Abstract
Interleukin-17A (IL-17A) is a newly emerging player in the pathogenesis of chronic lung diseases that amplifies inflammatory responses and promotes tissue remodeling. Stimulation of lung epithelial cells with IL-17A leads to activation of the transcription factor, nuclear factor kappa B (NF-κB), a key player in the orchestration of lung inflammation. We have previously demonstrated the importance of the redox-dependent post-translational modification, S-glutathionylation, in limiting activation of NF-κB and downstream gene induction. Under physiological conditions, the enzyme glutaredoxin 1 (Grx1) acts to deglutathionylate NF-κB proteins, which restores functional activity. In this study, we sought to determine the impacts of S-glutathionylation on IL-17A-induced NF-κB activation and expression of pro-inflammatory mediators. C10 mouse lung alveolar epithelial cells, or primary mouse tracheal epithelial cells exposed to IL-17A show rapid activation of NF-κB, and the induction of pro-inflammatory genes. Upon IL-17A exposure, sulfenic acid formation and S-glutathionylated proteins increased. Assessment of S-glutathionylation of NF-κB pathway components revealed S-glutathionylation of RelA (RelA-SSG) and inhibitory kappa B kinase alpha (IKKα-SSG) after stimulation with IL-17A. SiRNA-mediated ablation of Grx1 increased both RelA-SSG and IKKα-SSG and acutely increased nuclear content of RelA, and tended to decrease nuclear RelB. SiRNA mediated ablation or genetic ablation of Glrx1 decreased the expression of NF-κB regulated genes, KC and CCL20, in response to IL-17A, but conversely increased the expression of IL-6. Lastly, siRNAmediated ablation of IKKα attenuated nuclear RelA and RelB content and decreased expression of KC and CCL20 in response to IL-17A. Together, these data demonstrate a critical role for the S-glutathionylation/Grx1 redox axis in regulating IKKα and RelA S-glutathionylation and the responsiveness of epithelial cells to IL-17A.
Keywords: Interleukin-17A, glutaredoxin-1, protein S-glutathionylation, inhibitory kappa B kinase alpha, nuclear factor kappa B, redox
INTRODUCTION
The Interleukin-17 (IL-17) family is a newly emerging class of cytokines that plays a critical role in a variety of biological processes, including innate host defense and inflammation. This cytokine family consists of six members, IL-17A-F, with IL-17A the most extensively studied member to date. Upon binding of IL-17A to its heterodimeric receptors (IL-17RA/RC), various adaptor proteins containing unique interaction domains are recruited to the receptor complex, activating multiple pathways, which include mitogen activated protein kinases (MAPK) and nuclear factor kappa B (NF-κB). Aberrant IL-17 signaling has been functionally linked to the pathogenesis of a variety of autoimmune and inflammatory diseases, including multiple sclerosis, systemic lupus erythematosus, psoriasis, rheumatoid arthritis and asthma [1–5]. Although T lymphocytes are the main source of IL-17A, the expression of IL-17Rs appears to be more ubiquitous [6], and the role of structural cells, including epithelial cells, as major responders to IL-17A has been well documented [7–9]. Airway epithelial cells have been shown to upregulate many pro-inflammatory mediators and genes involved in host-defense following stimulation with IL-17A. These include beta defensins, mucin genes and dendritic cell and neutrophil chemoattractants [7, 10, 11], in part due to the activation of the transcription factor NF-κB.
Previous studies in our laboratory have demonstrated the importance of NF-κB activation within lung epithelial cells in the pathogenesis of allergic airways disease in mouse models [12–14]. Two parallel NF-κB activation pathways have been described to date: the classical and alternative pathways. In the classical pathway, inhibitor of kappa B kinase β (IKKβ) becomes activated via phosphorylation, allowing it to then phosphorylate inhibitor of kappa B α (IκBβ), marking it for ubiquitination and subsequent proteasomal degradation. This event facilitates nuclear entry of the transcription factor heterodimer RelA/p50, and subsequent gene induction. In the alternative NF-κB pathway, IKKα activation leads to the phosphorylation of p100 and its resultant processing to the active p52 form, and allows nuclear entry of the RelB/p52 heterodimer [15, 16]. The alternative pathway has typically been considered to function primarily in cells of the adaptive immune system and lymphoid organs, but more recent studies have demonstrated that both NF-κB pathways can cooperate to control pro-inflammatory responses in lung epithelial cells [17].
Given the central role of NF-κB in driving inflammatory responses, its activation is tightly regulated via protein-protein interactions as well as several post-translational modifications. Reversible oxidative modifications of cysteines, including sulfenic acid formation and protein S-glutathionylation (PSSG), have been shown to affect protein function [18–20]. PSSG represents the conjugation of the tripeptide antioxidant, glutathione (GSH), to reactive, oxidizable cysteines within proteins. This modification has considerable implications for biological processes, as it can alter the function of various proteins, and both PSSG-induced activation and inactivation have been reported, depending on the protein target [21]. Work by our laboratory and others has demonstrated that the NF-κB pathway, as well as upstream adaptor molecules such as TRAF6, are negatively regulated via PSSG [22–26]. Under physiological conditions, the thiol oxidoreductase glutaredoxin-1 (Grx1) can deglutathionylate proteins, thus restoring the native thiol form of the target cysteine residue [27]. Numerous studies initially focused on oxidant-induced PSSG, but more recent studies have demonstrated the role of this modification in the context of biologically relevant stimuli [22, 28–30].
Although IKKα acts as a positive regulator of IL-17A production in T-cells [31] and has also been shown to regulate IL-17A responses in synoviocytes, kidney cells and astrocytes [32], the role of epithelial IKKα in response to IL-17A is unclear. To date, it is also unknown whether IL-17A elicits redox perturbations in lung epithelial cells, and whether redox modifications alter pro-inflammatory responses to IL-17A. Because of the known regulation of the NF-κB pathway through PSSG, the ability of Grx1 to reverse this modification, and the possible link between IKKα and IL-17A, we focused herein on the role of the Grx1/PSSG redox axis in regulating the IKKα-driven responsiveness of lung epithelial cells to IL-17A.
MATERIALS AND METHODS
Antibodies
The following antibodies were used in this study: rabbit anti-RelA, rabbit anti-RelB (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-Histone H3, rabbit anti-IκBα (Cell Signaling Technology, Danvers, MA), mouse anti-GSH (Virogen, Watertown, MA), mouse anti-IKKα (Upstate, Millipore, Billerica, MA) Streptavidin conjugated-HRP (Jackson, West Grove, PA), goat anti-Grx1 (American Diagnostica, Stamford, CT) and mouse anti-β-actin (Sigma-Aldrich, Saint Louis, MO). The secondary HRP conjugated anti-rabbit and anti-mouse antibodies were from Amersham (Piscataway, NJ), anti-goat was from Jackson Laboratories (West Grove, PA). All fluorophore-conjugated antibodies were from Invitrogen (Carlsbad, CA).
Cell Culture
Primary mouse tracheal epithelial cells (MTECs) were isolated from WT mice or mice lacking the glutaredoxin-1 gene (referred herein as Glrx1 −/−), and cultured as previously described [33, 34]. A spontaneously transformed type II mouse lung alveolar epithelial cell line [35] (C10) was cultured as described previously [33]. Unless otherwise noted, 16 h prior to stimulation, C10 cells were switched to medium containing 0.5% FBS; MTECs were incubated for 16h in serum-free medium. Cells were stimulated with indicated concentrations of Interleukin-17A (IL-17A; R&D Systems, Minneapolis, MN) and harvested as previously described [36].
Transfection of siRNA
C10 cells were incubated with scrambled, non-targeting siRNA or Grx1 siRNA (Ambion, Carlsbad, CA) (all at 100 nM). C10 cells were incubated with SMARTpool scrambled nontargeting siRNA or SMARTpool IKKα siRNA (Dharmacon, Lafayette, CO) (all at 100nM). Cells were subsequently stimulated, harvested and analyzed as indicated.
Labeling of sulfenic acids using dimedone
Cells were switched to serum-free medium for 2 hours; in the final hour, cells were preincubated with 1 mM DCP-Bio1 (Kerafast, Boston, MA) in serum-free medium for 1h at 37° C. Medium containing DCP-Bio1 was removed, and fresh serum-free medium containing 100 ng/ml IL-17A was added to cells for indicated times. Cells were then fixed in 4% formalin and permeabilized with 0.2% Triton X100 in PBS for 10 min. The permeabilized cells were blocked in 5% BSA in PBS for 1h. The cells were then incubated with fluorescently labeled streptavidin (1:2000, SA alexa fluor 647) and the nucleus was counterstained with DAPI (1:4000). Cell images were acquired using a Zeiss LSM 510 META Confocal Laser Scanning Imaging System. All images were taken at 20× magnification. The image files were converted to tiff format and brightness and contrast were adjusted equally in all images.
Alternatively, cells were harvested in the presence of dimedone to detect oxidized cysteines in cell lysates according to a previously described method [37]. In brief, cells were serum-deprived for 2h, and then stimulated with IL-17A and lysed in buffer containing 1mM dimedone. Excess dimedone was removed using Micro Bio-spin 6 columns (Bio-Rad, Hercules, CA). Samples were analyzed by SDS-PAGE, and extent of sulfenic acid labeling was assessed using anti-dimedone antibody (Millipore, Darmstadt, Germany).
Detection of S-glutathionylated proteins (PSSG)
Cells were exposed to IL-17A as indicated, and at the selected times lysates were prepared as described before [38]. In brief, samples were immunoprecipitated (IP) using GSH antibody (2µg/ml) and precipitated proteins were subjected to polyacrylamide gel electrophoresis and subsequent immunoblotting for IKKα [38]. As a reagent control, samples were treated with 50mM DTT prior to IP, to decompose PSSG. In selected experiments, cells were incubated with 250µM biotinylated gluthathione ethyl ester (Bio-GEE) for 1h. Bio-GEE was prepared as previously described [39], and Bio-GEE-conjugated proteins were determined following nonreducing SDS-PAGE and Western blotting [39]. Western Blots were analyzed via densitometry, and
Total levels of PSSG in cell lysates were determined using the glutathione/glutathione reductase/NADPH/5,5’-dithiobis (2-nitrobenzoic acid) (DTNB) recycling assay, according to procedures described previously [40]. Briefly, lung tissue was homogenized in buffer containing 137 mM Tris·HCl, pH 8.0, 130 mM NaCl, and 1% NP-40. After determination of protein concentration, protein content was equalized and 500µg of protein was precipitated with acetone. Following centrifugation, the pellet was resuspended in buffer containing 0.1% Triton X-100 and 0.6% sulfasalicylic acid and freeze-thawed twice. Protein-associated glutathione was released with sodium borohydride, and total GSH levels were determined. The sodium borohydridesensitive fraction of GSH released from proteins was calculated and expressed as nanomoles GSH per milligram of protein.
mRNA analyses
Total RNA was isolated from cells and purified using the RNeasy kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA for taqman gene analysis using SYBR green (Bio-Rad, Hercules, CA). Expression values were normalized to the housekeeping gene cyclophilin. Primers used were: KC; forward GCTGGATTCACCTCAAGAA, reverse TGGGGACACCTTTTAGCATC, IL-6; forward CTGATGCTGGTGACAACCAC, reverse CAGAATTGCCATTGCACAAC, CCL20; forward AAGACAGATGGCCGATGAAG, reverse AGCCCTTTTCACCCAGTTCT, cyclophilin; forward TTCCTCCTTTCACAGAATTATTCCA, reverse CCAGTGCCATTATGG.
Enzyme-Linked Immunosorbent Assays (ELISA)
Cells were stimulated, and medium was assayed for KC, CCL-20 and IL-6 (R & D Systems, Minneapolis, MN) according to manufacturer’s instructions.
Statistical analyses
Statistical analyses were performed using Graphpad Prism software (GraphPad, San Diego, CA) using one- or two-way ANOVA with Bonferroni correction for multiple comparisons. All experiments were conducted at least three times and data are presented as mean values plus the standard error of the mean.
RESULTS
IL-17A activates classical and alternative NF-κB pathways
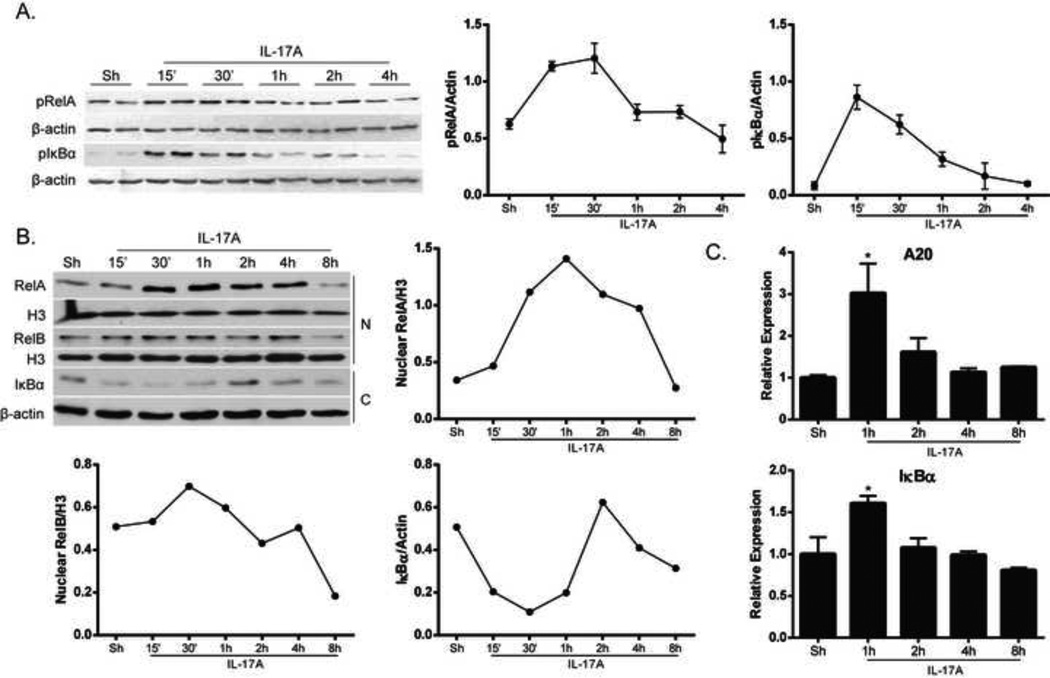
Recent work in our laboratory has provided insights into the interaction of the classical and alternative NF-κB pathways in controlling inflammatory responses in airway epithelial cells [17]. IL-17A-induced classical NF-κB activation in airway epithelial cells has been well documented [7, 10, 41, 42], but only recently was it demonstrated that IL-17A can also activate the alternative NF-κB pathway [17]. Results in Figure 1A and B demonstrate activation of the classical NF-κB pathway, as indicated by phosphorylated forms of RelA and IκBα (Fig. 1A) as well as IκBα degradation and increases in nuclear RelA content (Fig. 1B). Expression of IκBα and A20, negative regulators of NF-κB which are induced upon activation of NF-κB, was enhanced following IL-17A exposure (Fig. 1C). In response to IL-17A, slight increases in nuclear RelB content occurred over time, indicative of alternative NF-κB activation (Fig. 1B). These data demonstrate that IL-17A activates both classical and alternative NF-κB in lung epithelial cells.
Figure 1. Activation of classical and alternative NF-κB pathways in lung epithelial cells in response to IL-17A.
(A) Mouse alveolar type II cells (C10) were stimulated with IL-17A (100ng/ml) and NF-κB was assessed in whole cell lysates by measuring phosphorylated forms of RelA (pRelA) and IκBα (pIκBα). β-actin; loading control. Right panels: densitometric evaluation of pRelA and pIκBα. Data reflect arbitrary units, following normalization to loading control, β-actin. (B) Assessment of nuclear RelA and RelB content and degradation of cytosolic IκBα following stimulation with IL-17A (50ng/ml) in C10 cells. β-actin; loading control for cytosolic lysates, (labeled as C); Histone H3 (H3) was used as a loading control for nuclear lysates, (labeled as N). Right and bottom panels: densitometric evaluation of nuclear RelA, nuclear RelB and IκBα. Data reflect arbitrary units, following normalization to respective loading controls. (C) Evaluation of mRNA expression of NF-κB-dependent negative regulators A20 and IκBα via real-time PCR in C10 cells stimulated with IL-17A (50ng/ml). Results were normalized to the housekeeping gene cyclophilin and are presented as relative expression. *p≤0.05 (ANOVA), compared to untreated controls (Sh).
Cysteine oxidation and protein S-glutathionylation are increased following IL-17A exposure
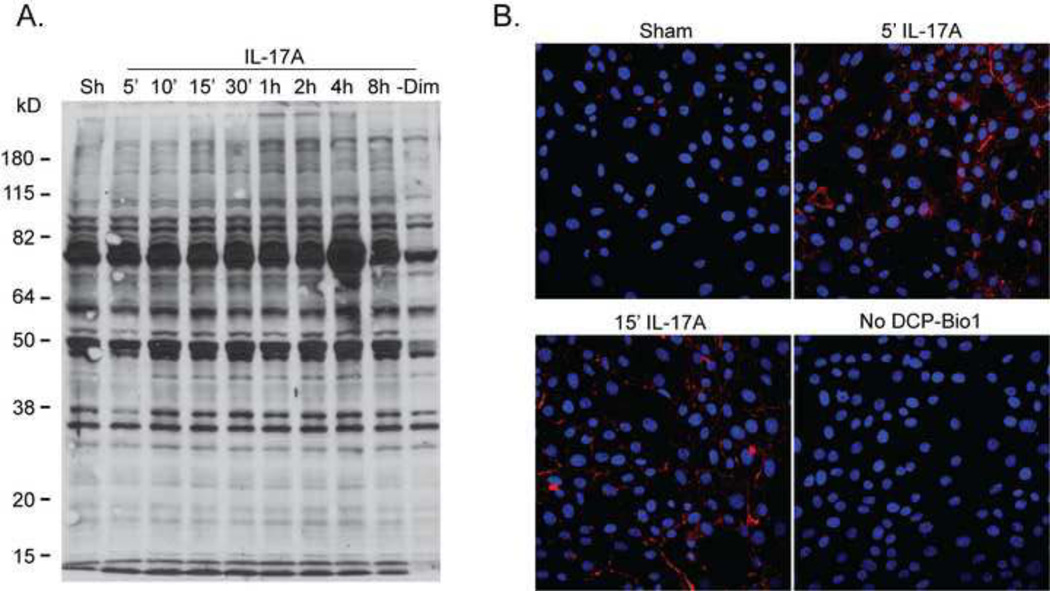
Given that modifications of cysteines play an important role in protein regulation, we next investigated whether IL-17A stimulation leads to perturbations in the cellular redox environment through determination of cysteine redox modifications. We utilized dimedone or a dimedone-based derivative (DCP-Bio1) to measure the extent of sulfenic acid formation following stimulation with IL-17A. Cells were either lysed in the presence of dimedone to trap sulfenic acid moieties or pre-incubated with DCP-Bio1 and then stimulated with IL-17A to visualize oxidation of cysteines via immunofluorescence. Results in Figure 2 demonstrate a rapid increase in sulfenic acid formation of numerous proteins based upon dimedone labeling and analysis via western blot (Fig. 2A) and immunofluorescence analysis (Fig. 2B). In cells stimulated with IL-17A, sulfenic acid residues were detectable predominantly in the periphery of the cell, suggestive of compartmentalization of sulfhydryl oxidations.
Figure 2. IL-17A increases cysteine oxidation in lung epithelial cells.
(A) C10 cells were stimulated with IL-17A (50ng/ml) for indicated times and subsequently harvested in the presence of dimedone (1mM) to trap the sulfenic acid form (SOH) of cysteines. Samples were analyzed via reducing SDS-PAGE, and overall sulfenic acid formation was determined using an anti-dimedone antibody (1:1000). −Dim, minus dimedone control. (B) Assessment of SOH formation (red) via immunofluorescence in C10 cells pre-incubated with DCP-Bio1 (1mM) for 1h and stimulated with 100ng/ml IL-17A for indicated times. An anti-biotin Alexafluor-647 secondary antibody was used to visualize DCP-Bio1- bound proteins. Nuclei are counterstained with DAPI (blue). No DCP-Bio1; omission of DCP-Bio1 label.
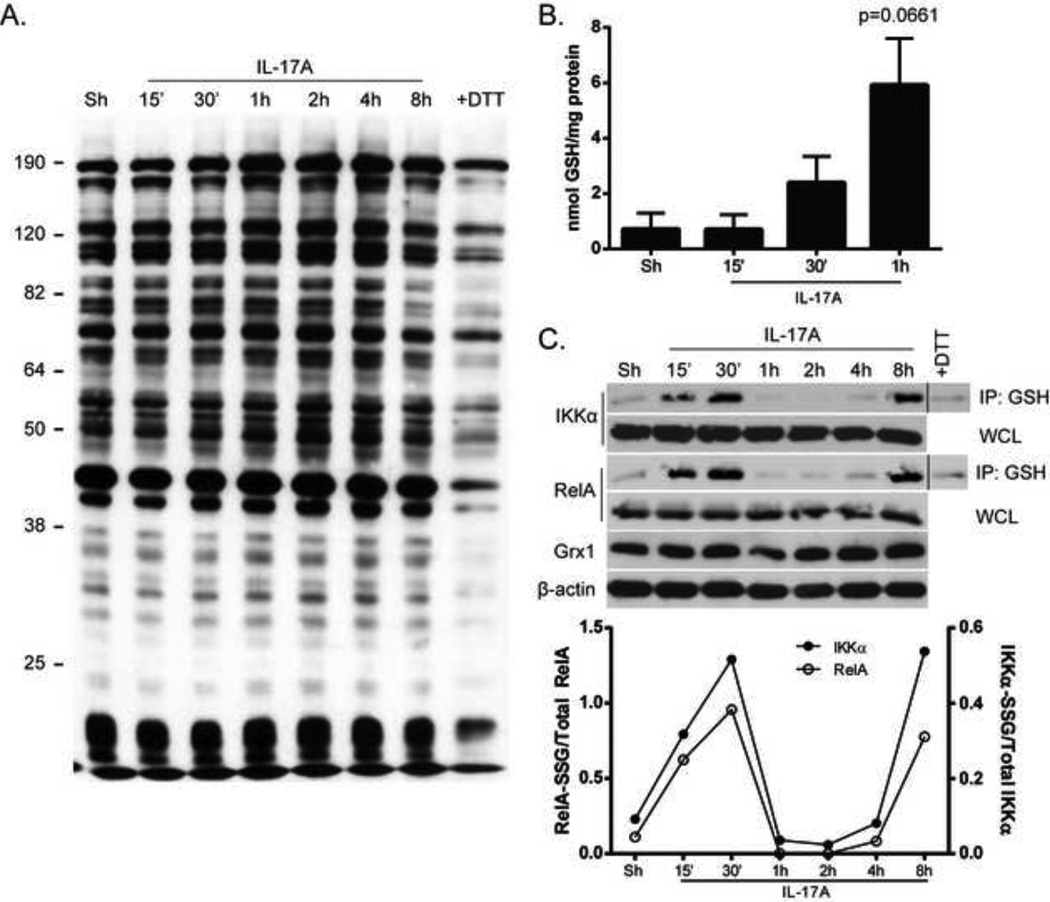
Because sulfenic acid formation is believed to be a gateway for further cysteine modifications, we next asked if S-glutathionylation (PSSG) was also increased in response to stimulation of cells with IL-17A. Glutathione-conjugated proteins from cell lysates were assessed by non-reducing SDS-PAGE analysis. Incubation with an anti-GSH antibody revealed a time-dependent increase in overall glutathione-adducted proteins in response to IL-17A (Fig. 3A). Overall PSSG content measured following release of GSH from precipitated proteins also showed trends towards increases in PSSG in epithelial cells stimulated with IL-17A at early time points (Fig. 3B). Extensive data exists to support a functional role for PSSG of NF-κB components in controlling the activity of this pathway [22–25], including work in our laboratory demonstrating the importance of IKKβ S-glutathionylation (IKKβ-SSG) [23, 43]. Therefore, we next examined the glutathionylation status of specific proteins in the NF-κB pathway after IL-17A stimulation. Although we were unable to consistently detect IKKβ-SSG in response to IL-17A (data not shown), we did detect increases in RelA-SSG and IKKα-SSG (Fig. 3C), which occurred in a biphasic manner. The bi-phasic changes in RelA-SSG and IKKα-SSG could reflect alterations in expression of Grx1. However, assessment of Grx1 protein content revealed no differences in expression (Fig. 3C) suggesting that time-dependent alterations of PSSG in response to IL-17A are not a mere reflection of differences in Grx1 content. Overall, these data indicate that IL-17A stimulation leads to a rapid increase in sulfenic acid and PSSG in lung epithelial cells, and that RelA and IKKα are targets for PSSG.
Figure 3. Increases in protein S-glutathionylation in C10 cells stimulated with IL-17A.
(A) C10 cells were stimulated with 50ng/ml IL-17A, and whole cell lysates were harvested at indicated times. SDS-PAGE analysis was performed under non-reducing conditions and GSH-conjugated proteins were assessed using an anti-GSH antibody (1:1000). A separate sample was incubated with (+DTT) to decompose S-glutathionylated proteins. (B) Following stimulation with 50ng/ml IL-17A, C10 cells were harvested at indicated times and overall S-glutathionylation was evaluated using the DTNB recycling assay. (C) C10 cells were stimulated with 50ng/ml IL-17A and harvested as indicated. S-glutathionylated proteins (200µg protein/sample) were immunoprecipitated (IP) using an anti-GSH antibody (2µg/ml) overnight at 4o C. Following IP, samples were subjected to SDS-PAGE and Western Blot analysis using RelA and IKKα antibodies. WCL: Whole cell lysates to confirm equal content of RelA and IKKα, or Grx1. +DTT, control sample incubated with DTT to decompose S-glutathionylated proteins. β-actin: loading control.
Bottom panel: densitometric evaluation of IKKα-SSG and RelA-SSG. Data reflect arbitrary units, following normalization to total IKKα or RelA.
Glutaredoxin 1 plays an important role in controlling epithelial cell responsiveness to IL-17A
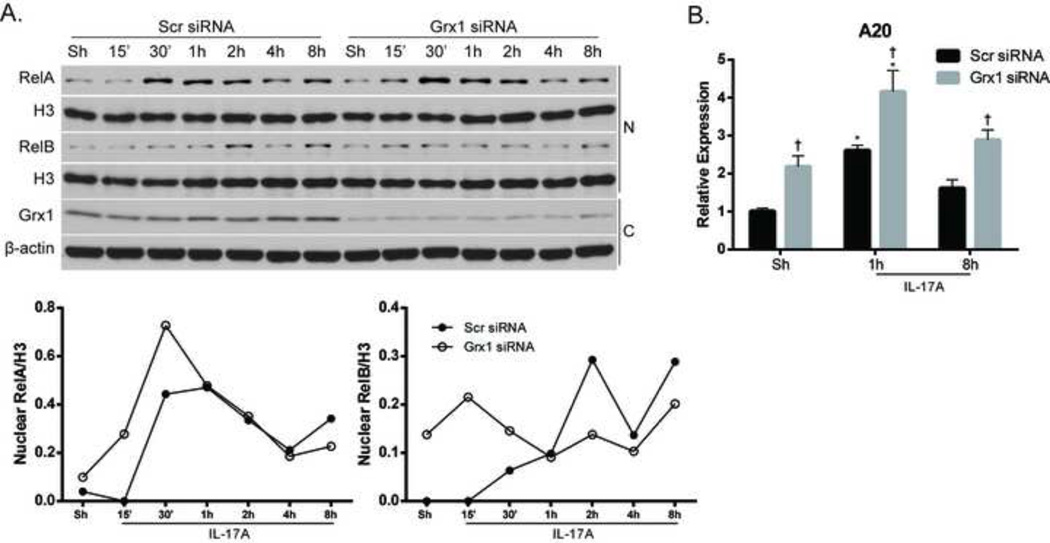
Under physiological conditions, the oxidoreductase Grx1 can catalyze the deglutathionylation of proteins, restoring their native structure and function, and IKKβ is known to be a substrate for this enzyme [23, 43]. Therefore, we hypothesized that Grx1 plays a role in IL-17A signaling by deglutathionylating NF-κB proteins, namely RelA and IKKα. First, we evaluated the effects of siRNA-mediated ablation of Grx1 on NF-κB activation by assessing nuclear content of RelA and RelB in response to IL-17A. RelA nuclear content was increased acutely after ablation of Grx1, compared to siRNA control cells exposed to IL-17A (Fig. 4A). In contrast, while nuclear content of RelB increased in response to IL-17A in siRNA control transfected cells, nuclear content of RelB in cells subjected to Grx1 ablation did not appear to change (Fig. 4A), indicating differential regulation of both NF-κB pathways by Grx1. The kinetics of nuclear RelA and RelB accumulation in response to IL-17A were considerably different as compared to Fig. 1B, likely the result of transfection of the cells. Interestingly, ablation of Grx1 also led to a baseline increase in mRNA expression of A20, which was further increased in response to IL-17A, compared to siRNA controls (Fig. 4B), indicating that certain facets of the NF-κB signaling pathway may be intrinsically dampened in the absence of Grx1.
Figure 4. Ablation of Grx1 alters NF-κB activation and mRNA expression of A20.
(A) Assessment of NF-κB activation following siRNA-mediated ablation of Grx1 or control siRNA and stimulation with IL-17A (50ng/ml). Nuclear (N) and cytosolic (C) lysates were harvested from C10 cells and nuclear content of RelA and RelB (top panels) was assessed. Histone H3 (H3); loading control. Bottom panels: Evaluation of Grx1 in cytosolic lysates to confirm siRNA-mediated ablation. β-actin; loading control. Graphs represent densitometric evaluation of nuclear RelA and RelB. Data reflect arbitrary units, following normalization to the loading Histone H3. (B) C10 cells were subjected to siRNA-mediated ablation of Grx1 or control siRNA, and stimulated with IL-17A for indicated times, and A20 mRNA expression was evaluated. Results were normalized to the housekeeping gene cyclophilin and are presented as relative expression.
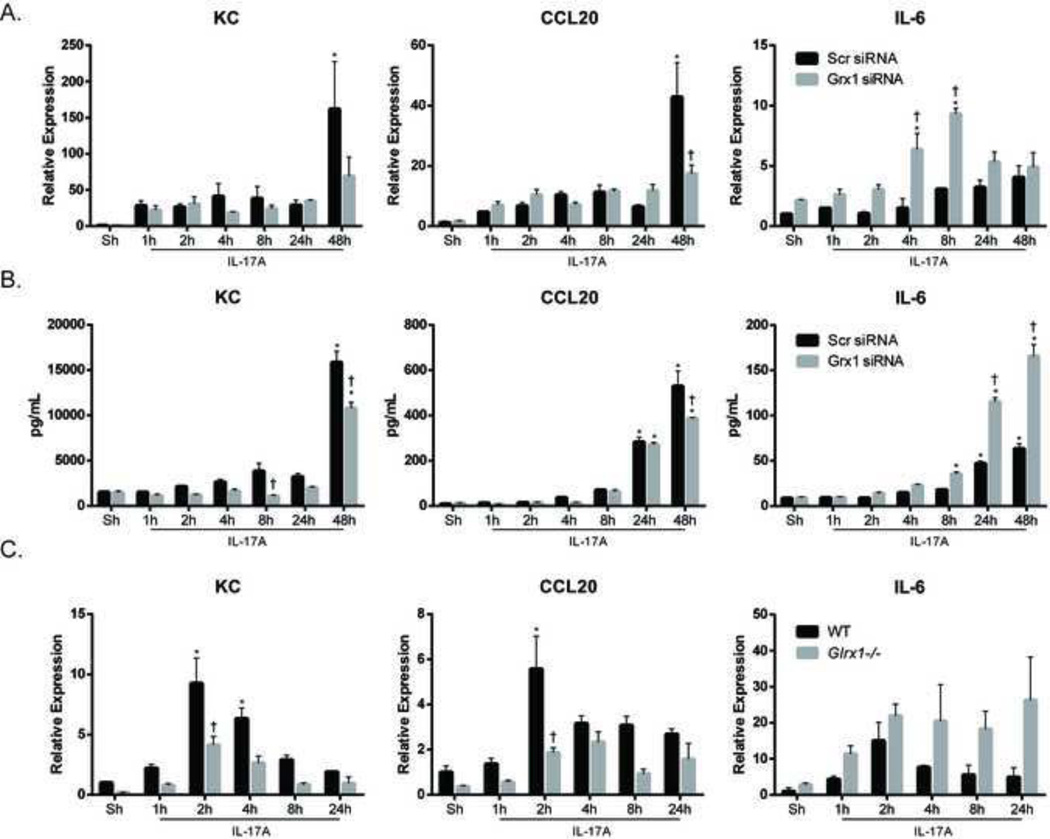
We next analyzed the impact of siRNA-mediated ablation of Grx1 on the expression of pro-inflammatory cytokines induced by IL-17A. IL-17A-induced mRNA expression of KC and CCL20 was substantially decreased following ablation of Grx1 (Fig. 5A), and similar decreases in content of these cytokines were apparent subsequent to Grx1 ablation (Fig. 5B), pointing to a requirement of Grx1 in augmenting the production of these cytokines. Intriguingly, the ability of IL-17A to increase expression of IL-6 mRNA and protein was markedly increased in C10s following siRNA mediated ablation of Grx1, in contrast to siRNA control transfected cells which demonstrated only small increases in IL-6 upon stimulation with IL-17A (Fig. 5A & B). Similar patterns of expression of KC, CCL20 and IL-6 were seen in primary mouse tracheal epithelial cells (MTECs) lacking the glutaredoxin-1 gene (Glrx1) following stimulation with IL-17A (Fig. 5C), further solidifying the importance of Grx1 in controlling epithelial cell responses to IL-17A. These data suggest an important functional role for Grx1 in controlling IL-17A-induced proinflammatory signaling. These findings also illuminate the unique impact of Grx1 in regulating pro-inflammatory responses to IL-17A in lung epithelial cells, which are highly dependent on the target gene.
Figure 5. Grx1 SiRNA or genetic ablation of Glrx1 alters pro-inflammatory responses in lung epithelial cells.
C10 cells stimulated with IL-17A (50ng/ml) for indicated times were analyzed for mRNA expression (A) and protein levels (B) of KC, CCL20 and IL-6 following siRNA-mediated ablation of Grx1 or control siRNA. (C) Expression of KC, CCL20 and IL-6 in WT or Glrx1−/− primary mouse tracheal epithelial cells (MTECs) following stimulation with IL-17A (20ng/ml). Expression values were normalized to the housekeeping gene cyclophilin, and data are presented as relative expression. * p≤0.05 (ANOVA) compared to untreated controls (Sh); † p≤0.05 (ANOVA) compared to scr siRNA groups at the same time point.
S-glutathionylation of RelA and IKKα is controlled by glutaredoxin 1
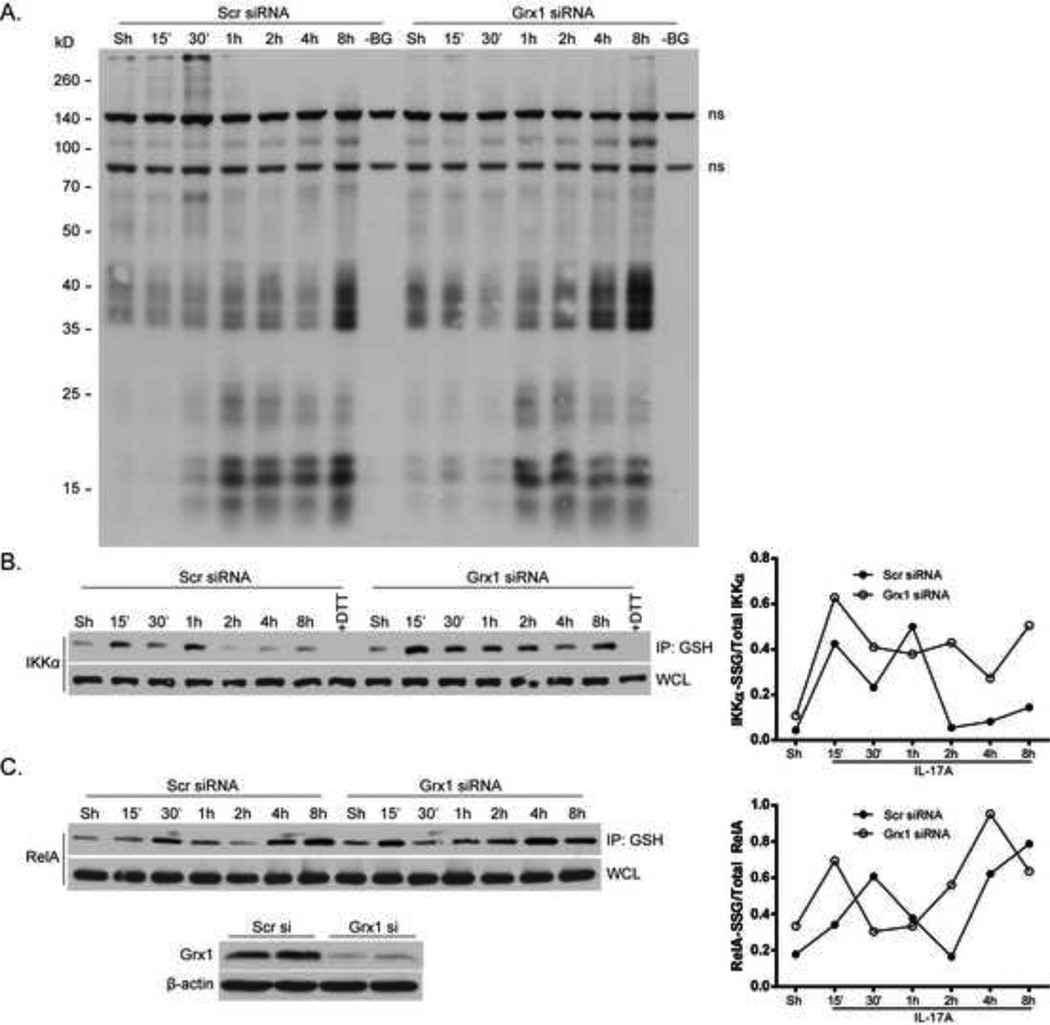
In order to further understand the mechanism by which Grx1 controls IL-17A-induced pro-inflammatory responses, we first evaluated the overall patterns of PSSG following ablation of Grx1. Cells were incubated with a biotinylated form of GSH (Bio-GEE) 1h prior to stimulation with IL-17A, and harvested at various time points. Results in Fig. 6A demonstrate a time-dependent increase in numerous Bio-GEE-conjugated proteins in response to IL-17A, some of which are further increased following Grx1 ablation, even in unstimulated control cells. We next determined whether RelA-SSG and IKKα-SSG are potential substrates for Grx1-mediated deglutathionylation, which may act to control IL-17A-induced pro-inflammatory signaling. Immunoprecipitation of S-glutathionylated proteins revealed a biphasic pattern of both RelA-SSG and IKKα-SSG in siRNA control transfected cells stimulated with IL-17A (Fig. 6B). The kinetics of increases in RelA-SSG and IKKα-SSG are somewhat different to earlier observations in Fig. 3C, likely due to the transfection protocol used. Nonetheless, IL-17A-mediated increases in RelA-SSG and IKKα-SSG were more pronounced subsequent to Grx1 ablation (Fig. 6B). Taken together, these data suggest a direct role for Grx1 in controlling the extent of both RelA-SSG and IKKα-SSG.
Figure 6. Grx1 ablation promotes S-glutathionylation of RelA and IKKα.
C10 cells were subjected to siRNA-mediated ablation of Grx1 or control siRNA. (A) Cells were pre-incubated with Bio-GEE (250µM) for 1h prior to stimulation with IL-17A (50ng/ml), and subsequently harvested at indicated times. SDS-PAGE performed under non-reducing conditions, followed by Western Blot analysis of Bio-GEE-conjugated proteins assessed via streptavidin-HRP. –BG; control wherein the Bio-GEE label was omitted. ns; non-specific bands. (B and C) Following siRNA-mediated ablation of Grx1, C10 cells were stimulated with IL-17A (50ng/ml) and harvested at indicated times. S-glutathionylated proteins were immunoprecipitated with GSH antibody (2µg/ml) and resolved via SDS-PAGE and western blotting for IKKα (B) or RelA (C). Bottom panel: western blot showing confirmation of Grx1 ablation. Right panels: densitometric evaluation of IKKα-SSG and RelA-SSG. Data reflect arbitrary units, following normalization to total IKKα or RelA.
Loss of IKKα results in dampened IL-17A responses in airway epithelial cells
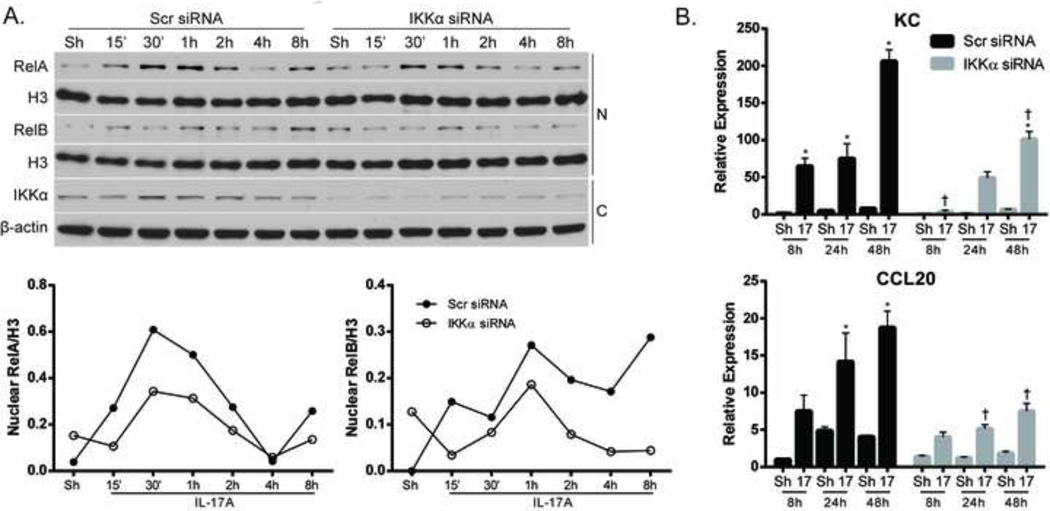
The relationship between IKKα and IL-17A has been established in various cell types, but the role of epithelial IKKα in controlling IL-17A responses is unknown. Therefore, we investigated the functional link between IKKα and IL-17A signaling in airway epithelial cells. Ablation of IKKα using siRNA resulted in small reductions in nuclear content of both RelA and RelB in response to IL-17A stimulation (Fig. 7A), in conjunction with diminished expression of KC and CCL20 (Fig. 7B). Ablation of IKKα using siRNA did not alter IL-6 expression in response to IL-17A (data not shown). These data collectively indicate a role for both Grx1 and IKKα in controlling airway epithelial cell responses to IL-17A stimulation, and that IKKα-SSG correlates with the magnitude and pattern of IL-17A- induced pro-inflammatory responses.
Figure 7. SiRNA-mediated ablation of IKKα modulates expression of pro-inflammatory mediators in lung epithelial cells.
(A) Assessment of nuclear RelA and RelB following siRNA-mediated ablation of IKKα or control siRNA and stimulation with IL-17A (50ng/ml). Nuclear (N) and cytosolic (C) lysates were harvested from C10 cells and nuclear content of RelA and RelB was assessed. Histone H3 (H3) was used as a loading control. Bottom panels: Assessment of IKKα in cytosolic lysates to confirm siRNA-mediated ablation. β-actin; loading control. Graphs represent densitometric evaluation of nuclear RelA and RelB. Data reflect arbitrary units, following normalization to the loading Histone H3. (B) Assessment of KC and CCL20 mRNA expression following siRNA-mediated ablation of IKKα and stimulation with IL-17A (50ng/ml) at indicated times. Values were normalized to the housekeeping gene cyclophilin, and data presented and relative expression. * p≤0.05 (ANOVA) compared to untreated controls (Sh); † p≤0.05 (ANOVA) compared to scr siRNA groups at the same time point.
DISCUSSION
The Interleukin-17 (IL-17) family of cytokines has garnered much recent attention. For example, a protective role of IL-17A has been demonstrated in host defense against bacterial infections [44–47]. Conversely, a pathogenic role of IL-17A has emerged in settings of autoimmune diseases, neutrophilic inflammation, cancer, and fibrotic tissue remodeling [48–51]. Therefore, an understanding of the molecular mechanisms whereby IL-17 family members elicit biological responses is of critical importance towards the development of therapeutics aimed at targeting this family of cytokines [6, 52, 53]. IL-17A is the most well characterized member, and proximal signaling events induced at the IL-17RA/C complex have been well characterized [6]. Thiol/redox perturbations are emerging as cardinal regulators of many biological pathways, yet it remains unclear whether IL17A elicits any redox changes and whether these events affect the cellular responses to IL-17A. In the present study we demonstrate that exposure to IL-17A induced rapid protein thiol oxidations, evidenced by increases in sulfenic acid formation and Sglutathionylated proteins. We also demonstrated that RelA and IKKα are both targets for IL-17Ainduced PSSG, and that IKKα contributes to pro-inflammatory signaling in lung epithelial cells. Furthermore, Glutaredoxin-1 (Grx1) controlled both RelA-SSG and IKKα-SSG, and regulated the profile and magnitude of pro-inflammatory responses. Overall these findings illuminate a new dimension of IL-17A-induced biological responses that involve a thiol/redox control mechanism.
Studies herein have focused on the response of airway epithelial cells to IL-17A. The airway epithelium is the first line of defense against inhaled insults and stimuli, and proper functioning of airway epithelial cells is necessary to maintain proper homeostasis in the lung [54, 55]. The airway epithelium also plays a vital role in responding to both external and internal signals to control the extent of inflammatory responses. Thus, airway epithelial cells are a major target of IL-17A, and have previously been shown to produce various host defense and pro-inflammatory mediators upon IL-17A exposure [7, 8, 10, 11]. IKKα plays a role in both classical and alternative NF-κB pathways, but also has NF-κB independent functions [56]. IKKα was originally demonstrated to play a role in adaptive immune responses and lymphoid organ development [57]. More recently, a functional role for IKKα in regulating polarization of IL-17-producing T helper 17 lymphocytes has been demonstrated [31] and IKKα has also been shown to regulate the responsiveness of synoviocytes, kidney cells and astrocytes to IL-17A [32]. The role of IKKα in regulating the responsiveness of epithelial cells to IL-17A had not been previously demonstrated. Results from the present study not only show the functional importance of IKKα in regulating the responsiveness of epithelial cells to IL-17A, but also show that the outcome of IL-17A signaling in epithelium is controlled by the Grx1/PSSG axis, in association with IKKα-SSG. These observations have broad implications not only for IL-17A-induced pro-inflammatory responses, but also for other stimuli that signal through IKKα, as these may also be regulated by Grx1-mediated PSSG. However, additional studies are needed to map the reactive cysteine within IKKα that is the target for PSSG induced by IL-17A, and to determine its functional importance.
The functional impact of RelA-SSG had been established previously, as studies have shown both inhibition of RelA nuclear entry [22, 58] and DNA binding [59] following RelA-SSG. Paradoxically, we demonstrate here that IL-17A-induced RelA-SSG corresponded to increases in nuclear RelA, which were further enhanced following ablation of Grx1. These results indicate that, in response to IL-17A, nuclear translocation of RelA may not be affected by its S-glutathionylation, but that RelA-SSG could interfere with DNA binding, as has been shown previously following oxidant-triggered PSSG in vitro [59]. Further investigation is needed to determine the functional role of RelA-SSG in response to IL-17A, which was beyond the scope of the current study.
It is plausible that other components of the IL-17 pathway, in addition to RelA and IKKα, may be targets for thiol/redox control. In this regard, TRAF6 was recently described as a target for Grx1-mediated deglutathionylation in the context of TLR/IL1R signaling [26]. Since the IL-17R complex also utilizes this adaptor molecule for the activation of NF-κB [6], it is plausible that TRAF6 is also a target for PSSG in cells stimulated with IL-17A. A recent study demonstrated the importance of A20 in negative feedback regulation of IL-17A-induced cellular responses [60]. A20 is one of several de-ubiquitinases that has been shown to be a target for reversible oxidations which regulate its activity [61]. The active site cysteine of A20 in particular was demonstrated as a target of hydrogen peroxide-induced oxidation, resulting in inhibition of its deubiquitinase activity [61]. It is plausible that PSSG also inhibits de-ubiquitinase activity of A20, thereby regulating the responsiveness of epithelial cells to IL-17A, scenarios that will require further investigation. It is worthy to mention that Grx1 status regulates mRNA expression of A20, and that absence of Grx1 led to a two fold increase of A20 mRNA. Despite this apparent link between A20 and Grx1, the specific mechanisms whereby Grx1 regulates A20 remain to be further elucidated.
The opposing outcome of IL-17A-induced KC and CCL20 expression compared to IL-6 expression following ablation of Grx1 is intriguing. While ablation of Grx1 attenuated IL-17Ainduced expression of KC and CCL20, ablation of Grx1 augmented the ability of IL-17A to induce IL-6, and similar effects were observed in two distinct epithelial cell cultures. These results clearly demonstrate that ablation of Grx1 does not dampen the overall pro-inflammatory response, but rather that ablation of Grx1 modifies the nature of the pro- inflammatory response of epithelial cells to IL-17A. It remains unclear whether the altered cellular responses to IL-17A following ablation of Grx1 are indeed due to altered function of NF-κB, and whether this involves RelA/p50 or RelB/p52 heterodimers, the latter of which are known to bind to a broader spectrum of kappaB sites [62]. Following ablation of Grx1, the overall nuclear content of RelA was acutely increased, whereas nuclear RelB content tended to decrease in response to IL-17A, as compared to respective siRNA controls. Thus, an altered balance of RelA/p50 or RelB/p52 dimers could account for the observed changes of pro-inflammatory responses to IL-17A. In this regard, prior studies have indicated that active RelB dimers can repress transcription of IL1B and IL12B genes [63], and that in association with decreased RelB, IL-6 levels increased [64]. C-Rel is important in the transcriptional regulation of IL6 [65], which raises the possibility that Grx1 may alter IL6 gene expression via c-Rel. Alternatively, it is possible that other transcription factors modulate pro-inflammatory gene expression following modulation of Grx1 which could account for the differential responses of IL-6 expression as compared to KC and CCL20. Future studies mapping transcription factor occupancy at the promoters of these cytokine genes will be required to fully understand these scenarios.
Results from the present study demonstrate increases in sulfenic acid formation and PSSG in response to IL-17A. Numerous studies have previously demonstrated these oxidative events under conditions of overt stress, or in response to direct exposure to an oxidant. The subtle patterns of oxidations demonstrated herein are reflective of a biological stimulus that elicits a cellular response without creating overt cellular stress, nor inducing cell death. The source of oxidants following IL-17A stimulation remains unknown. Increased sulfenic acid formation was observed as early as five minutes following IL-17A stimulation, and appeared to be localized primarily near the cell periphery (Fig. 2B). In the context of TLR/IL-1R signaling, previous studies have observed an oxidant-dependent recruitment of TRAF6 to the receptor complex, via non-phagocytic NADPH oxidase 2 (Nox2), which is necessary for signal propagation [66]. The pattern of sulfenic acid formation observed herein could potentially reflect a similar NADPH oxidase-induced signaling event that is localized to the IL-17R complex. Activation of Nox2 following engagement of the IL17R may potentially be responsible for the production of oxidants, which in turn lead to sulfenic-acid containing proteins. Indeed, further analyses will be required to fully determine the source of oxidants and the specific subcellular localization of these oxidative events.
The biochemical links between sulfenic acid intermediates and PSSG in biological settings remain poorly understood. Sulfenic acid intermediates can be unstable and give rise to further oxidations [67]. PSSG of sulfenic acid intermediates is believed to be important in the protection of cysteines against overoxidation [68]; therefore, it is plausible that sulfenic acid intermediates observed in response to IL-17A are the precursor to PSSG. The mechanisms that govern PSSG in response to IL-17A also remain unknown. PSSG can occur spontaneously, notably under conditions of oxidative stress when an abundance of oxidized glutathione (GSSG) is present [19]. Enzymatic catalysis of PSSG by glutathione S-transferase Pi (GSTP) has been demonstrated under conditions of oxidative stress [69], or in response to the death inducing ligand FasL [70]. It remains unknown at this time whether GSTP, a protein that is highly expressed in lung epithelial cells [71], plays a role in the catalysis of IKKα-SSG in response to IL-17A.
In summary, results from the present study demonstrate that the pro-inflammatory response elicited by IL-17A is controlled by the S-glutathionylation/Grx1 redox axis in association with RelA-SSG and IKKα-SSG. Therefore, avenues that target the protein thiol redox status can be exploited to harness the cellular response to IL-17A and have the potential to impact host defense and diseases associated with aberrant IL-17 signaling.
Highlights.
Thiol redox changes in response to IL-17A are unknown to date
IL-17A increases cysteine oxidation, S-glutathionylation, RelA-SSG and IKKα-SSG
IL-17A-induced RelA-SSG and IKKα-SSG is controlled by glutaredoxin-1 (Grx1)
IL-17A-induced pro-inflammatory gene expression is controlled by Grx1
Thiol redox-based regulatory mechanisms may control IL-17A- induced inflammation
ACKNOWLEDGEMENT
This work was supported by NIH Grants T32 ES007122-30, T32 HL076122, R01 HL60014, and an ATS unrestricted grant and a Parker B. Francis Fellowship (to V.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 2.Mok MY, Wu HJ, Lo Y, Lau CS. The relation of interleukin 17 (IL-17) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J Rheumatol. 2010;37:2046–2052. doi: 10.3899/jrheum.100293. [DOI] [PubMed] [Google Scholar]
- 3.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 4.Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 8.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 9.van den Berg A, Kuiper M, Snoek M, Timens W, Postma DS, Jansen HM, Lutter R. Interleukin-17 induces hyperresponsive interleukin-8 and interleukin-6 production to tumor necrosis factor-alpha in structural lung cells. Am J Respir Cell Mol Biol. 2005;33:97–104. doi: 10.1165/rcmb.2005-0022OC. [DOI] [PubMed] [Google Scholar]
- 10.Kao CY, Kim C, Huang F, Wu R. Requirements for two proximal NF-kappaB binding sites and IkappaB-zeta in IL-17A-induced human beta-defensin 2 expression by conducting airway epithelium. J Biol Chem. 2008;283:15309–15318. doi: 10.1074/jbc.M708289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol. 2009;183:6236–6243. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol. 2002;160:1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- 14.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, Irvin CG, Janssen-Heininger YM. Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 2008;177:959–969. doi: 10.1164/rccm.200707-1096OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 16.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tully JE, Nolin JD, Guala AS, Hoffman SM, Roberson EC, Lahue KG, van der Velden J, Anathy V, Blackwell TS, Janssen-Heininger YM. Cooperation between classical and alternative NF-kappaB pathways regulates proinflammatory responses in epithelial cells. Am J Respir Cell Mol Biol. 2012;47:497–508. doi: 10.1165/rcmb.2012-0014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalek RD, Nelson KJ, Holbrook BC, Yi JS, Stridiron D, Daniel LW, Fetrow JS, King SB, Poole LB, Grayson JM. The requirement of reversible cysteine sulfenic acid formation for T cell activation and function. J Immunol. 2007;179:6456–6467. doi: 10.4049/jimmunol.179.10.6456. [DOI] [PubMed] [Google Scholar]
- 19.Ghezzi P. Regulation of protein function by glutathionylation. Free Radic Res. 2005;39:573–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- 20.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tew KD, Manevich Y, Grek C, Xiong Y, Uys J, Townsend DM. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic Biol Med. 2011;51:299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin YC, Huang GD, Hsieh CW, Wung BS. The glutathionylation of p65 modulates NF-kappaB activity in 15-deoxy-Delta(1)(2),(1)(4)-prostaglandin J(2)-treated endothelial cells. Free Radic Biol Med. 2012;52:1844–1853. doi: 10.1016/j.freeradbiomed.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pineda-Molina E, Klatt P, Vazquez J, Marina A, Garcia de Lacoba M, Perez-Sala D, Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 25.Seidel P, Roth M, Ge Q, Merfort I, S'Ng CT, Ammit AJ. IkappaBalpha glutathionylation and reduced histone H3 phosphorylation inhibit eotaxin and RANTES. Eur Respir J. 2011;38:1444–1452. doi: 10.1183/09031936.00129610. [DOI] [PubMed] [Google Scholar]
- 26.Chantzoura E, Prinarakis E, Panagopoulos D, Mosialos G, Spyrou G. Glutaredoxin-1 regulates TRAF6 activation and the IL-1 receptor/TLR4 signalling. Biochem Biophys Res Commun. 2010;403:335–339. doi: 10.1016/j.bbrc.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25:332–346. [PMC free article] [PubMed] [Google Scholar]
- 28.Sakai J, Li J, Subramanian KK, Mondal S, Bajrami B, Hattori H, Jia Y, Dickinson BC, Zhong J, Ye K, Chang CJ, Ho YS, Zhou J, Luo HR. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 2012;37:1037–1049. doi: 10.1016/j.immuni.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 30.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Ruan Q, Hilliard B, Devirgiliis J, Karin M, Chen YH. Transcriptional regulation of the Th17 immune response by IKK(alpha) J Exp Med. 2011;208:787–796. doi: 10.1084/jem.20091346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, Shi Y, Harley JB, Shen N, Qian Y. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 33.Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci. 2008;121:1036–1045. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro. 1982;18:800–812. doi: 10.1007/BF02796504. [DOI] [PubMed] [Google Scholar]
- 35.Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines--tools for the study of differentiation and the neoplastic phenotype. Toxicology. 1997;123:53–100. doi: 10.1016/s0300-483x(97)00108-x. [DOI] [PubMed] [Google Scholar]
- 36.van der Velden JL, Schols AM, Willems J, Kelders MC, Langen RC. Glycogen synthase kinase 3 suppresses myogenic differentiation through negative regulation of NFATc3. J Biol Chem. 2008;283:358–366. doi: 10.1074/jbc.M707812200. [DOI] [PubMed] [Google Scholar]
- 37.Poole LB. Measurement of protein sulfenic acid content. Curr Protoc Toxicol. 2008;Chapter 17(Unit17):12. doi: 10.1002/0471140856.tx1702s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anathy V, Aesif SW, Guala AS, Havermans M, Reynaert NL, Ho YS, Budd RC, Janssen-Heininger YM. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol. 2009;184:241–252. doi: 10.1083/jcb.200807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan DM, Wehr NB, Fergusson MM, Levine RL, Finkel T. Identification of oxidant-sensitive proteins: TNF-alpha induces protein glutathiolation. Biochemistry. 2000;39:11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 41.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 42.Wong CK, Cao J, Yin YB, Lam CW. Interleukin-17A activation on bronchial epithelium and basophils: a novel inflammatory mechanism. Eur Respir J. 2010;35:883–893. doi: 10.1183/09031936.00088309. [DOI] [PubMed] [Google Scholar]
- 43.Aesif SW, Kuipers I, van der Velden J, Tully JE, Guala AS, Anathy V, Sheely JI, Reynaert NL, Wouters EF, van der Vliet A, Janssen-Heininger YM. Activation of the glutaredoxin-1 gene by nuclear factor kappaB enhances signaling. Free Radic Biol Med. 2011;51:1249–1257. doi: 10.1016/j.freeradbiomed.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aujla SJ, Dubin PJ, Kolls JK. Interleukin-17 in pulmonary host defense. Exp Lung Res. 2007;33:507–518. doi: 10.1080/01902140701756604. [DOI] [PubMed] [Google Scholar]
- 45.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian Y, Kang Z, Liu C, Li X. IL-17 signaling in host defense and inflammatory diseases. Cell Mol Immunol. 2010;7:328–333. doi: 10.1038/cmi.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaffen SL, Hernandez-Santos N, Peterson AC. IL-17 signaling in host defense against Candida albicans. Immunol Res. 2011;50:181–187. doi: 10.1007/s12026-011-8226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizutani N, Goshima H, Nabe T, Yoshino S. Complement C3a-induced IL-17 plays a critical role in an IgE-mediated late-phase asthmatic response and airway hyperresponsiveness via neutrophilic inflammation in mice. J Immunol. 2012;188:5694–5705. doi: 10.4049/jimmunol.1103176. [DOI] [PubMed] [Google Scholar]
- 49.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene. 2010;29:5653–5662. doi: 10.1038/onc.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G, Le Bert M, Quesniaux VF, Huaux F, Leite-de-Moraes M, Ryffel B, Couillin I. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PloS one. 2011;6:e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol. 2011;187:3003–3014. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 52.Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175–182. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 55.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, Cremin C, Sones J, Djukanovic R, Howarth PH, Collins JE, Holgate ST, Monk P, Davies DE. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556. doi: 10.1016/j.jaci.2011.05.038. e541-e512. [DOI] [PubMed] [Google Scholar]
- 56.Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol. 2009;19:404–413. doi: 10.1016/j.tcb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Xiao G, Rabson AB, Young W, Qing G, Qu Z. Alternative pathways of NF-kappaB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev. 2006;17:281–293. doi: 10.1016/j.cytogfr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Liao BC, Hsieh CW, Lin YC, Wung BS. The glutaredoxin/glutathione system modulates NF-kappaB activity by glutathionylation of p65 in cinnamaldehyde-treated endothelial cells. Toxicol Sci. 2010;116:151–163. doi: 10.1093/toxsci/kfq098. [DOI] [PubMed] [Google Scholar]
- 59.Qanungo S, Starke DW, Pai HV, Mieyal JJ, Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 60.Garg AV, Ahmed M, Vallejo AN, Ma A, Gaffen SL. The deubiquitinase a20 mediates feedback inhibition of interleukin-17 receptor signaling. Sci Signal. 2013;6:ra44. doi: 10.1126/scisignal.2003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kulathu Y, Garcia FJ, Mevissen TE, Busch M, Arnaudo N, Carroll KS, Barford D, Komander D. Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat Commun. 2013;4:1569. doi: 10.1038/ncomms2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fusco AJ, Huang DB, Miller D, Wang VY, Vu D, Ghosh G. NF-kappaB p52:RelB heterodimer recognizes two classes of kappaB sites with two distinct modes. EMBO reports. 2009;10:152–159. doi: 10.1038/embor.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 64.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakayama K, Shimizu H, Mitomo K, Watanabe T, Okamoto S, Yamamoto K. A lymphoid cell-specific nuclear factor containing c-Rel-like proteins preferentially interacts with interleukin-6 kappa B-related motifs whose activities are repressed in lymphoid cells. Mol Cell Biol. 1992;12:1736–1746. doi: 10.1128/mcb.12.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anathy V, Roberson E, Cunniff B, Nolin JD, Hoffman S, Spiess P, Guala AS, Lahue KG, Goldman D, Flemer S, van der Vliet A, Heintz NH, Budd RC, Tew KD, Janssen-Heininger YM. Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis. Mol Cell Biol. 2012;32:3464–3478. doi: 10.1128/MCB.00125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conklin DJ, Haberzettl P, Prough RA, Bhatnagar A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am J Phys Heart Circ Physiol. 2009;296:H1586–H1597. doi: 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]