize genome sequence (88), revealed the presence of not only the already reported expanded CPS, but also KSL, gene families. In addition, wheat (Triticum aestivum) recently has been shown to contain similarly expanded CPS and KSL gene families, with diverse biochemical function (117; 129). Intriguingly, investigation of OsKSL substrate range demonstrated some stereochemical promiscuity (i.e., of CPP), with two that react with the endogenous syn-CPP also able to react with CPP of normal stereochemistry, although this is not found in rice (55). However, the wheat ortholog of the rice syn-CPP producing OsCPS4 (i.e., TaCPS2), produces normal CPP instead (117), with similar normal and syn- CPP stereochemical promiscuity observed with the wheat TaKSL as well (129). Such metabolic plasticity may underlie the observed diversification of labdane-related diterpenoid metabolism in the cereal crop plant family, and potentially even more broadly.
Labdane-related diterpenoids in dicots: Examples from the Salvia genus
Although labdane-related diterpenoid biosynthesis has not been as extensively investigated in dicotyledonous plants, it is clear that at least some have diverse such natural products. The most extensive molecular information is available for species from the Salvia genus, although other dicots have been investigated as well (15; 84). Examples from Salvia include identification of the diterpene synthases responsible for biosynthesis of sclareol from Salvia sclarea (6; 19; 85). These are noteworthy for their illustration of the ability of terpene synthases, of both classes, to introduce water during the course of the catalyzed reaction (Figure 10), the importance of the resulting hydroxyl groups for increasing solubility and providing hydrogen-bonding potential will be discussed in more detail below. Another example stems from investigation of tashinone biosynthesis in the Chinese medicinal herb Danshen (Salvia miltiorrhiza), which provides insight into the production of phenolic diterpenoid natural products more generally. In particular, the relevant diterpene synthases have been identified and shown to produce a cyclohexa-1,4-diene isomer of abietadiene that is poised for aromatization (16). Strikingly, the various Salvia KSLs, although clearly most closely related to other KS(L)s, are more similar in size to prototypical plant class I terpene synthases, having lost the N-terminal γ-domain (33), the significance of which will be discussed in more detail below.
Figure 10.
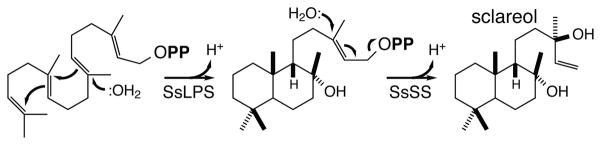
Reactions catalyzed by diterpene synthases from S. sclarea in production of sclareol (SsLPS, class II diterpene cyclase producing 8α-hydroxy-labdadienyl diphosphate, which is utilized by the class I diterpene synthase SsSS to produce sclareol). These reactions are noteworthy in their incorporation of water prior to concluding deprotonation.
Not to bicycle: Class I diterpene synthases acting directly on GGPP
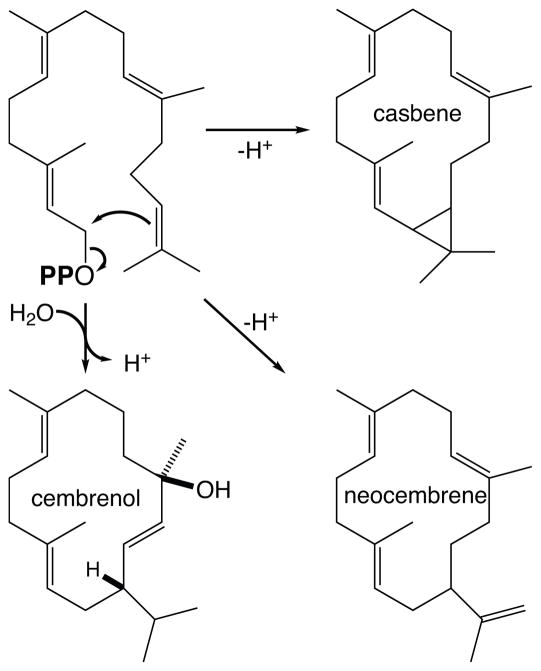
There are many diterpenoids whose biosynthesis is initiated by class I diterpene synthases that react directly with GGPP (i.e., rather than a bicyclic derivative such as CPP). However, given that the complexity of the end product is a function of the number of double-bonds in the precursor (11), surprisingly few such class I diterpene synthases have been identified, particularly by comparison to the multitude of functionally diverse mono- and sequi- terpene synthases already known (14). While a casbene synthase from castor bean (Ricinus communis) and a taxadiene synthase from a yew species (Taxus brevifolia) were among the first plant terpene synthases to be cloned (51; 114), only a few others are known. These include cembrenol and neocembrene synthases (44; 106), whose product only slightly differs from casbene (Figure 11). Interestingly, the three class I diterpene synthases producing casbene, cembrenol or neocembrene (respectively) are more closely related to mono- and sesqui- (class I) terpene synthases than angiosperm KS(L) – e.g., all of these fall into the TPS-a sub-family, rather than the KS(L) TPS-e/f sub-family (10). In addition, these three class I diterpene synthases are significantly smaller than the KS(L), being similar in size to the prototypical plant mono- and sesqui- terpene synthases. This reflects loss of the γ-domain (Figure 12), which forms half of the class II diterpene cyclase active site, and this loss appears to have occurred early in angiosperm evolution given the extensive and independent diversification of the relevant TPS sub-families (10). The β-domain that forms the other half of the class II diterpene cyclase active site is retained, which may reflect its extensive interface with the class I α-domain noted above. Indeed, loss of the γ-domain has been observed to independently occur at least two other times (33), suggesting that this is relatively facile. By contrast, it does not appear that further loss of the β-domain has occurred. While class I terpene synthases composed of only an α-domain have been recently reported from the lycophyte S. moellendorffii, these are more closely related to microbial terpene synthases rather than other plant terpene synthases, indicating a separate evolutionary origin (45), not derivation from the βα bi-domain terpene synthases.
Figure 11.
Macrocyclization reactions catalyzed by casbene, neocembrene, and cembrenol synthases.
Figure 12.
Loss of γ domain in prototypical (class I) plant terpene synthases. Illustrated by comparison of AgAS structure with that of the 5-epi-aristolochene (sesquiterpene) synthase (93). Domains are colored to assist visualization (γ, yellow; β, green; α, blue), with N-terminal helix that is retained during the ancient, but not more recent, γ domain loss event show in magenta. Note that the N-termini of AgAS (although not apparent in the crystal structure), as well epi-aristolochene synthase, fold back and form part of the class I active site in the C-terminal α domain.
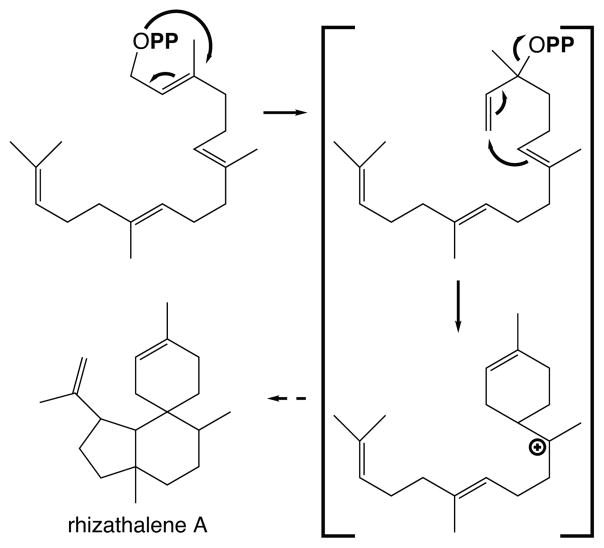
Much of the chemical diversity generated by sesqui- and particularly mono- terpene synthases requires rearrangement of the initial primary allylic diphosphate ester bond to a tertiary position, which removes the geometric barrier imposed by the trans 2,3-double bond to direct 1,6-bond formation of a cyclohexene ring (12). With GGPP the corresponding intermediate would be geranyllinalyl diphosphate. Accordingly, the discovery of a geranyllinalool synthase suggested the potential for analogous rearrangement of GGPP (32). This was substantiated by the more recent report of a rhizathalene synthase (103), which almost certainly proceeds through the corresponding rearranged geranyllinalyl diphosphate as an initial intermediate en route to initial cyclization to a 1,6-cyclohexene containing intermediate and, hence, to the more complex polycyclic rhizathalene (Figure 13). This then suggests that much more chemical diversity can be expected to arise from the action of class I diterpene synthases, which is supported by the diversity observed with the characterized microbial enzymes (92; 100).
Figure 13.
Rizathalene cyclization reaction demonstrates ability of class I diterpene synthases to rearrange GGPP to geranyllinalyl diphosphate (shown) en route to 1,6-cyclization to the shown cyclohexenyl carbocation intermediate.
Further transformations: Elaborating upon the hydrocarbon backbone
The diterpene synthases/cyclases described above most often produce a hydrocarbon olefin, which is, not surprisingly, highly hydrophobic (e.g., their partition coefficient or logP is ≥ 8.5). Accordingly, these must almost invariably be further elaborated by the introduction of oxygen, typically catalyzed by CYPs (EC 1.14.13.x), which are integral membrane proteins in eukaryotic organisms such as plants (62), enabling access to the presumably membrane embedded diterpenes. As discussed for GA biosynthesis above, these mono-oxygenases can catalyze multiple reactions with the same substrate, leading the incorporation of several atoms of oxygen. In addition, again from GA biosynthesis, it already is evident that these enzymes can alter the hydrocarbon backbone (e.g., the ring contraction catalyzed by KAO; Figure 4), leading to assignment of the resulting natural products to new structural families (e.g., gibberellins are not considered to be kauranoids per se, despite their common biosynthetic origins). However, most often the CYPs involved in diterpenoid natural products biosynthesis catalyze the hydroxylation reactions that are prototypical of these enzymatic super-family (62).
While the addition of a single hydroxyl group does increase solubility (e.g., decreasing logP by > 1), the resulting compounds resemble membrane components such as cholesterol, and most likely remain within the membrane. Accordingly, in order to provide solubility in a biological setting, it seems likely that two spatially separated hydroxyl groups are required, consistent with examination of the known bioactive diterpenoid natural products. At least in a few cases, this has been shown to decrease logP to ≤ 5 (116), which incidentally matches Lipinski’s rules for desirable pharmaceutical properties (46). Regardless, the incorporation of such polar groups also provides hydrogen-bonding potential, which would be expected to significantly increase the ability of the resulting natural product to specifically bind biological macromolecular targets. However, it should be noted that only in very few cases has it been established how multiple CYP operate in such biosynthesis, either order of action or even identification of the relevant CYP. Beyond GA, although several CYP have been identified from taxol biosynthesis, their order of action remains uncertain (18), and only for the relatively simple case of rice orzyalexin D & E biosynthesis, requiring only two CYPs, is such information available for diterpenoid natural products (116).
The relationship among CYPs can be inferred to some extent from the formal nomenclature. In particular, by the original definition, CYPs sharing more than 40% amino acid sequence identity are grouped into families, as designated by the number immediately following the CYP super-family designation, and CYPs sharing more than 55% amino acid sequence identity are grouped into sub-families designated by letter(s) following the family number, with individual CYP then finally identified by a concluding number, although some merging of (sub-)families has inevitably occurred as more molecular information becomes available (58). As an example of the nomenclature, the KO involved in GA biosynthesis from A. thaliana is CYP701A3 (30), with all the known KOs falling within the CYP701 family, and all those from angiosperms within the CYP701A sub-family (58). However, even such sub-family membership can not be used to assign biochemical function, as it has recently been shown that CYP701A8 from rice does not catalyze the prototypical KO reaction, but instead carries out hydroxylation at the neighboring C3α position in a variety of labdane-related diterpenes (110), consistent with assignment of KO activity in rice to the distinct paralog CYP701A6 (36; 83). The KAOs also involved in GA biosynthesis fall into the CYP88A sub-family (29), with no characterized family members yet found to operate in more specialized diterpenoid metabolism, although at least one member of different CYP88 sub-family does function in more specialized triterpenoid biosynthesis (89).
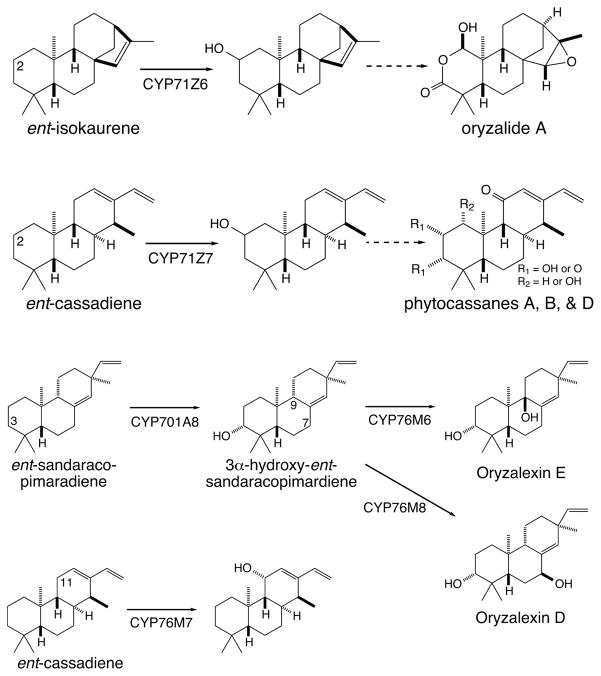
A number of other CYP sub-families are known to act in diterpenoid biosynthesis, with several members of the CYP725A sub-family assigned roles in taxol production (18), and at least two members of the CYP720B sub-family serving as promiscuous, yet multiply reactive oxidases in conifer resin acid metabolism (23; 81). In addition, two CYP families seem to contain multiple sub-families that function in diterpenoid natural products biosynthesis (Figure 14), albeit these are among the most extensive CYP families in plants. First, from the exceptionally large CYP71 family, a tobacco (Nicotiana tabacum) CYP71D sub-family member functions as a C6 hydroxylase in production of cembrendiol (107), although most of the member of this sub-family function in mono- or sesqui- terpenoid biosynthesis (22). At least two CYP71Z sub-family members seem to operate in rice labdane-related diterpenoid biosynthesis, both catalyzing C2-hydroxylation, with CYP71Z6 acting upon ent-isokaurene, presumably for production of the derived oryzadiones/oryzalides, while CYP71Z7 does so in phytocassane biosynthesis (115). In addition, at least one member of CYP99A, which is actually a sub-family of the CYP71 family (98), operates in momilactone biosynthesis, catalyzing the transformation of C19 from a methyl to the carboxylic acid necessary for 19,6β-lactone ring formation (109). Second, from the large CYP76 family, a number of rice CYP76M sub-family members have been shown play a role in more specialized labdane-related diterpenoid biosynthesis as well (108). For example, CYP76M7 catalyzes C11α-hydroxylation in phytocassane biosynthesis (98), while CYP76M6 and 8 catalyze C9β and C7β hydroxylation of 3α-hydroxy-ent-sandaracopimaradiene to produce oryzalexins E and D, respectively (116). Moreover, a CYP76AH sub-family member from Danshen seems to operate in tanshinone production, catalyzing C12-hydroxylation in formation of the intermediate ferruginol (20). Finally, members of the CYP714 family are known to function in GA metabolism (48; 126; 130), but no family members have yet been assigned roles in more specialized diterpenoid biosynthesis. Given the relatively few identified CYP from diterpenoid metabolism, it seems likely that other CYP (sub-)families will be found to operate in such natural product biosynthesis.
Figure 14.
Hydroxylation reactions carried out by CYP in biosynthesis of more specialized rice labdane-related diterpenoids, along with resulting (both indirect and direct) natural products.
Beyond the increased solubility and hydrogen-bonding potential imparted by the introduction of oxygen(s) catalyzed by the CYPs, these also offer opportunities for additional elaboration. This can be simply further oxidation, as catalyzed by short-chain dehydrogenases/reductases (SDR) such as that involved the final step of rice momilactone A biosynthesis (90), but perhaps more interesting is the addition of various functional groups, such as that catalyzed by the characterized transferases from taxol biosynthesis (18; 105). However, even less is known about the enzymes involved in such further elaboration than the initially operating CYPs.
BIOLOGICAL ACTIVITY
A range of roles for diterpenoid natural products drives their evolution
As described above, the GA phytohormones play critical roles in normal plant growth and development. Consistent with their prominent role in promoting stem elongation, as discussed above GA biosynthesis seems to have evolved during the differentiation of vascular plants, coupling function to evolution. As has been illustrated above, (di)terpenoid biosynthesis has evolved from that of GA, but these more specialized metabolites must have a selectable function in order to drive functional specialization and retention of the underlying enzymatic genes. In a few cases, biological roles have been assigned more specialized diterpenoid metabolites (Figure 15), providing some insight into the selective pressure leading to evolution of the relevant biosynthetic pathway. For example, conifer resin acids seem to function in defense against bark beetles, undergoing oxidative cross-linking following extrusion and volatilization of the mono- and sesqui- terpene components of the rosin to form a physical barrier, as well as exhibiting antifeedant and antibiotic activity (41). However, in the absence of genetic evidence, it is difficult to definitively assign more specific roles and/or physiological function. Hence, the focus here will be on diterpenoid natural products for which such genetic evidence is available. For example, it is clear from knock-out lines of the relevant diterpene synthase that rhizathalene acts as a semi-volatile antifeedant against root herbivory by the fungus gnat (Bradysia ssp) in A. thaliana (103). Interestingly, while the function of cembrendiol is not clear, suppression of the relevant CYP leads to accumulation of the cembrenol precursor instead, with the resulting tobacco plants exhibiting resistance to aphid colonization (107). In rice, analysis of knock-out lines of the upstream OsCPS4 and more specific OsKSL4 indicates that the primary function of the momilactones is allelochemical activity (i.e., suppression of the growth of other plant species)(118). Despite being the first rice phytoalexins isolated against the devastating fungal blast pathogen (Magneportha oryzae)(8; 9), these momilactone deficient knock-out lines are no more susceptible to infection by M. oryzae than their corresponding wild-type (parental) lines (118). However, suppression of OsCPS4 in a different genetic background does lead to increased susceptibility, as well as loss of allelopathy (101), perhaps due to a difference in timing of phytoalexin accumulation, which has been correlated with susceptibility (25). Indeed, alleochemical activity is attributable to momilactone B, which is selectively secreted from the roots, while momilactone A preferentially accumulates within the plant (e.g., upon infection), consistent with a role as a phytoalexin (39). These results highlight the complexity inherent in determining physiological function, as well as the differential activity of even closely related natural products. For example, momilactone B may be derived from momilactone A by a single biosynthetic step (64). On the other hand, such differential function then provides the selective pressure necessary for evolution of the relevant gene encoding the enzyme catalyzing this additional biosynthetic step. By contrast to the examples discussed above, despite the complexity and length of the relevant biosynthetic pathway, it is unclear what physiological function taxol serves in yew trees, and such uncertainty is prevalent among not only the diterpenoids, but all natural products. Nevertheless, the cases where such information is available demonstrates that diterpenoid activity spans plant-insect, plant-plant and plant-microbe interactions (Figure 15). According, these natural products serve in variety of roles, providing selectable advantages that drive continuing evolution of diterpenoid chemical diversity.
Figure 15.
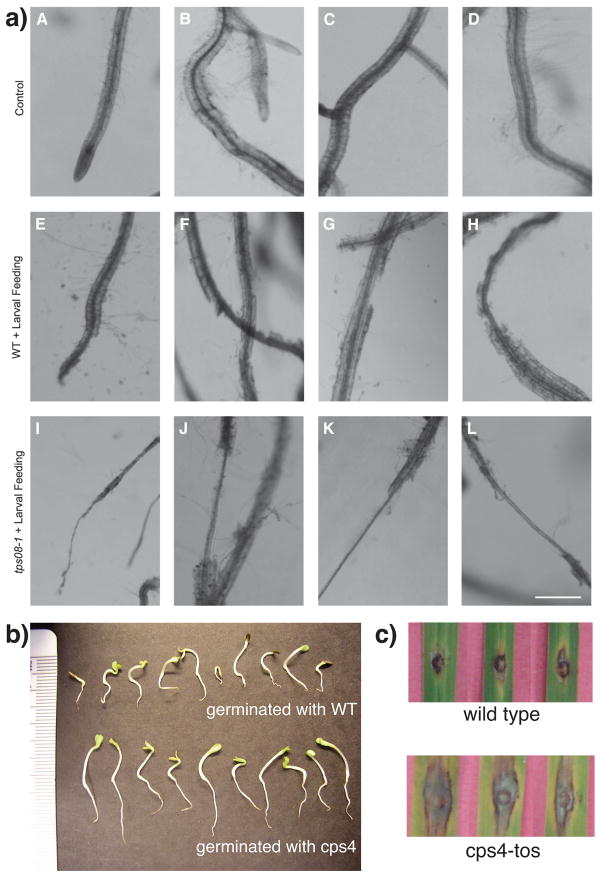
Illustrating the varied biological roles played by diterpenoid natural products. (a) The rhizathalenes produced by TPS08 act as antifeedants against herbivory by larva of the fungus gnat (Bradysia) in A. thaliana (103). Top row depicts undamaged roots (wild-type plants). Middle row depicts roots of wild-type plants after Bradysia feeding. Bottom row depicts roots from TPS08 knock-out (tps08-1) after Bradysia feeding. Reprinted with permission from ref. 103. (b) The momilactones, whose biosynthesis relies on OsCPS4, act as allelochemicals, suppressing the growth of other plants. Lettuce seedlings germinated in the presence of OsCPS4 knock-out (cps4) or its parental/wild-type (WT) rice seedlings. Reprinted with permission from ref. 118. This effect is most likely due to momilactone B (39). (c) The momilactones, most likely momilactone A, acts as an antibiotic phytoalexin against the rice fungal blast pathogen M. oryzae (101). Pictures of the lesions in the leaves of OsCPS knock-down (cps4-tos) or its parental/wild-type rice following infection with M. oryzae. Reprinted with permission from ref. 101.
The yin and yang of biosynthetic gene clusters in plants
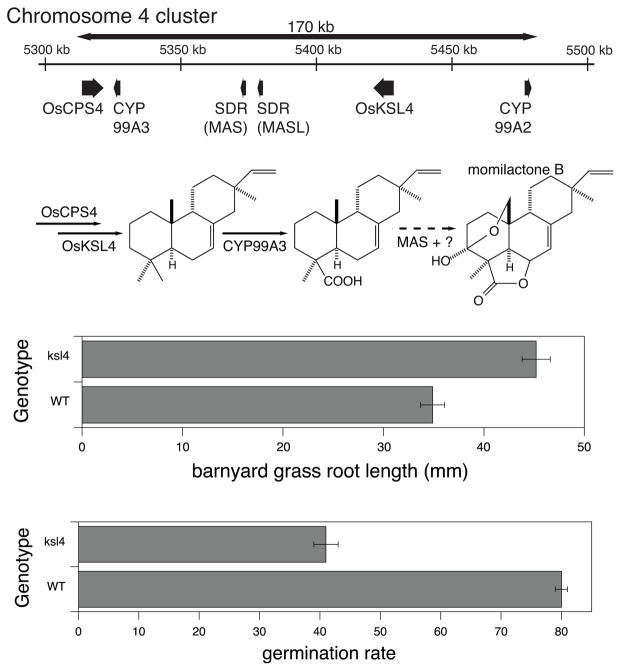
Finally, as observed in a so-far limited number of cases for plant natural products more generally (63), there also are diterpenoid biosynthetic gene clusters, investigation of which has offered some insight into the nature and assembly of these in plants versus microbes (where analogous gene clusters are shaped by horizontal gene transfer, which does not appear to be relevant in plants). In particular, it became evident at an early stage in the functional genomics based investigation of rice diterpenoid biosynthesis that both OsCPSs involved in more specialized metabolism were closely linked to OsKSL that acted on their enzymatic product – i.e., genes encoding sequentially acting enzymes were close together in the rice genome (74; 113). Moreover, the only other genes in these regions encoded CYP and, in one case, also an SDR (83). Given that all of the genes were clearly of plant origin and that their closest homologs are paralogs found elsewhere in the rice genome, these clusters do not appear to have arisen from any sort of horizontal gene transfer, but assembly during evolution of the rice genome via the usual vertical transmission. Nevertheless, later work demonstrated that, consistent with their physical linkage to the upstream OsCPS4 and OsKSL4, the nearby CYP99A2 and/or 3, as well as SDR, were involved in production of the momilactones, forming a dedicated biosynthetic gene cluster on rice chromosome 4 (Figure 16)(90). On the other hand, while the cluster on chromosome 2 has been primarily associated with phytocassane biosynthesis (98), CYP from this cluster seem to also participate in not only biosynthesis of the oryzadiones, for which the relevant OsKSL6 is found within the cluster, but also that of oryzalexins (108; 115; 116). Involvement in oryzalexin biosynthesis highlights the patchwork nature of this cluster, as the relevant OsKSL10 is found elsewhere in the rice genome. In addition, the KO paralog CYP701A8 also seems likely to function in phytocassane and oryzalexin biosynthesis, but is found elsewhere in the rice genome as well (albeit CYP701A8 is tightly linked in a tandem gene array with CYP701A6, encoding the essential KO required for GA production, ensuring its inheritance in any case)(110). While the patchwork coverage of biosynthetic pathways by these gene clusters is consistent with the absence of horizontal gene transfer, it is then somewhat unclear what drove their assembly. Although co-regulation via localized effect [e.g., on chromosomal state (111)] may provide pressure for maintenance of a cluster, the genes from the diterpenoid biosynthetic gene cluster on rice chromosome 2 are differentially regulated, with no obvious correlation to linear arrangement, arguing against a role for co-regulation in even cluster maintenance, at least in this case (98). More critically, it has been pointed out that co-regulation does not provide a driving force for assembly, which was suggested to require both positive selection (e.g., for production of a diterpenoid providing advantageous activity) and negative selection against incomplete inheritance (e.g., toxicity associated with a metabolic intermediate or derivative thereof)(99). Evidence for such dual push-pull selection pressure is evident from genetic dissection of the rice momilactone biosynthetic gene cluster, where the associated physiological functions in allelopathy and plant microbial defense provide positive selection pressure, but a striking 2-fold decrease in seed germination rate was observed with the OsKSL4 knock-out line, demonstrating a strong negative selection pressure against inheritance of OsCPS4 in the absence of OsKSL4 (118). Thus, the observed patchwork nature of natural product biosynthetic gene clusters in plants may result from the lack of negative selection against the loss of certain genes (i.e., those not found in the relevant cluster, such as OsKSL10, loss of which presumably does not have a significant deleterious effect).
Figure 16.
Yin and yang of plant biosynthetic gene clusters, as illustrated by that for diterpenoid (momilactone) biosynthesis in rice. Schematic of gene cluster on chromosome 4 in the rice genome and known roles of the encoded enzymes in momilactone biosynthesis, along with effect of knocking-out OsKSL4 (ksl4) relative to its parental/wild-type (WT) line on allelopathy (increased growth of the endemic rice paddy weed barnyard grass with ksl4) and germination rate (decreased with ksl4). The combination of positive and negative selection pressure (here allelopathy versus decreased germination rate) is hypothesized to drive gene clustering.
Supplementary Material
SUMMARY POINTS.
Diterpenoids, derived from GGPP, encompass over 12,000 known natural products, including many of significant importance, not only to the plants where the vast majority of these are found, but also to human industry.
While the diterpenoid natural products obviously encompass a vast array of chemical structures, these can nevertheless be grouped into structurally related families on the basis of their hydrocarbon backbones, which are largely produced by diterpene synthases/cyclases.
Chief among diterpenoid natural products are the gibberellin phytohormones, as it is from the requisite diterpene synthases/cyclases that evolution has drawn, via gene duplication and neofunctionalization, to yield the striking structural diversity observed not only with diterpenoid, but also terpenoid natural products more generally.
Diterpenoid natural products can be initially divided into two major super-families distinguished by whether or not ionization of the allylic diphosphate ester bond, catalyzed by prototypical (i.e., class I) terpene synthases is preceded by protonation-initiated bicyclization catalyzed by class II diterpene cyclases. Those in which such bicyclization occurs are termed labdane-related diterpenoids, which forms the largest super-family with ~7,000 known members.
The evolutionary relationship between gibberellin biosynthesis and more specialized diterpenoid metabolism is perhaps most evident in the labdane-related diterpenoids. Very early diverging examples of the relevant diterpene synthase/cyclases can be found in those operating in gymnosperm/conifer resin acid biosynthesis. More specialized labdane-related diterpenoid metabolism has been extensively diversified in the important cereal crop plant family, where it also has been most intensively studied, although examples can be found among dicots, where recent studies in the Salvia genus have begun to provide molecular information, as well.
While less is known about biosynthesis of diterpenoids outside of the labdane-related superfamily, recent reports demonstrate the range of chemical diversity that can be expected to result from the action of the relevant class I diterpene synthases acting directly on GGPP.
Even less is known about the enzymes catalyzing the further transformation necessary for production of the final/bioactive diterpenoid natural products. Thus, as for many other plant natural products, very few complete biosynthetic pathways have been elucidated for diterpenoids.
Evolution of the sometimes complex biosynthetic pathways and/or networks necessary for production of known diterpenoid natural products must be driven by selection pressure (i.e., physiological function derived from biological activity). However, again as is typical with plant natural products, the physiological function of very few diterpenoids has been determined with any degree of confidence. Nevertheless, there is an intriguing range of biological activity that has been ascribed to diterpenoid natural products.
Work on rice diterpenoid metabolism has provided some insights into the presence of biosynthetic gene clusters in plants, highlighting their complex nature, as well as supporting the hypothesis that both positive and negative selection pressure is required for their assembly.
FUTURE ISSUES.
It is clear that much more chemical diversity can be expected to arise from further investigation of diterpenoid metabolism. This includes elucidation of diterpenoid natural products derived from the recently discovered cisoid NNPP precursor, as well as characterization of additional diterpene synthases/cyclases. Moreover, the further elaboration required to generate the plethora of natural products found in each of the structurally related families of diterpenoids remains largely opaque, yet additional fascinating chemical transformations are required and still await elucidation in many cases.
Assignment of physiological function will help illuminate the selective pressure leading to evolution of the sometimes complex biosynthetic pathways and/or networks underlying the production of intricate diterpenoid natural products. This may be further expected to address the question of why certain species make so many diterpenoids (e.g., more than 20 are known in rice, but the majority of these were isolated as antibiotics against a single microbial pathogen – M. oryzae).
Even beyond identification of physiological function, only for GA is the molecular target and subsequent effect (i.e., signaling pathway in the case of GA) known (97). This provides an additional frontier for exploration with all natural products, including the diterpenoids.
Acknowledgments
We apologize to any colleagues whose work was not discussed due to the limitation in length of this review. Work in the Peters group on diterpenoid metabolism has been supported by grants from the USDA-NIFA-AFRI (2008-35318-05027), NSF (MCB0919735), and NIH (GM076324). R.J.P. gratefully acknowledges sabbatical fellowship support from the Alexander von Humboldt Foundation during preparation of this review.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Jiachen Zi, Email: jzi@iastate.edu.
Sibongile Mafu, Email: smafu@iastate.edu.
Reuben J. Peters, Email: rjpeters@iastate.edu.
LITERATURE CITED
- 1.Akhtar TA, Matsuba Y, Schauvinhold I, Yu G, Lees HA, et al. The tomato cis-prenyltransferase gene family. Plant J. 2013;73:640–52. doi: 10.1111/tpj.12063. [DOI] [PubMed] [Google Scholar]
- 2.Anterola A, Shanle E, Mansouri K, Schuette S, Renzaglia K. Gibberellin precursor is involved in spore germination in the moss Physcomitrella patens. Planta. 2009;229:1003–7. doi: 10.1007/s00425-008-0875-1. [DOI] [PubMed] [Google Scholar]
- 3.Banks JA, Nishiyama T, Hasebe M, Bowman JL, Gribskov M, et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science. 2011;332:960–3. doi: 10.1126/science.1203810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, et al. Cloning and characterization of the maize An1 gene. Plant Cell. 1995;7:75–84. doi: 10.1105/tpc.7.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomke C, Tudzynski B. Diversity, regulation, and evolution of the gibberellin biosynthetic pathway in fungi compared to plants and bacteria. Phytochemistry. 2009;70:1876–93. doi: 10.1016/j.phytochem.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Caniard A, Zerbe P, Legrand S, Cohade A, Valot N, et al. Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant Biol. 2012;12:119. doi: 10.1186/1471-2229-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao R, Zhang Y, Mann FM, Huang C, Mukkamala D, et al. Diterpene cyclases and the nature of the isoprene fold. Proteins. 2010;78:2417–32. doi: 10.1002/prot.22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartwright DW, Langcake P, Pryce RJ, Leworthy DP, Ride JP. Chemical activation of host defence mechanisms as a basis for crop protection. Nature. 1977;267:511–3. [Google Scholar]
- 9.Cartwright DW, Langcake P, Pryce RJ, Leworthy DP, Ride JP. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry. 1981;20:535–7. [Google Scholar]
- 10.Chen F, Tholl D, Bohlmann J, Pichersky E. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–29. doi: 10.1111/j.1365-313X.2011.04520.x. [DOI] [PubMed] [Google Scholar]
- 11.Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem Rev. 2006;106:3412–42. doi: 10.1021/cr050286w. [DOI] [PubMed] [Google Scholar]
- 12.Christianson DW. Unearthing the roots of the terpenome. Curr Opin Chem Biol. 2008;12:141–50. doi: 10.1016/j.cbpa.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criswell J, Potter K, Shephard F, Beale MB, Peters RJ. A single residue change leads to a hydroxylated product from the class II diterpene cyclization catalyzed by abietadiene synthase. Org Lett. 2012;14:5828–31. doi: 10.1021/ol3026022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degenhardt J, Kollner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70:1621–37. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Falara V, Pichersky E, Kanellis AK. A copal-8-ol diphosphate synthase from the angiosperm Cistus creticus subsp. creticus is a putative key enzyme for the formation of pharmacologically active, oxygen-containing labdane-type diterpenes. Plant Physiol. 2010;154:301–10. doi: 10.1104/pp.110.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao W, Hillwig ML, Huang L, Cui G, Wang X, et al. A functional genomics approach to tanshinone biosynthesis provides stereochemical insights. Org Lett. 2009;11:5170–3. doi: 10.1021/ol902051v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, Honzatko RB, Peters RJ. Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat Prod Rep. 2012;29:1153–75. doi: 10.1039/c2np20059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerra-Bubb J, Croteau R, Williams RM. The early stages of taxol biosynthesis: an interim report on the synthesis and identification of early pathway metabolites. Nat Prod Rep. 2012;29:683–96. doi: 10.1039/c2np20021j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunnewich N, Higashi Y, Feng X, Choi KB, Schmidt J, Kutchan TM. A diterpene synthase from the clary sage Salvia sclarea catalyzes the cyclization of geranylgeranyl diphosphate to (8R)-hydroxy-copalyl diphosphate. Phytochemistry. 2012;91:93–9. doi: 10.1016/j.phytochem.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Zhou YJ, Hillwig ML, Shen Y, Yang L, et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc Natl Acad Sci U S A. 2013;110:12108–13. doi: 10.1073/pnas.1218061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall DE, Zerbe P, Jancsik S, Quesada AL, Dullat H, et al. Evolution of conifer diterpene synthases: diterpene resin acid biosynthesis in lodgepole pine and jack pine involves monofunctional and bifunctional diterpene synthases. Plant Physiol. 2013;161:600–16. doi: 10.1104/pp.112.208546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamberger B, Bak S. Plant P450s as versatile drivers for evolution of species-specific chemical diversity. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2013;368:20120426. doi: 10.1098/rstb.2012.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamberger B, Ohnishi T, Seguin A, Bohlmann J. Evolution of diterpene metabolism: Sitka spruce CYP720B4 catalyzes multiple oxidations in resin acid biosynthesis of conifer defense against insects. Plant Physiology. 2011;157:1677–95. doi: 10.1104/pp.111.185843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris LJ, Saparno A, Johnston A, Prisic S, Xu M, et al. The maize An2 gene is induced by Fusarium attack and encodes an ent-copalyl diphosphate synthase. Plant Mol Biol. 2005;59:881–94. doi: 10.1007/s11103-005-1674-8. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa M, Mitsuhara I, Seo S, Imai T, Koga J, et al. Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol Plant Microbe Interact. 2010;23:1000–11. doi: 10.1094/MPMI-23-8-1000. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi K, Horie K, Hiwatashi Y, Kawaide H, Yamaguchi S, et al. Endogenous diterpenes derived from ent-kaurene, a common gibberellin precursor, regulate protonema differentiation of the moss Physcomitrella patens. Plant Physiol. 2010;153:1085–97. doi: 10.1104/pp.110.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi K, Kawaide H, Notomi M, Sakigi Y, Matsuo A, Nozaki H. Identification and functional analysis of bifunctional ent-kaurene synthase from the moss Physcomitrella patens. FEBS Lett. 2006;580:6175–81. doi: 10.1016/j.febslet.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Hedden P, Thomas SG. Gibberellin biosynthesis and its regulation. Biochem J. 2012;444:11–25. doi: 10.1042/BJ20120245. [DOI] [PubMed] [Google Scholar]
- 29.Helliwell CA, Chandler PM, Poole A, Dennis ES, Peacock WJ. The CYP88A cytochrome P450, ent-kaurenoic acid oxidase, catalyzes three steps of the gibberellin biosynthesis pathway. Proc Natl Acad Sci, USA. 2001;98:2065–70. doi: 10.1073/pnas.041588998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helliwell CA, Poole A, Peacock WJ, Dennis ES. Arabidposis ent-kaurene oxidase catalyzes three steps of gibberellin biosynthesis. Plant Physiol. 1999;119:507–10. doi: 10.1104/pp.119.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helliwell CA, Sullivan JA, Mould RM, Gray JC, Peacock WJ, Dennis ES. A plastid envelope location of Arabidopsis ent-kaurene oxidase links the plastid and endoplasmic reticulum steps of the gibberellin biosynthesis pathway. Plant J. 2001;28:201–8. doi: 10.1046/j.1365-313x.2001.01150.x. [DOI] [PubMed] [Google Scholar]
- 32.Herde M, Gartner K, Kollner TG, Fode B, Boland W, et al. Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT. Plant Cell. 2008;20:1152–68. doi: 10.1105/tpc.106.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillwig ML, Xu M, Toyomasu T, Tiernan MS, Gao W, et al. Domain loss has independently occurred multiple times in plant terpene synthase evolution. Plant J. 2011;68:1051–60. doi: 10.1111/j.1365-313X.2011.04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano K, Nakajima M, Asano K, Nishiyama T, Sakakibara H, et al. The GID1-mediated gibberellin perception mechanism is conserved in the Lycophyte Selaginella moellendorffii but not in the Bryophyte Physcomitrella patens. Plant Cell. 2007;19:3058–79. doi: 10.1105/tpc.107.051524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iijima Y, Gang DR, Fridman E, Lewinsohn E, Pichersky E. Characterization of geraniol synthase from the peltate glands of sweet basil. Plant Physiol. 2004;134:370–9. doi: 10.1104/pp.103.032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, et al. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol Biol. 2004;54:533–47. doi: 10.1023/B:PLAN.0000038261.21060.47. [DOI] [PubMed] [Google Scholar]
- 37.Kanno Y, Otomo K, Kenmoku H, Mitsuhashi W, Yamane H, et al. Characterization of a rice gene family encoding type-A diterpene cyclases. Biosci Biotechnol Biochem. 2006;70:1702–10. doi: 10.1271/bbb.60044. [DOI] [PubMed] [Google Scholar]
- 38.Kato T, Kabuto C, Sasaki N, Tsunagawa M, Aizawa H, et al. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973;14:3861–4. [Google Scholar]
- 39.Kato-Noguchi H, Peters RJ. The role of momilactones in rice allelopathy. J Chem Ecol. 2013;39:175–85. doi: 10.1007/s10886-013-0236-9. [DOI] [PubMed] [Google Scholar]
- 40.Kawaide H, Hayashi K, Kawanabe R, Sakigi Y, Matsuo A, et al. Identification of the single amino acid involved in quenching the ent-kauranyl cation by a water molecule in ent-kaurene synthase of Physcomitrella patens. FEBS J. 2011;278:123–33. doi: 10.1111/j.1742-4658.2010.07938.x. [DOI] [PubMed] [Google Scholar]
- 41.Keeling CI, Bohlmann J. Diterpene resin acids in conifers. Phytochemistry. 2006;67:2415–23. doi: 10.1016/j.phytochem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Keeling CI, Dullat HK, Yuen M, Ralph SG, Jancsik S, Bohlmann J. Identification and functional characterization of monofunctional ent-copalyl diphosphate and ent-kaurene synthases in white spruce reveal different patterns for diterpene synthase evolution for primary and secondary metabolism in gymnosperms. Plant Physiol. 2010;152:1197–208. doi: 10.1104/pp.109.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keeling CI, Weisshaar S, Ralph SG, Jancsik S, Hamberger B, et al. Transcriptome mining, functional characterization, and phylogeny of a large terpene synthase gene family in spruce (Picea spp.) BMC Plant Biol. 2011;11:43. doi: 10.1186/1471-2229-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirby J, Nishimoto M, Park JG, Withers ST, Nowroozi F, et al. Cloning of casbene and neocembrene synthases from Euphorbiaceae plants and expression in Saccharomyces cerevisiae. Phytochemistry. 2010;71:1466–73. doi: 10.1016/j.phytochem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Li G, Kollner TG, Yin Y, Jiang Y, Chen H, et al. Nonseed plant Selaginella moellendorffi [corrected] has both seed plant and microbial types of terpene synthases. Proc Natl Acad Sci U S A. 2012;109:14711–5. doi: 10.1073/pnas.1204300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 47.Mafu S, Hillwig ML, Peters RJ. A novel labda-7,13e-dien-15-ol-producing bifunctional diterpene synthase from Selaginella moellendorffii. ChemBioChem. 2011;12:1984–7. doi: 10.1002/cbic.201100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magome H, Nomura T, Hanada A, Takeda-Kamiya N, Ohnishi T, et al. CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1215788110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mann FM, Prisic S, Davenport EK, Determan MK, Coates RM, Peters RJ. A single residue switch for Mg2+-dependent inhibition characterizes plant class II diterpene cyclases from primary and secondary metabolism. J Biol Chem. 2010;285:20558–63. doi: 10.1074/jbc.M110.123307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin DM, Faldt J, Bohlmann J. Functional characterization of nine norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol. 2004;135:1908–27. doi: 10.1104/pp.104.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mau CJD, West CA. Cloning of casbene synthase cDNA: Evidence for conserved structural features among terpenoid cyclases in plants. Proc Natl Acad Sci U S A. 1994;91:8497–501. doi: 10.1073/pnas.91.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyazaki S, Katsumata T, Natsume M, Kawaide H. The CYP701B1 of Physcomitrella patens is an ent-kaurene oxidase that resists inhibition by uniconazole-P. FEBS Lett. 2011;585:1879–83. doi: 10.1016/j.febslet.2011.04.057. [DOI] [PubMed] [Google Scholar]
- 53.Morrone D, Chambers J, Lowry L, Kim G, Anterola A, et al. Gibberellin biosynthesis in bacteria: Separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum. FEBS Lett. 2009;583:475–80. doi: 10.1016/j.febslet.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 54.Morrone D, Chen X, Coates RM, Peters RJ. Characterization of the kaurene oxidase CYP701A3, a multifunctional cytochrome P450 from gibberellin biosynthesis. Biochem J. 2010;431:337–44. doi: 10.1042/BJ20100597. [DOI] [PubMed] [Google Scholar]
- 55.Morrone D, Hillwig ML, Mead ME, Lowry L, Fulton DB, Peters RJ. Evident and latent plasticity across the rice diterpene synthase family with potential implications for the evolution of diterpenoid metabolism in the cereals. Biochem J. 2011;435:589–95. doi: 10.1042/BJ20101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrone D, Jin Y, Xu M, Choi S-Y, Coates RM, Peters RJ. An unexpected diterpene cyclase from rice: Functional identification of a stemodene synthase. Arch Biochem Biophys. 2006;448:133–40. doi: 10.1016/j.abb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Morrone D, Xu M, Fulton DB, Determan MK, Peters RJ. Increasing complexity of a diterpene synthase reaction with a single residue switch. J Am Chem Soc. 2008;130:5400–1. doi: 10.1021/ja710524w. [DOI] [PubMed] [Google Scholar]
- 58.Nelson D, Werck-Reichhart D. A P450-centric view of plant evolution. Plant J. 2011;66:194–211. doi: 10.1111/j.1365-313X.2011.04529.x. [DOI] [PubMed] [Google Scholar]
- 59.Nualkaew N, De-Eknamkul W, Kutchan TM, Zenk MH. Membrane-bound geranylgeranyl diphosphate phosphatases: purification and characterization from Croton stellatopilosus leaves. Phytochemistry. 2006;67:1613–20. doi: 10.1016/j.phytochem.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Nualkaew N, Guennewich N, Springob K, Klamrak A, De-Eknamkul W, Kutchan TM. Molecular cloning and catalytic activity of a membrane-bound prenyl diphosphate phosphatase from Croton stellatopilosus Ohba. Phytochemistry. 2013;91:140–7. doi: 10.1016/j.phytochem.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Oldfield E, Lin FY. Terpene biosynthesis: modularity rules. Angew Chem Int Ed Engl. 2012;51:1124–37. doi: 10.1002/anie.201103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortiz de Montellano PR. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem Rev. 2010;110:932–48. doi: 10.1021/cr9002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osbourn A. Gene clusters for secondary metabolic pathways: an emerging theme in plant biology. Plant Physiol. 2010;154:531–5. doi: 10.1104/pp.110.161315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peters RJ. Uncovering the complex metabolic network underlying diterpenoid phytoalexin biosynthesis in rice and other cereal crop plants. Phytochemistry. 2006;67:2307–17. doi: 10.1016/j.phytochem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Peters RJ. Two rings in them all: The labdane-related diterpenoids. Nat Prod Rep. 2010;27:1521–30. doi: 10.1039/c0np00019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters RJ. Gibberellin phytohormone metabolism. In: Bach T, Rohmer M, editors. Isoprenoid synthesis in plants and microorganisms: New concepts and experimental approaches. New York: Springer; 2013. pp. 233–49. Number of 233–49 pp. [Google Scholar]
- 67.Peters RJ, Carter OA, Zhang Y, Matthews BW, Croteau RB. Bifunctional abietadiene synthase: Mutual structural dependence of the active sites for protonation-initiated and ionization-initiated cyclizations. Biochemistry. 2003;42:2700–7. doi: 10.1021/bi020492n. [DOI] [PubMed] [Google Scholar]
- 68.Peters RJ, Croteau RB. Abietadiene synthase catalysis: Conserved residues involved in protonation-initated cyclization of geranylgeranyl diphosphate to (+)-copalyl diphosphate. Biochemistry. 2002;41:1836–42. doi: 10.1021/bi011879d. [DOI] [PubMed] [Google Scholar]
- 69.Peters RJ, Croteau RB. Abietadiene synthase catalysis: Mutational analysis of a prenyl diphosphate ionization-initiated cyclization and rearrangement. Proc Natl Acad Sci USA. 2002;99:580–4. doi: 10.1073/pnas.022627099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peters RJ, Flory JE, Jetter R, Ravn MM, Lee H-J, et al. Abietadiene synthase from grand fir (Abies grandis): Characterization and mechanism of action of the “pseudomature” recombinant enzyme. Biochemistry. 2000;39:15592–602. doi: 10.1021/bi001997l. [DOI] [PubMed] [Google Scholar]
- 71.Peters RJ, Ravn MM, Coates RM, Croteau RB. Bifunctional abietadiene synthase: Free diffusive transfer of the (+)-copalyl diphosphate intermediate between two distinct active sites. J Am Chem Soc. 2001;123:8974–8. doi: 10.1021/ja010670k. [DOI] [PubMed] [Google Scholar]
- 72.Prisic S, Peters RJ. Synergistic substrate inhibition of ent-copalyl diphosphate synthase: A potential feed-forward inhibition mechanism limiting gibberellin metabolism. Plant Physiol. 2007;144:445–54. doi: 10.1104/pp.106.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prisic S, Xu J, Coates RM, Peters RJ. Probing the role of the DXDD motif in class II diterpene cyclases. ChemBioChem. 2007;8:869–74. doi: 10.1002/cbic.200700045. [DOI] [PubMed] [Google Scholar]
- 74.Prisic S, Xu M, Wilderman PR, Peters RJ. Rice contains two disparate ent-copalyl diphosphate synthases with distinct metabolic functions. Plant Physiol. 2004;136:4228–36. doi: 10.1104/pp.104.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ravn MM, Coates RM, Flory J, Peters RJ, Croteau R. Stereochemistry of the cyclization-rearrangement of (+)-copalyl diphosphate to (-)-abietadiene catalyzed by recombinant abietadiene synthase from Abies grandis. Org Lett. 2000;2:573–6. doi: 10.1021/ol991230p. [DOI] [PubMed] [Google Scholar]
- 76.Ravn MM, Coates RM, Jetter R, Croteau R. Stereospecific intramolecular proton transfer in the cyclization of geranylgeranyl diphosphate to (-)-abietadiene catalyzed recombinant cyclase from grand fir (Abies grandis) Chem Commun (Camb) 1998;1998:21–2. [Google Scholar]
- 77.Ravn MM, Jin Q, Coates RM. Synthesis of allylic isoprenoid diphosphates by SN2 displacement of diethyl phosphate. Eur J Org Chem. 2000:1401–10. [Google Scholar]
- 78.Ravn MM, Peters RJ, Coates RM, Croteau RB. Specificity and mechanism of abietadiene synthase catalysis: Stereochemistry and stabilization of the cryptic pimarenyl carbocation intermediate. J Am Chem Soc. 2002;124:6998–7006. doi: 10.1021/ja017734b. [DOI] [PubMed] [Google Scholar]
- 79.Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–9. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 80.Ro D-K, Bohlmann J. Diterpene resin acid biosynthesis in loblolly pine (Pinus taeda): functional characterization of abietadiene/levopimaradiene synthase (PtTPS-LAS) cDNA and subcellular targeting of PtTPS-LAS and abietadienol/abietadienal oxidase (PtAO, CYP720B1) Phytochemistry. 2006;67:1572–8. doi: 10.1016/j.phytochem.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 81.Ro DK, Arimura G, Lau SY, Piers E, Bohlmann J. Loblolly pine abietadienol/abietadienal oxidase PtAO (CYP720B1) is a multifunctional, multisubstrate cytochrome P450 monooxygenase. Proc Natl Acad Sci U S A. 2005;102:8060–5. doi: 10.1073/pnas.0500825102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roy A, Roberts FG, Wilderman PR, Zhou K, Peters RJ, Coates RM. 16-Aza-ent-beyerane and 16-Aza-ent-trachylobane: Potent Mechanism Based Inhibitors of Recombinant ent-Kaurene Synthase from Arabidopsis thaliana. J Am Chem Soc. 2007;129:12453–60. doi: 10.1021/ja072447e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004;134:1642–53. doi: 10.1104/pp.103.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sallaud C, Giacalone C, Topfer R, Goepfert S, Bakaher N, et al. Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Plant J. 2012 doi: 10.1111/j.1365-313X.2012.05068.x. epub. [DOI] [PubMed] [Google Scholar]
- 85.Schalk M, Pastore L, Mirata MA, Khim S, Schouwey M, et al. Towards a Biosynthetic Route to Sclareol and Amber Odorants. J Am Chem Soc. 2012;134:18900–3. doi: 10.1021/ja307404u. [DOI] [PubMed] [Google Scholar]
- 86.Schepmann HG, Pang J, Matsuda SP. Cloning and characterization of Ginkgo biloba levopimaradiene synthase, which catalyzes the first committed step in ginkolide biosynthesis. Arch Biochem Biophys. 2001;392:263–9. doi: 10.1006/abbi.2001.2438. [DOI] [PubMed] [Google Scholar]
- 87.Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, et al. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc Natl Acad Sci U S A. 2011;108:5455–60. doi: 10.1073/pnas.1014714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, et al. The B73 maize genome: complexity, diversity, and dynamics. Science. 2009;326:1112–5. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- 89.Seki H, Ohyama K, Sawai S, Mizutani M, Ohnishi T, et al. Licorice beta-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14204–9. doi: 10.1073/pnas.0803876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimura K, Okada A, Okada K, Jikumaru Y, Ko K-W, et al. Identification of a biosynthetic gene cluster in rice for momilactones. J Biol Chem. 2007;282:34013–8. doi: 10.1074/jbc.M703344200. [DOI] [PubMed] [Google Scholar]
- 91.Silverstone AL, Chang C-W, Krol E, Sun T-P. Developmental regulation of the gibberellin biosynthetic gene GA1 in Arabidopsis thaliana. Plant J. 1997;12:9–19. doi: 10.1046/j.1365-313x.1997.12010009.x. [DOI] [PubMed] [Google Scholar]
- 92.Smanski MJ, Peterson RM, Huang SX, Shen B. Bacterial diterpene synthases: new opportunities for mechanistic enzymology and engineered biosynthesis. Curr Opin Chem Biol. 2012;16:132–41. doi: 10.1016/j.cbpa.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Starks CM, Back K, Chappell J, Noel JP. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science. 1997;277:1815–20. doi: 10.1126/science.277.5333.1815. [DOI] [PubMed] [Google Scholar]
- 94.Stofer Vogel B, Wildung MR, Vogel G, Croteau R. Abietadiene synthase from grand fir (Abies grandis) J Biol Chem. 1996;271:23262–8. doi: 10.1074/jbc.271.38.23262. [DOI] [PubMed] [Google Scholar]
- 95.Sugai Y, Ueno Y, Hayashi K, Oogami S, Toyomasu T, et al. Enzymatic (13)C labeling and multidimensional NMR analysis of miltiradiene synthesized by bifunctional diterpene cyclase in Selaginella moellendorffii. J Biol Chem. 2011;286:42840–7. doi: 10.1074/jbc.M111.302703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun T-P, Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell. 1994;6:1509–18. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun TP. The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Current biology : CB. 2011;21:R338–45. doi: 10.1016/j.cub.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 98.Swaminathan S, Morrone D, Wang Q, Fulton DB, Peters RJ. CYP76M7 is an ent-cassadiene C11α-hydroxylase defining a second multifunctional diterpenoid biosynthetic gene cluster in rice. Plant Cell. 2009;21:3315–25. doi: 10.1105/tpc.108.063677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takos AM, Rook F. Why biosynthetic genes for chemical defense compounds cluster. Trends Plant Sci. 2012;17:383–8. doi: 10.1016/j.tplants.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 100.Toyomasu T. Recent Advances Regarding Diterpene Cyclase Genes in Higher Plants and Fungi. Biosci Biotechnol Biochem. 2008;72:1168–75. doi: 10.1271/bbb.80044. [DOI] [PubMed] [Google Scholar]
- 101.Toyomasu T, Usui M, Sugawara C, Otomo K, Hirose Y, et al. Reverse-genetic approach to verify physiological roles of rice phytoalexins: characterization of a knockdown mutant of OsCPS4 phytoalexin biosynthetic gene in rice. Physiologia plantarum. 2013 doi: 10.1111/ppl.12066. [DOI] [PubMed] [Google Scholar]
- 102.Vandermoten S, Haubruge E, Cusson M. New insights into short-chain prenyltransferases: structural features, evolutionary history and potential for selective inhibition. Cellular and molecular life sciences : CMLS. 2009;66:3685–95. doi: 10.1007/s00018-009-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vaughan MM, Wang Q, Webster FX, Kiemle D, Hong YJ, et al. Formation of the unusual semivolatile diterpene rhizathalene by the Arabidopsis class I terpene synthase TPS08 in the root stele is involved in defense against belowground herbivory. Plant Cell. 2013;25:1108–25. doi: 10.1105/tpc.112.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vranova E, Coman D, Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol. 2013;64:665–700. doi: 10.1146/annurev-arplant-050312-120116. [DOI] [PubMed] [Google Scholar]
- 105.Walker K, Croteau R. Taxol biosynthetic genes. Phytochemistry. 2001;58:1–7. doi: 10.1016/s0031-9422(01)00160-1. [DOI] [PubMed] [Google Scholar]
- 106.Wang E, Wagner GJ. Elucidation of the functions of genes central to diterpene metabolism in tobacco trichomes using posttranscriptional gene silencing. Planta. 2003;216:686–91. doi: 10.1007/s00425-002-0904-4. [DOI] [PubMed] [Google Scholar]
- 107.Wang E, Wang R, DeParasis J, Loughrin JH, Gan S, Wagner GJ. Suppression of a P450 hydroxylase gene in plant trichome glands enhances natural-product-based aphid resistance. Nat Biotechnol. 2001;19:371–4. doi: 10.1038/86770. [DOI] [PubMed] [Google Scholar]
- 108.Wang Q, Hillwig ML, Okada K, Yamazaki K, Wu Y, et al. Characterization of CYP76M5–8 indicates metabolic plasticity within a plant biosynthetic gene cluster. J Biol Chem. 2012;287:6159–68. doi: 10.1074/jbc.M111.305599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Q, Hillwig ML, Peters RJ. CYP99A3: Functional identification of a diterpene oxidase from the momilactone biosynthetic gene cluster in rice. Plant J. 2011;65:87–95. doi: 10.1111/j.1365-313X.2010.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Q, Hillwig ML, Wu Y, Peters RJ. CYP701A8: A rice ent-kaurene oxidase paralog diverted to more specialized diterpenoid metabolism. Plant Physiol. 2012;158:1418–25. doi: 10.1104/pp.111.187518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wegel E, Koumproglou R, Shaw P, Osbourn A. Cell type-specific chromatin decondensation of a metabolic gene cluster in oats. Plant Cell. 2009;21:3926–36. doi: 10.1105/tpc.109.072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wilderman PR, Peters RJ. A single residue switch converts abietadiene synthase into a pimaradiene specific cyclase. J Am Chem Soc. 2007;129:15736–7. doi: 10.1021/ja074977g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilderman PR, Xu M, Jin Y, Coates RM, Peters RJ. Identification of syn-pimara-7,15-diene synthase reveals functional clustering of terpene synthases involved in rice phytoalexin/allelochemical biosynthesis. Plant Physiol. 2004;135:2098–105. doi: 10.1104/pp.104.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wildung MR, Croteau RB. A cDNA clone for taxadiene synthase, the diterpene cyclase that catalyzes the committed step of taxol biosynthesis. J Biol Chem. 1996;271:9201–4. doi: 10.1074/jbc.271.16.9201. [DOI] [PubMed] [Google Scholar]
- 115.Wu Y, Hillwig ML, Wang Q, Peters RJ. Parsing a multifunctional biosynthetic gene cluster from rice: Biochemical characterization of CYP71Z6 & 7. FEBS Lett. 2011;585:3446–51. doi: 10.1016/j.febslet.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Y, Wang Q, Hillwig ML, Peters RJ. Picking sides: Distinct roles for CYP76M6 and -8 in rice oryzalexin biosynthesis. Biochem J. 2013;454:209–16. doi: 10.1042/BJ20130574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu Y, Zhou K, Toyomasu T, Sugawara C, Oku M, et al. Functional characterization of wheat copalyl diphosphate synthases elucidates the early evolution of labdane-related diterpenoid metabolism in the cereals. Phytochemistry. 2012;84:40–6. doi: 10.1016/j.phytochem.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu M, Galhano R, Wiemann P, Bueno E, Tiernan M, et al. Genetic evidence for natural product-mediated plant-plant allelopathy in rice (Oryza sativa) New Phytol. 2012;193:570–5. doi: 10.1111/j.1469-8137.2011.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu M, Wilderman PR, Morrone D, Xu J, Roy A, et al. Functional characterization of the rice kaurene synthase-like gene family. Phytochemistry. 2007;68:312–26. doi: 10.1016/j.phytochem.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 120.Xu M, Wilderman PR, Peters RJ. Following evolution's lead to a single residue switch for diterpene synthase product outcome. Proc Natl Acad Sci USA. 2007;104:7397–401. doi: 10.1073/pnas.0611454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–51. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 122.Yamaguchi S, Saito T, Abe H, Yamane H, Murofushi N, Kamiya Y. Molecular cloning and characterization of a cDNA encoding the gibberellin biosynthetic enzyme ent-kaurene synthase B from pumpkin (Cucurbita maxima L.) Plant J. 1996;10:101–11. doi: 10.1046/j.1365-313x.1996.10020203.x. [DOI] [PubMed] [Google Scholar]
- 123.Yamane H. Biosynthesis of phytoalexins and regulatory mechanisms of it in rice. Biosci Biotechnol Biochem. 2013;77:1141–8. doi: 10.1271/bbb.130109. [DOI] [PubMed] [Google Scholar]
- 124.Yasumura Y, Crumpton-Taylor M, Fuentes S, Harberd NP. Step-by-step acquisition of the gibberellin-DELLA growth-regulatory mechanism during land-plant evolution. Current biology : CB. 2007;17:1225–30. doi: 10.1016/j.cub.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 125.Zerbe P, Chiang A, Yuen M, Hamberger B, Draper JA, et al. Bifunctional cis-abienol synthase from Abies balsamea discovered by transcriptome sequencing and its implications for diterpenoid fragrance production. J Biol Chem. 2012;287:12121–31. doi: 10.1074/jbc.M111.317669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y, Zhang B, Yan D, Dong W, Yang W, et al. Two Arabidopsis cytochrome P450 monooxygenases, CYP714A1 and CYP714A2, function redundantly in plant development through gibberellin deactivation. Plant J. 2011;67:342–53. doi: 10.1111/j.1365-313X.2011.04596.x. [DOI] [PubMed] [Google Scholar]
- 127.Zhou K, Gao Y, Hoy JA, Mann FM, Honzatko RB, Peters RJ. Insights into diterpene cyclization from the structure of the bifunctional abietadiene synthase. J Biol Chem. 2012;287:6840–50. doi: 10.1074/jbc.M111.337592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou K, Peters RJ. Electrostatic effects on (di)terpene synthase product outcome. ChemComm. 2011;47:4074–80. doi: 10.1039/c0cc02960b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou K, Xu M, Tiernan MS, Xie Q, Toyomasu T, et al. Functional characterization of wheat ent-kaurene(-like) synthases indicates continuing evolution of labdane-related diterpenoid metabolism in the cereals. Phytochemistry. 2012;84:47–55. doi: 10.1016/j.phytochem.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu Y, Nomura T, Xu Y, Zhang Y, Peng Y, et al. ELONGATED UPPERMOST INTERNODE Encodes a Cytochrome P450 Monooxygenase That Epoxidizes Gibberellins in a Novel Deactivation Reaction in Rice. Plant Cell. 2006;18:442–56. doi: 10.1105/tpc.105.038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.