Abstract
Advances in genome sequencing technologies have begun to revolutionize neurogenetics allowing the full spectrum of genetic variation to be better understood in relationship to disease. Exome sequencing of hundreds to thousands of samples from patients with autism spectrum disorder, intellectual disability, epilepsy, and schizophrenia provide strong evidence of the importance of de novo and gene-disruptive events. There are now several hundred new candidate genes and targeted resequencing technologies that allow screening of dozens of genes in tens of thousands of individuals with high specificity and sensitivity. The decision of which genes to pursue depends on numerous factors including recurrence, prior evidence of overlap with pathogenic copy number variants, the position of the mutation within the protein, the mutational burden among healthy individuals, and membership of the candidate gene within disease-implicated protein networks. We discuss these emerging criteria for gene prioritization and the potential impact on the field of neuroscience.
INTRODUCTION
Recent exome (and genome) sequencing studies of families have aimed to comprehensively discover genetic variation in order to identify the most likely causal mutation in patients with disease. Sequencing studies of parent-proband trios with intellectual disability (ID)1,2, autism spectrum disorder (ASD)3-7, schizophrenia (SCZ)8-10, and epilepsy11 have all suggested that de novo point mutations play an important role in pediatric and adult disorders of brain development (Table 1). The relative contribution of de novo mutations to each disorder remains to be determined but appears to correlate well with the degree of reduced fitness/fecundity of the given condition12. However, not only de novo events but also rare inherited CNVs can have an effect on fecundity, their overall effect on fecundity is however still debated13. Biologically, 75–80% of de novo point mutations arise paternally3,14 likely due to increasing numbers of cell divisions in the male germline lineage when compared to the female lineage. These findings are consistent with some epidemiological data which find advancing paternal age as a significant predictor of ASD, ID and SCZ15-17 and argue for the need to properly control for paternal age when comparing mutation rates between probands and siblings. The importance of de novo and private rare mutations is especially important clinically as there are now reports of diagnostic yields ranging from 10–55% for select (usually the most severe) groups of patients with ID1,2 and epilepsy18 in addition to resolution of unsolved Mendelian disorders19. It is clear that next-generation sequencing approaches have provided powerful tools for candidate gene identification. Deciding which genes to pursue, however, is not always self-evident since follow-up research and diagnostic studies are critical to understand the full contribution of a particular mutation to its respective phenotype.
Table 1.
A summary of major (exome) sequencing studies (n = 11). Summary of de novo mutation discovery, including: size of study, number of de novo mutations and severity for each group.
| Number of coding and splice site de novo point mutations in probands |
Number of coding and splice site de novo point mutations in controls/siblings |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Phenotype | Number of patients |
Number of controls (c)/ siblings (s) |
Total | LoF | Codon indels (in- frame) |
Missen se |
Synony mous |
Nonsyno nymous/s ynonymo us ratio |
Total | LoF | Codon indels (in- frame) |
Missen se |
Synony mous |
Nonsyno nymous/s ynonymo us ratio |
| Rauch et al. | ID | 51 | 20(c) | 91 | 21 | 0 | 58 | 12 | 6.6 (79/12) | 27 | 3 | 0 | 17 | 7 | 2.9 (20/7) |
| de Ligt et al. | ID | 100 | 0 | 79 | 15 | 0 | 48 | 16 | 3.9 (63/16) | NA | NA | NA | NA | NA | NA |
| O’Roak et al. | ASD | 209 | 50(s) | 260 | 38 | 0 | 154 | 68 | 2.8 (192/68) |
50 | 3 | 0 | 31 | 16 | 2.1 (34/16) |
| Sanders et al. * | ASD | 225 | 200(s) | 172 | 15 | 3 | 125 | 29 | 4.9 (143/29) |
125 | 5 | 0 | 82 | 38 | 2.3 (87/38) |
| Neale et al. | ASD | 175 | 0 | 169 | 18 | 0 | 101 | 50 | 2.4 (119/50) |
NA | NA | NA | NA | NA | NA |
| Iossifov et al.** |
ASD | 343 | 343(s) | 362 | 61 | 7 | 209 | 85 | 3.3 (277/85) |
314 | 30 | 8 | 203 | 73 | 3.3 (241/73) |
| Jiang et al. | ASD | 32 | 0 | 42 | 5 | 0 | 28 | 9 | 3.7 (33/9) | NA | NA | NA | NA | NA | NA |
| Allen et al. | EE | 264 | 0 | 289 | 36 | 1 | 196 | 56 | 4.2 (233/56) |
NA | NA | NA | NA | NA | NA |
| Gulsuner et al. | SCZ | 105 | 84(c) | 100 | 12 | 0 | 57 | 31 | 2.2 (69/31) | 67 | 11 | 0 | 37 | 19 | 2.5 (48/19) |
| Xu et al.*** | SCZ | 231 | 34 | 164 | 20 | 4 | 115 | 25 | 5.6 (139/25) |
17 | 1 | 0 | 11 | 5 | 2.4 (12/5) |
| Fromer et al. | SCZ | 623 | 0 | 640 | 65 | 9 | 410 | 156 | 3.1 (484/156) |
NA | NA | NA | NA | NA | NA |
|
| |||||||||||||||
| Sum: | 2358 | 731 | 2368 |
306
(12.9%) |
24
(1.0%) |
1501
(63.4%) |
537
(22.7%) |
3.4
(1831/537) |
600 |
53
(8.8%) |
8
(1.3%) |
381
(63.5%) |
158
(26.3%) |
2.8
(442/158) |
|
Putative LoF includes nonsense, frameshift and canonical splice site mutations based on gene annotation
study did not consider indels
not all de novo mutations were validated by Sanger sequencing
excluded splice site variants if not in canonical di-nucleotide of splice site. For some studies the numbers deviate from the numbers given in the main publication; numbers considered here were retrieved from de novo mutation overviews from supplementary tables and re-annotated using Seattle-Seq. When considering all studies together the total amount of de novo mutations identified in patients vs. controls is different (Fisher’s exact test: 0.0012), this may however rather reflect the technical differences between the individual studies. The most significant difference is seen for the number of LoF de novo mutations is significantly higher in patients than in controls (Fisher’s exact test: 0.0062); similarly the total amount of nonsynonymous vs. synonymous variants is higher in cases than in controls, however this does not reach significance (Fisher’s exact test: 0.066).
In this review, we will discuss the prioritization of candidate genes, show emerging trends, and highlight potential strategies for subsequent functional characterization of these neurodevelopmental genes. We focus on lessons learned from eleven recent studies that report 2,368 de novo mutations from a total of 2,358 probands and 600 de novo mutations from 731 controls (Table 1). The bulk of the data originate from sequencing studies of parents and probands with ASD, ID and epileptic encephalopathies (EE) but more recent studies have also highlighted the importance of de novo mutations in SCZ. There is evidence that de novo mutations, particularly disruptive mutations, occur in the same genes despite the nosological distinction for these different diseases. For the purpose of this review, we collectively term these diseases as ‘neurodevelopmental disorders’ but recognize that especially adult-onset diseases such as SCZ have etiologic components that are not neurodevelopmental in origin.
1) Recurrently mutated genes
One of the frequently used concepts in considering possible ‘new disease genes’ responsible for a given neurodevelopmental phenotype is the recurrence of de novo mutations in the same gene as well as the absence of such mutations in healthy controls. This rule follows the precedent established for the discovery of pathogenic de novo copy number variants (CNVs) during the last decade with the highest priority given to recurrent mutations that lead to a complete loss of function of one of the parental copies of the gene. Up to ten independent reports of de novo mutations in SCN2A and nine independent reports of de novo mutations in SCN1A and STXBP1 have been described (Tables 2 and 3). Strikingly de novo mutations in those genes are found, to date, exclusively in probands but never in controls. Simulation data suggest that at least two but certainly three or more recurrent de novo loss-of-function (LoF) events are unlikely to occur by chance, making such genes outstanding candidates (Table 2)7.
Table 2.
Recurrent and overlapping genes—de novo mutations in same genes observed between ID, ASD, EE and SCZ. Genes reported with recurrent (≥4) nonsynonymous de novo mutations identified in eleven studies on four different neurodevelopmental phenotypes.
| Gene | Total observations | Mutation type | Neurodevelopmental disorder | ||||
|---|---|---|---|---|---|---|---|
| LoF (nonsense, fs, splice) | missense | ASD | ID | EE | SCZ | ||
| SCN2A | 11 | 7 | 4 | 4 | 4 | 2 | 1 |
| SCN1A | 9 | 4 | 5 | 1 | 0 | 8 | 0 |
| STXBP1 | 9 | 2 | 7 | 1 | 3 | 5 | 0 |
| GABRB3 | 5 | 0 | 5 | 1 | 0 | 4 | 0 |
| TRIO | 5 | 0 | 5 | 2 | 2 | 1 | 0 |
| POGZ | 4 | 3 | 1 | 2 | 0 | 0 | 2 |
| MYH9 | 4 | 1 | 3 | 1 | 1 | 0 | 2 |
| SYNGAP1 | 4 | 4 | 0 | 0 | 3 | 0 | 1 |
TTN and MUC5B were excluded from this table due to high variant load in controls and unlikely involvement in the phenotypes discussed.
Table 3. Details of recurrent de novo mutations in SCN2A identified in seven studies with three different neurodevelopmental phenotypes.
| Gene | Coding Effect | Mutation (genomic DNA level) | Mutation (cDNA level) | Mutation (protein level) | Study | Disorder |
|---|---|---|---|---|---|---|
| SCN2A | frameshift | Chr2(GRCh37):g.166170553_166170584del | NM_021007.2:c.1318_1349del | p.Glu440Argfs*20 | Jiang et al. | ASD |
| SCN2A | frame shift | Chr2(GRCh37):g.166172105dup | NM_021007.2:c.1508dup | p.Asn503Lysfs*19 | Rauch et al. | ID |
| SCN2A | frameshift | Chr2(GRCh37):g.166179825_166179826del | NM_021007.2:c.1831_1832del | p.Leu611Valfs*35 | Rauch et al. | ID |
| SCN2A | missense | Chr2 (GRCh3 7):g.166198975G>A | NM_021007.2:c.2558G>A | p.Arg853Gln | Allen et al. | EPI |
| SCN2A | missense | Chr2(GRCh37):g.166198975G>A | NM_021007.2:c.2558G>A | p.Arg853Gln | Allen et al. | EPI |
| SCN2A | missense | Chr2(GRCh37):g.166201311C>T | NM_021007.2:c.2809C>T | p.Arg937Cys | Rauch et al. | ID |
| SCN2A | nonsense | Chr2(GRCh3 7):g.166201379C>A | NM_021007.2:c.2877C>A | p.Cys959* | Sanders et al. | ASD |
| SCN2A | nonsense | Chr2(GRCh3 7):g.166210819G>T | NM_021007.2:c.3037G>T | p.Gly1013* | Sanders et al. | ASD |
| SCN2A | nonsense | Chr2(GRCh37):g.166231415G>A | NM_021007.2:c.4193G>A | p.Trp1398* | de Ligt et al. | ID |
| SCN2A | missense | Chr2(GRCh37):166234111C>T | NM_021007.2:c.4259C>T | p.Thr1420Met | Iossifov et al. | ASD |
| SCN2A | splice site | Chr2(GRCh37):g.166187838A>G | NM_001040142.1:c.2150–2A>G | p.? | Fromer et al. | SCZ |
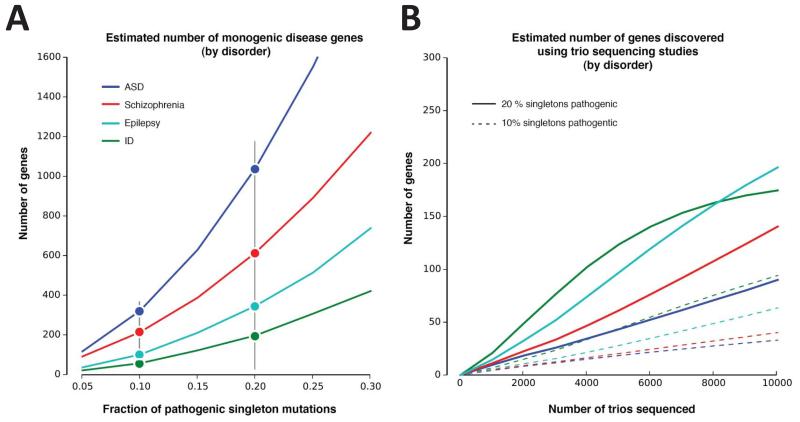
The frequency of recurrence is dependent on the extent of locus heterogeneity associated with each disease. Leveraging observed recurrences of de novo mutations (Fig. 1), diseases such simplex autism and SCZ are thought to arise from >500 genes, while studies of severe ID and epileptic encephalopathies (infantile spasms and Lennox-Gastaux subtypes) suggest lower heterogeneity. Such estimates should be considered only rough approximations at this point since they are highly dependent upon the fraction of de novo mutations that are in fact pathogenic as well as ascertainment biases in sample collection (Fig. 1). In this regard it is interesting that several of these top-scoring candidate genes have been observed in patients with epilepsy11, ID1,2, and ASD3-7. This may not be surprising in light of the comorbidity of these diseases and if one accepts that the level of heterogeneity is lower for epilepsy-related disorders than for ASD, SCZ, or ID. In such a scenario, sequencing of even a modest number of epilepsy cases delivers recurrent mutations more frequently than more broadly defined DD (developmental delay) or ASD11,18. One study of SCZ, for example, highlighted four recurrently mutated genes, but maybe more remarkable the same study identified overlapping genes with ASD when focusing on prenatally expressed genes9. The stronger overlap between ASD, ID and epilepsy and yet limited overlap with SCZ (Table 2) could be largely because only a subset of the disease stems from a neurodevelopmental origin.
Figure 1. Genes with recurrent de novo mutations in four neurodevelopmental disorders.
(A) We estimate the number of fully penetrant disease genes based on a de novo model using the “Unseen Species Problem”. We consider all recurrent missense or loss-of-function de novo mutations pathogenic, as well as a defined fraction of mutations in genes observed just once (because all de novo mutations are unlikely to be pathogenic). The ratio between genes mutated recurrently and the rate of “singleton” mutations suggests an estimate for the “true” number of pathogenic genes. Including more singleton mutations increases the fraction of each disorder explained by single de novo SNVs at the “cost” of including more pathogenic genes. Initial exome sequencing studies of epilepsy and ID focused on specific pediatric subtypes or the most severe cases; thus, the number of generalized epilepsy or ID genes is likely to be much higher. (B) Expected hit rate (or sensitivity) of true positive genes discovered using trio sequencing studies (under a family-wise error rate of 5%, i.e. each gene passes exome-wide significance of 2.6e–6). We estimate the power of trio sequencing to detect statistically significant associations for disease genes, under the assumption that 10% or 20% of singleton mutations could be fully penetrant genes (vertical black bar in (A)). We assume the distribution of these genes is uniform within each disorder and that they do not differ significantly from all genes in terms of length and mutability, although these are taken into account when determining significance.
Perhaps, the most striking examples are recurrent identical de novo mutations within the same gene. Across the various studies, such identical recurrences have already been observed for six genes (ALG13, KCNQ3, SCN1A, CUX2, DUSP15 and SCN2A; Table 4). Such events are exceedingly unlikely with estimates of identical recurrences in ALG13 and SCN2A calculated at p = 7.77 × 10−12 and p = 1.14 × 10−9, respectively, in the case of epilepsy11. Most of these estimates of significance, however, assume a random mutation process. Yet mutational hotspots certainly exist and recurrence of the same mutation cannot be taken as proof positive of an association.
Table 4. Recurrent identical de novo mutations in six genes identified in eleven published exome studies with different neurodevelopmental phenotypes.
| Gene | Coding Effect | Mutation (genomic DNA level) | Mutation (cDNA level) | Mutation (protein level) | Study | Disorder |
|---|---|---|---|---|---|---|
| ALG13 | Missense | ChrX(GRCh37):g.110928268A>G | NM_001099922.2:c.320A>G | p.Asn107Ser | de Ligt et al. | ID |
| ALG13 | Missense | ChrX(GRCh37):g.110928268A>G | NM_001099922.2:c.320A>G | p.Asn107Ser | Allen et al. | EE |
| ALG13 | Missense | ChrX(GRCh37):g.110928268A>G | NM_001099922.2:c.320A>G | p.Asn107Ser | Allen et al. | EE |
| KCNQ3 | Missense | Chr 8(GRCh3 7):g.133192493 G> A | NM_001204824.1:c.328C>T | p.Arg110Cys | Rauch et al. | ID |
| KCNQ3 | Missense | Chr 8(GRCh3 7):g.133192493 G> A | NM_001204824.1:c.328C>T | p.Arg110Cys | Allen et al. | EE |
| SCN1A | splice donor | LRG_8:g.24003G>A | NM_006920.4:c.602+1G>A | p.? | Allen et al. | EE |
| SCN1A | splice donor | LRG_8:g.24003G>A | NM_006920.4:c.602+1G>A | p.? | Allen et al. | EE |
| CUX2 | Missense | Chr 12(GRCh3 7):g.111748354G> A | NM_015267.3:c.1768G>A | p.Glu590Lys | Rauch et al. | ID |
| CUX2 | Missense | Chr 12 (GRCh3 7):g.111748354G>A | NM_015267.3:c.1768G>A | p.Glu590Lys | Allen et al. | EE |
| SCN2A | Missense | Chr2(GRCh37):g.166198975 G>A | NM_021007.2:c.2558G>A | p.Arg853Gln | Allen et al. | EE |
| SCN2A | Missense | Chr2(GRCh37):g.166198975 G>A | NM_021007.2:c.2558G>A | p.Arg853Gln | Allen et al. | EE |
| DUSP15 | Missense | Chr20(GRCh37):g.30450489G>A | NM_080611.2:c.320C>T | p.Thr107Met | Neale et al. | ASD |
| DUSP15 | Missense | Chr20(GRCh37):g.30450489G>A | NM_080611.2:c.320C>T | p.Thr107Met | Fromer et al. | SCZ |
The significance of the majority of de novo mutations remains unclear. For most genes, only a single de novo mutation has been identified. Nevertheless, based on the observation that de novo LoF mutations occur 2–3 times more frequently in ASD probands when compared to unaffected siblings, it is now estimated that a large fraction of these singletons will be relevant to disease etiology. When considering all studies in aggregate, de novo LoF mutations are observed significantly more in cases than in controls (Table 1; Fisher’s exact test: p-value = 0.0062). The interpretation of recurrent missense mutations, however, represents a greater challenge. Sanders et al.4 estimated that four missense de novo mutations in the same genes would be required in simplex autism to exceed a chance finding; this was based on a cohort size of up to 2,000 with an estimated locus heterogeneity of 1,000 ASD risk loci. Given the extreme locus heterogeneity of diseases such as ASD and ID, other strategies have been adopted to prioritize likely causal genes. High-throughput targeted multiplexed resequencing technologies, such as molecular inversion probes, have been employed to screen ~50 candidate genes in thousands of patients and controls3,18. Such approaches are scalable, inexpensive (<$1 per gene per sample), sensitive, and specific increasing by an order of magnitude the number of patients that can be screened. The strategy was particularly useful in discovering a burden of de novo LoF mutation of CHD8 associated with ASD20. The relatively ease in detecting de novo mutations allows rapid identification of potential candidate genes; the number of cases that are required to make these findings statistically significant (Fig. 1) can be lower for the genes that are mutated exclusively in a large number of patients when compared to standard case-control studies21.
2) Prior evidence of overlap with pathogenic CNVs
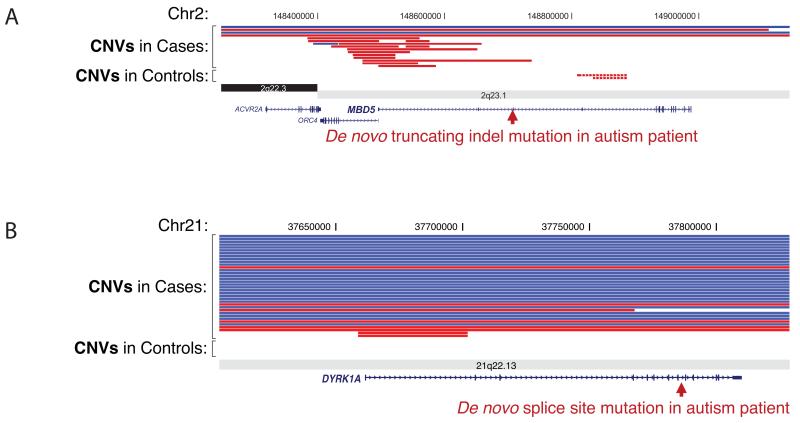
Another strategy has been to compare patterns of CNVs in patient and control populations to prioritize genes (Fig. 2)22. Extensive CNV morbidity maps have been developed for tens of thousands of children with autism, ID and epilepsy helping to define pathogenic regions of dosage imbalance in the human genome23-26. Overlapping deletions in such collections occasionally refine the smallest region of overlap highlighting a modest number of candidate genes. Recurrent de novo point mutations in a gene within such a region with CNV burden significantly increases the likelihood that LoF of the gene is responsible for a phenotype. O’Roak et al.3 and Rauch et al.2 each discovered, for example, LoF point mutations for SETBP1—a gene where a significant enrichment for deletion CNVs has been seen in patients with overlapping neurodevelopmental phenotypes but not in controls. Similar patterns have recently been observed for DYRK1A and MBD5 (Fig. 2), including reports of balanced but gene-disrupting chromosomal translocations27. Such information has been used to compute haploinsufficiency scores2,28 to strengthen the case of ’causality’ of de novo LoF mutations (Fig. 2).
Figure 2. CNV and exome intersections define candidate genes.
Deletion (red) and duplication (blue) burden for DD/ID cases and controls for two genes (A) DYRK1A and (B) MBD5 as compared to sporadic LoF mutations based on exome sequencing of 209 autism simplex trios. DYRK1A is a strong candidate gene for cognitive deficits associated with Down syndrome; LoF mutations are associated with minibrain phenotype in Drosophila65, autism-like behavior in mouse64, and deletion syndrome in humans27,63. MBD5 has been implicated as the causal gene for the 2q23.1 deletion syndrome associated with epilepsy, autism, and ID91,92.
3) Position of the mutation within the protein
The bulk of de novo mutations discovered from exome sequencing projects are missense mutations, with more than 60% in probands and controls (Table 1). Distinguishing pathogenic signal from the background of benign mutations is an active area of research. For genes for which previous CNVs or LoF mutations were described, ‘severe’ missense mutations are also likely to result in a dosage effect. However, there are examples, usually from clinically well-defined neurodevelopmental syndromes, showing that missense mutations result in different outcomes either based on the protein domain they affect, the position in the gene (e.g., the amino- or carboxy-terminus of the resulting protein)29, or their potential to modify the normal function of the protein. Examples for the latter include gain-of-function (GoF) mutations and LoF mutations in the same gene that result in different phenotypic outcomes30,31. SETBP1 GoF in a degron sequence (ubiquitination motif) results in the rare but well-defined Schinzel-Giedion syndrome32 while deletions or LoF mutations may result in a distinct and milder phenotype comprising ASD/ID with speech delay and other features2,3,33,34. Other examples include different phenotypic effects dependent on the location of the mutation; early truncating and missense mutations in NOTCH2 are known to cause Alagille syndrome35 while truncating events restricted to the last exon escape nonsense mediated decay (NMD) and result in Hajdu-Cheney syndrome36,37.
4) Mutational burden among healthy individuals
One approach to prioritizing missense mutations leverages evolutionary conservation by assigning ‘mutability scores’ per gene or even at the base pair level. O’Roak et al.3, for example, established an evolutionary mutation score per human gene based on the human-chimpanzee divergence and the size of a gene. Similarly the Epi4k Consortium used a gene-specific mutation rate based on a per-base score38; this score was, however, not based on human-chimpanzee evolution but made use of human-specific polymorphism data. A recent publication used rare variant data from healthy individuals and offers a new integrative annotation tool for noncoding variants39. The wealth of available control exome sequence data can also be used to estimate the (rare) variant load per gene (and distribution). For example, the analysis of data generated from sequencing 6,500 ‘control’ exomes as part of the ESP6500 has been used to define the load of LoF mutations per gene2 and to prioritize >4,000 human genes that are most intolerant to variation11. Another approach uses random mutation modeling40 to calculate the likelihood that observed (de novo) mutations have a damaging effect; similar prioritizations are provided by tools that score individual mutation severity (SIFT, PolyPhen2, MutationTaster, MutPred, CONDEL, etc.), some of which can be adapted to a gene-based prioritization score from genome-wide data41. These population data provide a powerful unbiased approach to hone in on genes that are likely to be among the most penetrant because of the complete absence of disruptive variation in the general population (e.g., CHD8 or DYRK1A). A critical aspect of such analyses is the reliability of a particular gene model. Most human genes show evidence of alternative splice forms—many of which have no known function. Apparent hotspots of mutation for a particular exon (often 5′ or 3′) in both cases and controls may suggest mis-annotation, the presence of a processed pseudogene, or an alternative nonfunctional splice form.
5) Pathway enrichment and links to cancer biology
Another popular approach to suss out the most important gene candidates for further characterization has been to identify specific biological networks of genes enriched in cases as compared to controls. Although this approach cannot be used unequivocally to define causality, membership of a specific gene in a particular protein-protein interaction (PPI) or co-expression network may increase the likelihood of its association with disease. Numerous studies have reported significant enrichment of both de novo CNV and SNV mutations in particular pathways3,4,42,43. O’Roak et al., for example, reported a significant enrichment of de novo disruptive autism mutations among proteins associated with chromatin remodeling, beta-catenin and WNT signaling—a finding that was replicated in a follow-up resequencing study of more than 2,400 probands. One instance, in which membership of a new candidate gene within a PPI network led to the discovery of an autism gene, is the recent example of ADNP. A single ADNP LoF mutation was initially observed from exome sequencing studies. Although the gene did not reach statistical significance when comparing cases and controls20, it was strongly implicated in the PPI network originally defined by O’Roak et al. Targeted resequencing experiments combined with clinical exome sequencing identified several additional cases with de novo mutations and remarkably similar phenotypes representing a new SWI-SNF-related autism syndrome (Fig. 3)44. Notably, many of the genes implicated in the beta-catenin pathway have also been described as mutated in patients with ID1 but not in patients with SCZ. Similarly, an enrichment of genes interacting with FMRP—the gene responsible for Fragile X syndrome—has been reported with de novo mutations in ASD5, epilepsy11 and, most recently, SCZ10,45. Whether this observation is due to the relative high degree of cases that also presented with comorbid ID remains to be determined.
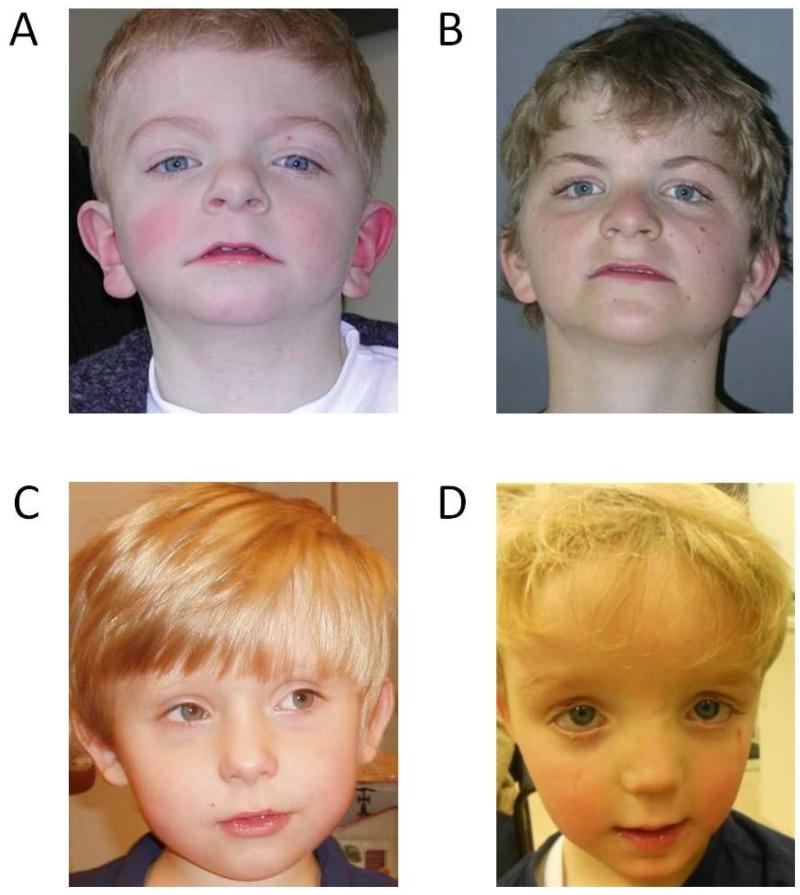
Figure 3. Phenotypic similarity of two patients with identical PACS1 de novo mutations and two patients with similar ADNP mutations.
(A and B) These two unrelated patients show identical de novo point mutations (c.607C>T; p.(Arg203Trp)) mutation in PACS1 (RefSeq NM_018026.2)53. The striking similarity in clinical phenotype include low anterior hairline, highly arched eyebrows, synophrys, hypertelorism with downslanted palpebral fissures, long eyelashes, a bulbous nasal tip, a flat philtrum with a thin upper lip, downturned corners of the mouth, and low-set ears. (C and D) These two unrelated patients both show LoF mutations in ADNP (c.2496_2499delTAAA; p.(Asp832Lysfs*80) and c.2157C>G; p.(Tyr719*))44 resulting in a new SWI-SNF related autism syndrome. Patients present with clinical similarities, including a prominent forehead, a thin upper lip and a broad nasal bridge.
In addition to PPI networks, studies of co-expression have shown enrichment for specific spatio-temporal patterns of expression. A study of co-expressed genes affected by de novo mutations reported an enrichment in fetal prefrontal cortical network in SCZ8, which is in line with the finding by Xu et al.9 that genes with higher expression in early fetal life have significant contribution to SCZ by de novo mutations. Similarly, Willsey et al.46 working with a few high-confidence sets of ‘ASD genes’ as seeds reported a convergence of deep layer cortical projection neurons (layers 5 and 6) in mid-fetal development. Another analysis using a larger set of ASD and ID risk genes suggested translational regulation by FMRP and an enrichment in superficial cortical layers43. Implicit in these types of analyses is the notion that while more than 1,000 genes may be responsible for ASD or ID, in the end the genes will converge on a few highly enriched networks of related genes. It is possible that molecular therapies targeted to the network at a specific stage of development as opposed to the individual gene may be beneficial to specific groups of patients.
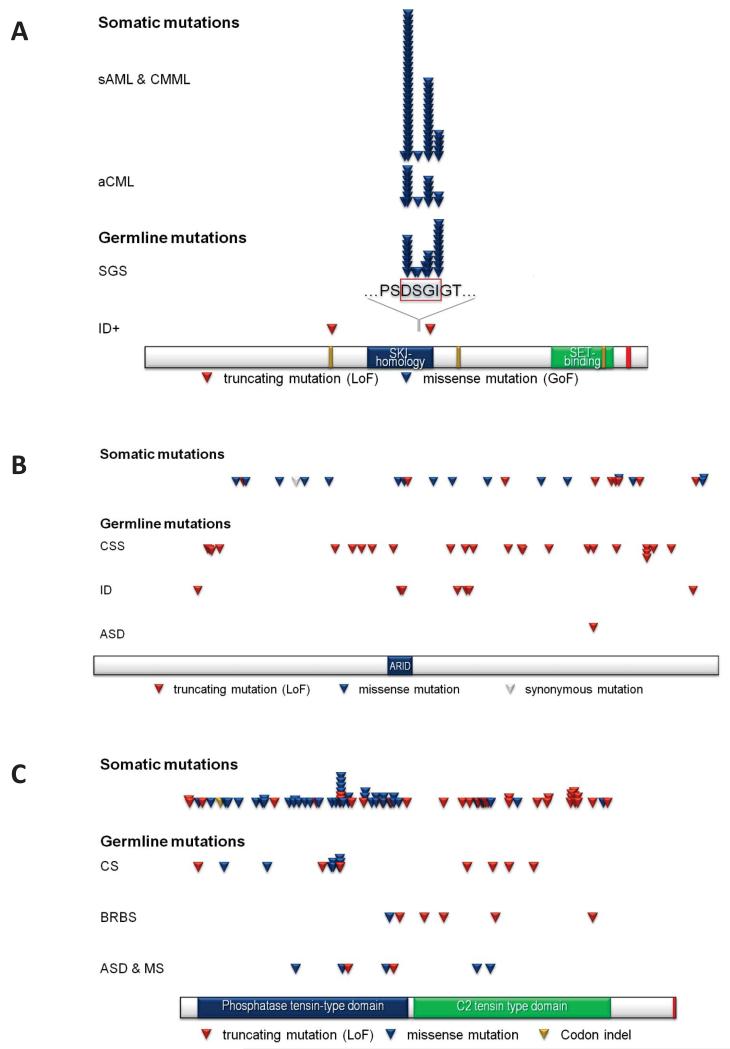
Related to this, it is intriguing that several recurring genes and pathways that have been implicated in neurodevelopmental disease have also been associated with different forms of cancer (Fig. 4)47. While clear-cut examples like the mutation of tumor suppressor genes, PTEN (Cowden syndrome) or ARID1B (Coffin-Siris syndrome), and neurodevelopmental disease have been extensively reviewed48, more recent exome sequencing data from patients with neurodevelopmental disease suggests potentially new links. The most striking observation here is the identical point mutations reported to cause cancer when mutated somatically and (severe) neurodevelopmental syndromes when mutated in the germline. Examples include the identical mutations in SETBP132, ASXL149, and EZH250, as well as several genes of the RAS-MAP-kinase pathway associated with parental-age effect Mendelian disorders51 (Table 5). It is important to stress that this is an observation at an individual gene level and should not be translated to an epidemiological link, i.e., this cannot be generalized to speculate that patients with neurodevelopmental disorders in these specific genes will all be at a higher risk for certain cancer types. Instead, it is likely that this convergence represents a selection of genes that play a fundamental role in cell biology (e.g., cell proliferation and/or membership in multi-subunit complexes associated with chromatin remodeling). There is also the distinct possibility of pleiotropy; i.e., genes and pathways have completely unrelated functions explaining developmental defects and cancer independently. Therefore, de novo mutations in those genes can result in different outcomes depending on timing, genetic background, and cellular context. Nevertheless, there may be advantages to integrating sequence data from patients with neurodevelopmental disease and massive sequencing programs devoted to the discovery of somatic mutations within tumors, e.g., the International Cancer Genome Project52. It is possible that these intersections will help to further prioritize genes important in both cellular development and neurodevelopment.
Figure 4. Coincidental de novo mutations in cancer and neurodevelopmental disorders.
Examples: SETBP1 (Figure 2A), ARID1B (Figure 2B) and PTEN (Figure 2C).
(A) Mutation spectrum of SETBP1. sAML & CMML = secondary acute myeloid leukemia & chronic myelomonocytic leukemia93 [1× p.Asp868Tyr, 28× p.Asp868Asn, 1× p.Ser869Asn, 15× p.Gly870Ser, 5× p.Ile871Thr]; aCML = atypical chronic myeloid leukemia94 [7× p.Asp868Asn, 1× p.Ser869Gly, 5× p.Gly870Ser, 2× p.Ile871Thr]; SGS = Schinzel-Giedion syndrome32 [Hoischen et al. unpublished: 1× p.Asp868Ala, 7× p.Asp868Asn, 1× p.Ser869Arg, 1× p.Ser869Asn, 4× p.GGly870Ser, 2× p.Gly870Asn, 10× p.Ile871Thr]; ID+ = intellectual disability with other features2,3 [p.Leu592* & p. 906fs]. (B) Mutation spectrum of ARID1B. Somatic mutations retrieve from COSMIC database. Only ‘somatic validated’ and ‘previously described’ somatic mutations with PubMed entry were considered.
CSS = Coffin-Siris syndrome48,55 [p.Gln408Profs*127, p.Ser413Valfs*122, p.Asn420Lysfs*115, p.Pro449Argfs*53, p.Tyr867Thrfs*47, p.Met935Asnfs*7, p.Ser959Argfs*9, p.Ala1000Argfs*5, p.Arg1075*, p.Gly1283Trpfs*38, p.Arg1337*, p.Tyr1366*, p.Pro1489Leufs*10, p.Tyr1540*, p.Gln1541Argfs*35, p.Trp1637Cysfs*6, p.Lys1777*, p.Phe1798Leufs*52, p.Asp1879Thrfs*95, p.Arg1990*, p.Arg1990*, p.Arg1990*, p.Trp2013*, p.Pro2078Leufs*21]; ID = intellectual disability54 [p.Arg372Profs*163, p.Arg1102*, p.Lys1108Argfs*9, p.Gln1307*, p.Tyr1346*, p.Arg1338Argfs*76, p.Ser2155Leufs*33]; ASD = autism spectrum disorder3 [p.Phe1798Leufs*52]; splice site mutations not considered. (C) Mutation spectrum of PTEN. Somatic mutations retrieved from COSMIC database. Only ‘somatic validated’ and ‘previously described’ somatic mutations with at least five independent entries are displayed. CS = Cowden syndrome; ASD & MS = autism spectrum disorder and macrocephaly syndrome; BRBS = Bannayan-Riley-Ruvalcana syndrome [based on OMIM entries]; splice site mutations not considered.
Table 5. Genes implicated in cancer (somatic mutations) and developmental disorders (germline mutations).
| Gene name | Neurodevelopmental disorder |
PubMed
ID |
Mutation
types |
Cancer
types/malignan cies |
PubMed
ID |
Mutation types |
|---|---|---|---|---|---|---|
| Isolated neurodevelopmental phenotypes and cancer | ||||||
| PTEN | ASD, Cowden S.* | 23160955, 9259288 |
LoF, missense |
Many types | 21252315 | missense, LoF |
| CTCF | ID | 23746550 | LoF | Breast cancer, Prostate cancer |
9591631 | deletion |
| ARID1B (SWI/SNF complex) | ID, ASD, Coffin-Siris S. | 22426309, 22426308 |
LoF | Many types | 22426308 | LoF |
| Clinically defined neurodevelopmental syndromes and cancer | ||||||
| MED12 | Lujan-Fryns S., Ohdo S., Opitz-Kaveggia S. | 17369503, 23395478, |
missense | Prostate cancer | 22610119 | missense |
| MLL2 (KMT2D) | Kabuki S. | 20711175 | LoF, CNV, missense |
Many types | 21796119, 21163964 |
LoF, missense |
| CREBBP | Rubinstein-Taybi S.; epilepsy | 7630403, 11331617 |
LoF, CNV, missense |
ALL | 21390130 | LoF, missense |
| ATRX | X-linked α-thalassaemia/mental retardation S. | 7697714 | Missense, LoF |
PanNET, Glioblastoma |
21252315, 22286061 |
missense, LoF |
| Identical mutations in neurodevelopmental phenotypes and cancer | ||||||
| ASXL1 | Bohring Opitz S. | 21706002 | LoF | Myeloid malignancies |
19388938 | LoF |
| SETBP1 | Schinzel Giedion S. | 20436468 | GoF | Leukemia | 23222956 | GoF |
| EZH2 | Weaver S.* | 22177091 | GoF | B-cell lymphoma | 20081860 | GoF |
| ‘Paternal age effect disorders’ (FGFR2, FGFR3, HRAS, PTPN11, BRAF, MAP2K1) |
Apert S., Crouzon/Pfeiffer S., achondroplasia, Muenke S., Costello S.*, Noonan S.*, cardio-facio-cutaneous S. |
22325359 | GoF | Many types | 22325359 | GoF |
| Different mutation types observed in neurodevelopmental phenotypes and cancer | ||||||
| CTNNB1 | ID/ASD | 23033978 | LoF | Many types | 12060769 | activating?, LoF? duplications, CNV, |
| CHD7 | CHARGE S. | 15300250 | CNVs, LoF, missense |
Small-cell lung cancer |
20016488 | amplifications, translocations |
| MYCN | Feingold S. | 15821734 | LoF, missense |
Neuroblastoma | 6197179 | amplification |
| ABCC9 | Cantu S. | 22610116, 22608503 |
GoF | Endometrial cancer |
23104009 | missense |
Patients have increased risk of cancer;
Abbreviations: PanNET – Pancreatic neuroendocrine tumors; ALL – acute lymphoblastic leukemia; AML – Acute myeloid leukemia; S. – Syndrome
Phenotypic similarity of recurrent de novo mutations
Statistical support of recurrent mutations is not the sole arbiter in determining pathogenicity of particular mutations and genes. In particular, it is important to consider the phenotypic presentation and overlap of the individuals with the same presumptive underlying genetic lesion. In this regard, we note that many of the initial studies are likely enriching for the most severe cases since ascertainment is clinical as opposed to population based. As a result, initial estimates of penetrance may be overestimated and phenotypic heterogeneity underestimated. Nevertheless, identification of clinically recognizable syndromes or sets of phenotypic features has historically been used to strengthen the case of a particular gene’s involvement. In the past, gene discovery was usually driven by detailed description of a particular syndrome (e.g., Fragile X or Rett syndrome) followed by a systematic hunt for the mutated gene. Recognition of clinical subtypes, however, is now beginning to occur after mutation and gene discovery. In the case of PACS153, for example, identical de novo mutations result in patients with a strikingly similar phenotypic outcome (Fig. 3). Such clinical discernment now, more than ever, requires the expertise of the clinician.
It should be noted, however, that not all genes when mutated will show a phenotypic convergence but rather may be much more variable in their phenotypic presentation. For example, mutations in ARID1B can either lead to isolated ID54 or syndromic forms of ID with a recognizable phenotype known as Coffin-Siris syndrome48,55. The type of mutation may be critical in this regard. It is noteworthy that patients with LoF mutations of SCN2A described in autism cohorts4 do not present with epilepsy in contrast to multiple recurrent missense mutations identified among epileptic encephalopathies, or more specifically infantile spasms or Lennox-Gastault subtype. Similarly, de novo missense mutations in CHD2, SETD5, and SLC6A1 have been reported in patients with ASD yet frameshift mutations in the same genes are seen in patients with ID but without ASD features2. There are several considerations regarding genotype-phenotype correlations.
Some of the classically defined neurodevelopmental clinically defined syndromes may present with broader (or milder) phenotypes as defined by initial clinical case reports2. There is evidence that the genetic background upon which these mutations occur significantly influences phenotypic outcome56-58.
‘Genotype-first’ approaches using current genomic technologies followed by ‘reverse phenotyping’ are beginning to define more subtle syndromes that are still opaque within large umbrella cohorts such as ASD or ID13. Some examples include macrocephalic subtypes of ASD/ID caused by mutations in PTEN and CHD820,59 and DD/ID and epilepsy caused by de novo mutations in SCN2A1,2,18.
After discovery of potential causative mutations, more detailed and standardized phenotyping assessments are necessary to eliminate disease ascertainment biases. Since patients with a specific mutation will be individually rare, greater coordination, including patient recontact, will need to occur across clinical research centers.
Detailed phenotypic characterization of patients is an important first step in modeling mutations in other organisms. Indeed, additional support for a gene’s involvement in disease often is provided by related pathologies in these model organisms and may be used to rapidly prioritize genes for further study as well as to provide additional insight into function. In many cases, mouse models60 or Drosophila mutant lines61 already exist and neurologic phenotypes have been, at least, partially documented. For example, recurrent LoF mutations of ADNP (activity dependent neuroprotective peptide) were recently described in patients with autism and ID3. Heterozygous knockout mice show a neuronal glial pathology associated with reduced cognitive function62 and this phenotype was recognized in mouse models prior to the association in human disease. Similarly, heterozygous deletions or mutations of DYRK1A in humans20,63, mice64 and fruitflies65 all show a phenotype of reduced brain volume associated with microcephaly. In this regard, it is interesting that the DYRK1A LoF were the last to be documented with the models preexisting the discovery of human genetic diseases. With new resources like the Zebrafish Mutation Project66 and the International Knockout Mouse Consortium60, more systematic and high-throughput genome-wide approaches for model organisms may be achieved in particular for LoF mutations.
Limitations and future directions
Despite the great success of recent exome studies, it is important to note that most of the analyses, to date, have been restricted to the protein-coding portion of the genome—a very small fraction (1.5%) of all human genetic variation. Furthermore, the definition of the protein-coding portion is far from perfect. Portions of the reference genome67,68 and gene annotation69 are incomplete especially in relation to isoforms specifically expressed in the brain. Regulatory variation and its impact are currently ignored. Even though genome sequencing costs have reduced, discovery and interpretation of genetic variation remain significant hurdles. Unlike protein-coding sequencing, defining the functional regions and the type of mutations that will abrogate such function remain active areas of research. Nevertheless, the genes where dosage imbalance have been found to strongly associate with disease represent a logical starting point to begin to interrogate regulatory mutations as well as epigenetic effects that may have a similar effect. Targeted resequencing of the entire genomic loci as well as full genome sequencing will undoubtedly discover new mutations and further improve our understanding of the phenotype-genotype relationship. Despite the recent emphasis on de novo mutations, their contribution to disease can only be understood in the context of the full spectrum of genetic variation of each individual25,57.
Even for the protein-coding component, sequence discovery is incomplete with 5–10% of the exons either being missed or insufficiently captured to call genetic variation. The bias is particularly pronounced for genes mapping to high GC content regions of the genome where as many as 20–30% of the exons may be insufficiently covered. The sequencing technology also introduces biases against certain types and classes of mutation. The discovery of indels is largely regarded as incomplete because of difficulties associated with mapping short sequence reads in low complexity regions70. Although there have been recent methods to call smaller CNVs, validation experiments indicating specificity and sensitivity are still far from ideal71,72. The development of new sequencing chemistries and platforms that can cheaply and in a high-throughput manner access these regions of the genome should remain a high priority.
There is another level of reduced sensitivity, related to the timing of de novo mutations. It is increasingly recognized that postzygotic de novo mutations, i.e., mutations in somatic state, may play an important role in neurodevelopmental disorders73. While the importance of somatic de novo mutations has been recognized for many years in the field of cancer genetics52,74-76, we are only starting to appreciate its prevalence in neurodevelopmental disease77,78. Several exome studies report that individual de novo mutations likely occurred postzygotically with estimates ranging from 1–2% of new mutations based on analysis of DNA derived from blood. O’Roak et al.3, for example, have shown that ~4% (9/209 cases) of de novo mutations likely occurred postzygotically. Mosaic mutations have been observed as a more general theme for CNVs, but not yet linked systematically to disease79. More sensitive technologies80,81 are required to identify lower-level mosaics as well as access to clinically more relevant tissue types. For defined disorders with isolated neurologic involvement, this may never be possible if the mosaicism is restricted to neuronal subtypes in the brain.
Next to technical hurdles, there is the daunting prospect of the extreme locus heterogeneity of these diseases. This raises the distinct possibility that a recurrent de novo mutation in a second patient will never be seen again in the same clinic. This can be partially overcome by developing new models for data sharing (e.g., de novo variant databases) and generating larger sample collections of patients (>50,000) that may be screened in follow-up targeted resequencing experiments. This requires a shift toward a more integrated and collaborative model of clinical and basic research. Successful models of clinical lab cooperation and standardization have already been established for the exchange of CNV data, e.g., the ISCA (International Standards for Cytogenomic Arrays) Consortium, and there is momentum to do the same for exome and genome sequence datasets, e.g., the ICCG (International Collaboration for Clinical Genomics)24. The sheer size of the dataset (multiple Petabytes), ever-changing advances in sequencing technology, and the importance of standardized call sets, however, pose major challenges moving forward.
Although sporadic mutations have been the focus of this review, the importance of inherited mutations should not be underestimated. There is in fact compelling evidence that such variation contributes significantly to these diseases45,72,82,83. While specific gene effects are much more difficult to tease apart in the general population owing to the genetic heterogeneity of these diseases45, other approaches such as studies of consanguineous families have identified numerous candidate risk genes under a recessive disease model84-86. It should also be noted that the effect size and penetrance for many of the recurrent de novo mutations is not yet known. For autism, de novo mutations have been thought to collectively increase risk 10– to 20–fold for up to 20% of cases with disease. It is likely that in some cases a rare variant will be necessary but not sufficient to confer phenotype requiring that both inherited and de novo mutations be jointly considered in order to understand their impact as has been noted for some CNV risk variants57,87. Understanding the gender bias, which is particularly pronounced for ASD and ID, will require integrating inherited and de novo mutations from both the X chromosome and autosomes. Data from CNVs as well as SNVs suggest that the carrier burden of males and females differ significantly42,72,88,89. The evidence suggests that females are more likely to be carriers of deleterious mutations and, therefore, protected against such diseases perhaps sex dependent modifiers map genetic architecture to diagnostic boundaries differently between the sexes.
Since many of the genes linked to disease will likely have minimal functional annotation in the human genome, it will be necessary to perform systematic studies to understand their specific role in neurodevelopment. The sheer volume of high-impact genes will probably necessitate large-scale model organism knockouts in Drosophila, zebrafish, and mouse60,66, industrial-level development of iPSC cell lines and neuronal differentiation protocols90, as well as massive screens using mass spectrometry to identify protein-interacting partners. All of these approaches have their own limitations. For example, it is an open question how well knockouts will model neurodevelopmental diseases such as ASD or ID since most of the known effects in humans occur in the heterozygous state and most knockout phenotypes are typically studied as homozygous LoF. Many phenotypic aspects of complex neuropsychiatric and neurobehavioral disease will not be amenable to model systems further limiting such functional approaches. Notwithstanding these challenges, it is a golden age of ‘neurogene’ discovery which promises to improve not only our understanding of disease but provide fundamental insight into the biology of human brain development.
Supplementary Material
ACKNOWLEDGEMENTS
We grateful to Tonia Brown and Christian Gilissen for assistance during manuscript preparation; Frank Kooy for early preprint access and Han G. Brunner for sharing patient photographs used for Figure 3. A.H. is supported by a ZonMW grant (916–12–095); E.E.E. is supported by a National Institute of Mental Health (NIMH) grant (1R01MH101221–01) and is an investigator of the Howard Hughes Medical Institute.
Footnotes
CONFLICT OF INTEREST STATEMENT
E.E.E. is on the scientific advisory board (SAB) of DNAnexus, Inc. and was an SAB member of Pacific Biosciences, Inc. (2009–2013) and SynapDx Corp. (2011–2013).
REFERENCES
- 1.De Ligt J, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–9. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 2.Rauch A, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–82. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 3.O’Roak BJ, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–50. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders SJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–41. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iossifov I, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–99. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y-H, et al. Detection of Clinically Relevant Genetic Variants in Autism Spectrum Disorder by Whole-Genome Sequencing. Am. J. Hum. Genet. 2013;93:249–263. doi: 10.1016/j.ajhg.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neale BM, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–5. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulsuner S, et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154:518–29. doi: 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu B, et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat. Genet. 2012;44:1365–9. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fromer M, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014 doi: 10.1038/nature12929. doi:10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen AS, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–21. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veltman J. a, Brunner HG. De novo mutations in human genetic disease. Nat. Rev. Genet. 2012;13:565–75. doi: 10.1038/nrg3241. [DOI] [PubMed] [Google Scholar]
- 13.Stefansson H, et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–6. doi: 10.1038/nature12818. [DOI] [PubMed] [Google Scholar]
- 14.Kong A, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488:471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malaspina D, et al. Advancing paternal age and the risk of schizophrenia. Arch. Gen. Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 16.Hultman CM, Sandin S, Levine SZ, Lichtenstein P, Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiatry. 2011;16:1203–1212. doi: 10.1038/mp.2010.121. [DOI] [PubMed] [Google Scholar]
- 17.McGrath JJ, et al. A Comprehensive Assessment of Parental Age and Psychiatric Disorders. JAMA Psychiatry. 2014;4072:1–9. doi: 10.1001/jamapsychiatry.2013.4081. [DOI] [PubMed] [Google Scholar]
- 18.Carvill GL, et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat. Genet. 2013;45:825–30. doi: 10.1038/ng.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, et al. Clinical Whole-Exome Sequencing for the Diagnosis of Mendelian Disorders. N. Engl. J. Med. 2013 doi: 10.1056/NEJMoa1306555. 131002140031007. doi:10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Roak BJ, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–22. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Roak BJ, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–22. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Roak BJ, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper GM, et al. A copy number variation morbidity map of developmental delay. Nat. Genet. 2011;43:838–46. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaminsky EB, et al. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet. Med. 2011;13:777–84. doi: 10.1097/GIM.0b013e31822c79f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefansson H, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vulto-van Silfhout AT, et al. Clinical significance of de novo and inherited copy number variation. Hum. Mutat. 2013 doi: 10.1002/humu.22442. doi:10.1002/humu.22442. [DOI] [PubMed] [Google Scholar]
- 27.Møller RS, et al. Truncation of the Down Syndrome Candidate Gene DYRK1A in Two Unrelated Patients with Microcephaly. 2008:1165–1170. doi: 10.1016/j.ajhg.2008.03.001. doi:10.1016/j.ajhg.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang N, Lee I, Marcotte EM, Hurles ME. Characterising and predicting haploinsufficiency in the human genome. PLoS Genet. 2010;6:e1001154. doi: 10.1371/journal.pgen.1001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Bokhoven H, Brunner HG. Splitting p63. Am. J. Hum. Genet. 2002;71:1–13. doi: 10.1086/341450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowen ME, et al. Loss-of-function mutations in PTPN11 cause metachondromatosis, but not Ollier disease or Maffucci syndrome. PLoS Genet. 2011;7:e1002050. doi: 10.1371/journal.pgen.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tartaglia M, Gelb B. Noonan Syndrome and Related Disorders. Annu. Rev. Genomics Hum. Genet. 2005;6:45–68. doi: 10.1146/annurev.genom.6.080604.162305. [DOI] [PubMed] [Google Scholar]
- 32.Hoischen A, et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat. Genet. 2010;42:483–5. doi: 10.1038/ng.581. [DOI] [PubMed] [Google Scholar]
- 33.Filges I, et al. Reduced expression by SETBP1 haploinsufficiency causes developmental and expressive language delay indicating a phenotype distinct from Schinzel-Giedion syndrome. J. Med. Genet. 2011;48:117–22. doi: 10.1136/jmg.2010.084582. [DOI] [PubMed] [Google Scholar]
- 34.Marseglia G, et al. 372 kb microdeletion in 18q12.3 causing SETBP1 haploinsufficiency associated with mild mental retardation and expressive speech impairment. Eur. J. Med. Genet. 2012;55:216–21. doi: 10.1016/j.ejmg.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Kamath BM, et al. NOTCH2 mutations in Alagille syndrome. J. Med. Genet. 2012;49:138–44. doi: 10.1136/jmedgenet-2011-100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isidor B, et al. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat. Genet. 2011;43:306–8. doi: 10.1038/ng.778. [DOI] [PubMed] [Google Scholar]
- 37.Simpson M. a, et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat. Genet. 2011;43:303–5. doi: 10.1038/ng.779. [DOI] [PubMed] [Google Scholar]
- 38.Kryukov GV, Pennacchio L. a, Sunyaev SR. Most rare missense alleles are deleterious in humans: implications for complex disease and association studies. Am. J. Hum. Genet. 2007;80:727–39. doi: 10.1086/513473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khurana E, et al. Integrative annotation of variants from 1092 humans: application to cancer genomics. Science. 2013;342:1235587. doi: 10.1126/science.1235587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter H, Douville C, Stenson PD, Cooper DN, Karchin R. Identifying Mendelian disease genes with the variant effect scoring tool. BMC Genomics. 2013;14(Suppl 3):S3. doi: 10.1186/1471-2164-14-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilman SR, et al. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parikshak NN, et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. doi: 10.1016/j.cell.2013.10.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helsmoortel C, et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat. Genet. 2014 doi: 10.1038/ng.2899. doi:10.1038/ng.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell SM, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014 doi: 10.1038/nature12975. doi:10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willsey AJ, et al. Co-expression networks implicate human mid-fetal deep cortical projection neurons in the pathogenesis of autism. Cell. doi: 10.1016/j.cell.2013.10.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ronan JL, Wu W, Crabtree GR. From neural development to cognition: unexpected roles for chromatin. Nat. Rev. Genet. 2013;14:347–59. doi: 10.1038/nrg3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santen GWE, et al. Coffin-Siris Syndrome and the BAF Complex: Genotype-Phenotype Study in 63 Patients. Hum. Mutat. 2013 doi: 10.1002/humu.22394. doi:10.1002/humu.22394. [DOI] [PubMed] [Google Scholar]
- 49.Hoischen A, et al. De novo nonsense mutations in ASXL1 cause Bohring-Opitz syndrome. Nat. Genet. 2011;43:729–31. doi: 10.1038/ng.868. [DOI] [PubMed] [Google Scholar]
- 50.Gibson WT, et al. Mutations in EZH2 cause Weaver syndrome. Am. J. Hum. Genet. 2012;90:110–8. doi: 10.1016/j.ajhg.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goriely A, Wilkie AOM. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am. J. Hum. Genet. 2012;90:175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudson TJ, et al. International network of cancer genome projects. Nature. 2010;464:993–8. doi: 10.1038/nature08987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuurs-Hoeijmakers JHM, et al. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am. J. Hum. Genet. 2012;91:1122–7. doi: 10.1016/j.ajhg.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoyer J, et al. Haploinsufficiency of ARID1B, a member of the SWI/SNF-a chromatin-remodeling complex, is a frequent cause of intellectual disability. Am. J. Hum. Genet. 2012;90:565–72. doi: 10.1016/j.ajhg.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santen GWE, et al. Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat. Genet. 2012;44:379–80. doi: 10.1038/ng.2217. [DOI] [PubMed] [Google Scholar]
- 56.Girirajan S, et al. Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am. J. Hum. Genet. 2013;92:221–37. doi: 10.1016/j.ajhg.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girirajan S, et al. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N. Engl. J. Med. 2012;367:1321–31. doi: 10.1056/NEJMoa1200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Classen CF, et al. Dissecting the genotype in syndromic intellectual disability using whole exome sequencing in addition to genome-wide copy number analysis. Hum. Genet. 2013;132:825–41. doi: 10.1007/s00439-013-1296-1. [DOI] [PubMed] [Google Scholar]
- 59.Zaidi S, et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature. 2013;498:220–3. doi: 10.1038/nature12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skarnes WC, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–42. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tweedie S, et al. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 2009;37:D555–9. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vulih-shultzman I, et al. Activity-Dependent Neuroprotective Protein Snippet NAP Reduces Tau Hyperphosphorylation and Enhances Learning in a Novel Transgenic Mouse Model. 2007;323:438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- 63.Van Bon BWM, et al. Intragenic deletion in DYRK1A leads to mental retardation and primary microcephaly. Clin. Genet. 2011;79:296–9. doi: 10.1111/j.1399-0004.2010.01544.x. [DOI] [PubMed] [Google Scholar]
- 64.Fotaki V, et al. Dyrk1A Haploinsufficiency Affects Viability and Causes Developmental Delay and Abnormal Brain Morphology in Mice Dyrk1A Haploinsufficiency Affects Viability and Causes Developmental Delay and Abnormal Brain Morphology in Mice. 2002 doi: 10.1128/MCB.22.18.6636-6647.2002. doi:10.1128/MCB.22.18.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tejedor F, et al. minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron. 1995;14:287–301. doi: 10.1016/0896-6273(95)90286-4. [DOI] [PubMed] [Google Scholar]
- 66.Kettleborough RNW, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496:494–7. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Genovese G, et al. Using population admixture to help complete maps of the human genome. Nat. Genet. 2013;45:406–14. 414e1–2. doi: 10.1038/ng.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sudmant PH, et al. Diversity of human copy number variation and multicopy genes. Science. 2010;330:641–6. doi: 10.1126/science.1197005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beyer K, et al. New brain-specific beta-synuclein isoforms show expression ratio changes in Lewy body diseases. Neurogenetics. 2012;13:61–72. doi: 10.1007/s10048-011-0311-8. [DOI] [PubMed] [Google Scholar]
- 70.Karakoc E, et al. Detection of structural variants and indels within exome data. Nat. Methods. 2012;9:176–8. doi: 10.1038/nmeth.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fromer M, et al. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am. J. Hum. Genet. 2012;91:597–607. doi: 10.1016/j.ajhg.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krumm N, et al. Transmission Disequilibrium of Small CNVs in Simplex Autism. Am. J. Hum. Genet. 2013;93:595–606. doi: 10.1016/j.ajhg.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lupski JR. Genetics. Genome mosaicism--one human, multiple genomes. Science. 2013;341:358–9. doi: 10.1126/science.1239503. [DOI] [PubMed] [Google Scholar]
- 74.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 77.Banka S, et al. MLL2 mosaic mutations and intragenic deletion-duplications in patients with Kabuki syndrome. Clin. Genet. 2013;83:467–71. doi: 10.1111/j.1399-0004.2012.01955.x. [DOI] [PubMed] [Google Scholar]
- 78.Huisman S. a, Redeker EJW, Maas SM, Mannens MM, Hennekam RCM. High rate of mosaicism in individuals with Cornelia de Lange syndrome. J. Med. Genet. 2013;50:339–44. doi: 10.1136/jmedgenet-2012-101477. [DOI] [PubMed] [Google Scholar]
- 79.Rodríguez-Santiago B, et al. Mosaic uniparental disomies and aneuploidies as large structural variants of the human genome. Am. J. Hum. Genet. 2010;87:129–38. doi: 10.1016/j.ajhg.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hiatt JB, Pritchard CC, Salipante SJ, O’Roak BJ, Shendure J. Single molecule molecular inversion probes for targeted, high-accuracy detection of low-frequency variation. Genome Res. 2013;23:843–54. doi: 10.1101/gr.147686.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shiroguchi K, Jia TZ, Sims P. a, Xie XS. Digital RNA sequencing minimizes sequence-dependent bias and amplification noise with optimized single-molecule barcodes. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1347–52. doi: 10.1073/pnas.1118018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klei L, et al. Common genetic variants, acting additively, are a major source of risk for autism. Mol. Autism. 2012;3:9. doi: 10.1186/2040-2392-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee SH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu TW, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77:259–73. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrow EM, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–23. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Najmabadi H, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- 87.He X, et al. Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes. PLoS Genet. 2013;9:e1003671. doi: 10.1371/journal.pgen.1003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levy D, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–97. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 89.Jacquemont S, et al. A Higher Mutational Burden in Females Supports a “‘Female Protective Model’” in Neurodevelopmental Disorders. Am. J. Hum. Genet. 2014 doi: 10.1016/j.ajhg.2014.02.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 91.Van Bon BWM, et al. The 2q23.1 microdeletion syndrome: clinical and behavioural phenotype. Eur. J. Hum. Genet. 2010;18:163–70. doi: 10.1038/ejhg.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Talkowski ME, et al. Assessment of 2q23.1 microdeletion syndrome implicates MBD5 as a single causal locus of intellectual disability, epilepsy, and autism spectrum disorder. Am. J. Hum. Genet. 2011;89:551–63. doi: 10.1016/j.ajhg.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Makishima H, et al. Somatic SETBP1 mutations in myeloid malignancies. Nat. Genet. 2013;45:942–6. doi: 10.1038/ng.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Piazza R, et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat. Genet. 2013;45:18–24. doi: 10.1038/ng.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.