Abstract
Indometacin, an inhibitor of cyclooxygenase-2 (COX-2), has been shown to exert anticancer effects in a variety of cancers. However, the effect and mechanism of indometacin on high glucose (HG)-induced proliferation and invasion of pancreatic cancer (PC) cells remain unclear. Multiple lines of evidence suggest that a large portion of pancreatic cancer (PC) patients suffer from either diabetes or HG which contributing PC progression. In this study, we report that indometacin down-regulated HG-induced proliferation and invasion via up-regulating E-cadherin but not COX-2 in PC cells. Additionally, the E-cadherin transcriptional repressors, Snail and Slug, were also involved in the process. Furthermore, the proliferation and invasion of PC cells, incubated in HG medium and treated with indometacin were significantly increased when E-cadherin was knocked down (Si-E-cad). Moreover, the protein levels of MMP-2, MMP-9, and VEGF were increased in PC cells transfected with Si-E-cad. Finally, the activation of the PI3K/AKT/GSK-3β signaling pathway was demonstrated to be involved in indometacin reversing HG-induced cell proliferation and invasion in PC cells. In conclusion, these results suggest that indometacin plays a key role in down-regulating HG-induced proliferation and invasion in PC cells. Our findings indicate that indometacin could be used as a novel therapeutic strategy to treat PC patients who simultaneously suffer from diabetes or HG.
Keywords: Cancer progression, COX-2, E-cadherin, high glucose, indometacin, invasion, pancreatic cancer, PI3K/AKT, proliferation, therapeutic strategy
INTRODUCTION
Pancreatic cancer (PC) is one of the most aggressive malignancies in the world and its 5-year survival rate is less than 5%. Difficulty in early diagnosis, frequent local invasion, and rapid progression are the major causes of low survival rate, the mortality almost equal to its incidence [1, 2]. Recent investigation showed that approximately 80% of PC patients with impaired glucose tolerance or diabetes mellitus may develop as an early clinical manifestation of PC [3]. Case-control studies have shown that patients with PC have an increased risk of developing diabetes, especially within 3 years of cancer diagnosis [4]. Furthermore, the PC patients with high glucose (HG) in blood have a poorer prognosis than those with normal blood glucose [5]. Our previous studies also showed that HG promoted cell proliferation via transactivating EGFR and enhancing the expression of glial cell line-derived neurotrophic factor (GDNF) and its tyrosine kinase receptor RET in PC cells [6, 7]. In addition, HG could promote the perineural invasion (PNI) of PC cells in vitro and in vivo [8]. However, the exact molecular mechanisms underlying this dismal clinical course remain largely unknown.
E-cadherin is a calcium-dependent cell-cell adhesion protein and the function of E-cadherin has been linked with cancer metastasis, peritoneal dissemination, and poor prognosis [9, 10]. In epithelial cells, the cytoplasmic tail of E-cadherin forms a dynamic complex with catenins and regulates several intracellular signal transduction pathways, including Wnt/β-catenin, PI3K/AKT, Rho GTPase, and NF-κB signalling. Several lines of evidence indicate that the engagement of E-cadherin results in the activation of PI3K and AKT in carcinoma cells [11].
Indometacin, a well-known anti-inflammatory drug and a non-selective inhibitor of cyclooxygenase-2 (COX-2), has previously been demonstrated to have anticancer activities against many types of neoplastic diseases [12-15]. The mechanism of its anti-cancer activities may be by inhibiting cell growth, inducing apoptosis [16] and suppressing the process of tumor invasion through regulating the expression of adhesion molecule such as E-cadherin. In the present study, we aim to determine the effect of indometacin on HG-induced proliferation and invasion of PC cells and the underlying mechanism.
MATERIALS AND METHODS
Cell Culture and Reagents
The human PC cell lines, BXPC-3 and Panc-1, were obtained from the American Type Culture Collection (Rockville, MD, USA). They were grown in DMEM containing 10% fetal bovine serum, penicillin G (100 units/mL), and streptomycin (100 μg/mL) in a humidified atmosphere of 5% CO2 at 37 °C. Indometacin, dimethylsulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) were acquired from Sigma Chemicals (St. Louis, MO, USA). A stock solution of indometacin was dissolved in DMSO at 50 g/L. Cell culture media were purchased from Gibco BRL (Grand Island, NY, USA). E-cadherin antibodies were purchased from BD Biosciences (San Jose, CA, USA). AKT, phospho-AKT (Ser473), GSK-3β, phospho-GSK-3β, and COX-2 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). The β-actin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), as was the horseradish peroxidase-conjugated donkey anti-goat IgG. Horseradish peroxidase -conjugated goat anti-rabbit IgG and goat anti-mouse IgG were obtained from Bio-Rad Laboratories (Hercules, CA, USA). Matrigel (BD Biosciences, CA, USA) and 24-well transwells (Corning, NY, USA) were also used. Other reagents were purchased from common commercial sources. All drug solutions were freshly prepared on the day of testing.
MTT Assay
Proliferation rates were measured by using MTT assays as described previously [6]. Briefly, BXPC-3 and Panc-1 cells were seeded in 96-well plates at a density of 1×104 cells per well and incubated overnight in the medium containing 10% FBS. The cells were then treated with LG (5.5 mM), mannitol (osmotic group), HG (25 mM) or HG + indometacin (0, 50, 100 or 150 mg/L). The DMSO concentration was adjusted to 0.4%. Cells incubated in serum-free medium were used as the control group. After incubation for 24, 48 and 72 h at 37 °C, 20 μl of MTT solution (5 mg/ml in phosphate buffered saline [PBS]) was added to each well, and the cells were incubated for an additional 4 h at 37 °C. Next, 100 μl DMSO was added into each well at 37 °C. The optical density (OD) value was determined using a spectrophotometer (Bio-Rad, CA, USA) at 490 nm. The proliferation rate was defined as OD (cell plate) / OD (blank plate). At minimum, each experiment was performed in triplicate, and the results were presented as the percentages relative to their controls.
Matrigel Invasion Assay
The invasion assay was performed as reported previously with some modifications [17]. Briefly, 2.0×104 cells were suspended in 500 μl of serum-free medium supplemented with 0.1% BSA and seeded into the upper compartment of Matrigel-coated transwell chambers (8 μm pore size, Bio-Coat Matrigel Invasion Chambers, Falcon 24-well culture plates, BD Biosciences Labware). DMEM supplemented with 10% FBS was placed in the lower compartment as a chemoattractant. The cells were removed from the upper surface of the filter by scraping with a cotton swab after 24 h in culture. The invaded cells were fixed in methanol and stained with crystal violet. Mean values of the data obtained from three separate chambers were presented. All experiments were performed in triplicate.
Real-Time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, CA, USA), and cDNA was synthesized using a PrimeScript RT reagent Kit (TaKaRa, Dalian, China). The real-time experiments were conducted on an iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using a SYBR Green Real-time PCR Master Mix (TaKaRa, CA, USA). The amplification of target genes were performed at following conditions: 30 s at 95 °C followed by 35 cycles of denature at 95 °C for 5 s, annealing at 60 °C for 30 s and elongation at 72 °C for 30 s. The primers used for SYBR Green RT-qPCR were as follows: for human E-cadherin, sense, 5′-ACA GCC CCG CCT TAT GAT T-3′ and antisense, 5′-TCG GAA CCG CTT CCT TCA-3′; for Snail, sense, 5′-CCC CAA TCG GAA GCC TAA CT-3′ and antisense, 5′-GCT GGA AGG TAA ACT CTG GAT TAG A-3′; for Slug, sense, 5′-TTC GGA CCCACA CAT TAC CT-3′ and antisense, 5′-GCA GTG AGG GCA AGA AAA AG-3′; for Twist, sense, 5′-GGA GTC CGC AGT CTT ACG AG-3′ and antisense, 5′-TCT GGA GGA CCT GGT AGA GG-3′; for ZEB1, sense, 5′-GCA CCT GAA GAG GAC CAG AG-3′ and antisense, 5′-TGC ATC TGG TGT TCC ATT TT-3′; for COX-2, sense, 5′-TTC AAA TGA GAT TGT GGG AAA ATT GCT-3′ and antisense, 5′-GTG CAT CAA CAC AGG CGC CTC TTC-3′; and for β-actin, sense, 5′-ATC GTG CGT GAC ATT AAG GAG AAG-3′ and antisense, 5′-AGG AAG AAG GCT GGA AGA GTG-3′. The comparative C (T) method was used to quantify the expression of each target gene using β-actin as the normalization control.
Western Blotting Analysis
Cells were harvested in lysis buffer [50 mM Tris (pH7.5), 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 1 mM EDTA, and 0.1% SDS] containing protease inhibitor cocktail (Sigma–Aldrich), and protein concentrations were determined using the DC Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein (40 μg) was electrophoresed on 7.5% SDS-polyacrylamide gels, transferred to nitrocellulose membranes (Amersham Bioscience), and incubated with specific primary antibodies at 4 °C overnight. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Immunoreactive bands were visualized using an enhanced chemiluminescence kit (Millipore, MA, USA).
siRNA Transfections
siRNA against E-cadherin and a negative control siRNA were purchased from GenePharm (Shanghai, China). Cells (0.2 × 106 wells) seeded in six-well plates were transfected with 100 nM siRNA using Lipofectamine RNAi MAX Reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. The cells were used for further experiments at 24 h after transfection.
Statistical Analysis
All experiments were performed at least three times. The results were expressed as the mean ± SD, Differences were analyzed using a one-way ANOVA with the LSD post hoc test for multiple comparisons with GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA). p < 0.05 was considered as significant difference.
RESULTS
Effect of HG on Proliferation and Invasion in PC Cells
As a first step toward analyzing the role of HG in PC cells proliferation and invasion, cells were incubated in different glucose concentrations of HG (25 mM) and LG (5.5 mM). However, incubated PC cells contained equivalent concentrations of mannitol as osmotic control.
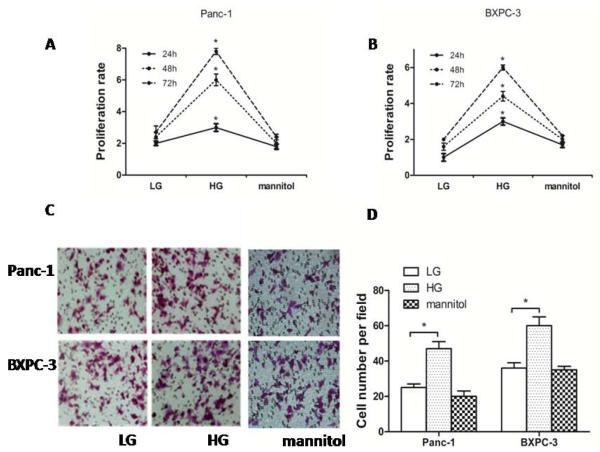
To determine the effect of glucose concentration on PC cells, we demonstrated that, in BXPC-3 cells and Panc-1 cells, the proliferation rate was increased in a dose dependent manner with increasing glucose concentrations at 24 h, 48 h, and 72 h, respectively, while mannitol did not affect cell proliferation (p <0.05) (Fig. 1A and Fig. 1B). The cells’ invasive ability was determined using Matrigel-coated transwell chambers. The invasive ability of the cells was dramatically enhanced by higher glucose concentrations but mannitol had no effect (Fig. 1C and Fig. 1D). These results suggest that HG modulates the cells proliferation and invasion not through mechanisms other than the altering osmotic pressure.
Fig. (1). HG increased the proliferation rate and invasion of PC cells.
(A) Panc-1 cells were seeded in a 96-well plate for 12 h and then treated with LG (5.5 mM) and HG (25 mM) for 24, 48 or 72 h, and equivalent concentrations of mannitol were used as osmotic control. Proliferation rate was measured by MTT assay. (B) The proliferation rate of BXPC-3 cells was detected with the MTT assay. (C) The effects of LG and HG on PC cells invasion (mannitol as osmotic control). (D) The number of migrated cells was quantified by counting the number of cells from 10 random fields at ×200 magnification. * p < 0.05 compared to normal controls. Results are represented by mean ± SEM (n = 3; values without a common letter are significantly different, p < 0.05). Column: mean; bar: SD.
Indometacin Down-Regulated the Effect of HG-Induced Proliferation and Invasion in PC Cells
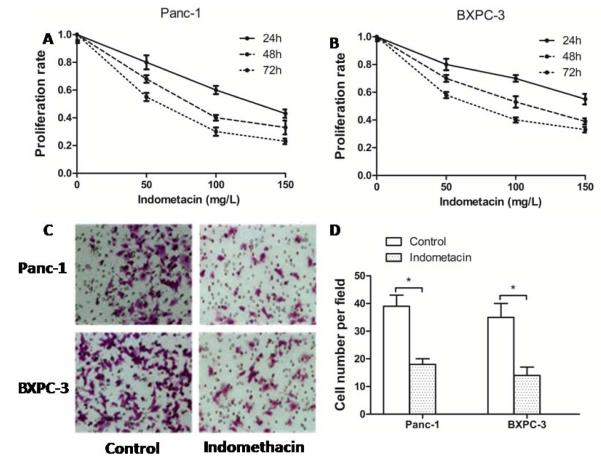
To explore the role of indometacin in PC progression, we investigated the effect of indometacin on the cell proliferation rate and invasion. Cells, which were incubated in HG culture, were then treated with a series of gradually increasing concentrations of indometacin (0 as a control, 50, 100 and 150 mg/L) for 24, 48, and 72 h. Our results showed that indometacin decreased the rate of cell proliferation in a dose-dependent manner (Fig. 2A and 2B). According to the results of the MTT assay, we chose the concentrations of 100 mg/L and time point of 48 h as an adequate intervention for subsequent experiments. Similarly, the statistical analysis showed that indometacin (100 mg/L) remarkably decreased HG-induced cell invasive ability compared to the control group (0 mg/L) in BXPC-3 cells and Panc-1 cells (Fig. 2C and 2D).
Fig. (2). Indometacin down-regulated the effect of HG-induced proliferation and invasion in PC cells.
(A, B) Panc-1 and BXPC-3 cells were seeded in a 96-well plate with HG for 12 h and treated with gradually increasing concentrations of indometacin (0 as a control and 50, 100 or 150 mg/L) for 24 h, 48 h, 72 h. Proliferation rate was measured with the MTT assay. (C) Panc-1 and BXPC-3 cells were incubated with HG for 48 h. The invasive ability of cells was detected in DMEM containing 1% FBS. Cells were seeded in the matrigel-coated transwell upper chambers. (D) The number of migrated cells was quantified by counting the number of cells from 10 random fields at ×200 magnification. * p < 0.05 compared to control (Indometacin 0 mg/L). The data represent the results of three independent experiments. Column: mean; bar: SD.
Involvement of E-Cadherin but not COX-2 in the Event of Indometacin Reversing HG-Induced Effect in PC Cells
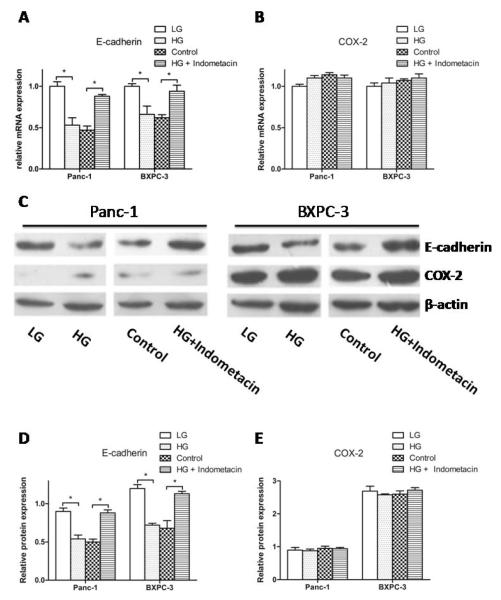
To determine the mechanism underlying the indometacin inhibition effect on HG-induced cell proliferation and invasion, we monitored the expression levels of E-cadherin and COX-2 by real time RT-PCR and Western blotting. Without indometacin intervention, HG markedly suppressed the mRNA expression of E-cadherin in comparison with LG. Treatment with indometacin (100 mg/L) markedly diminished the HG-induced down-regulation of E-cadherin compared with the control group (0 mg/L) in BXPC-3 cells and Panc-1 cells (Fig. 3A). However, we didn’t find significant alterations in the expression of COX-2 at the varying glucose concentrations. More importantly, as a traditional inhibitor for COX-2, indometacin had no effect on the mRNA expression of COX-2 in HG condition (Fig. 3B). The protein levels of E-cadherin and COX-2 showed similar trend (Fig. 3C, 3D and 3E). These results suggest that indometacin most likely modulates HG-induced PC cell proliferation and invasion through E-cadherin.
Fig. (3). Involvement of E-cadherin but not COX-2 in the event of indometacin reversing HG-induced effect in PC cells.
(A, B) The mRNA expression of E-cadherin and COX-2 was estimated by real-time RT-PCR in four groups. The expression of each target gene was quantified using β-actin as a normalization control. (C) The protein expression of E-cadherin and COX-2 was evaluated by Western blotting. The blots were then re-probed with β-actin as a loading control. (D, E) The relative protein expression of E-cadherin and COX-2 in different group. * p < 0.05 compared to controls. The data represent the results of three independent experiments. Column: mean; bar: SD.
The Effect of Knockdown E-Cadherin on Proliferation and Invasion in PC Cells
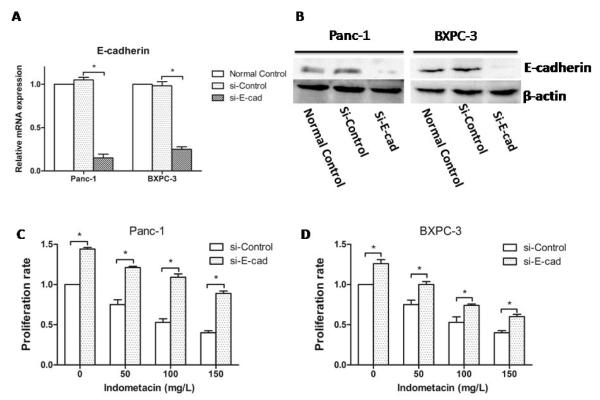
To further investigate the molecular mechanism through which indometacin inhibits HG-induced proliferation and invasion, we used siRNA to knockdown E-cadherin in PC cells (Fig. 4A and 4B) and evaluated cell proliferation and invasion.
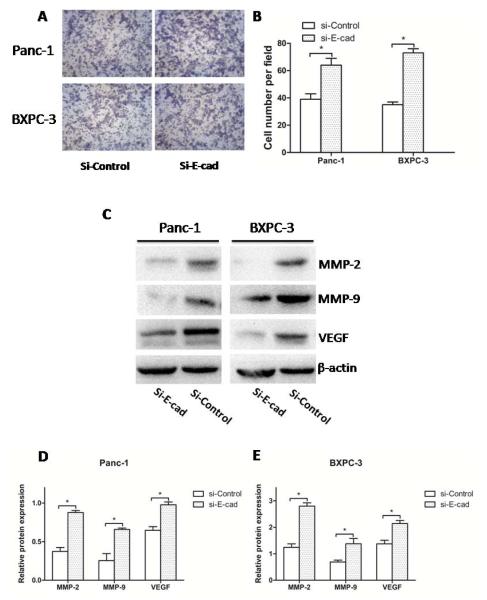
Fig. (4). The effect of knockdown E-cadherin on proliferation in PC cells.
(A) The knockdown of E-cadherin by siRNA for 48 h was confirmed by real-time RT-PCR. (B) The knockdown of E-cadherin by siRNA for 48 h was confirmed by Western blotting. (C, D) Panc-1 and BXPC-3 cells were seeded in a 96-well plate with HG for 12 h and treated with a series of gradually increasing concentrations of indometacin (0 as a control and 50, 100 or 150 mg/L) for 48 h. Proliferation rate was shown by MTT assay. * p < 0.05 compared with Si-control. The data represent the results of three independent experiments. Column: mean; bar: SD.
We found that the proliferation rate of PC cells incubated in HG for 48 h was significantly increased in cells with Si-E-cadherin (Si-E-cad) compared to Si-Control at each concentration of indometacin. Furthermore, the proliferation rate after knockdown was present in a dose-dependent manner, which was parallel to the Si-Control group in BXPC-3 cells and Panc-1 cells (Fig. 4C and 4D).
Through detecting the cells’ invasive ability, we found that the invasion of PC cells was significantly increased in the condition of Si-E-cad compared with Si-control. In addition, to determine the possible mechanism of the effect of E-cadherin on the PC invasion and metastasis, we found that the protein levels of metastasis-related factors (MMP-2, MMP-9, VEGF), which were increased in PC cells after Si-E-cad and treatment with indometacin 100 mg/L for 48 h. (Fig. 5C). This result shows that E-cadherin not only plays a key role in the process of indometacin inhibiting HG-induced invasion, but also regulates the expressions of MMP-2, MMP-9, VEGF as well as their involvement in invasion (Fig. 5D and 5E).
Fig. (5). The effect of knockdown E-cadherin on invasion in PC cells.
(A) Panc-1 and BXPC-3 cells were incubated with HG for 48 h. The invasive ability by DMEM containing 1% FBS seeded in matrigel-coated transwell upper chambers. (B) The number of migrated cells was quantified by counting the number of cells from 10 random fields at ×200 magnification. (C) The expression of MMP-2, MMP-9, and VEGF at protein level was evaluated by Western blotting in two groups. The blots were then re-probed with β-actin as a loading control. (D, E) The relative protein expression of MMP-2, MMP-9, and VEGF in two groups. * p < 0.05 compared with Si-control. The data represent the results of three independent experiments. Column: mean; bar: SD.
The Effect of Indometacin on E-Cadherin Transcriptional Repressors Expression in PC Cells
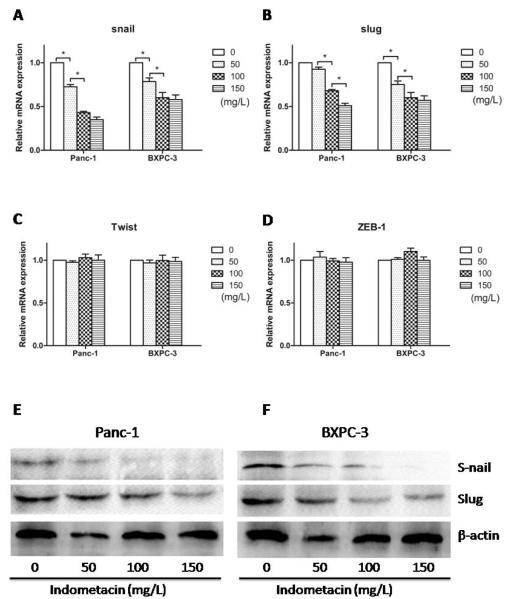
To investigate whether indometacin up-regulates E-cadherin expression by modulating the transcriptional regulation of E-cadherin, we used real time RT-PCR and Western blotting to examine the levels of E-cadherin transcriptional repressors, Snail, Slug, Twist, and ZEB1. Treatment with different doses of indometacin (for 48 h) down-regulated Snail and Slug mRNA levels in a dose-dependent manner (Fig. 6A and 6B) but without significantly influence on Twist and ZEB1 mRNA levels (Fig. 6C and 6D). The protein levels of Snail and Slug were also measured by Western blotting. The similar results of protein were also observed in Fig. 6E and 6F. The protein levels of Twist and ZEB1 were similar with real time RT-PCR (data not shown). These results demonstrated that indometacin could modulate E-cadherin expression through the transcriptional repressors.
Fig. (6). The effect of indometacin on E-cadherin transcriptional repressors expression in PC cells.
(A, B, C, D) The expression of Snail, Slug, Twist and ZEB1 at mRNA level was estimated with real-time RT-PCR. The expression of each target gene was quantified using β-actin as a normalization control. (E, F) Panc-1 and BXPC-3 cells were incubated with HG for 12 h and then treated with a series of gradually increasing concentrations of indometacin (0 as a control and 50, 100 or 150 mg/L) for 48 h. The expression of Snail and Slug at protein level was evaluated by Western blotting. The blots were then re-probed with β-actin as a loading control. * p < 0.05 compared to controls. The data represent the results of three independent experiments. Column: mean; bar: SD.
The Activation of the PI3K/AKT/GSK-3β Signaling Pathway is Critical for Indometacin Reversing HG-Induced Cell Proliferation and Invasion in PC Cells
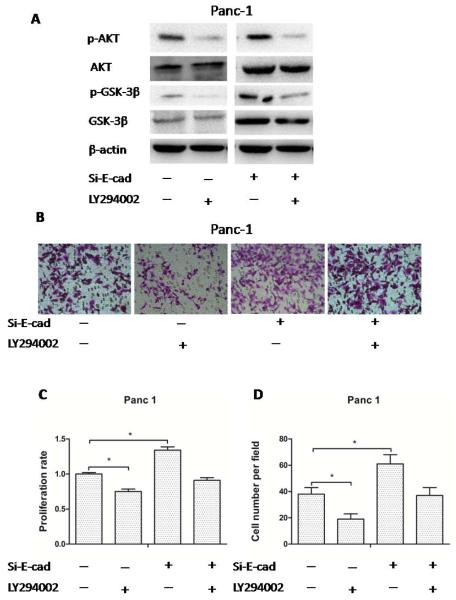
To determine the mechanism of indometacin on HG-induced proliferation and invasion, we analyzed the role of the PI3K/AKT/GSK-3β signaling pathways when indometacin treated cells were added with the AKT pathways inhibitor (LY294002) and/or Si-E-cad in HG medium. LY294002 treatment reduced the phosphorylation of AKT and GSK-3β, accompanied with decrease of proliferation rate and invasion in Panc-1 and BXPC-3 cells. When Si-E-cad was used alone, the phosphorylation of AKT and GSK-3β, and cell proliferation rate were increased significantly. Combining with LY294002 and Si-E-cad, the phosphorylation of AKT and GSK-3β were neutralized compared with the control group [Si-E-cad (−) and LY294002 (−)] (Fig. 7A). And the combination demonstrated no obvious regulation for proliferation rate and invasion compared with control. To investigate the molecular effect of combining with PI3K/AKT/GSK-3β signaling pathway and Si-E-cad on indometacin intervention cells proliferation and invasion in HG medium, we detected the proliferation rate by MTT assay in Panc-1 cells. We found that AKT inhibitor treatment reduced the proliferation rate in Panc-1 cells. When Si-E-cad was used alone, the proliferation rate was increased significantly. Combination of AKT pathways inhibitor and Si-E-cad demonstrated no obvious regulation for proliferation rate compared with control on the MTT assay [Si-E-cad (−) and LY294002 (−)] (Fig. 7C). What’s more, we found that the HG-induced invasion of Panc-1 cells was significantly abolished in the presence of AKT inhibitor. In addition, combined with AKT pathway inhibitor and Si-E-cad, the invasive ability displayed weaker change than control (Fig. 7B and 7D). Also, the similar results occurred in the BXPC-3 cells.
Fig. (7). The activation of the PI3K/AKT/GSK-3β signaling pathway is critical for indometacin reversing HG-induced cell proliferation and invasion in PC cells.
(A) The expression of p-AKT, AKT, p-GSK-3β, GSK-3β and β-actin at protein level was evaluated by Western blotting in four groups. The blots were then re-probed with β-actin as a loading control. (B) Panc-1 cells were incubated with HG for 48 h. The invasive ability by DMEM containing 1% FBS seeded in matrigel-coated Transwell upper chambers. The cells were treated with an AKT pathway inhibitor (LY294002) and/or Si-E-cad. (C) Panc-1 cells were seeded in a 96-well plate with HG for 12 h and then treated with AKT pathways inhibitor (LY294002) and/or Si-E-cad for 48 h. Proliferation rate was determined with the MTT assay. (D) The number of migrated cells was quantified by counting the number of cells from 10 random fields at ×200 magnification. Control: [Si-E-cad (−) and LY294002 (−)]. * p < 0.05 compared to controls. The data represent the results of three independent experiments. Column: mean; bar: SD.
DISCUSSION
We have previously demonstrated that HG promotes cell proliferation and perineural invasion (PNI), enhanced GDNF and RET expression in vitro, and promoted PNI in nude mice xenograft tumor models [6-8]. Although the promotion effect of HG on cancers is common and definite, the treatment methods to the HG-induced cancers are still scarce and inaccurate. In the present study, we compared the effects of various treatment conditions: low glucose (LG): 5.5 mM; osmotic control: mannitol; HG: 25 mM; HG + indometacin (0, 50, 100, 150 mg/L) for various periods of time (24 h, 48 h, and 72 h) on the proliferation and invasion of PC cells in vitro. We found that HG promoted proliferation and invasion significantly in every period of time. The proliferation rate of HG group was significantly higher than LG group in these two cell lines. However, the proliferation rate of osmotic control group did not change consistently in both cell lines, suggesting that osmotic pressure has little effect on proliferation.
In a study about the factors responsible for thymic involution in a model of STZ-induced diabetes, administration of indometacin concomitantly with STZ reduced thymic involution but did not prevent the onset of hyperglycemia. This finding suggested that COX-2 inhibition may retard the thymic involution by reducing serum glucocorticoids in STZ-induced diabetes [18]. In our study, we investigate the function and mechanism of indometacin on HG-induced cell behavior. Concomitantly, indometacin exhibited anti-proliferative and anti-invasion activity on PC cells (BXPC-3 and Panc-1) in HG condition in a dose-dependent manner. The proliferation rate of the two cell lines decreased with prolonged incubation time despite identical indometacin concentration. Indometacin inhibited proliferation of the PC cells in HG condition in a dose-dependent manner. Similarly, the results of transwell showed that HG promoted the invasion of PC cells, and indometacin inhibited this invasive ability of cancer cells incubated in HG condition. Our data support the idea that exogenous administration of indometacin could reverse the HG-induced cell behavior.
E-cadherin is best characterized as adherens junction protein, which through homotypic interactions contributes to the maintenance of the epithelial barrier function. Much research showed that HG could induce epithelial-mesenchymal transition and the loss of epithelial phenotype E-cadherin was involved in the progress [19, 20]. Our results also found that HG could decrease the expression of E-cadherin compared with LG in two PC cells. Some results suggested the antiproliferative effect of indometacin may contribute to enhanced cell adhesion through increased expression of E-cadherin and translocation of beta-catenin from the nucleus to the cell membrane in HT-29 colon cancer cells [21]. Indometacin induced cellular morphological change and migration via E-cadherin in A549 human lung cancer cells [22]. In our study, treatment with indometacin more markedly diminished the HG-induced down-regulation of E-cadherin than control group in BXPC-3 cells and Panc-1 cells.
A previous study found that HG could increase expression of COX-2 in human pancreatic islets of diabetic mice [23].We also evaluated the effects of HG and indometacin on the expressions of COX-2. The results showed that the expression of COX-2 in BXPC-3 cells was positive and negative in Panc-1 which was consistent with previous reports [24]. There were no significant changes in the COX-2 mRNA/protein expression between HG and LG in both cell lines. In addition, indometacin didn’t alter the expression of COX-2 in HG condition. This demonstrates that indometacin inhibits the progress of PC through a COX-2 independent mechanism in HG condition. Our data support the idea that indometacin promotes adipogenesis of mesenchymal stem cells through a COX independent way [25]. However, the mechanism of independent COX-2 involved in indometacin regulation the PC cells behavior is not studied in this research. It will be explored in next work.
E-cadherin regulates cell growth in a cell adhesion-independent manner. To further investigate the molecular mechanism by which indometacin inhibits HG-induced proliferation and invasion process, siRNA was used to knockdown E-cadherin. We found that the proliferation rate and invasion of PC cells treated with indometacin were significantly increased with Si-E-cad in comparison with Si-Control. To determine the possible mechanism involved in the effect of E-cadherin on the PC invasion and metastasis, we found that the regulation of the expression levels of MMP-2, MMP-9, and VEGF by E-cadherin may be contributed in PC invasion.
In cancer cells, the loss of E-cadherin gene expression was mainly due to an overexpression of transcriptional repressors including Snail, Slug, Twist and ZEB1 [26]. A previous study about ovarian cancer demonstrated that the overexpression of Snail and Slug resulted in the down-regulation of E-cadherin, in enhanced cell motility, and in cell invasiveness [27]. Slug expression promotes invasion and metastasis of human PC cells through upregulation of MMP-9 [28]. In the study, indometacin down-regulated Snail and Slug mRNA and protein levels in a dose-dependent manner but had no significant influence on Twist and ZEB1 in PC cells. This suggested that indometac may regulate the expression of E-cadherin through these two repressors. Of course, E-cadherin can’t be the only regulatory factor for the mechanism of indometacin regulation PC progress, although it play an important role in the course. Thus, more other research is necessary in the future.
Class PI3Ks are heterodimers consisting of a catalytic subunit in complex with a regulatory subunit (p85, also existing in distinct isoforms) [29], which are involved in a broad variety of cellular functions, including proliferation and differentiation, growth, survival, and motility. The activation of PI3K is often linked to the PI3K-mediated activation of protein kinase B or AKT. Of note, the phosphatase PTEN catalyzes the reverse reaction and shuts down PI3K/AKT signaling. Several studies indicate that the engagement of E-cadherin results in the activation of PI3K and AKT in keratinocytes, epithelial cells, and carcinoma cells [30, 31]. We monitored the role of the PI3K/AKT/GSK-3β signaling pathways when cells were treated with AKT pathway inhibitor and/or Si-E-cad. The results suggest AKT pathway inhibitor was more impactful than Si-E-cad for the activation of PI3K/AKT/GSK-3β signaling pathways. In addition, the regulation of AKT pathway inhibitor and/or Si-E-cad for proliferation rate and invasion were assessed. AKT pathways inhibitor counteracts the promotive function of Si-E-cad in the event of indometacin reversing HG-induced cell behavior. These results showed that PI3K/AKT/GSK-3β signaling pathways were involved in the progress of indometacin reversing HG-induced cell proliferation and invasion. This study elaborates the inhibition effect of indometacin on PC in HG condition. However, indometacin is only one of the COX-2 inhibitors. Whether other similar drugs have the same function and mechanism will be needed a new study in future. In addition, this study was completed in vitro. Thus, future in vivo experiments and clinical trials should validate the significance of indometacin in cancer progression, treatment and therapy.
CONCLUSION
This study suggests that indometacin inhibits HG-induced proliferation and invasion via up-regulating of E-cadherin, but not COX-2 in PC cells. The activation of the PI3K/AKT/GSK-3β signaling pathway is critical for indometacin reversing the HG-induced cell proliferation and invasion of PC cells. Indometacin may be an effective adjuvant therapy for the treatment of PC patients with hyperglycemia.
ACKNOWLEDGEMENTS
This work was financially supported by Grants from China National Natural Science Foundation of China (No. 81172360, 81201824), the Clinical Innovation Funds of the 1st Affiliated Hospital of XJTU (10ZD01), and project grants from the NCRR (P20 RR020151) and the NIGMS (P20 GM103505 and P30 GM103332-01) from the NIH. The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of China National Natural Science Foundation, the US NIH, National Center for Research Resources (NCRR), or National Institute of Resources (NCRR), or National Institute of General Medical Sciences (NIGMS).
ABBREVIATIONS
- COX-2
Cyclooxygenase-2
- DMSO
Dimethylsulfoxide
- GDNF
Glial cell line-derived neurotrophic factor
- HG
High glucose
- LG
Low glucose
- PC
Pancreatic cancer
- PNI
Perineural invasion
- Si-E-cad
Si-E-cadherin
Footnotes
CONFLICT OF INTEREST The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- [2].Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morrison M. Pancreatic cancer and diabetes. Adv. Exp. Med. Biol. 2012;771:229–239. doi: 10.1007/978-1-4614-5441-0_18. [DOI] [PubMed] [Google Scholar]
- [4].Slim I, Ach K, Chefii R, Trimech-Ajmi S, Landolsi A, Chadli-Chaieb M, Maaroufi-Beizig A, Chaieb L. Diabetes mellitus as an early symptom of pancreatic cancer diagnosed three years later. Ann. Endocrinol. 2009;70(1):76–79. doi: 10.1016/j.ando.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [5].Hwang A, Narayan V, Yang YX. Type 2 diabetes mellitus and survival in pancreatic adenocarcinoma: a retrospective cohort study. Cancer. 2013;119(2):404–410. doi: 10.1002/cncr.27731. [DOI] [PubMed] [Google Scholar]
- [6].Liu H, Ma Q, Li J. High glucose promotes cell proliferation and enhances GDNF and RET expression in pancreatic cancer cells. Mol. Cell. Biochem. 2011;347(1-2):95–101. doi: 10.1007/s11010-010-0617-0. [DOI] [PubMed] [Google Scholar]
- [7].Han L, Ma Q, Li J, Liu H, Li W, Ma G, Xu Q, Zhou S, Wu E. High glucose promotes pancreatic cancer cell proliferation via the induction of EGF expression and transactivation of EGFR. PLoS One. 2011;6(11):e27074. doi: 10.1371/journal.pone.0027074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li J, Ma Q, Liu H, Guo K, Li F, Li W, Han L, Wang F, Wu E. Relationship between neural alteration and perineural invasion in pancreatic cancer patients with hyperglycemia. PLoS One. 2011;6(2):e17385. doi: 10.1371/journal.pone.0017385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin. Exp. Metastas. 2008;25(6):643–655. doi: 10.1007/s10585-008-9171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68(7):2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Van den Bossche J, Malissen B, Mantovani A, De Baetselier P, Van Ginderachter JA. Regulation and function of the E-cadherin/catenin complex in cells of the monocyte-macrophage lineage and DCs. Blood. 2012;119(7):1623–1633. doi: 10.1182/blood-2011-10-384289. [DOI] [PubMed] [Google Scholar]
- [12].Kubatka P, Kalicka K, Bojkova B, Ahlers I, Ahlersova E, Pec M. Neoplastic effect of indomethacin in N-methyl-N-nitrosourea induced mammary carcinogenesis in female rats. Klin. Onkol. 2012;25(5):359–363. [PubMed] [Google Scholar]
- [13].Lange A, Gustke H, Glassmeier G, Heine M, Zangemeister-Wittke U, Schwarz JR, Schumacher U, Lange T. Neuronal differentiation by indomethacin and IBMX inhibits proliferation of small cell lung cancer cells in vitro. Lung cancer. 2011;74(2):178–187. doi: 10.1016/j.lungcan.2011.03.017. [DOI] [PubMed] [Google Scholar]
- [14].de Groot DJ, van der Deen M, Le TK, Regeling A, de Jong S, de Vries EG. Indomethacin induces apoptosis via a MRP1-dependent mechanism in doxorubicin-resistant small-cell lung cancer cells overexpressing MRP1. Brit. J. Cancer. 2007;97(8):1077–1083. doi: 10.1038/sj.bjc.6604010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Das A, Banik NL, Ray SK. Methylprednisolone and indomethacin inhibit oxidative stress mediated apoptosis in rat C6 glioblastoma cells. Neurochem. Res. 2007;32(11):1849–1856. doi: 10.1007/s11064-007-9371-4. [DOI] [PubMed] [Google Scholar]
- [16].Setia S, Vaish V, Sanyal SN. Chemopreventive effects of NSAIDs as inhibitors of cyclooxygenase-2 and inducers of apoptosis in experimental lung carcinogenesis. Mol. Cell. Biochem. 2012;366(1-2):89–99. doi: 10.1007/s11010-012-1286-y. [DOI] [PubMed] [Google Scholar]
- [17].Meng Y, Lu Z, Yu S, Zhang Q, Ma Y, Chen J. Ezrin promotes invasion and metastasis of pancreatic cancer cells. J. Transl. Med. 2010;8:61. doi: 10.1186/1479-5876-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mic AA, Mic FA, Tatu CA, Ionac M, Ordodi VL, Paunescu V. Indomethacin inhibits thymic involution in mice with streptozotocin-induced diabetes. Comparative Med. 2007;57(5):476–481. [PubMed] [Google Scholar]
- [19].Lei P, Jiang Z, Zhu H, Li X, Su N, Yu X. Poly(ADP-ribose) polymerase-1 in high glucose-induced epithelial-mesenchymal transition during peritoneal fibrosis. Int. J. Mol. Med. 2012;29(3):472–478. doi: 10.3892/ijmm.2011.859. [DOI] [PubMed] [Google Scholar]
- [20].Liu R, Wang Y, Xiao Y, Shi M, Zhang G, Guo B. SnoN as a Key Regulator of the High Glucose-Induced Epithelial-Mesenchymal Transition in Cells of the Proximal Tubule. Kidney Blood Press. R. 2012;35(6):517–528. doi: 10.1159/000339172. [DOI] [PubMed] [Google Scholar]
- [21].Kapitanovic S, Cacev T, Antica M, Kralj M, Cavric G, Pavelic K, Spaventi R. Effect of indomethacin on E-cadherin and beta-catenin expression in HT-29 colon cancer cells. Exp. Mol. Pathol. 2006;80(1):91–96. doi: 10.1016/j.yexmp.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [22].Kato T, Fujino H, Oyama S, Kawashima T, Murayama T. Indomethacin induces cellular morphological change and migration via epithelial-mesenchymal transition in A549 human lung cancer cells: a novel cyclooxygenase-inhibition-independent effect. Biochem. Pharmacol. 2011;82(11):1781–1791. doi: 10.1016/j.bcp.2011.07.096. [DOI] [PubMed] [Google Scholar]
- [23].Shanmugam N, Todorov IT, Nair I, Omori K, Reddy MA, Natarajan R. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (AGER), and in islets from diabetic mice. Diabetologia. 2006;49(1):100–107. doi: 10.1007/s00125-005-0065-7. [DOI] [PubMed] [Google Scholar]
- [24].El-Rayes BF, Ali S, Sarkar FH, Philip PA. Cyclooxygenase-2-dependent and -independent effects of celecoxib in pancreatic cancer cell lines. Mol. Cancer Ther. 2004;3(11):1421–1426. [PubMed] [Google Scholar]
- [25].Styner M, Sen B, Xie Z, Case N, Rubin J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J. Cell. Biochem. 2010;111(4):1042–1050. doi: 10.1002/jcb.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- [27].Kurrey NK, K A, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol. Oncol. 2005;97(1):155–165. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- [28].Zhang K, Chen D, Jiao X, Zhang S, Liu X, Cao J, Wu L, Wang D. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab. Invest. 2011;91(3):426–438. doi: 10.1038/labinvest.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [29].Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell. Biol. 2010;11(5):329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- [30].McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, Yap AS. E-cadherin adhesion activates c-Src signaling at cell-cell contacts. Mol. Biol. Cell. 2007;18(8):3214–3223. doi: 10.1091/mbc.E06-12-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Georgopoulos NT, Kirkwood LA, Walker DC, Southgate J. Differential regulation of growth-promoting signalling pathways by E-cadherin. PLoS One. 2010;5(10):e13621. doi: 10.1371/journal.pone.0013621. [DOI] [PMC free article] [PubMed] [Google Scholar]