Abstract
We conducted a phase I trial to examine the maximally tolerated dose (MTD) of the oral protease inhibitor nelfinavir (NFV) in combination with temozolomide and concurrent radiotherapy in patients with glioblastoma and to gather preliminary data for response. The study was conducted in patients with newly diagnosed glioblastoma after surgical resection. Patients were treated with standard radiotherapy (6,000 cGy to the gross tumor volume), temozolomide (75 mg/m2 daily) together with daily oral NFV starting 7–10 days prior to chemoradiotherapy continuing for the duration of chemoradiation for 6 weeks. Temozolomide (150–200 mg/m2) was resumed 4 weeks after completion of chemoradiotherapy. Two dose levels of NFV were investigated: 625 mg twice daily (bid) and 1,250 mg bid in a cohort escalation design. A total of 21 patients were enrolled. At the maximum tolerated dose, 18 subjects were enrolled to further evaluate toxicity and for preliminary estimate of efficacy for further phase II study. No dose-limiting toxicity was noted at 625 mg bid. At 1,250 mg bid, 3 dose-limiting episodes of hepatotoxicity were noted and one dose-limiting episode of diarrhea. The MTD for this study was 1,250 mg bid. NFV (1,250 mg bid) concurrent with temozolomide and radiotherapy is tolerated in most patients with glioblastoma. At the 1,250 mg bid dose level, patients should be monitored for hepatotoxicity and GI side effects.
Keywords: Nelfinavir, Glioblastoma, Malignant glioma, Radiation therapy, Radiosensitizer
Introduction
Glioblastoma multiforme (GBM) is the most frequent primary malignant brain tumor in adults. The standard of care of newly diagnosed glioblastoma following surgical resection to the extent safely feasible is concurrent temozolomide with radiotherapy followed by monthly temozolomide for 5 days each month for at least 6 cycles [1]. This course of therapy results in a median survival of 14.6 months and a progression-free survival of about 6.9 months as compared with median survival of 12.1 months with radiotherapy alone [1]. However, this standard therapy still only results in a modest survival benefit, hence a clear need for new approaches exists. The addition of a molecularly-targeted therapy to the standard treatment is one such approach.
GBM has been notably resistant to therapies, and in particular, radiotherapy. Ideal radiotherapy for the treatment of tumors, including GBM, is selective treatment effect on tumor cells, with relatively minimal effect on normal cells. Radiosensitizers are agents that enhance selective targeting of tumor cells using radiotherapy. One way radiosensitizers may have this differential effect is through selective targeting of pathways more highly expressed in tumor cells.
In GBM, poorer prognosis is associated with PTEN and EGFR or EGFRvIII (truncated EGFR) tumor mutations, which occur in a third of patients and in up to 40 % of patients respectively [2–4]. EGFR and Ras have been shown to modulate tumor radiosensitivity [5–7]. EGFR has a number of downstream effectors, including Ras and PI3K. EGFR and Ras-mediated radioresistance is mediated, at least in part by PI3K, and phosphorylated Akt (PAkt) is a good marker for this effect [8]. We have previously shown in head and neck cancer that P-Akt is a good predictor of (worse) clinical response to radiation [9]. We and others have shown that blocking PI3K-Akt pathway enhances radiation response in vitro and in vivo [8, 10, 11]. Radiosensitization occurs (when this pathway is blocked) in cells in which this pathway is constitutively activated but does not occur in cells (such as normal tissues) in which this pathway is not activated [8, 9, 11]. Inhibition of this pathway, therefore, is a promising approach for radiosensitization. One difficulty in implementing this therapeutic strategy has been obtaining the means to block this deregulated Akt signaling pathway in patients.
We have found that one class of commonly used drugs, the HIV protease inhibitors (HPIs) interfere with PI3K-Akt signaling. These drugs given in combination with reverse transcriptase inhibitors are the mainstay of the current therapeutic regimens for HIV infected patients. The HPIs are peptidomimetics that inhibit the HIV aspartyl protease, a retroviral enzyme that cleaves the viral gag-pol poly-protein and is necessary for the production of infectious viral particles. One prominent side effect of HPI treatment is insulin resistance [12, 13]. Since Akt plays a key role in the coordinated regulation of growth and metabolism by the insulin/IGF—signaling pathway [14, 15], we explored the possibility that HPIs might inhibit PI3K-Akt signaling in tumor cells and radiosensitize human tumors as a result.
Nelfinavir (NFV) is an inhibitor of the human immunodeficiency virus protease. HPIs have been used continuously in patients with well-characterized pharmacokinetics. The apparent volume of distribution was 2–7 L/kg and the terminal half-life in plasma was typically 3.5–5 h. NFV has an excellent tolerability and safety profile characterized by low rates of toxicity [16]. The most common acute side effects of NFV are gastrointestinal and are mostly mild or moderate in intensity and generally easily managed. Patients who have been on long-term NFV for 1–2 years can have hyperlipidemia, insulin resistance, and fat redistribution. It inhibits cytochrome p450 resulting in high serum concentrations of drugs metabolized by this pathway. There are reports of HIV patients on protease inhibitors who have received radiation therapy; no increase in side effects from the radiation have been reported and clinical outcome may be improved [17]. The standard dose of NFV in HIV patients is 1,250 mg bid given orally [18].
We tested NFV and found that it inhibited Akt at concentrations that are routinely achieved in patients. NFV also sensitized tumor cells both in vitro and in vivo to radiation [19]. NFV acted synergistically with radiation to delay GBM xenograft tumor growth in mice [19]. Therefore, it is a clear potential agent for treatment of glioblastoma. There is a high frequency of Akt activation in GBM, linked to the pathogenesis of the tumor [19]. NFV has been shown to be distributed in brain tissue while on therapy and is likely to have increased brain penetrance during fractionated radiotherapy when disruption of the blood–brain-barrier may occur. In addition, it has not been shown to sensitize normal tissues to radiation [19]. NFV has been safely administered to HIV positive patients over the last decade with minimal side effects. Thus, if NFV could safely be combined with the standard chemoradiation protocol, the possibility of enhanced efficacy could be explored. We conducted a phase I study of the combination of NFV with temozolamide and radiation therapy in patients with glioblastoma to identify the maximally tolerated dose (MTD), define the toxicities, and explore potential clinical benefit.
Methods
Study design
This is a phase I study using a cohort escalation design of NFV in combination with temozolomide and radiotherapy in patients with patients with glioblastoma. The study design was approved by the University of Pennsylvania Institutional Review Board (IRB) and is compliant with local and national guidelines. All patients gave written informed consent prior to study entry.
Three subjects were enrolled in the first cohort, followed by 3 in the second cohort which allowed an additional 15 subjects enrolled at the defined maximum tolerable dose. Eligible patients were aged ≥18 years, with a new diagnosis of histologically confirmed supratentorial WHO Grade IV glioma with ECOG performance status 0–2 who had had maximally achievable surgical resection. Treatment with radiotherapy began within 5 weeks of surgery. Treatment with NFV started 7–10 days prior to radiation and temozolomide and continued throughout the radiation course.
Patients were required to have adequate bone marrow (absolute neutrophil count ≥1,500 per mm3, platelet count ≥100,000 per mm3), renal (serum creatinine <1.5 times the upper limit of normal) and hepatic function (serum AST or ALT <2 times the upper limit of normal, serum bilirubin <1.5 mg/dl). Patients who were receiving corticosteroids had to be receiving a stable or decreasing dose for at least 14 days before randomization. All patients with reproductive potential must have been using effective contraception.
Exclusion criteria included infection with HIV, any prior cranial radiotherapy, pregnant or lactating women, or having received any other investigation agents during or within 1 month prior to treatment. Patients receiving the following drugs that are contraindicated with NFV were excluded: anti-arrhythmics (amiodarone, quinidine), antimycobacterial (rifampin), ergot derivatives (dihydroergotamine, ergonovine, ergotamine, methylergonovine), herbal products (St. John’s wort), HMG-CoA reductase inhibitors (lovastatin, simvastatin), neuroleptic (pimozide), and sedatives/hypnotics (midazolam, triazolam). Patients receiving the following drugs were clinically evaluated as to whether dosage/medication could be changed to permit the patient on study: anti-convulsants (carbamazepine, phenobarbital, phenytoin), anti-mycobacterial (rifabutin), erectile dysfunction agent (sildenafil), HMG-CoA reductase inhibitor (atorvastatin), immunosuppressants (cyclosporine, tacrolimus, sirolimus), narcotic analgesic (methadone), oral contraceptive (ethinyl estradiol), and macrolide antibiotic (azithromycin).
Dosing
Two dose levels of NFV were studied: 625 and 1,250 mg twice daily. Provided no limiting toxicity was encountered, the highest dose level was expanded to include six patients as an extended safety phase. Patients were advised to take NFV orally with food preferably high in fat because the bioavailability increases under the influence of a high fat meal. Treatment with NFV began 7–10 days prior to starting radiation and chemotherapy and continued for the duration of chemoradiation.
For both NFV levels, chemotherapy consisting of temozolomide was administered concurrently with radiation. This was the same regimen used in the Stupp trial [1]. Temozolomide 75 mg/m2 was administered daily starting on the first day of radiation and ending on the last day of radiation. After a 4-week break, patients then received six cycles of adjuvant temozolomide according to the standard 5-day schedule every 28 days. The dose is 150 mg/m2 for the first cycle and is increased to 200 mg/m2 beginning with the second cycle, so long as there are no hematologic toxic effects.
The radiation was conventional treatment and dosing. This consists of treatment to the tumor plus a generous margin for a total of 6,000 cGy in 30 fractions. Only patients with supratentorial tumors were eligible for this study to ensure that the radiation techniques are similar in each patient.
Treatment continued until there was evidence of recurrent disease, when the constraints of the protocol were detrimental to the subject’s health, or the patient refused to continue.
Dose escalation
The study used a classical 3 + 3 dose escalation design. Three patients were enrolled at the low dose level and observed for at least 1 month after completion of radiation. When no patient experienced dose-limiting toxicity (DLT), escalation to the next dose level was allowed. At the second dose level, three subjects were entered at a time. If at most one of six patients developed DLT, then the second dose was defined as MTD. Otherwise, if two or more of the six patients developed DLT, the MTD was exceeded and the first dose level declared the MTD. Patients at any particular dose level could be entered simultaneously. Once the MTD was defined, 12 additional subjects were enrolled, for a total of 18 patients, to provide preliminary exploratory efficacy data.
Assessment
Toxicities were graded according to the National Cancer Institute’s Common Toxicity Criteria (CTCAE) v. 3.0. DLT was defined as any grade 3 or higher non-hematologic or grade 4 or higher hematologic toxicity. Any radiation treatment delay longer than 2 weeks due to NFV was considered a DLT. Due to delayed toxicities attributable to radiotherapy, all toxicities observed within 1 month of chemoradiotherapy were included in the DLT assessment.
Evaluations during treatments
Within 6 weeks prior to start of study drug, screening evaluations consisting of history and physical exam, preand post-op MRI brain with contrast, and target radiation volume delineation were completed. Within 30 days prior to start of study drug, laboratory examination consisting of complete blood count with differential, serum chemistries and liver function tests, and pregnancy test as indicated were completed. 7–10 days after study entry, repeat laboratory exam including CBC with differential and basic serum chemistries was performed and continued weekly with weekly clinical evaluation from weeks 3 to 6. During weeks 10–11, subjects had imaging studies as clinically indicated. MRI brain with contrast were performed at 1, 2, 6 months, and in subsequent follow-up as clinically indicated. CBC and chemistries were performed weekly during radiotherapy and as clinically indicated during follow-up. All patients were followed up for survival.
Dose modification
The DLT for which NFV was held was any of the following adverse events related to the combination treatment: hematologic toxicities including grade 4 neutropenia, ≥grade 3 thrombocytopenia, and non-hematologic toxicities including ≥grade 2 neurotoxicity, ≥grade 3 nausea, vomiting, or diarrhea if uncontrolled by maximal antiemetic/anti-diarrheal therapy, total bilirubin >2.0 mg/dl, AST/ALT >5 times upper limit normal, or any other non-hematologic adverse event ≥grade 3, except alopecia. After any toxicities ≤grade 2, consideration for restarting NFV was only made after discussion with the principal investigator. If restarted during radiotherapy, NFV was reduced to a lower dose level.
Statistical considerations
The primary endpoint was the determination of MTD. Secondary endpoints were efficacy outcomes and time to progression and overall survival. Overall survival was calculated from time of surgical resection until death. Progression free survival was determined from time of surgical resection until radiographic or clinical evidence of tumor progression or recurrence.
Results
From July 2009 through April 2011, 21 patients were entered into the study at the Abramson Cancer Center at the University of Pennsylvania. Their characteristics are given in Table 1. All patients completed the study and were evaluable. None were lost to follow-up. Four patients discontinued treatment because of toxicity (three patients with grade 3 hepatotoxicity and one patient with grade 3 diarrhea). Otherwise, discontinuation was attributable to progressive disease: one patient was dose discontinued after being hospitalized for severe agitation associated with tumor progression and another patient with grade 4 DVT/PE. At progression of disease, patients received standard salvage therapy which included interventions such as reresection, bevacizumab infusion, and reirradiation.
Table 1.
Patient demographic and clinical characteristics
| Characteristic | All patients | 625 mg bid | 1,250 mg bid |
|---|---|---|---|
| Number of patients | 21 | 3 | 18 |
| Gender | |||
| Male | 11 | 3 | 8 |
| Female | 10 | 0 | 10 |
| Age (year) | |||
| Median | 58 | 52 | 59 |
| Range | 29–81 | 38–58 | 29–81 |
| ECOG performance status | |||
| 0 | 5 | 1 | 4 |
| 1 | 12 | 1 | 11 |
| 2 | 4 | 1 | 3 |
| Extent of resection | |||
| Biopsy | 2 | 1 | 1 |
| Incomplete resection | 11 | 2 | 9 |
| Gross total resection | 8 | 0 | 8 |
| Recursive partitioning | |||
| Analysis class | |||
| III | 3 | 1 | 2 |
| IV | 12 | 1 | 11 |
| V | 6 | 1 | 5 |
| Antiepileptic drugs used | |||
| EIAED | 6 | 1 | 5 |
| Non-EIAED | 15 | 2 | 13 |
| No antiepileptic drug | 4 | 1 | 3 |
A schematic of treatment delivery is depicted in Fig. 1. During the first part of the trial, no DLT was observed at the 625 mg twice daily dose level in the first 3 patients treated at that dose level (Table 2). No DLT was seen in the first 6 patients treated at 1,250 mg twice daily dose level. The 1,250 mg twice daily dose level was then expanded to include a total of 18 patients. In this cohort, four DLTs were observed. Three patients experienced grade 3 hepatotoxicity and one patient grade 3 diarrhea (Table 3). Of the three patients affected by hepatotoxicity, two were receiving NFV and the study drug was discontinued. The third patient experienced elevated liver enzymes 2 weeks after NFV treatment was completed. Liver enzymes of all three patients reverted to normal without additional specific treatment.
Fig. 1.

Schematic of study treatment timeline
Table 2.
Toxicities by dose level
| Toxicities | Dose level |
|||
|---|---|---|---|---|
| 625 mg bid |
1,250 mg bid |
|||
| Grade 1–2 |
Grade 3–4 |
Grade 1–2 |
Grade 3–4 |
|
| Hemoglobin | 2 | |||
| Leukopenia | 1 | |||
| Lymphopenia | 2 | |||
| Neutropenia | 1 | |||
| Thrombocytopenia | 1 | |||
| Pruritis | 2 | |||
| Rash | 3 | |||
| Anorexia | 4 | |||
| Constipation | 2 | |||
| Dehydration | 1 | |||
| Diarrhea | 2 | 10 | 1 | |
| Distention/bloating | 2 | |||
| Flatulence | 1 | |||
| Dyspepsia | 4 | |||
| Anal incontinence | 3 | |||
| Nausea | 1 | 10 | ||
| Salivary gland changes/ metallic taste |
1 | |||
| Vomiting | 2 | |||
| Alkaline phosphatase | 1 | |||
| ALT | 2 | 5 | ||
| AST | 3 | 3 | ||
| Hyperbilirubinemia | 1 | 1 | ||
| Hypocalcemia | 1 | |||
| Hyperglycemia | 2 | 6 | ||
| Hypokalemia | 1 | |||
| Hyponatremia | 2 | |||
| Photophobia | 1 | |||
| Pain | 1 | |||
Table 3.
Potentially attributable toxicities by dose level
| Toxicities | Dose level |
|||
|---|---|---|---|---|
| 625 mg bid |
1,250 mg bid |
|||
| Grade 1–2 |
Grade 3–4 |
Grade 1–2 |
Grade 3–4 |
|
| Anorexia | 4 | |||
| Constipation | 2 | |||
| Diarrhea | 2 | 10 | 1 | |
| Distention/bloating | 2 | |||
| Flatulence | 1 | |||
| Dyspepsia | 4 | |||
| Anal incontinence | 3 | |||
| Nausea | 1 | 10 | ||
| Vomiting | 2 | |||
| Alkaline phosphatase |
1 | |||
| ALT | 2 | 5 | ||
| AST | 3 | 3 | ||
| Hyperbilirubinemia | 1 | 1 | ||
| Hyperglycemia | 2 | 6 | ||
The only probable treatment related non-hepatotoxic grade 3/4 related adverse event recorded was grade 3 diarrhea in one patient. Serious non-hepatotoxic adverse events which may or may not have led to dose change or discontinuation occurred in 10 total events in 8 patients. These were attributable to diarrhea in 1, deep venous thrombosis/pulmonary embolus in 3, hyperbilirubinemia in 1, seizure in 1, transient ischemic event in 1, hyperkalemia in 1, and agitation requiring hospitalization in 2. The transient ischemic event, hyperkalemia, seizure and agitation were considered to be not related to treatment, but it was not thought possible to fully assess the relationship of the remaining events to treatment. In particular, however, there was no excess of thrombotic/embolic events, no hemorrhagic events grade ≥2, and no cardiac or hypertensive adverse events.
Although the study was not designed specifically to look at drug efficacy, this preliminary data was collected. The median overall survival was 13.7 months (range 3.2–39.2 months). The median PFS was 7.2 months (range 2.6–18.9 months). The patterns of progression included out of treatment field recurrence in 3 patients among which two patients had diffuse leptomeningeal disease (Fig. 2), and local tumor progression in 18 patients. The mean PFS among the three patients with out of field (OOF) recurrence was 14.5 months.
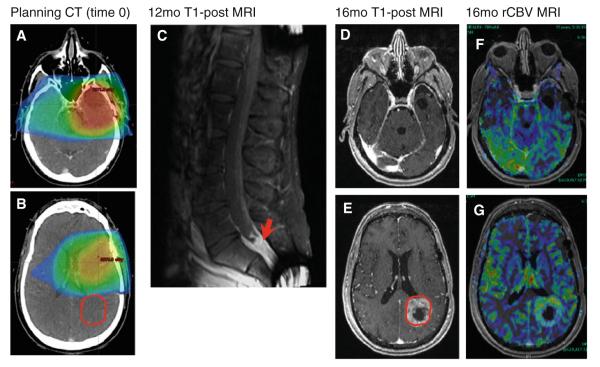
Fig. 2.
Treatment field and OOF recurrence in patient with 13 months PFS. A Planning CT showing primary tumor bed and treatment volumes. B Recurrent tumor GTV (in red) superimposed on initial treatment bed shows no overlap. C T1-post MRI at 1 year shows drop metastases in spinal cord. D–G T1-post MRI shows OOF recurrence (tumor circled in red on E) with corresponding increased rCBV as shown in G, suggesting active tumor. Decreased rCBV is seen in original tumor bed/treatment volume (original treatment area depicted in D) as shown in F as area of decreased perfusion in the left temporal lobe, suggesting inactive tissue
Discussion
The present study was intended to investigate DLT’s at two NFV dose levels (625 and 1,250 mg bid) in combination with standard dose temozolomide and radiotherapy and establish the MTD. Treatment in the NFV 625 mg bid cohort was well-tolerated, with no DLT seen in the patients treated at this dose level. At the 1,250 mg bid dose level, four DLT’s were identified, three of which were hepatotoxicity related and one gastrointestinal, giving a DLT rate of 22 % (4 of 18). In addition to the four DLT’s, there were two other serious adverse events for which dose was discontinued—one patient with DVT/PE and another with grade 3 agitation due to tumor progression which was not attributable to the treatment regimen.
At 625 mg twice daily, there did not seem to be a greater level of toxicity than that expected with temozolomide alone. In the first 6 patients enrolled at the 1,250 mg bid dose level, there were no DLTs, defining this dose as the MTD. Although only the patients in the 625 mg bid group and first 6 patients enrolled in the 1,250 mg bid group were used to define MTD in our study, treatment and evaluation were continued thereafter. At 1,250 mg twice daily, the total number of NFV doses delayed or discontinued for reasons of hepatotoxicity, diarrhea, or DVT/PE was 5. NFV was approved for clinical use in HIV patients in 1997 [20]. The standard dose of NFV in HIV patients is 1,250 mg bid given orally [18]. The pharmacokinetic properties of NFV were evaluated in healthy volunteers and HIV-infected patients; no substantial differences were observed between the two groups [18]. To date, NFV has had an excellent tolerability and safety profile that is characterized by low rates of toxicity and related discontinuations [16]. The most common acute side effects of NFV are gastrointestinal (GI). The diarrhea related to NFV is mostly mild or moderate in intensity, occurs at the initiation of therapy and is generally easily managed with loperamide and oral hydration. In a 48-week study of NFV administered at 1,250 mg bid, however, 18 % of patients experienced moderate or severe diarrhea; although, for the majority of cases, this adverse effect resolved [21]. In our study, although many patients experienced GI side effects such as diarrhea or nausea/vomiting, most were mild and easily managed with loperamide and oral hydration. Only one patient at the 1,250 mg bid dose level experienced dose-limiting diarrhea. To date, there are little toxicity and efficacy data available in cancer patients who have taken NFV. Even though the number of DLTs at the higher dose regimen was increased, 1,250 mg NFV twice daily in combination with temozolomide (75 mg/m2 daily) and radiotherapy is the recommended dose given appropriate concurrent monitoring of liver function tests.
Three patients experienced OOF tumor recurrence, two of whom had leptomeningeal disease. The percentage of patients with OOF recurrences was 14.3 %, and previous studies report between 4.2 and 20 % [22, 23]. The PFS among the three patients with OOF recurrence is more than double the overall mean PFS suggesting the possibility that better local tumor control for a longer period led to increased likelihood of first recurrence elsewhere. It is tempting to speculate that NFV may alter the natural history of OOF tumor progression, but a larger study would be needed to show a statistically significant effect. Genetic and biologic correlates of tumor typing may help identify patients that would benefit from the addition of NFV to standard therapy.
This study was primarily intended to establish safety, but we report preliminary data on efficacy which is difficult to interpret given the small number of patients. Another limitation to the interpretation of our efficacy data is lack of analysis of MGMT methylation status in these patients. Since MGMT-methylated patients tend to have better outcomes after treatment with temozolomide, this may be a confounding factor in patients with longer survival and PFS in our study. In addition, further conclusions about efficacy in a small number of patients would have been enhanced by the characterization of other molecular markers for these tumors, such as phospho-Akt, which might directly determine the clinical outcomes for these patients on NFV. The observed patterns of progression/recurrence were mostly local tumor progression within the treatment field. Further study of NFV in combination with temozolomide and radiation is warranted. This phase I trial provides toxicity data to facilitate such future studies.
Conclusion
The combination of NFV (1,250 mg twice daily dose) with temozolomide (75 mg/m2 daily) in combination with radiotherapy is feasible and relatively well tolerated. At the 1,250 mg twice daily dose, main dose-limiting toxicities observed were hepatotoxicity and GI related side effects such as diarrhea. The recommended dose of NFV is 1,250 mg twice daily with weekly monitoring of liver function tests.
Acknowledgments
This work was supported by the Department of Radiation Oncology, University of Pennsylvania, ASCO Young Investigator Award, Burroughs Wellcome Career Award for Medical Scientists (1006792) and the National Institute of Health/National Institute of Neurologic Disorders and Stroke (K08 NS076548-01). We acknowledge the Department of Radiation Oncology Clinical Research Coordinators for their assistance with the study. The clinical trial protocol was formulated and written at the ASCO/AACR: Methods in Clinical Cancer Research (Vail) Workshop.
Footnotes
Conflict of interest The University of Pennsylvania has a patent pending on the use of compounds of the type like NFV in combination with radiation. Under the policies of the University of Pennsylvania, the University may receive financial benefits based on the results of this study. There are no conflicts of interest to disclose for any of the authors.
References
- 1.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa R, Sugiyama T, Narita Y, Fumari F, Cavenee WK, Matsutani M. Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol. 2004;21(2):53–56. doi: 10.1007/BF02484510. [DOI] [PubMed] [Google Scholar]
- 3.Moldvay J, Scheid P, Wild P, Nabil K, Siat J, Borrelly J, et al. Predictive survival markers in patients with surgically resected non-small cell lung carcinoma. Clin Cancer Res. 2000;6(3):1125–1134. [PubMed] [Google Scholar]
- 4.Schneider PM, Praeuer HW, Stoeltzing O, Boehm J, Manning J, Metzger R, Fink U, Wegerer S, Hoelscher AH, Roth JA. Multiple molecular marker testing (p53, C-Ki-ras, c-erbB-2) improves estimation of prognosis in potentially curative resected non-small cell lung cancer. Br J Cancer. 2000;83(4):473–479. doi: 10.1054/bjoc.2000.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harari PM, Huang SM. Head and neck cancer as a clinical model for molecular targeting of therapy: combining EGFR blockade with radiation. Int J Radiat Oncol Biol Phys. 2001;49(2):427–433. doi: 10.1016/s0360-3016(00)01488-7. [DOI] [PubMed] [Google Scholar]
- 6.McKenna WG, Weiss MC, Endlich B, Ling CC, Bakanauskas VJ, Kelsten ML, Muschel RJ. Synergistic effect of the v-myc oncogene with H-ras on radioresistance. Cancer Res. 1990;50(1):97–102. [PubMed] [Google Scholar]
- 7.Bernhard EJ, Stanbridge EJ, Gupta S, Gupta AK, Soto D, Bakanauskas VJ, Cerniglia GJ, Muschel RJ, McKenna WG. Direct evidence for the contribution of activated N-ras and Kras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60(23):6597–6600. [PubMed] [Google Scholar]
- 8.Gupta AK, Bakanauskas VJ, Cerniglia GJ, Cheng Y, Bernhard EJ, Muschel RJ, McKenna WG. The Ras radiation resistance pathway. Cancer Res. 2001;61(10):4278–4282. [PubMed] [Google Scholar]
- 9.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, Machtay M, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8(3):885–892. [PubMed] [Google Scholar]
- 10.Gupta AK, Cerniglia GJ, Mick R, Ahmed MS, Bakanauskas VJ, Muschel RJ, McKenna WG. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys. 2003;56(3):846–853. doi: 10.1016/s0360-3016(03)00214-1. [DOI] [PubMed] [Google Scholar]
- 11.Grana TM, Rusyn EV, Zhou H, Sartor CI, Cox AD. Ras mediates radioresistance through both phosphatidylinositol 3-kinase-dependent and Raf-dependent but mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-independent signaling pathways. Cancer Res. 2002;62(14):4142–4150. [PubMed] [Google Scholar]
- 12.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353(9170):2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 13.Powderly WG. Long-term exposure to lifelong therapies. J Acquir Immune Defic Syndr. 2002;29(Suppl 1):S28–S40. doi: 10.1097/00126334-200202011-00005. [DOI] [PubMed] [Google Scholar]
- 14.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278(49):49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 15.Cross DA, Watt PW, Shaw M, Downes CP, van der Kaay J, Holder JC, Cohen P. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. 1997;406(1–2):211–215. doi: 10.1016/s0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- 16.Saag MS, Tebas P, Sension M, Conant M, Myers R, Chapman SK, Anderson R. Randomized, double-blind comparison of two nelfinavir doses plus nucleosides in HIV-infected patients (Agouron study 511) AIDS. 2001;15(15):1971–1978. doi: 10.1097/00002030-200110190-00009. [DOI] [PubMed] [Google Scholar]
- 17.Stadler RF, Gregorcyk SG, Euhus DM, Place RJ, Huber PH, Simmang CL. Outcome of HIV-infected patients with invasive squamous-cell carcinoma of the anal canal in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2004;47(8):1305–1309. doi: 10.1007/s10350-004-0584-1. [DOI] [PubMed] [Google Scholar]
- 18.Thomson Healthcare . Physicians’ desk reference. 58th edn Thomson PDR; Montvale, NJ: 2004. [Google Scholar]
- 19.Jiang Z, Pore N, Cerniglia GJ, Mick R, Georgescu M, Bernhard EJ, Hahn SM, Gupta AK, Maity A. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res. 2007;67(9):4467–4473. doi: 10.1158/0008-5472.CAN-06-3398. [DOI] [PubMed] [Google Scholar]
- 20.James JS. Nelfinavir (Viracept) approved: fourth protease inhibitor available. AIDS Treat News. 1997;267:1–2. [PubMed] [Google Scholar]
- 21.Rodriguez-French A, Boghossian J, Gray GE, Nadler JP, Quinones AR, Sepulveda GE, Millard JM, Wannamaker PG. The NEAT study: a 48-week open-label study to compare the antiviral efficacy and safety of GW433908 versus nelfinavir in antiretroviral therapy—naïve HIV-1-infected patients. J Acquir Immune Defic Syndr. 2004;35(1):22–32. doi: 10.1097/00126334-200401010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Chang EL, Akyurek S, Avalos T, Rebueno N, Spicer C, Garcia J, Famiglietti R, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007;68(1):144–150. doi: 10.1016/j.ijrobp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Milano MT, Okunieff P, Donatello RS, Mohile NA, Sul J, Walter KA, Korones DN. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys. 2010;78(3):1147–1155. doi: 10.1016/j.ijrobp.2009.09.018. [DOI] [PubMed] [Google Scholar]