Abstract
Metformin, a biguanide drug used in the treatment of type II diabetes, was evaluated alone and in combination with amifostine, captopril, MESNA or N-acetyl-cysteine (NAC) for its ability to protect when administered 24 h after irradiation. Mouse embryo fibroblasts (MEF), human microvascular endothelial cells (HMEC) and SA-NH mouse sarcoma cells were exposed to 4 Gy in vitro. C3H mice were exposed to 7 Gy and evaluated utilizing an endogenous spleen colony assay system. Amifostine and WR1065, administered 30 min prior to irradiation, were used as positive controls. Treatment of MEF, HMEC and SA-NH cells with metformin elevated survival levels by 1.4-, 1.5- and 1.3-fold compared to 1.9-, 1.8- and 1.6-fold for these same cells treated with WR1065, respectively. Metformin (250 mg/kg) was effective in protecting splenic cells from a 7 Gy dose in vivo (protection factor = 1.8). Amifostine (400 mg/kg), administered 30 min prior to irradiation resulted in a 2.6-fold survival elevation, while metformin administered 24 h after irradiation in combination with NAC (400 mg/kg), MESNA (300 mg/kg) or captopril (200 mg/kg) enhanced survival by 2.6-, 2.8- and 2.4-fold, respectively. Each of these agents has been approved by the FDA for human use and each has a well characterized human safety profile. Metformin alone or in combination with selected sulfhydryl agents possesses radioprotective properties when administered 24 h after radiation exposure comparable to that observed for amifostine administered 30 min prior to irradiation making it a potentially useful agent for radiation countermeasures use.
Introduction
Following the horrific events of September 11, 2001, there has been a concerted effort to protect the population from radiological terrorism. A major focus of this effort has been in the development of chemical agents that can protect against the toxic effects of ionizing radiation. As a result of the 2004 report from a National Cancer Institute Workshop (1), the development of radioprotective agents was subdivided into three categories: prophylactic agents that protect if administered prior to radiation exposure; mitigator agents that are administered during or after irradiation that can prevent or lessen radiation toxicity; and therapeutic agents that are administered after irradiation to treat and enhance recovery from radiation-induced damage. While these terms represent three distinct classes of radioprotectors, it is possible that some radioprotective agents can exert protective effects across all three of these artificial categories (2, 3). At present there is only one prophylactic radioprotector that has been approved by the U.S. Food and Drug Administration (FDA) and that is amifostine, which is used for protection against xerostomia induced by radiation exposure in the treatment of head and neck cancer (4).
There is currently considerable interest in identifying and developing an effective radiation countermeasure agent that can be administered 24 h after radiation exposure since it is anticipated that aid to many potential victims of a radiological accident or terrorist attack would be delayed due to the resulting chaos and confusion that would exist. Furthermore, the rapid development and deployment of such an agent for radiation countermeasures use would be facilitated if it were already approved by the FDA for other uses along with having a robust and well documented safety profile. One such agent that is evoking such interest is the biguanide drug metformin that is currently in use as the first line of treatment for type II diabetics. Metformin has been demonstrated to moderately protect mouse embryo fibroblasts from radiation-induced toxicity while being directly cytotoxic to human cancer cells (5). This potential enhancement in therapeutic ratio for metformin combined with radiation therapy has been the focus of numerous studies in which it has been reported to not only directly kill cancer cells and inhibit tumor growth, but also improve tumor oxygenation and enhance radiotherapy response (6– 9). Coupled with metformin's apparent effects on radiation response are reports that it can down-regulate age related oxidative stress (10), protect against endogenous reactive oxygen species and associated DNA damage (11, 12), inhibit cell growth and reduce protein synthesis (13) and exhibit chemopreventive properties in reducing the risk of carcinogenesis (14). For these reasons we identified metformin as a potential mitigator/therapeutic radiation protector for use as a radiation countermeasure agent.
The wide spread use of metformin around the world as the first line treatment drug for type II diabetes and its well characterized safety profile make this agent an ideal candidate for investigation regarding its ability to reduce or protect against radiation-induced toxicity when administered after exposure. Keeping in line with the proposed requirement that such an agent be effective when administered 24 h after radiation exposure we evaluated metformin alone or in combination with other well characterized cytoprotective agents that have been approved by the FDA and have well known safety profiles. Captopril is an angiotensin converting enzyme inhibitor that is used to treat high blood pressure, MESNA is a detoxifying agent used to inhibit hemorrhagic cystitis induced by ifosfamide in cancer treatment, and N-acetyl-cysteine (NAC) is a modified form of cysteine which has been used as an anti-mucolytic agent. Each of these drugs contains a sulfhydryl group and has exhibited cytoprotective properties. Amifostine or its active free thiol form WR1065 were used under the standard prophylactic conditions of administration 30 min prior to radiation exposure to serve as positive controls for radiation protection and as a standard for comparison of the effectiveness of metformin administered alone or in combination with captopril, MESNA, or NAC when administered 24 h after irradiation (4).
Methods and Materials
Cells and Culture Conditions
Mouse embryo fibroblasts (MEF) were isolated from 14–16-day-old pregnant female C57BL/6 WT mice following a method described in detail elsewhere (15). Mice were euthanized, and the uterus was removed and placed in a culture dish containing sterile phosphate-buffered saline (PBS, Invitrogen Life Technologies, Carlsbad, CA). Organs, tail, limbs and head were removed for genotyping. Embryos were placed in PBS with 0.25% trypsin (Invitrogen Life Technologies) and finely minced with scissors. Minced tissues were incubated for 15 min at 37°C and pipetted to dissociate the tissue. This process was repeated two to three times after which supernatants were collected and centrifuged. Cells were re-suspended in culture medium containing (1:1) Dulbecco's modified Eagle's medium (DMEM):F12 (Invitrogen Life Technologies), 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), 100 units/ml penicillin and 100 mg/ml streptomycin (Invitrogen Life Technologies) and plated in 100 mm diameter dishes at a density of 106 cells/dish. MEF were then transformed with c-myc and H-Ras to immortalize them according to a method described in detail elsewhere (15). SA-NH cells derived from an SA-NH murine sarcoma tumor and adapted for in vitro growth were grown and cultured as described elsewhere (16). Human microvascular endothelial cells (HMEC) from human dermis immortalized with SV40 were maintained in endothelial basal medium MCDB131 (Invitrogen Life Technologies) as described elsewhere (17). All cell cultures were maintained at 37°C in a humidified environment containing 5% CO2 and were grown to confluence for irradiation and drug treatment.
In Vitro Cell Survival Assay
All cells were irradiated with 4 Gy X rays using a Philips RT250 X-ray generator operating at 250 kVp and 15 mA at a dose rate of 0.368 Gy/min (15). Unirradiated cells served as controls. Immediately after irradiation, cells were trypsinized, counted, diluted and known numbers seeded into 100 mm diameter tissue culture dishes to allow the development of 50–150 colonies per dish. Colonies were stained with 20% crystal violet and scored 12 days after plating. Five dishes per experimental point were used and experiments were repeated three times (15–17).
Mice
Female C3H mice between 8 and 11 weeks old were supplied by Harlan Laboratories (Indianapolis, IN) and allowed a minimum of 1 week to acclimate in The University of Chicago animal facility. They were provided standard laboratory rodent chow and clean water ad libitum. Mice were housed five to a cage under standard conditions (12 h light/dark cycle at 48% relative humidity and a constant temperature of 22°C). The care and treatment of animals was in accordance with institutional guidelines and adherence to the NIH Guide for the Care and Use of Laboratory Animals.
Drugs
Metformin (1,1-Dimethylbiguanide hydrochloride from Sigma-Aldrich, St. Louis, MO) was dissolved in PBS and sterilized using a 0.22 μm syringe filter. Concentrations of metformin for studies with cells in culture ranged from 1–20 mM. No cytotoxicity was observed at any of these concentrations. A 5 mM concentration of metformin was chosen for routine use in all subsequent in vitro studies. WR1065 (Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Cancer Institute, Bethesda, MD) was used either alone prior to irradiation as a positive control at its maximum cytoprotective concentration of 4 mM (18) or in combination with metformin at select times after irradiation.
For in vivo studies, mice were injected i.p. with a final volume of 0.2 ml for all single and drug combinations used. Metformin was administered at a final concentration of 250 mg/kg body weight. Captopril was injected at a concentration of 200 mg/kg body weight, MESNA was injected at a concentration of 300 mg/kg body weight and NAC was injected at a concentration of 400 mg/kg body weight. These concentrations were chosen because they were nontoxic. Amifostine (Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Cancer Institute) was administered i.p. 30 min prior to irradiation at a concentration of 400 mg/kg body weight, a dose known to afford maximum radioprotection in the C3H mouse model (4).
In Vivo Irradiation and Drug Treatments
Mice at 8–11 weeks of age were placed in a cylindrical clear-plastic holder and exposed to X rays at room temperature using an X-ray generator operated at 250 kVp and 15 mA at a dose rate of 1.33 Gy/min. Based on early studies, a dose of 7 Gy X rays produced on average 10 nodules per spleen using the endogenous spleen colony assay of Till and McCulloch (19). All drug preparations were made just prior to their use. Amifostine was injected 30 min prior to irradiation to serve as a positive control while metformin alone or in combination with each of the sulfhydryl-containing drugs was injected 24 h after irradiation.
Spleen Colony Assay
The classical endogenous spleen colony assay was used to assess radioprotector efficacy (19). Mice were euthanized 13 days after irradiation and drug treatment to remove spleens and place them in Bouin's solution (Sigma-Aldrich) and allowed to soak for a minimum of 30 min before being examined for nodules appearing on the surface of the spleens.
Statistical Analysis
Means and standard errors were calculated for all data points from at least three independent experiments. Pairwise comparisons of cell survival frequencies between each of the experimental conditions were performed using a Student's two-tailed t test (SigmaPlot 11.0, SPSS, Chicago, IL).
Results
In Vitro Toxicity Assessment of Metformin
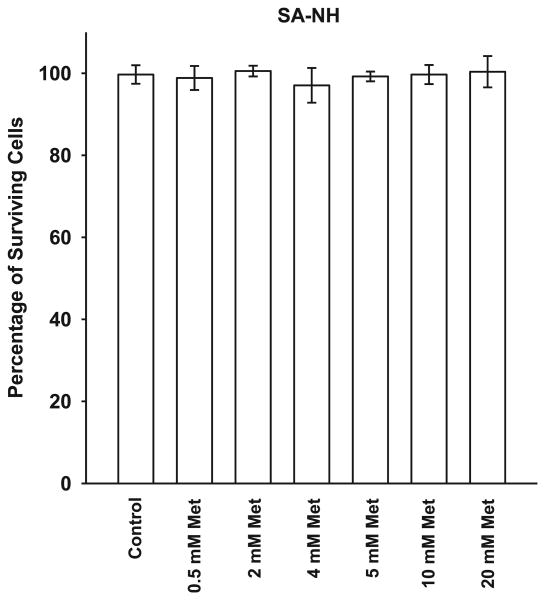
Shown in Fig. 1 is a toxicity assessment for a 1 h exposure of SA-NH sarcoma cells to metformin over a 0.5–20 mM concentration range. Metformin exhibited no cytotoxicity throughout this dose range. A concentration of 5 mM was chosen for all subsequent experiments.
FIG. 1.
The toxicity profile for metformin (Met) in SA-NH murine sarcoma cells treated with concentrations ranging of 0.5 to 20 mM after a 1 h exposure. Each experiment was performed three times and error bars represent the standard error of the mean (SEM).
In Vitro Time Course Evaluation of Metformin Effectiveness after Radiation Exposure
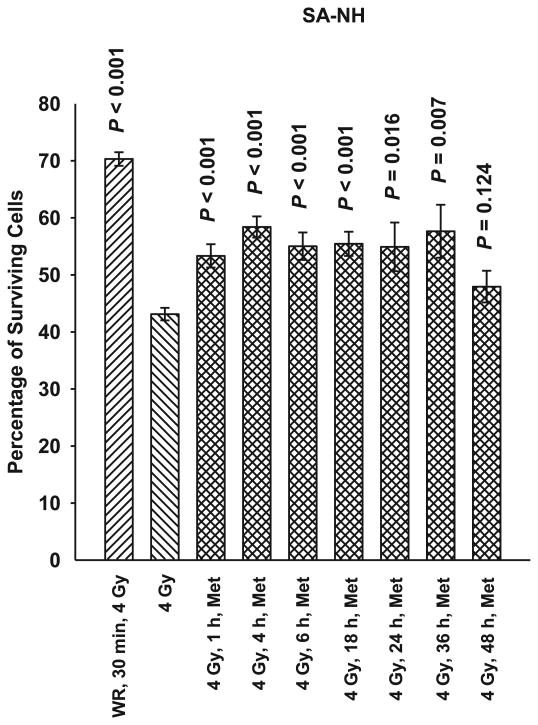
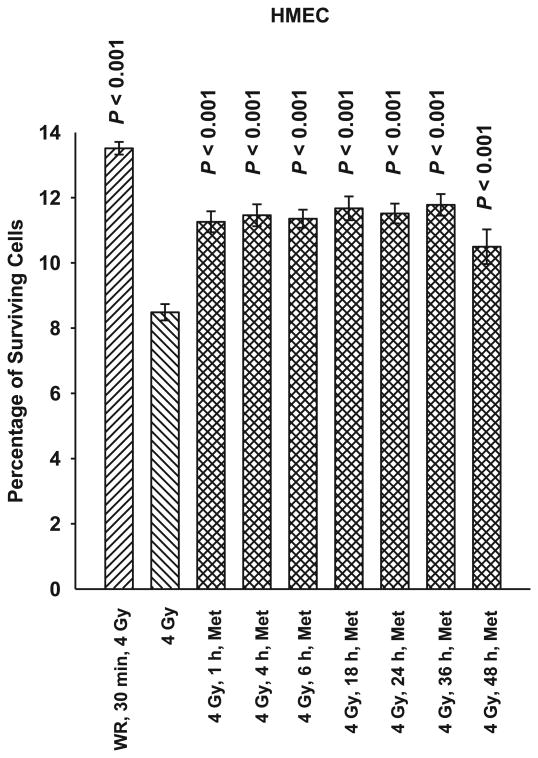
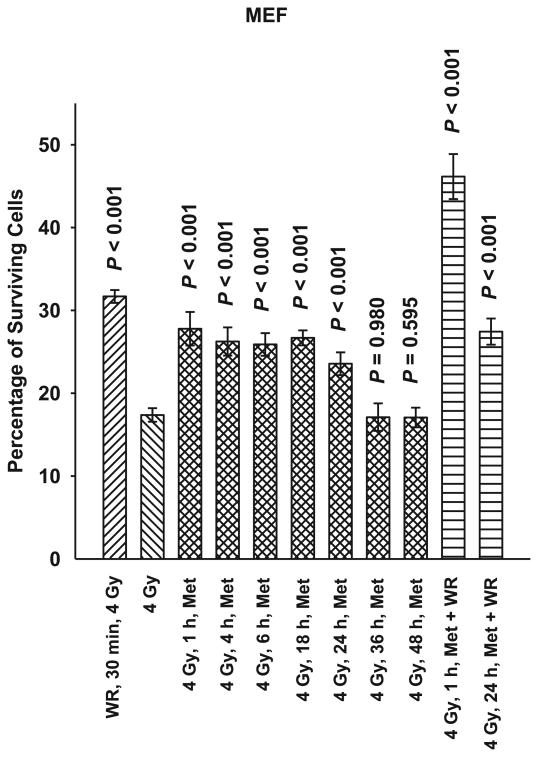
SA-NH, HMEC and MEF cells were each exposed to 5 mM metformin for 1 h at time intervals of 1, 4, 6, 18, 24, 36 or 48 h after exposure to 4 Gy. As demonstrated in Figs. 2, 3 and 4, metformin was effective in protecting against radiation-induced cell killing of SA-NH with protection factors ranging from 1.2 to 1.3. Protection factors ranging from 1.3 to 1.5 were observed for HMEC, and 1.4 to 1.6 for MEF cells treated with metformin at times ranging from 1–24 h after irradiation. Protection was extended in SA-NH by a factor of 1.3 and HMEC by a factor of 1.5 when metformin was added 36 h later, while only HMEC exhibited elevated radiation resistance if metformin was added 48 h after irradiation (PF = 1.3). While each of these elevated survival levels reached significance levels of P ≤ 0.007 compared to cells only exposed to 4 Gy, none of the metformin only treatments afforded the level of protection observed in SA-NH, HMEC, or MEF treated with WR1065 30 min prior to irradiation, e.g., protection factors of 1.6, 1.8 and 1.9, respectively.
FIG. 2.
A time course of the radioprotective effects of metformin (Met) in SA-NH murine sarcoma cells at a concentration of 5 mM and an exposure time of 1 h administered from 1 h to 48 h after irradiation with 4 Gy. Amifostine's active free thiol form WR1065 (WR) was administered at a concentration of 4 mM 30 min prior to irradiation to serve as a positive control for radioprotective comparison. Each experiment was repeated three times and error bars represent the SEM. P values comparing cell survival at 4 Gy only to those following treatment with Met or WR are presented for comparison.
FIG. 3.
A time course of the radioprotective effects of metformin (Met) in human microvascular endothelial cells (HMEC) at a concentration of 5 mM and an exposure time of 1 h administered from 1 h to 48 h after irradiation with 4 Gy. WR1065 (WR) was administered at a concentration of 4 mM 30 min prior to irradiation to serve as a positive control for radioprotective comparison. Each experiment was repeated three times and error bars represent the SEM. P values comparing cell survival at 4 Gy only to those following treatment with Met or WR are presented for comparison.
FIG. 4.
A time course of the radioprotective effects of metformin (Met) in mouse embryo fibroblasts (MEF) at a concentration of 5 mM and an exposure time of 1 h administered from 1 h to 48 h after irradiation with 4 Gy. WR1065 (WR) was administered at a concentration of 4 mM either 30 min before irradiation as a positive control for radioprotective comparison or in combination with Met 1 h or 24 h after irradiation. Each experiment was repeated three times and error bars represent the SEM. P values comparing cell survival levels exposed to 4 Gy only with those exposed to Met and WR alone or in combination are presented for comparison.
The combination of metformin and WR1065 was evaluated after irradiation only in MEF cells. If this combination of drugs was added 1 h after irradiation, cell survival was significantly enhanced (PF = 2.7) (see Fig. 4). This combined drug enhancement in protective effectiveness over WR1065 preirradiation treatment alone was not observed, however, if the combination of drugs was added 24 h later, e.g., protection factor of 1.6.
In Vivo Assessment of Metformin Alone or in Combination as a Radiation Mitigator
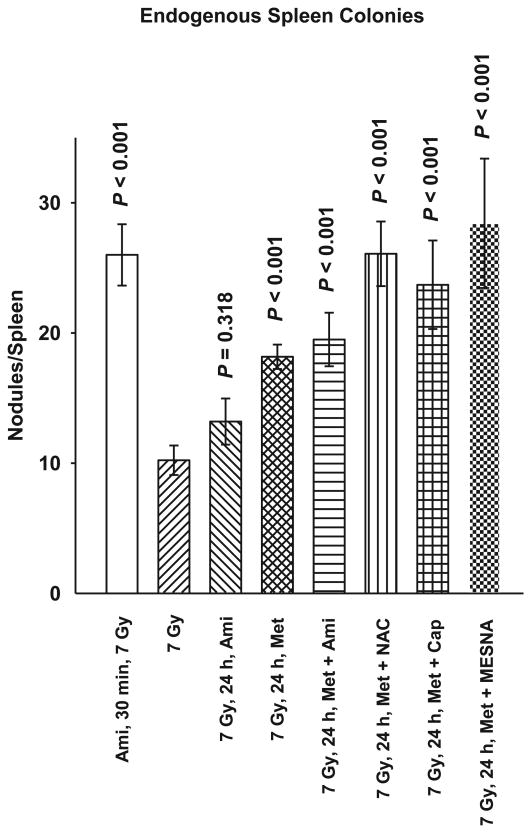
To assess the efficacy of metformin as a mitigator when used alone or in combination with select FDA approved sulfhydryl-containing drugs in protecting against radiation-induced cytotoxicity, a classical in vivo model system for normal tissue toxicity was used. Current regulations at The University of Chicago preclude the use of classical LD50 or LD70 assays to evaluate drugs for their radioprotective effectiveness. For this reason we chose the well characterized endogenous spleen colony assay first described by Drs. Till and McCulloch in 1961 as a direct measurement of the radiation sensitivity of mouse splenocytes (19). C3H mice 8 to 11 weeks of age were exposed to a 7 Gy whole body dose of ionizing radiation. Amifostine administered 30 min prior to or 24 h after irradiation was used as a positive control for radioprotector effectiveness. Metformin alone or in combination with amifostine, NAC, Captopril, or MESNA was administered 24 h after irradiation and the data are shown in Fig. 5. A dose of 7 Gy resulted in an average of 10 regenerating nodules on the surface of the spleens. Amifostine administered before irradiation significantly protected the spleens as evidenced by an average of 26 nodules per spleen, resulting in a protection factor of 2.6. Amifostine administered 24 h following irradiation afforded no significant elevation in protection (P = 0.318). Metformin alone administered 24 h after irradiation significantly protected irradiated animals (P < 0.001). The average number of spleen nodules observed was 18 giving rise to a protection factor of 1.8. The combination of metformin with amifostine, NAC, Captopril or MESNA resulted in greater elevations in protection (PF = 2.0, 2.6, 2.4 and 2.8, respectively), approaching or exceeding that observed for amifostine only when administered 30 min prior to irradiation, e.g., protection factor of 2.6.
FIG. 5.
A comparison of the radioprotective effects of metformin (Met) at a dose of 250 mg/kg alone or in combination with amifostine (Ami) 400 mg/kg, captopril (Cap) 200 mg/kg, MESNA 300 mg/kg and N-acetyl-cysteine (NAC) 400 mg/kg administered 24 h after a 7 Gy dose of whole body ionizing radiation using a C3H mouse model and the endogenous spleen colony assay are presented. Ami alone (400 mg/kg), administered 30 min prior to or 24 h after 7 Gy irradiation are included as positive controls. The number of colonies growing on the surface of spleens from mice in each experimental group 13 days after radiation exposure were counted and serve as a measure of the relative radio-protectiveness of the various experimental treatments. Error bars represent the SEM. P values comparing the number of spleen colonies following the various treatment groups with that after 7 Gy only are presented for comparison.
Discussion
For decades metformin has been a first line drug for the treatment of type II diabetes. Over 120 million prescriptions for its use are written each year (20). The result of the long and wide spread use of this drug is a robust drug safety profile that makes it a viable candidate for development for novel uses. Interest in metformin for use outside of the area of diabetes control was elevated in 2005 with the seminal observation that metformin treatment reduced the risk of cancer in diabetic patients (21). Since then, numerous studies have been initiated to both better understand the drug's mechanism(s) of action (10–13) and its potential for novel uses in cancer treatment and chemoprevention (6–9, 14). The current study was prompted by the demonstration by several groups that metformin could affect the response of mammalian cells exposed to ionizing radiation both by moderately protecting normal tissue as well as sensitizing tumor cells (5–9).
To address the potential of metformin for use as an effective radiation countermeasures drug for postirradiation administration, both nonmalignant and malignant cell lines were used in this study. Immortalized mouse embryo fibroblasts (MEF) transfected with c-myc and H-Ras and human microvascular endothelial cells (HMEC) immortalized with SV40 were assayed along with a mouse sarcoma tumor designated SA-NH. A toxicity profile for metformin was generated using SA-NH tumor cells since reports in the literature suggested that malignant cells would be preferentially more sensitive to its toxic effects (6, 7). No toxicity was observed, however, over the dose range of 0.5 to 20 mM after a 1 h exposure time (see Fig. 1). The lack of cytotoxicity may be the result of the short exposure time chosen or simply a reflection of the lack of toxicity over the concentration range tested. Regardless, a 5 mM concentration of metformin was chosen for all in vitro studies to characterize its potential protective effects when administered as a function of time after radiation exposure. To facilitate the evaluation of metformin as a potential radiation countermeasures agent it was important to identify a standard to which it could be compared. Amifostine was chosen for this role since it is the only FDA approved radiation protector for use in radiation therapy. Its mechanism of action is well worked out, as well as the time/dosing parameters required for its maximum level of radioprotection under both in vitro and in vivo conditions (4). For this reason we chose a concentration of 4 mM of WR1065, the active free thiol form of amifostine, administered 30 min prior to irradiation for all in vitro experiments and a concentration of 400 mg/kg amifostine administered 30 min prior to radiation exposure for all animal experiments. As shown in Figs. 2–4, WR1065 offered the greatest enhancement in cell survival for all three cell lines tested. While metformin was not as effective in protecting cells, it did confer significant radiation protection for all three cell lines when administered from 1–24 h after radiation exposure. This is a highly significant observation since it is well known that WR1065 is ineffective when administered after irradiation because its primary mechanism of action as a radiation protector is mediated through its ability to scavenge free radicals. Metformin's effectiveness in elevating cell survival when administered after irradiation suggests that it is working through a mechanism of action other than free radical scavenging. While all three cell lines could be protected when metformin was administered 24 h later, SA-NH cells could still be protected at 36 h while HMEC appeared to be the most susceptible to metformin's protective abilities as evidenced by its highly significant enhanced survival even if metformin was administered 48 h following irradiation. This suggests that the robustness and magnitude of metformin's protective ability may be cell type specific. When WR1065 was added with metformin 1 h after irradiation, an enhanced level of protection was observed in MEF cells (Fig. 4). The interaction of these two agents suggests that metformin's protective effectiveness could be enhanced by the addition of a sulfhydryl-containing drug. That was the impetus for evaluating it alone or in combination with other FDA approved sulfhydryl drugs in the animal study.
C3H mice, a minimum of 10 per experimental group, were used to assess the postirradiation protective effects of metformin used in combination with the sulfhydryl-containing FDA approved drugs captopril, MESNA and NAC. Thirty mice per experimental group were used to assess the effects of 7 Gy whole body irradiation with or without post-radiation exposure to metformin only using the endogenous spleen colony assay (see Fig. 5). The magnitude of post-radiation protection by metformin was greater in magnitude when assessed under these in vivo conditions than was observed when testing was done in vitro. This relative increase in magnitude of protection could be due simply to the unique response of splenic stem cells or it could be the result of a more complex protective effect of metformin that is expressed only under in vivo conditions. As an example, it has been proposed that metformin can reduce endogenous reactive oxygen species associated DNA damage through its ability to activate 5′-AMP-activated protein kinase (AMPK) and thus inhibit mTOR signaling that results in an attenuation of constitutive H2AX phosphorylation and resulting ATM activation (10– 12). This ability of metformin to affect a survival associated cell signaling process as well as to slow down cellular proliferation, which in turn would allow for an increased time for the repair of radiation damage before it becomes fixed at cell division (5, 10), offers a reasonable hypothesis to account for its protective abilities under both in vitro and in vivo conditions. However, under in vivo conditions metformin is also known to affect cellular metabolism in a number of organ systems, and in particular it has the ability to decrease insulin and insulin-like growth factor levels systemically. Since insulin is a well known growth factor, its reduction in vivo could add an additional mechanism through which metformin might allow for slower cell renewal progression and as a result an increased time for repair. Radiation-sensitive renewal cells such as splenic stem cells which are the effector cells measured using the endogenous spleen colony assay system might well be reflecting this added element of metformin protection compared to what was observed using the three in vitro cell lines (19, 20).
Metformin's post-radiation protective effectiveness was augmented to varying degrees through the addition of amifostine, captopril, MESNA and NAC, with the latter two agents appearing to be the most effective under the conditions tested (see Fig. 5). While the primary protective mechanism attributed to drugs containing sulfhydryl groups is that of free radical scavenging, these agents have the ability to activate a pro-survival NFκB signaling process that leads to elevated intra-mitochondrial manganese superoxide dismutase (SOD2) levels (16–18). This effect is known to elevate radiation resistance and has been described as a thiol-induced delayed radioprotector effect (16–18). When coupled with metformin's ability to affect cellular metabolism and inhibit proliferation, the added ability of thiols to activate a NFκB prosurvival pathway mediated through elevated SOD2 could be reflected in the augmented elevation in cell survival observed when both types of agents were combined.
The focus of the countermeasures program has been the development of novel agents capable of protecting proliferating stem cells in self renewing radiation sensitive tissues such as bone marrow and gastro-intestinal tissues when administered at times after radiation exposure. Many of the approaches have focused on agents or natural products that enhance survival through the mechanism of inhibiting apoptosis in these sensitive stem cell target populations. While success has been recognized using this approach, a caveat remains regarding the potential of these damaged stem cells to proliferate with a high propensity for the future development of malignancies. Metformin offers a new paradigm in the approach to develop a post-radiation countermeasures drug. Not only does it have the ability to elevate cell survival, but it also has well recognized properties of being an anti-carcinogenesis agent that is currently being evaluated in clinical trials.
Acknowledgments
The authors thank the excellent technical assistance of Mr. Kenneth Baker in the performance of the in vitro and in vivo studies. This work was supported in part by the DOE Low Dose Program/Project Grant DE-SC0001271 and NIH NCI R01-CA132998 awarded to Dr. David J. Grdina. Dr. Jeffrey S. Murley is a minority equity partner in Pinnacle Oncology LLC and Dr. David J. Grdina is a paid consultant to Pinnacle Biologics and a minority equity partner in Pinnacle Oncology LLC.
References
- 1.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, et al. Models for evaluating agents intended for the prophylaxis, mitigation, and treatment of radiation injuries. Radiat Res. 2004;162:711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 2.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Modulation of radiation injury. Science. 2004;304:693–4. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 3.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009;85(7):539–73. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- 4.Grdina DJ, Kataoka Y, Murley JS. Amifostine: Mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Inter. 2000;16:237–79. doi: 10.1515/dmdi.2000.16.4.237. [DOI] [PubMed] [Google Scholar]
- 5.Muaddi H, Chowdhury S, Vellanki R, Zamiara P, Koritzinsky M. Contributions of AMPK and p53 dependent signaling to radiation response in the presence of metformin. Radiotherapy and Oncol. 2013;108:446–50. doi: 10.1016/j.radonc.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Song CW, Lee H, Dings RPM, Williams B, Powers J, Santos TD, et al. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep. 2012;2:362. doi: 10.1038/srep00362. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108(10):2021–32. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zannella V, Dal Pra A, Muaddi H, McKee T, Stapleton S, Sykes J, et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res. 2013 Oct 18; doi: 10.1158/1078-0432.CCR-13-1787. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Skinner HD, Crane CH, Garrett CR, Eng C, Chang GJ, Skibber JM, et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med. 2013;2(1):99–107. doi: 10.1002/cam4.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Na HJ, Park JS, Pyo JH, Lee SH, Jeon HJ, Kim YS, et al. Mechanism of metformin: Inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev. 2013;134:381–90. doi: 10.1016/j.mad.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Algire C, Moiseeva O, Deschenes-Simard X, Amrein L, Petruccelli L, Birman E, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res. 2012;5(4):536–43. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 12.Halicka HD, Zhao H, Li J, Traganos F, Zhang S, Lee M, et al. Genome protective effect of metformin as revealed by reduced level of constitutive DNA damage signaling. Aging. 2011;3(10):1–11. doi: 10.18632/aging.100397. www.impactaging.comopen access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tosca L, Rame C, Chabrolle C, Tesseraud S, Dupont J. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction. 2010;139:409–18. doi: 10.1530/REP-09-0351. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferrraros C, Menendez JA. Metformin and cancer. Cell Cycle. 2010;9:1057–64. doi: 10.4161/cc.9.6.10994. [DOI] [PubMed] [Google Scholar]
- 15.Grdina DJ, Murley JS, Miller RC, Mauceri HJ, Sutton HG, Thirman MJ, et al. A manganese superoxide dismutase (SOD2)-mediated adaptive response. Radiat Res. 2013;179:115–24. doi: 10.1667/RR3126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murley JS, Kataoka Y, Weydert CJ, Oberley LW, Grdina DJ. Delayed cytoprotection after enhancement of Sod2 (MnSOD) gene expression in SA-NH mouse sarcoma cells exposed to WR-1065, the active metabolite of amifostine. Radiat Res. 2002;158:101–9. doi: 10.1667/0033-7587(2002)158[0101:dcaeos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Murley JS, Kataoka Y, Weydert CJ, Oberley LW, Grdina DJ. Delayed radioprotection by nuclear transcription factor kB-mediated induction of manganese superoxide dismutase in human microvascular endothelial cells after exposure to the free radical scavenger WR1065. Free Rad Biol Med. 2006;40:1004–16. doi: 10.1016/j.freeradbiomed.2005.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murley JS, Kataoka Y, Cao D, Li JJ, Oberley LW, Grdina DJ. Delayed radioprotection by NFkB-mediated induction of Sod2 (MnSOD) in SA-NH tumor cells after exposure to clinically used thiol-containing drugs. Radiat Res. 2004;162:536–46. doi: 10.1667/rr3256. [DOI] [PubMed] [Google Scholar]
- 19.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–22. [PubMed] [Google Scholar]
- 20.Taubes G. Cancer prevention with a diabetes pill? Science. 2012;335:29. doi: 10.1126/science.335.6064.29. [DOI] [PubMed] [Google Scholar]
- 21.Evans JMM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. Br Med J. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]