Abstract
The autosomal recessive form of the Hyper IgE syndrome (AR-HIES) with dedicator of cytokinesis 8 (DOCK8) deficiency is associated with difficult to treat persistent viral skin infections, including papilloma virus infection. Type I interferons play an important role in the defense against viruses. We examined the effect of therapy with IFN–α 2b in an 11-year old boy with DOCK8 deficiency due to a homozygous splice donor site mutation in DOCK8 intron 40. His unremitting warts showed dramatic response to IFN–α 2b therapy. Immunological studies revealed decreased circulating plasmacytoid dendritic cells (pDCs) and profound deficiency of IFN-α production by his peripheral blood mononuclear cells in response to treatment with CpG oligonucleotides. These findings indicate that underlying pDC deficiency and impaired IFN-α production may predispose to chronic viral infections in DOCk8 deficiency. IFN–α 2b therapy maybe useful in controlling recalcitrant viral infections in these patients.
Keywords: Combined Immunodeficiency, Dedicator of Cytokinesis 8, DOCK8, Hyper Immunoglobulin E syndrome, interferon–α 2b, Warts, Papilloma Virus
Introduction
Dedicator of cytokinesis 8 (DOCK8)1 deficiency is an autosomal recessive form of hyper-immunoglobulin E (HIES) syndrome that manifests recurrent sinopulmonary infections, viral skin infections, recurrent abscesses, mucocutaneous candidiasis, eczema, hepatic disorders, asthma, multiple food allergies and increased risk for malignancy and autoimmune diseases [1, 2]. DOCK8-deficient patients are highly susceptible to viral skin infections including molluscum contagiosum and human papilloma virus that usually progressive and unfortunately may cause squamous cell carcinomas in some cases [1-3]. The laboratory evaluation usually reveals marked elevations in serum immunoglobulin E (IgE) level, eosinophilia and T-cell lymphopenia with impaired proliferative responses to mitogens [1-3]. We describe an 11 year-old boy with DOCK8 deficiency due to a splice junction mutation in DOCK8 who presented with severe generalized warts that responded to interferon– alpha 2b (IFN–α 2b) therapy.
Materials And Methods
PCR and sequence analysis
Genomic DNA and total RNA were prepared from blood, and cDNA was synthesized as previously described [1]. Exons and flanking intron/exon boundaries from DOCK8 were amplified from genomic DNA by PCR according to standard protocols with Taq polymerase. PCR products were purified and the DNA was sequenced with the ABI PRISM BigDye Terminator kit V3.1 (Applied Biosystems, Foster City, Calif), the 3130xl Applied Biosystems Genetic Analyzer, DNA Sequencing Analysis software, version 5.2 (Applied Biosystems), and Sequencher, version 5.0 (Gene Codes Corp, Ann Arbor, Mich).
Immunoblotting
Epstein Barr virus (EBV)-transformed B cells were derived and lysed in lysis buffer, as described [4]. Whole cell lysates (50 μg/lane) were resolved by SDS-PAGE and transferred to nitrocellulose filters and immunoblotted with polyclonal rabbit anti- DOCK8 and monoclonal anti-human actin antibodies (Sigma-Aldrich). The blots were developed by using horseradish peroxidase–conjugated secondary antibodies and enzyme-linked chemiluminescence, and exposed to film.
Flow cytometry
For detection of pDCs, PBMCs were stained with anti-human CD123 (AC145) and BDCA-4 (AD5-17F6) mAbs (Miltenyi) with the appropriate isotype controls as described [4]. Standard flow cytometric methods were used for staining. Data collected with a LSRFortessa cell analyzer (BD Biosciences) were analyzed in the lymphocyte gate with FlowJo software (TreeStar).
IFN-α production
Human PBMCs were isolated from heparinized blood obtained from patients and normal donors using Ficoll-Hypaque (Pharmacia Biotech). Cells were suspended in RPMI-1640 containing 10% heat-inactivated FCS (Hyclone), 2 mM L-glutamine, 50 mg/ml streptomycin and 100 U/ml penicillin. PBMCs (2×106 cells/ml) were either unstimulated or stimulated with class A CpG ODN 2216 Invivogen). IFN-α culture supernatants was measured after 24 h by ELISA (Mabtech) according to the manufacturer instructions [4].
Results
The patient was born to first-degree consanguineous parents (Figure 1A). During the first year of life he started to manifest eczema and uncontrolled asthma that required frequent emergency visits and hospitalizations. He had recurrent skin abscesses requiring surgical drainage and intravenous antibiotics. He had one episode of molluscum contagiosum in the neck and trunk and one episode of clinical sepsis. He had recurrent purulent otitis media that required several courses of antibiotics and four sets of bilateral ventilating ear tubes that failed to overcome his ear discharges. At the age of three years he developed severe chickenpox involving the oral mucosa that mandated hospitalization for more than two weeks. Starring at age 4 he started suffering from progressive generalized unremitting warts over his face, neck, limbs and trunk that did not respond to cimetidine and cryotherapy. In addition, he suffered from multiple episodes of anaphylaxis provoked by different foods. Egg, peanut, sesame, kiwi and banana allergy was confirmed by positive skin prick tests. At the age of 9 years he was referred to the immunology clinic at King Abdulaziz Medical City, Jeddah, Saudi Arabia for evaluation of unresolved extensive warts.
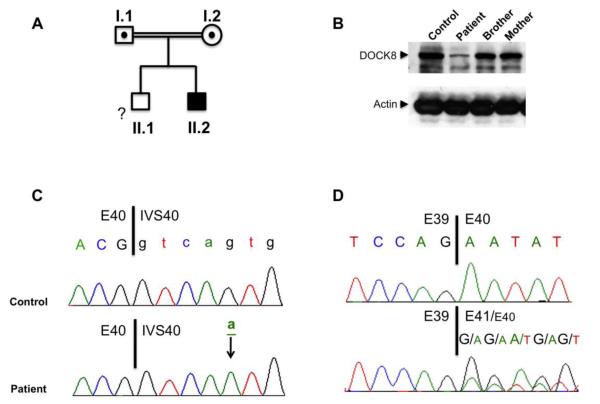
Figure 1.
A. Pedigree of the patient’s family. Each generation is designated by a Roman numeral (I–II), and each individual by an Arabic numeral. Squares, males; circle, female (mother). Filled symbol: patient. Dotted symbol: heterozygote carrier. The double lines connecting the parents denote consanguinity. The genetic status of the brother, who was otherwise healthy, was not determined and is indicated by ‘?’. B. Immunoblot analysis of DOCK8 protein expression in EBV cell lines derived from a control subject, the patient, his brother and mother. The blots were also probed for actin as a protein loading control. C. Sanger sequencing chromotograms of DOCK8 exon (E)40/intervening sequence (IVS)40 5′ splice junction. Exonic sequence is in upper case and the intronic sequence in lower case. The mutant base (g→a) is underlined. D. Sanger sequencing chromotograms of DOCK8 cDNA at the junction of E39/E40 sequences. For patient sequence, the dominant E39:E41 trace is shown in upper caps while that of the minor E39:E40 trace in smaller caps.
Significant clinical findings included severe fungating generalized warts involving the face, neck, truck and limbs, and bilateral purulent yellowish ear discharge. His immunologic investigation showed lymphopenia, eosinophilia, extreme elevation of IgE; 28,375 IU/ml, hypergammaglobulinemia and reduced lymphocyte response to phytohemagglutinin (PHA) stimulation, which was 40% of normal control on repeated occasions (Table. I). Despite prior full immunization, he showed very low antibody titers to diphtheria, pertussis, tetanus, measles, mumps and rubella. Thereafter, he was given two extra doses of diphtheria, tetanus and 13-valent – adsorbed pneumococcal polysaccharide conjugate vaccine and one dose of MMR vaccines, but persisted to have a poor antibody response to these vaccines (Table. I).
Table 1.
Laboratory findings.
| Blood cells | Patient | Normal range |
|
| ||
| WBC (cells/μl) | 5,100 | 4,000 – 12,000 |
|
| ||
| Neutrophils (cells/μl) | 2,550 | 1,100 – 7,200 |
|
| ||
| Eosinophils (cells/μ) | 740 | 100 – 700 |
|
| ||
| Hemoglobin (g/dl) | 13.1 | 11 – 14.5 |
|
| ||
| Platelet counts (cell/μl) | 214,000 | 150,000 – 450,000 |
|
| ||
| Total lymphocytes (cells/μl) | 1,390 | 1,300 – 7,200 |
|
| ||
| CD3+ (cells/μl) | 960 | 1,000 – 2,000 |
|
| ||
| CD3+ /CD4+ (cells/μl) | 650 | 500 – 1,300 |
|
| ||
| CD3+ /CD8+ (cells/μl) | 290 | 300 – 800 |
|
| ||
| CD19+ (cells/μl) | 330 | 200 – 700 |
|
| ||
| CD16+ NK (cells/μl) | 70 | 100 – 700 |
|
| ||
| CD4+CD45RA+ (% of CD4+cells) | 18 | 27-41 |
|
| ||
| CD4+CD45RO+ (% of CD4+cells) | 73 | 59-73 |
|
| ||
| % CD69+ T cells post PHA stimulation | 40% | 90 – 100 % |
|
| ||
| Immunoglobulin (Ig) levels | ||
|
| ||
| IgG (g/L) | 20.5 | 6.5 – 16.0 |
|
| ||
| IgA (g/L) | 2.3 | 0.35 – 2.5 |
|
| ||
| IgM (g/L) | 0.31 | 0.5 – 2.5 |
| IgE (IU/ml) | 28,375 | < 200 |
|
| ||
| Antibody titer (normal titer): | Baseline | Post-2 booster doses* |
|
| ||
| Tetanus (0.11– 5.0 IU/ml) | 0.07 | 0.1 |
| Diphtheria (0.1– 5.0 IU/ml) | < 0.1 | 0.1 |
| Pneumococcal (mg/L) | 8.6 | 13.6 |
| Measles (>12 U/ml) | 1 | 1 |
| Mumps (>12 U/ml) | 10 | 5 |
| Rubella (>10 IU/ml) | N/A | 202 |
The Patient’s clinical features and laboratory findings prompted an evaluation for DOCK8 deficiency. Immunoblotting of cell lysates derived from an EBV– immortalized B-cell line generated from the patient’s B cells revealed profoundly decreased expression (~10%) of an otherwise apparently normal-sized DOCK8 protein as compared to lysates of EBV cell lines derived from his mother, brother and an unrelated normal control (Figure 1B). Sequencing of the DOCK8 gene identified a homozygous mutation in intervening sequence (IVS) 40 splice donor site: c.5223 +5 g>A (Figure 1C). The parents of the patient were heterozygous for this mutation (data not shown). cDNA sequence analysis revealed that the mutation impaired RNA splicing, leading to leaky exon 40 skipping (Figure 1D). Starting at the end of exon (E) 39, a dominant out-of-frame cDNA species emerged that directly linked E39 and E41 sequences while skipping that of E40. This out-of frame-transcript would be predicted to terminate prematurely eight codons downstream of the E39 sequence, leading to the absence of protein expression due to the degradation of the mutant transcript by the process of nonsense-mediated decay. These findings are consistent with the residual DOCK8 protein expression in the patient emanating from the translation of the minor normal DOCK8 cDNA species that escapes the hypomorphic splicing defect (Figure 1D).
The patient’s chronic generalized warts prompted us to investigate the number and function of plasmacytoid dendritic cells (pDC), which play an important role in clearing viruses [5]. pDCs express TLR7 and TLR9 and secrete copious amount of type I interferons in response to recognition of viral RNA and DNA [6]. Type I IFNs upregulate major histocompatibility complex molecules (MHC) I and II, and enhance the presentation of viral peptides to cytotoxic T cells by conventional DCs; they also promote NK function [6]. IFN–α therapy was used for laryngeal papillomatosis with variable responses [7]. We have recently found that pDCs are severely and significantly diminished in DOCK8 deficiency and that their ability to secrete IFN-α was also decreased [8]. This was also the case in our patient, whose pDCs were decreased by more than 60% as compared to control subjects while the production of IFN-α by his PBMC in response to CpG treatment, which is primarily mediated by pDCs, was decreased by more than 10 folds (Figure 2A, B).
Figure 2.
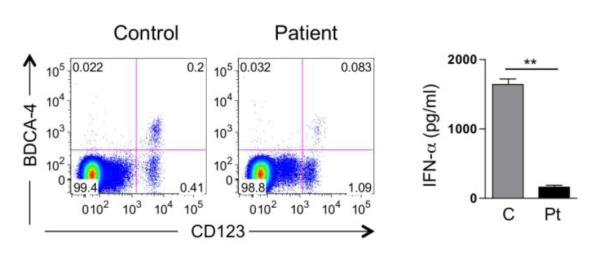
A. Representative FACS analysis for BDCA-4+ CD123+ pDCs in the lymphocyte gate of PBMCs from a control subject and the DOCK8-deficient subject. B. IFN-α production in supernatants of CpG-stimulated PBMCs from controls (n=2) and patients (n=2 independent determinations). **P<0.01.
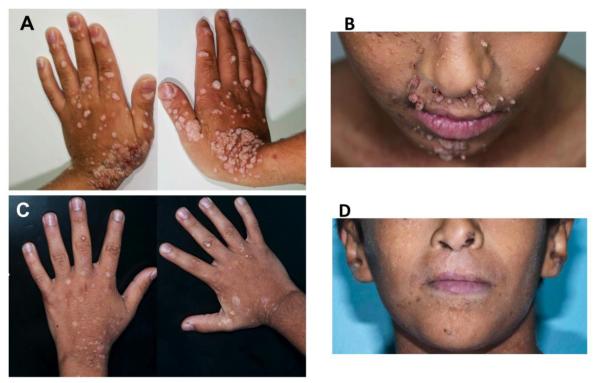
Due to the severe generalized warts in our patient, we started him on pegylated IFN–α 2b therapy 40μg subcutaneous once weekly to possibly alleviate skin disease and to prevent spread to the eyes and nasopharyngeal space. After 6-weeks the generalized warts showed progressive response to IFN–α 2b treatment and five months later his warts almost completely resolved, leaving healing scars (Figure 3A-D). His purulent ear discharges also disappeared without recurrence.
Figure 3.
Representative pictures of the lesions in face and hands of the patient before IFN-α 2b therapy (A, B), and 4 months into IFN-α 2b therapy (C, D),
Discussion
In this report, we describe the efficacy of IFN-α 2b therapy in the treatment of severe warts in a patient with DOCK8 deficiency. The dramatic response to therapy DOCK8 deficiency was associated with paucity of circulating pDCs and a profound decrease in the production by his PBMC of IFN-α in response to stimulation with CpG, indicative of a state of IFN-α deficiency.
Mechanisms by which DOCK8 deficiency precipitate circulating pDC depletion and decreased IFN-α production may include an defective pDC development and/or mobilization in the periphery in response to chemokine signals, as well as impaired response to activation by toll-like receptor ligands. Studies on the related DOCK family member DOCK2 revealed that it plays a critical role in the migration of plasmacytoid DCs (pDCs) into peripheral lymphoid tissues in response to chemokine signals [9, 10]. DOCK8 may also play a similar role, evidence by its requirement for interstitial dendritic cell migration during immune responses [11]. Our own studies have demonstrated that DOCK8 mediates the response of B cells to CpG stimulation by linking TLR9, the target of CpG activation, to MyD88 and downstream signaling pathways [4]. Thus, DOCK8 deficiency may also impair the response of pDCs to TLR activating signals.
In addition to impaired IFN-α production, other factors may contribute to the persistence of warts in DOCK8-deficient subjects, including deficiency and impaired function and persistence of cytotoxic T cells, ineffective killing by NK cells and defective DOCK8 and MyD88-dependent Toll-like receptor signaling [4, 12-14]. Significantly, IFN–α upregulates the activities of several of the aforementioned pathways, including NK and NKT cell cytolytic activities, which may explain the dramatic resolution of the warts in our patient in response to IFN-α 2b therapy.
In summary, this case report suggests that IFN–α 2b may be a useful therapy for extensive warts in DOCK8-deficient subjects. While hematopoietic stem cell transplantation is the currently the definitive curative therapy for DOCK8 deficiency, the availability of adjunct therapies including IFN-α should enable management of disease in preparation of bone marrow transplantation or in those cases where no available donor marrow is available [15].
Highlights.
– A child with Dedicator of Cytokinesis 8 (DOCK8) deficiency who suffered from chronic, recalcitrant warts had decreased circulating plasmacytoid dendritic cells and profoundly deficient IFN-α production.
–Therapy with interferon–α 2b was effective in inducing the resolution of the warts.
– Plasmacytoid dendritic cell insufficiency may contribute to chronic viral infections in DOCK8 deficiency.
Acknowledgment
The work was supported by the National Institutes of Health (NIH) grants 5R01AI065617 and 1R21AI087627 to T.A.C., and 1R01AI100315 and NIH/NIAID Atopic Dermatitis Research Network HHSN272201000020C to R.S.G. It was also supported by a grant from the Scientific and Technological Research Council of Turkey (1059B191300622 to S.K.). We thank the laboratory staff members at King Abdulaziz Medical City–WR for their technical support and Dr. Janet Chou, MD, Harvard Medical School for insights and discussions.
Footnotes
Abbreviations: DOCK8; dedicator of cytokinesis 8 deficiency; DT; diphtheria and tetanus; EBV; Epstein-Barr virus; HIES; hyper-immunoglobulin E.; IFN–α 2b; interferon– alpha 2b; MMR; measles, mumps and rubella; TLR; Toll-like receptors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, Chen A, Kim HS, Lloret MG, Schulze I, Ehl S, Thiel J, Pfeifer D, Veelken H, Niehues T, Siepermann K, Weinspach S, Reisli I, Keles S, Genel F, Kutukculer N, Camcioglu Y, Somer A, Karakoc-Aydiner E, Barlan I, Gennery A, Metin A, Degerliyurt A, Pietrogrande MC, Yeganeh M, Baz Z, Al-Tamemi S, Klein C, Puck JM, Holland SM, McCabe ER, Grimbacher B, Chatila TA. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–1302. e1284. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, Davis J, Turner ML, Uzel G, Holland SM, Su HC. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chu EY, Freeman AF, Jing H, Cowen EW, Davis J, Su HC, Holland SM, Turner ML. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79–84. doi: 10.1001/archdermatol.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, Rauter I, Benson H, Schneider L, Baxi S, Recher M, Notarangelo LD, Wakim R, Dbaibo G, Dasouki M, Al-Herz W, Barlan I, Baris S, Kutukculer N, Ochs HD, Plebani A, Kanariou M, Lefranc G, Reisli I, Fitzgerald KA, Golenbock D, Manis J, Keles S, Ceja R, Chatila TA, Geha RS. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13:612–620. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- [7].Leventhal BG, Kashima HK, Mounts P, Thurmond L, Chapman S, Buckley S, Wold D. Long-term response of recurrent respiratory papillomatosis to treatment with lymphoblastoid interferon alfa-N1. Papilloma Study Group. N Engl J Med. 1991;325:613–617. doi: 10.1056/NEJM199108293250904. [DOI] [PubMed] [Google Scholar]
- [8].Keles S, Jabara HH, Reisli R, McDonald DR, Barlan I, Hanna-Wakim R, Dbaibo R, Lefranc R, Al-Herz W, Geha RS, Chatila TA. Plasmacytoid Dendritic Cell Depletion in DOCK8 Deficiency: Rescue of Severe Herpetic Infections with Interferon Alpha-2b Therapy. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.03.032. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gotoh K, Tanaka Y, Nishikimi A, Inayoshi A, Enjoji M, Takayanagi R, Sasazuki T, Fukui Y. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood. 2008;111:2973–2976. doi: 10.1182/blood-2007-09-112169. [DOI] [PubMed] [Google Scholar]
- [10].Gotoh K, Tanaka Y, Nishikimi A, Nakamura R, Yamada H, Maeda N, Ishikawa T, Hoshino K, Uruno T, Cao Q, Higashi S, Kawaguchi Y, Enjoji M, Takayanagi R, Kaisho T, Yoshikai Y, Fukui Y. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J Exp Med. 2010;207:721–730. doi: 10.1084/jem.20091776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, Duan X, Uruno T, Nishikimi A, Sanematsu F, Yokoyama S, Stein JV, Kinashi T, Fukui Y. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119:4451–4461. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, Crockford TL, Lockstone HE, Freeman A, Arkwright PD, Smart JM, Ma CS, Tangye SG, Goodnow CC, Cerundolo V, Godfrey DI, Su HC, Randall KL, Cornall RJ. DOCK8 is critical for the survival and function of NKT cells. Blood. 2013;122:2052–2061. doi: 10.1182/blood-2013-02-482331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, Tan A, Arkwright PD, Al Suwairi W, Lugo Reyes SO, Yamazaki-Nakashimada MA, Garcia-Cruz Mde L, Smart JM, Picard C, Okada S, Jouanguy E, Casanova JL, Lambe T, Cornall RJ, Russell S, Oliaro J, Tangye SG, Bertram EM, Goodnow CC. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, Belohradsky BH, Heinz V, Freeman AF, Sullivan KE, Holland SM, Torgerson TR, Al-Herz W, Chou J, Hanson IC, Albert MH, Geha RS, Renner ED, Orange JS. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131:840–848. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gatz SA, Benninghoff U, Schutz C, Schulz A, Honig M, Pannicke U, Holzmann KH, Schwarz K, Friedrich W. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:552–556. doi: 10.1038/bmt.2010.169. [DOI] [PubMed] [Google Scholar]