Summary
Cadherins are Ca2+-dependent cell-cell adhesion proteins with an extracellular region of five domains (EC1 to EC5). Adhesion is mediated by “strand-swapping” of a conserved tryptophan residue in position 2 between EC1 domains of opposing cadherins, but the formation of this structure is not well understood. Using single molecule Fluorescence Resonance Energy Transfer (FRET) and single molecule force measurements with the Atomic Force Microscope (AFM), we demonstrate that cadherins initially interact via EC1 domains without swapping tryptophan-2 to form a weak Ca2+ dependent initial encounter complex that has 25% of the bond strength of a strand-swapped dimer. We suggest that cadherin dimerization proceeds via an induced fit mechanism where the monomers first form a tryptophan-2 independent initial encounter complex and then undergo subsequent conformational changes to form the final strand-swapped dimer.
Introduction
Cadherins are Ca2+-dependent cell-cell adhesion proteins that mediate dynamic cellular processes during embryonic development and tissue remodeling, and are essential for maintaining the structural integrity of solid tissues (Gumbiner, 2005; Halbleib and Nelson, 2006; Pokutta and Weis, 2007; Takeichi, 2007). Disruption of cadherin binding is common in metastatic cancers (Cowin et al., 2005).
Classical cadherins are single-pass transmembrane proteins with an extracellular region comprised of five cadherin (EC) domains separated by interdomain Ca2+ binding sites (Pokutta and Weis, 2007). X-ray crystallography (Boggon et al., 2002; Haussinger et al., 2004; Patel et al., 2006; Pertz et al., 1999; Shapiro et al., 1995), NMR (Haussinger et al., 2004; Miloushev et al., 2008; Overduin et al., 1995), electron microscopy (Al-Amoudi et al., 2007; He et al., 2003) and single molecule measurements (Zhang et al., 2009) have shown that opposing cadherins interact via N-terminal EC1 domains to form trans dimers; the probability of adhesion is increased cooperatively by increasing the density of cadherins (Zhang et al., 2009). X-ray crystallography and NMR of trans dimers indicates that there is an exchange of N terminal β-strands between opposing EC1 residues which leads to the insertion of a conserved Tryptophan-2 side chain (W2) into a hydrophobic pocket on their adhesive partner (Boggon et al., 2002; Haussinger et al., 2004; Miloushev et al., 2008; Patel et al., 2006; Pokutta and Weis, 2007); in contrast, the W2 residue is intramolecularly packed in a majority of cadherin monomers (Haussinger et al., 2004; Miloushev et al., 2008). Mutational data supports the importance of the strand-exchange interface in cadherin-cadherin adhesion. Mutation of W2 to alanine (W2A), or mutations in the pocket that binds to W2, both abolish cell-cell adhesion (Kitagawa et al., 2000; Shan et al., 2000; Tamura et al., 1998). Furthermore, the introduction of cysteine residues at positions that would form a disulfide bond in a strand-swapped trans-dimer produces cross-linked molecules from cell extracts (Harrison et al., 2005). Despite extensive studies, the molecular events involving N-terminal β-strands swapping and insertion of W2 side chains is not well understood.
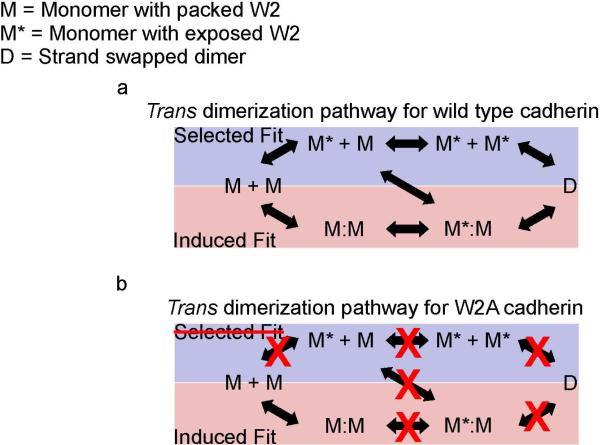
Two alternative pathways for tryptophan exchange and formation of strand-exchange dimers have been proposed (Figure 1a, adapted from (Miloushev et al., 2008)). In the induced-fit pathway, two cadherin monomers with intramolecularly packed W2 form an initial encounter complex. The initial dimer contact then allows the release of each W2 residue followed by their insertion into the opposing monomer to form the strand-swapped dimer. In the selected-fit pathway, cadherin monomers adopt an active conformation that exposes the W2 residues before binding. Subsequent collisions between activated cadherin monomers result in the formation of a stand-swapped dimer.
Figure 1. Alternative pathways for the formation of strand-exchange dimers in wild type cadherin and W2A cadherin.
Figure 1a has been adopted from (Miloushev et al., 2008) (a)Wild type cadherin: In the minimal selected-fit pathway (blue box), a population of cadherin monomers (M) adopt an active conformation (M*) by exposing their W2 residues. Collisions between activated cadherin monomers result in the formation of a stand swapped dimer (D). In the minimal induced-fit pathway (pink box), two cadherin monomers with intramolecularly packed W2 first form an initial encounter complex (M:M). A conformational change then results in the swapping of W2 residues and the formation of a strand-swapped dimer. (b)W2A cadherin: Mutating the W2 residue eliminates the selected-fit pathway for formation of a strand-swapped dimer. Interactions between individual W2 mutants would occur only if the cadherins dimerize via an induced-fit mechanism. Since the interacting W2 mutant cadherins cannot proceed to form a strand swapped dimer, they stall at the initial encounter complex which can be detected and characterized using single molecule techniques.
NMR measurements of the isolated EC1 domain of Type II cadherin-8 identified three cadherin conformations; monomers with intramolecularly packed W2 residues, monomers with exposed W2 residues, and strand-swapped dimers. However, an initial encounter complex was not detected. Based on these experiments, it was proposed that the EC1 domain of cadherin-8 dimerizes via a selected-fit mechanism (Miloushev et al., 2008). The dimerization mechanism for the complete cadherin extracellular region (EC1 - EC5) may differ from the isolated EC1 domain used in the NMR experiment, because the isolated EC1 domain lacks the interdomain Ca2+ binding sites (Miloushev et al., 2008). Mutagenesis studies have shown that disruption of Ca2+ coordination in the EC1-EC2 interface abolish strand-exchange (Chitaev and Troyanovsky, 1998). Furthermore allosteric coupling between Ca2+ binding sites, Ca2+ concentration and W2 docking has been measured (Harrison et al., 2005; Prakasam et al., 2006). NMR analysis of dimerization of E-cadherin EC1-EC2 fragments has revealed distinct conformations for the Ca2+-free monomer, Ca2+-bound monomer, and Ca2+-bound dimer (Haussinger et al., 2004).
One strategy to resolve the mechanism of strand dimerization is to block the selected fit pathway for cadherin dimerization and test whether trans dimers are formed by an induced fit mechanism. This can be accomplished by mutating the W2 residue thereby eliminating the selected-fit pathway for the formation of a strand-swapped dimer (Figure 1b). Interactions between the individual W2 mutants would occur only if the cadherins dimerize via an induced-fit mechanism. Since the interacting W2 mutant cadherins cannot proceed to completion and form a strand-swapped dimer, this strategy would capture the initial encounter complex which can be detected and characterized using single molecule techniques (Figure 1b). Here, we use a series of in vitro assays involving single molecule Fluorescence Resonance Energy Transfer (FRET) and single molecule force measurements with the Atomic Force Microscope (AFM) to demonstrate that cadherins form trans dimers via an induced-fit mechanism.
Results
Single molecule FRET measurements on cross-linked W2A cadherin
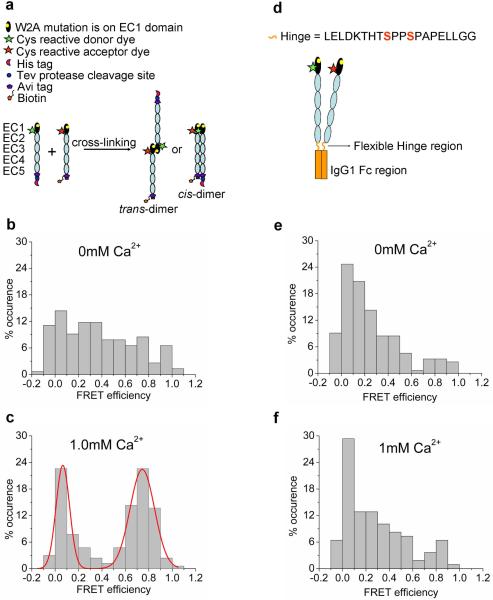
To detect the formation of an initial cadherin-cadherin encounter complex and identify EC domains that form the initial encounter complex, we measured FRET between purified, full-length E-cadherin extracellular domains (EC1 - EC5) in which W2 was mutated to alanine (W2A) (Chien et al., 2008; Kitagawa et al., 2000; Prakasam et al., 2006; Tamura et al., 1998; Tsuiji et al., 2007) (Figure 2a). Biological activity of the W2A cadherin extracellular domain was monitored using Ca2+-dependent aggregation of protein-coated beads (Figure 1S, & 2S, Supporting Information). For the single molecule FRET experiments, Cy3 (donor) and Alexa-647 (acceptor) FRET dye labels were attached to a cysteine residue engineered at position 20 on the EC1 domain, and the C-terminal of the EC5 domain was fused with a hexa-His affinity tag for protein purification and a biotin for functional surface immobilization (Figure 2a).
Figure 2. Characterizing the structure of the initial encounter complex using single molecule FRET.
(a) Steps involved in cross-linking EC1 labeled W2A cadherin monomers. W2A cadherin monomers engineered with a His-affinity tag or a biotin-tag were labeled with a donor Cy3 fluorophore or acceptor Alexa-647 fluorophore respectively at N20C. Donor- and acceptor-labeled cadherins were cross-linked in solution using amine reactive BS3 cross-linker. Cross-linked dimer yields in 0mM Ca2+ and 1mM Ca2+ were 45% and 20%, respectively. The higher cross-linking yield in 0mM Ca2+ was attributed to the high levels of nonspecific interaction of W2A cadherin in the absence of Ca2+ (see below). (b) Histogram of FRET efficiencies for W2A cadherin dimers cross-linked at 0mM Ca2+ shows a broad distribution of FRET efficiencies without a pronounced peak. This distribution arises because of the nonspecific interaction of the EC1 domains of the W2A mutants in the absence of Ca2+. (c) Histogram of FRET efficiencies for W2A cadherin dimers cross-linked at 1mM Ca2+ shows a pronounced peak at a FRET efficiency of 0.74 with 84% of the co-localized fluorescence spots have FRET values greater than 0.5. A FRET value of 0.74 corresponds to a distance of approximately 4nm between the donor and acceptor fluorophores and arises due to the interaction of the EC1 domains in the initial encounter complex. (d) Schematic of W2A cadherin-Fc dimer construct engineered by fusing the COOH terminus of the cadherin extracellular region to the Fc domain of human IgG1. Engineered cysteines (N20C) on the EC1 domains of the cadherins were dual labeled with donor and acceptor fluorophores. The cysteine residues in the core hinge region had been mutated to serines to prevent nonspecific labeling of the Fc dimer. (e-f) Histogram of FRET efficiencies for W2A cadherin-Fc dimers cross-linked at 0mM and 1mM Ca2+. W2A cadherin-Fc shows a broad distribution of FRET efficiencies without a pronounced peak due to the nonspecific interaction of the EC1 domains when the W2A cadherin are constrained in a cis orientation.
Since cadherin-cadherin binding has a low affinity (KD ~ 720μM in 1mM Ca2+ and KD ~ 10mM in 0mM Ca2+ (Haussinger et al., 2004)), we used chemical cross-linking in solution with Bis(sulfosuccinimidyl)suberate (BS3) to stabilize transient interactions between donor- and acceptor-labeled W2A cadherin monomers and trap the cadherin-cadherin initial encounter complex (Figure 2a). Cross-linked W2A cadherins were oriented and immobilized on a surface and observed in Total Internal Reflection geometry with single molecule sensitivity. FRET data was analyzed only from molecules containing co-localized donor and acceptor fluorophores determined using sequential two color excitation. Cross-linking yields determined from gel filtration (Figure 3S, Supporting Information) at 0mM Ca2+ and 1mM Ca2+ were 45% and 20% respectively; the higher cross-linking yield in 0mM Ca2+ is attributed to nonspecific interaction of W2A cadherin in the absence of Ca2+ (see text below and Figure 2b and 2e).
W2A cadherins cross-linked in 0mM Ca2+ showed a broad distribution of FRET efficiencies without a pronounced peak (Figure 2b). This distribution corresponds to a wide range of interaction distances (ranging from < 2.5nm to > 8nm) between the donor and acceptor fluorophores on the cadherin EC1 domains. This indicates that in the absence of Ca2+, the EC1 domains of W2A cadherins are `sticky' and interact in many nonspecific orientations. In contrast, a majority of wild-type cadherin monomers crosslinked in 0mM Ca2+ showed a FRET efficiency of 0 (i.e., the wild type cadherins do not interact via their outermost EC1 domains) (Zhang et al., 2009), suggesting that W2 may play a role in preventing nonspecific binding between EC1 domains in the absence of Ca2+. Recent Molecular Dynamics simulations indicate that in the absence of Ca2+, wild type cadherin occasionally adopts a conformation where the W2 side chain extends away from the trans binding interface (Sotomayor and Schulten, 2008). The exposed W2 residue may sterically hinder the nonspecific interactions of the EC1 domains. This explains our previous findings that a majority of wild-type cadherin monomers crosslinked in 0mM Ca2+ showed a FRET efficiency of 0 (Zhang et al., 2009). While the nonspecific binding between EC1 domains could arise due to the substitution of a tryptophan with an alanine residue, this is unlikely since the interaction is Ca2+ dependent.
Unlike the FRET distributions observed in the absence of Ca2+, a majority of W2A cadherin cross-linked in 1mM Ca2+ exhibit a pronounced peak at a FRET value of 0.74 and another peak at FRET~ 0. The FRET~ 0.74 peak corresponds to a distance of approximately 4nm between the donor and acceptor fluorophores on the EC1 domains (Figure 2c). This indicates that W2A cadherins interact specifically via their EC1 domains to form an initial encounter complex that is not mediated by W2. In 1mM Ca2+, 84% of the cross-linked W2A cadherins had a FRET efficiency greater than 0.5 (Figure 2c).The FRET distribution measured with the W2A mutants is similar to the FRET values at 0 and 0.8 that was measured for wild type cadherins that interact via their EC1 domains to form trans dimers (Zhang et al., 2009).
Single molecule FRET measurements on W2A cadherin-Fc constructs
The cross-linking experiments described in the previous section were performed in solution prior to surface immobilization, and the high FRET values could have arisen through proteins interacting via their EC1 domains in either a parallel or anti-parallel orientation (Figure 2a). It has been proposed that the EC1 domains of highly curved cadherin pairs presented from opposing cells interact in a parallel orientation and exchange their W2 residues even though the overall orientation of the protein is an anti-parallel trans dimer (Boggon et al., 2002; He et al., 2003; Pokutta and Weis, 2007).
To distinguish between parallel and anti-parallel orientations, the COOH terminus of the W2A cadherin extracellular domain was fused to the Fc domain of Human IgG1 (Figure 2d). The 20 amino acid hinge region linking the cadherin monomers to the dimerized Fc serves as a flexible tether that allows the cadherin pair to form an overall cis-orientation through the dimerized Fc domain (Zhang et al., 2009) so that the EC1 domains on the highly curved cadherin extracellular region can interact only in an anti-parallel orientation. In order to monitor the distance between EC1 domains of the cadherin-Fc dimers, EC1 domains were dual labeled with Cy3 donor and Alexa-647 acceptor FRET dyes attached to cysteine residues engineered at position 20 (Figure 2d). The W2A cadherin-Fc constructs were oriented and immobilized on a surface and their FRET signal was monitored at 0mM (Figure 2e) or 1mM Ca2+ (Figure 2f). At both Ca2+ concentrations, a broad distribution of FRET efficiencies was measured similar to the W2A cadherin monomers cross-linked in 0mM Ca2+ (Figure 2b). This confirms that in the absence of Ca2+, the EC1 domains of the W2A cadherin constructs are `sticky' and interact in a range of nonspecific orientations. In contrast, the EC1 domains of wild-type cadherin-Fc do not interact in either 0.1mM or 1.0mM Ca2+ (Zhang et al., 2009).
From the lack of a pronounced peak at a high FRET value with the W2A cadherin-Fc dimer constructs, we conclude that the high FRET signal observed with cross-linked W2A cadherin monomers arose through proteins forming a trans- rather than a cis-dimer encounter complex.
Single molecule AFM force measurements with W2A cadherin monomers
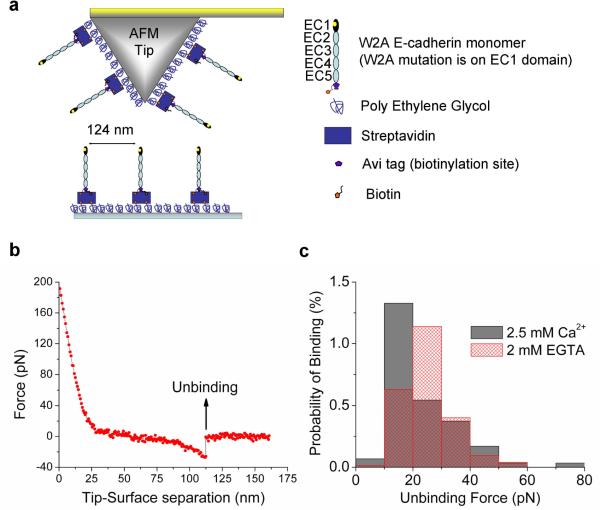
To characterize the bond strength of the W2A cadherin initial encounter complex, we measured binding between opposing W2A-cadherins at the single molecule level using an Atomic Force Microscope (AFM) (Figure 3a) and directly compared it to the unbinding force for wild type cadherins previously measured at a similar loading rate (Zhang et al., 2009). This comparison is valid only if the reaction coordinate for the forced unbinding of the wild type and W2A cadherins are similar which is likely considering that the two proteins differ by a single amino acid.
Figure 3. Characterizing the bond-strength of the initial encounter complex using single molecule AFM force measurements.
(a) Schematic of Atomic Force Microscope (AFM) tip and substrate functionalized with biotinylated W2A cadherin monomers for single molecule force measurements. The tip and surface were functionalized with Poly Ethylene Glycol (PEG) linkers, some of which were decorated with Streptavidin. Biotinylated W2A cadherin monomers were bound to Streptavidin on the tip and surface; flexible PEG linkers enable unhindered interactions between opposing cadherins during tip-substrate encounters. (b) A typical force curve showing the unbinding of a single W2A cadherin molecule. The tip and the substrate decorated with cadherins were bought into contact so that cadherins on the tip and substrate formed an adhesive complex. When the tip was withdrawn from the substrate a force was exerted and above a critical force the adhesive complex ruptured. Forces were measured 2937 times in 2.5mM Ca2+ yielding 75 binding events and 7467 times in 2mM EGTA yielding 173 binding events. (c) Histogram of W2A cadherin monomer binding events measured in 2.5mM Ca2+ (solid gray bars) and 2mM EGTA (hatched red bars). The binding events measured in 2.5mM Ca2+ were fitted to a Gaussian distribution with a peak force of 17 pN ± 10pN. The probability of binding did not change in the absence of Ca2+ (hatched red bar).
The W2A cadherin monomers were bound to polymer tethers on the AFM tip and to the substrate, and the binding of single cadherin molecules was measured in 2.5mM Ca2+ or in the absence of Ca2+ (Figures 3b and 3c). The W2A-cadherin initial encounter complex in 2.5mM Ca2+ had a bond strength of 17pN ± 10pN at a loading rate of 12.9nN/nm ± 1.7nN/nm (Figure 3c). This binding force is significantly weaker than the bond strength of 64pN ± 27pN that was measured with wild-type cadherin monomers in 2.5mM Ca2+ at a similar loading rate of 17.9nN/nm ± 1.4nN/nm (Zhang et al., 2009).
The bond-strength of the initial encounter complex formed by the interaction of opposing W2A-cadherin monomers is ~25% the bond strength of a strand swapped dimer formed by the interaction of opposing wild type cadherin monomers. Thus, while the W2 residue is not required to form a weakly adhesive initial encounter complex, it is essential to strengthen the final trans-dimer. A weak interaction between W2A cadherin constructs has been previously measured in Surface Force Apparatus (Prakasam et al., 2006) and micropipette force measurements (Chien et al., 2008).
In contrast to measurements with wild type cadherin monomers (Zhang et al., 2009), the frequency of binding events with the W2A cadherin monomers did not decrease in the absence of Ca2+ (Figure 3c). We interpret this finding as further evidence of nonspecific interactions between the EC1 domains of opposing W2A cadherin constructs measured in the FRET experiments (Figures 2b, 2e and 2f).
Discussion
We have tested two specific models of how trans-dimers form by “strand swapping” of conserved tryptophan residues in position 2 (W2) of the EC1 domain of opposed cadherins. The central question is whether cadherin monomers form an initial encounter complex and then induce a conformational change to swap their W2 residues (induced-fit), or if monomers with complementary conformations are selected and stabilized from a pre-existing equilibrium of monomers with pre-exposed and packed W2 residues (selected-fit) (Figure 1) (Miloushev et al., 2008).
To resolve the mechanism of trans dimerization, we blocked the selected-fit pathway by mutating the W2 residue to alanine (W2A) and tested whether the initial cadherin encounter complex formed by an induced-fit or selected fit mechanism. We identified the EC domains that mediate initial encounter complex formation by measuring FRET between W2A mutant E-cadherin extracellular domains. Since cadherin-cadherin binding has a low affinity, we used chemical cross-linking in solution to capture the initial encounter complex. A majority of W2A cadherin crosslinked in 1mM Ca2+ interact via their EC1 domains to form an initial encounter complex. Since this interaction between EC1 domains was not observed when the W2A cadherins were constrained to interact in a cis orientation, we conclude that the initial encounter complex was formed by W2A cadherins formed in a trans conformation. Our data strongly suggest that formation of cadherin trans dimers occurs via an induced-fit mechanism.
Previous NMR measurements of only the EC1 domain of Type II cadherin-8 concluded that dimers formed via a selected-fit mechanism (Miloushev et al., 2008). Our data show that the dimerization mechanism for the complete extracellular region of a Type-I cadherin, E-cadherin, is different. A likely reason for this difference is that the EC1 domain used in the NMR experiment lacked the interdomain Ca2+ binding sites that are essential for strand-exchange interaction (Chitaev and Troyanovsky, 1998). Alternatively, dimerization mechanism may be different between Type-I and Type-II cadherins. While our experiments show that the induced-fit mechanism for trans-dimerization is important, we cannot exclude the possibility that additional trans-dimerization pathways exist.
It is also noteworthy that our FRET measurements indicate that the orientation of the EC1 domains in the initial encounter complex is similar to that in a strand-swapped trans dimer. This implies that the structure of the initial encounter complex is not significantly altered when W2 residues are swapped to form the final trans-dimer. While we use the term “induced” fit to reference previous models for strand-swapping (Miloushev et al., 2008), the mechanism of W2 exchange might better reflect a “permissive” fit. The bond-strength of the initial encounter complex is however ~25% of the bond strength of a strand swapped trans dimer, measured with our single molecule atomic force microscopy assay. This indicates that while the W2 residue is not required to form the initial encounter complex, it is essential to strengthen the adhesion of the final trans dimer. Based on our measurements, we propose that the initial, weakly bound structure (the M:M in Fig. 1a) induces the release of the intramolecularly packed W2's without significant relative movement of the EC1 domains. A weak binding between W2A cadherin constructs has also been measured previously (Chien et al., 2008; Prakasam et al., 2006) and explains why the initial encounter complex formed by the W2A cadherin constructs form small aggregates in bulk bead binding assays.
Our results also suggest that W2 plays a role in preventing nonspecific binding of the EC1 domains of cadherin in the absence of calcium. In 0mM Ca2+, the EC1 domains of the W2A cadherin monomer and W2A cadherin-Fc dimer mutants are `sticky' and interact in a range of nonspecific orientations. In contrast, the wild-type cadherin monomers do not interact via their outermost EC1 domains in 0mM Ca2+. While the nonspecific binding between EC1 domains might be attributed to the substitution of W2 with an alanine residue, this is unlikely since the measured “stickiness” is Ca2+ dependent. Previous studies indicated an allosteric coupling between Ca2+ concentration and W2 docking (Harrison et al., 2005; Prakasam et al., 2006). Recent Molecular Dynamics simulations indicate that the side chain of W2 fluctuates between an exposed, and partially buried conformation in the absence of Ca2+ (Sotomayor and Schulten, 2008). In wild type cadherins, the exposed W2 residue could sterically hinder the nonspecific sticking of the cadherin EC1 residues in the absence of Ca2+.
Experimental Procedures
Cloning, expression and purification of E-cadherin constructs
Cloning, expression and purification of the E-cadherin monomer with a C-terminal Avi-tag, Tev sequence and His-tag (E-cadherin/ATH), and the E-cadherin/Fc construct with the extracellular domain of E-cadherin fused to a hinge and constant region of human IgG1 have been described previously (Zhang et al., 2009). The engineered proteins contained a surface accessible cysteine (N20C) introduced on the EC1 domain of both E-cadherin/Fc and E-cadherin/ATH. The only difference from the previously described constructs is that the tryptophan-2 residue on the EC1 domain of both E-cadherin/Fc and E-cadherin/ATH was mutated to alanine (W2A) using QuikChange kit (Stratagene).
As described previously (Zhang et al., 2009), the cadherin sequences were transfected into HEK 293 cells and the protein was purified from the conditioned growth media. W2A Fc-cadherin dimers were purified by passing the filtered conditioned media over a protein-A coated CL-4B sepharose resin column (GE Healthcare), eluted with 0.2M Glycine buffer at pH 2.6 and neutralized immediately by 1M Tris buffer at pH 8.0. Filtered conditioned media containing His-tagged W2A E-cadherin monomers were incubated with Nickel NTA resin (Invitrogen Corp) for 2 hours, washed with 50mM Imidizole to remove nonspecifically bound protein and eluted with 250mM imidizole. The W2A Fc-dimer and W2A E-cadherin/ATH constructs were further purified by running through Superdex200 10/300 GL size exclusion column (GE Healthcare) equilibrated with 25mM HEPES buffer at pH 7.4 containing 100mM NaCl, 10mM KCl and 1mM CaCl2.
Bead aggregation assays
Biological adhesive activity of the cadherin constructs were tested by monitoring the aggregation and disaggregation of cadherin functionalized beads in the presence or absence of Ca2+ respectively. The bead aggregation assay was identical to the previously described protocol for cadherin monomers and cadherin-Fc dimers (Zhang et al., 2009). W2A E-cadherin monomers were bound to Cobalt based Dynabeads Talon (Invitrogen Corp) and incubated in either a Ca2+ free buffer (50mM Tris buffer at pH 7.4, 100mM NaCl, 10mM KCl and 0.2% w/v BSA) or buffer containing 1.8 mM Ca2+ (50mM Tris buffer at pH 7.4, 1.8 mM Ca2+, 100mM NaCl, 10mM KCl and 0.2% w/v BSA) for 1 hour with constant agitation. The adhesion of W2A cadherin-Fc dimers was assayed by monitoring the aggregation of protein-A decorated Dynabeads (Invitrogen Corp.) in the presence of 1.8 mM Ca2+ (50mM Tris buffer at pH 7.4, 1.8 mM Ca2+, 100mM NaCl, 10mM KCl and 0.2% w/v BSA) or 2mM EGTA (50mM Tris buffer at pH 7.4, 2mM EGTA, 100mM NaCl, 10mM KCl and 0.2% w/v BSA).
The bead images were taken with a 20X objective and a Coolsnap HQ2 camera on a Leica microscope system. The W2A E-cadherin monomer coated beads incubated in 1.8 mM Ca2+ formed small aggregates while W2A E-cadherin monomer coated beads incubated in a Ca2+ free buffer showed no aggregation (Figure S1a and S1b, Supporting Information). Similarly, the W2A cadherin-Fc coated beads incubated in 1.8 mM Ca2+ formed small aggregates while W2A cadherin-Fc coated beads incubated in a 2mM EGTA showed no aggregation (Figure S1c and S1d, Supporting Information). A similar weak aggregation of W2A cadherin coated beads has been reported previously (Prakasam et al., 2006)
In order to quantitatively analyze the bead aggregation data, the images were converted to binary images and processed with an erosion and a dilation operator to identify clusters of connected beads. The fractional area occupied by each cluster was calculated using a range of cutoff sizes to differentiate aggregated clusters from non-aggregated beads. Both the W2A E-cadherin monomer and W2A cadherin Fc coated beads showed aggregation at all cutoff sizes in the presence of Ca2+ (Figure S2, Supporting Information).
Fluorescent labeling of E-cadherin constructs
The protocol for labeling the W2A cadherin constructs with a single fluorophore is identical to the protocol described previously (Zhang et al., 2009). All fluorescent dye labeling was done in a 25mM HEPES buffer at pH 7.4 with 100mM NaCl, 10mM KCl and 1mM CaCl2. The N20C residue on W2A cadherin was labeled with a 10 to 20 fold molar excess of either Cy3 maleimide (GE Healthcare) or Alexa-647 maleimide (Invitrogen Corp). W2A-Fc-Cadherin dimer molecules were dual labeled with Cy3 maleimide and Alexa647 maleimide dyes in a 1:1 molar ratio. Prior to labeling, the Cys residues were reduced using a ten-fold molar excess of pH neutral TCEP (Pierce Biotechnology). Dye labeled protein was separated from free dye using a Superdex 200 10/300 GL column at 4°C. Labeling efficiency was quantified by measuring the protein (absorption at 280nm) and dye concentrations (absorption maxima of 550nm for Cy3 and 651nm for Alexa647 dyes). The dye to protein labeling efficiency was 60%-90%.
Biotinylation and cross-linking of W2A cadherin monomers
Using previously described protocols (Zhang et al., 2009), a 40μM solution of W2A cadherin monomers was biotinylated with BirA enzyme (BirA500 kit, Avidity LLC) in a pH 7.4 buffer containing 25mM HEPES 5mM NaCl 1mM CaCl2. Equimolar concentrations of His tagged W2A cadherin monomers labeled with Cy3 dye and biotinylated W2A cadherin monomers labeled with Alexa-647 dye were concentrated to 80μM and crosslinked over ice for 30 minutes using 1mM amine reactive BS3 crosslinker (Pierce Biotechnology). Prior to cross-linking, the His-tag was cleaved from the biotinylated cadherin using AcTev protease (Invitrogen Corp). The cross-linking reaction was quenched using 1M pH 8.0 Tris buffer. Cross-linked dimers and non-cross-linked monomers were separated by Superdex 200 10/300 GL column chromatography. The cross-linked dimer yield was measured by absorption at 280nm as the protein eluted off the sizing column (Figures S3a and S3b, Supporting Information). Cross-linking reactions were carried out in buffers containing 0mM Ca2+ and 1mM Ca2+. The crosslinked dimer yields at these calcium concentrations were 45% and 20% respectively. The higher cross-linking yield in 0mm Ca2+ can be attributed to the high levels of nonspecific interaction of W2A cadherin in the absence of Ca2+ (Figure 2b, 2e, 2f and 3c).
Single molecule FRET experiments
Sample preparation for the single molecule Total Internal Reflection Fluorescence microscopy experiments has been described previously (Zhang et al., 2009). The cadherin constructs were immobilized on a freshly cleaned quartz surface functionalized with nonspecifically bound biotinylated BSA (0.2mg/ml for 15mins). The biotins were decorated with Streptavidin molecules (Pierce Biotechnology) which was used to immobilize crosslinked cadherin dimers. The Fc-cadherin dimers were immobilized on biotinylated protein-G (Pierce Biotechnology) that was bound to the Streptavidin molecules.
Fluorescence lifetimes of Cy3 and Alexa647 dyes were increased by using protocatechuic acid (PCA)/protocatechuate-3,4-dioxygenase (PCD) (Sigma-Aldrich) oxygen scavenger system (Aitken et al., 2008) plus a triplet state quencher/blinking suppressant Trolox (Rasnik et al., 2006). The molecules were imaged in a pH 7.4 buffer containing 25mM HEPES, 100mM NaCl, 10mM KCl, 2.5mM PCA, 50nM PCD and 2mM trolox.
FRET data was acquired only from molecules containing co-localized donor and acceptor fluorophores. Colocalized fluorophores were identified by initially locating the acceptor fluorophores using a 0.5 sec excitation with a red laser, then exciting the donor fluorophores and observing FRET using a 30~100 sec excitation with the green laser and finally confirming the location of the acceptor using a 3 sec illumination with the red laser. Each co-localized molecule was assigned a FRET value by fitting the histogram of the FRET time trace or by averaging the FRET values of all time points prior to photobleaching. Final FRET histograms were built based on assigned FRET values of all co-localized molecules.
Single molecule force measurements
Sample preparation for single molecule AFM force measurements has been described previously (Zhang et al., 2009). Briefly, biotinylated W2A cadherin monomers were immobilized on glass coverslips and the Si tip of an AFM cantilever (Olympus) that was silanized and functionalized with polyethylene glycol spacers (PEG 5000, Laysan Bio). One percent of the PEG spacers presented biotin molecules on their other end. These biotins were incubated with Streptavidin and the biotinylated W2A cadherin monomers were bound to the streptavidin molecules on the AFM tip and surface.
The spring constants of the AFM cantilevers were measured with the thermal fluctuation method (Hutter and Bechhoefer, 1993). Forces between single cadherin molecules were measured with an Agilent 5500 AFM in a pH 7.5 buffer (10mM Tris, 100mM NaCl, 10mM KCl) in either 2.5mM CaCl2 or 2mM EGTA. The tip and the substrate decorated with cadherins were bought into contact for either 1100ms, 340ms or 115ms so that cadherins on the tip and substrate formed an adhesive complex. Three different tip-surface contact times were chosen to allow a direct comparison with previous single molecule AFM force measurements of wild type cadherins (Zhang et al., 2009). This is a valid approach since no conclusion on cadherin binding probability was made in these experiments. When the tip was withdrawn from the substrate (at a constant velocity of 316nm/s) a force was exerted on the bound cadherins and above a critical force the adhesive complex ruptured. Forces were measured 2937 times in 2.5mM Calcium yielding 75 binding events and 7467 times in 2mM EGTA yielding 173 binding events.
Supplementary Material
Acknowledgements
Work in the Chu lab is supported by grants from NSF, NASA and AFOSR. Work in the Nelson lab is supported by NIH RO1 GM35527. We thank Agilent Technologies for their generous loan of an AFM 5500.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitken CE, Marshall RA, Pulglisi JD. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys. J. 2008;94:1826–1835. doi: 10.1529/biophysj.107.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amoudi A, Diez DC, Betts MJ, Frangakis AS. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832–U838. doi: 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- Chien YH, Jiang N, Li F, Zhang F, Zhu C, Leckband D. Two stage cadherin kinetics require multiple extracellular domains but not the cytoplasmic region. J. Biol. Chem. 2008;283:1848–1856. doi: 10.1074/jbc.M708044200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM. Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation. J. Cell Biol. 1998;142:837–846. doi: 10.1083/jcb.142.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr. Opin. Cell Biol. 2005;17:499–508. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Corps EM, Berge T, Kilshaw PJ. The mechanism of cell adhesion by classical cadherins: the role of domain 1. J. Cell Sci. 2005;118:711–721. doi: 10.1242/jcs.01665. [DOI] [PubMed] [Google Scholar]
- Haussinger D, Ahrens T, Aberle T, Engel J, Stetefeld J, Grzesiek S. Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography. EMBO J. 2004;23:1699–1708. doi: 10.1038/sj.emboj.7600192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WZ, Cowin P, Stokes DL. Untangling desmosomal knots with electron tomography. Science. 2003;302:109–113. doi: 10.1126/science.1086957. [DOI] [PubMed] [Google Scholar]
- Hutter JL, Bechhoefer J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993;64:1868–1873. [Google Scholar]
- Kitagawa M, Natori M, Murase S, Hirano S, Taketani S, Suzuki ST. Mutation analysis of cadherin-4 reveals amino acid residues of EC1 important for the structure and function. Biochem. Biophys. Res. Commun. 2000;271:358–363. doi: 10.1006/bbrc.2000.2636. [DOI] [PubMed] [Google Scholar]
- Miloushev VZ, Bahna F, Ciatto C, Ahlsen G, Honig B, Shapiro L, Palmer AG. Dynamic properties of a type II cadherin adhesive domain: Implications for the mechanism of strand-swapping of classical cadherins. Structure. 2008;16:1195–1205. doi: 10.1016/j.str.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin M, Harvey TS, Bagby S, Tong KI, Yau P, Takeichi M, Ikura M. Solution Structure of the Epithelial Cadherin Domain Responsible for Selective Cell-Adhesion. Science. 1995;267:386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L. Type II cadherin ectodomain structures: Implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Pertz O, Bozic D, Koch AW, Fauser C, Brancaccio A, Engel J. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 1999;18:1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure and mechanism of Cadherins and catenins in cell-cell contacts. Annu. Rev Cell Dev. Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Prakasam A, Chien YH, Maruthamuthu V, Leckband DE. Calcium site mutations in cadherin: Impact on adhesion and evidence of cooperativity. Biochemistry. 2006;45:6930–6939. doi: 10.1021/bi060213m. [DOI] [PubMed] [Google Scholar]
- Rasnik I, McKinney SA, Ha T. Nonblinking and longlasting single-molecule fluorescence imaging. Nat. Methods. 2006;3:891–893. doi: 10.1038/nmeth934. [DOI] [PubMed] [Google Scholar]
- Shan WS, Tanaka H, Phillips GR, Arndt K, Yoshida M, Colman DR, Shapiro L. Functional cis-heterodimers of N- and R-cadherins. J. Cell Biol. 2000;148:579–590. doi: 10.1083/jcb.148.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Alsnielsen J, Colman DR, Hendrickson WA. Structural Basis of Cell-Cell Adhesion by Cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Sotomayor M, Schulten K. The allosteric role of the Ca2+ switch in adhesion and elasticity of C-cadherin. Biophys. J. 2008;94:4621–4633. doi: 10.1529/biophysj.107.125591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat. Rev. Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- Tsuiji H, Xu L, Schwartz K, Gumbiner BM. Cadherin conformations associated with dimerization and adhesion. J. Biol. Chem. 2007;282:12871–12882. doi: 10.1074/jbc.M611725200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sivasankar S, Nelson WJ, Chu S. Resolving cadherin interactions and binding cooperativity at the single molecule level. Proc. Natl. Acad. Sci. U. S. A. 2009;106:109–114. doi: 10.1073/pnas.0811350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.