Abstract
Glutamine has been implicated as an immunomodulatory nutrient, but how glutamine uptake is mediated during T-cell activation is poorly understood. We have shown that naïve T-cell activation is coupled with rapid glutamine uptake, which depended on the amino acid transporter ASCT2. ASCT2 deficiency impaired the induction of T helper-1 (Th1) and Th17 cells and attenuated inflammatory T-cell responses in mouse models of immunity and autoimmunity. Mechanistically, ASCT2 was required for T cell receptor (TCR)-stimulated activation of the metabolic kinase mTORC1. We have further shown that TCR-stimulated glutamine uptake and mTORC1 activation also required a TCR signaling complex composed of the scaffold protein CARMA1, the adaptor molecule BCL10, and the paracaspase MALT1. This function was independent of IKK kinase, a major downstream target of the CARMA1 complex. These findings highlight a mechanism of T-cell activation involving ASCT2-dependent integration of the TCR signal and a metabolic signaling pathway.
Keywords: Glutamine uptake, TCR signaling, ASCT2, mTORC1, CARMA1, T-cell differentiation, EAE
INTRODUCTION
T cells serve as a central cellular component of adaptive immunity. Following their maturation in the thymus, naïve T cells circulate in the periphery searching for specific antigens. Upon activation by an antigen, naïve CD4+ T cells proliferate and differentiate into various subsets of T helper (Th) cells, including Th1, Th2, and Th17 cells, which produce different cytokines and mediate distinct effector functions (Zhu et al., 2010). In addition to their critical involvement in immune responses against infections, Th1 and Th17 cells also play an important role in mediating the pathogenesis of autoimmune inflammatory disorders, such as experimental autoimmune encephalomyelitis (EAE) (Simmons et al., 2013). The activated naïve CD4+ T cells can also produce Foxp3+ regulatory T (Treg) cells with immunosuppressive functions (Zhu et al., 2010). Thus, deregulated CD4+ T-cell activation and differentiation are a hallmark of autoimmune and inflammatory diseases.
The activation and differentiation of T cells require signals elicited by the T-cell receptor (TCR) and costimulatory receptors, particularly CD28, as well as signals stimulated by various cytokines (Yamane and Paul, 2012). Moreover, emerging evidence suggests that proper activation and differentiation of T cells are also influenced by other signals derived from the immune microenviroment, including metabolic stimuli (Maciver et al., 2013; van der Windt and Pearce, 2012; Waickman and Powell, 2012). A signaling molecule that integrates the environmental stimuli to mediate T-cell function is protein kinase mammalian target of rapamycin (mTOR) (Chi, 2012; Waickman and Powell, 2012). mTOR exists in two major signaling complexes, mTOR complex 1 (mTORC1) and mTORC2, which differ in their mechanism of activation and signaling functions (Sarbassov et al., 2005). In particular, mTORC1 phosphorylates two regulators of protein synthesis, the S6 kinase 1 (S6K1, also known as p70 S6K) and 4 elongation factor-binding protein 1 (4E-BP1), thereby promoting cell growth and proliferation (Fingar et al., 2002; Holz and Blenis, 2005; Sarbassov et al., 2005). In T cells, mTORC1 is activated by both the TCR and CD28 signals and environmental cues, including those derived amino acids (Chi, 2012). Amino acids activate mTORC1 by inducing its translocation to lysosomal membranes in a mechanism that depends on a specific regulator complex (Sancak et al., 2010). Ligation of TCR and CD28 triggers the activation of mTORC1, which is important for preventing T-cell anergy (Gorentla et al., 2011; Zheng et al., 2007). The TCR signal induces leucine uptake via a System L amino-acid transporter, composed of Slc7a5 and CD98, which is important for activation of mTORC1 and metabolic reprogramming of T cells (Sinclair et al., 2013).
Glutamine is the most abundant amino acid in the bloodstream and has been implicated as an immunomodulatory nutrient (Karinch et al., 2001; Lund and Williamson, 1985; Newsholme, 2001). It is generally thought that glutamine, although not an essential amino acid, provides fuel for rapidly dividing cells, such as lymphocytes and enterocytes. However, precisely how glutamine regulates immune responses is incompletely understood. Moreover, although several amino acid transporters have been shown to mediate glutamine transportation in cancer cell lines (McGivan and Bungard, 2007), whether any of them is required for glutamine uptake in T cells is obscure. In the present study, we found that stimulation of naïve CD4+ T cells via the TCR and CD28, triggered rapid uptake of glutamine. Furthermore, glutamine was critically required for naïve CD4+ T-cell differentiation to Th1 and Th17 inflammatory T cells but not to Th2 or Treg cells. By employing a gene targeting approach, we identified the amino acid transporter ASCT2 (also known as Slc1a5) (Utsunomiya-Tate et al., 1996) as a crucial mediator of TCR-stimulated glutamine uptake in naïve CD4+ T cells. Although ASCT2 was dispensable for T-cell proliferation and IL-2 induction, it was essential for the generation of Th1 and Th17 cells under both in vitro and in vivo conditions. Consistently, Slc1a5−/− mice were refractory to induction of the T-cell dependent autoimmunity, EAE. We also obtained genetic evidence that ASCT2 coupled the TCR and CD28 signals to the activation of the metabolic kinase mTORC1. Thus, our data suggest a mechanism of T-cell activation that involves ASCT2-dependent integration of the TCR and CD28 signals with glutamine uptake and activation of a metabolic signaling pathway.
RESULTS
ASCT2 regulates lymphocyte homeostasis
To study the physiological function of ASCT2, we employed a conventional gene targeting approach (Figures S1A–S1C). The Slc1a5−/− mice were born with expected Mendelian ratios and did not show obvious abnormalities in growth or survival (data not shown). These mutant animals also had normal thymocyte development, as revealed by the comparable frequencies of thymocyte sub-populations and total thymocyte numbers in the Slc1a5+/+ and Slc1a5−/− mice (Figures S1D and S1E).
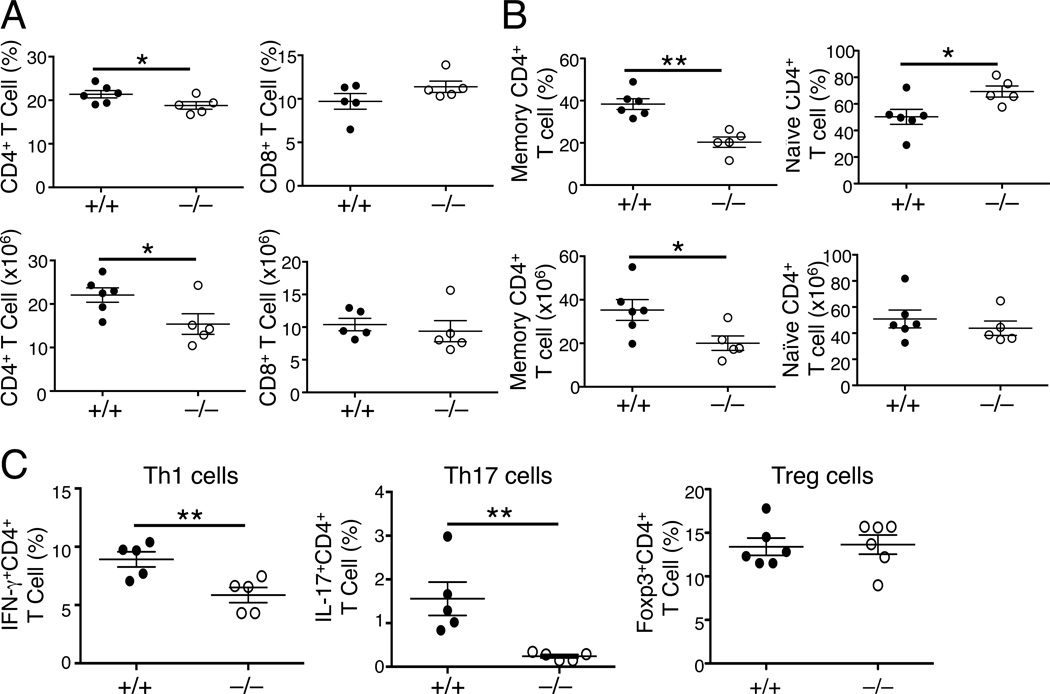
To examine the role of ASCT2 in regulating peripheral T-cell homeostasis, we examined the frequency of T cells in the spleen of the Slc1a5+/+ and Slc1a5−/− mice. At young ages (6–7 wk), the Slc1a5−/− mice had a moderate, but statistically significant, increase in the frequency of T cells and decrease in the frequency of B cells in the spleen (Figure S2A). This result was not because of an increase in the number of T cells but rather due to the reduced B-cell numbers (Figure S2B). The frequency and absolute number of memory and naïve CD4+ T cells was also largely comparable between young Slc1a5+/+ and Slc1a5−/− mice (Figures S2C and S2D). However, older ASCT2-deficient mice (5–6 mon) had reduced frequency and numbers of CD4+ T cells (Figure 1A), reflecting a profound reduction in the CD44hiCD62Llo memory population (Figure 1B). In contrast, the Slc1a5−/− mice had increased frequency and normal numbers of the CD44loCD62Lhi naïve CD4+ T cells compared to the Slc1a5+/+ mice (Figure 1B). No obvious abnormalities were found in CD8+ T cells (Figure 1A). Among the CD4+ memory T cells, there was a dramatic reduction in the percentage of interferon-γ (IFN-γ)-producing Th1 and IL-17-producing Th17 cells; however, the ASCT2 deficiency did not alter the frequency of Treg cells (Figure 1C). These results suggest that although ASCT2 is dispensable for thymocyte development, it is required for regulating the homeostasis and possibly the differentiation of peripheral CD4+ T cells.
Figure 1. ASCT2 deficiency perturbs T-cell homeostasis.
Flow cytometry analyses of T-cell populations in the spleen of age-matched Slc1a5+/+ (+/+) and Slc1a5−/− (−/−) mice (5 month old). Data are mean ± SD values of multiple mice (each circle represents a mouse).
(A and B) Frequency (upper) and absolute number (lower) of CD4+ and CD8+ T cells (A) and the memory (CD44hiCD62Llo) and naïve (CD44loCD62Lhi) CD4+ T cells (B).
(C) Frequency of IFN-γ-producing Th1 and IL-17-producing Th17 cells (gated on CD4+CD44hiCD62Llo CD4+ T cells) as well as Treg cells (CD4+CD25+Foxp3+). Data are representative of four independent experiments. *P < 0.05, **P < 0.01.
Also see Figures S1 and S2
ASCT2 is required for naïve CD4+ T-cell differentiation in vitro
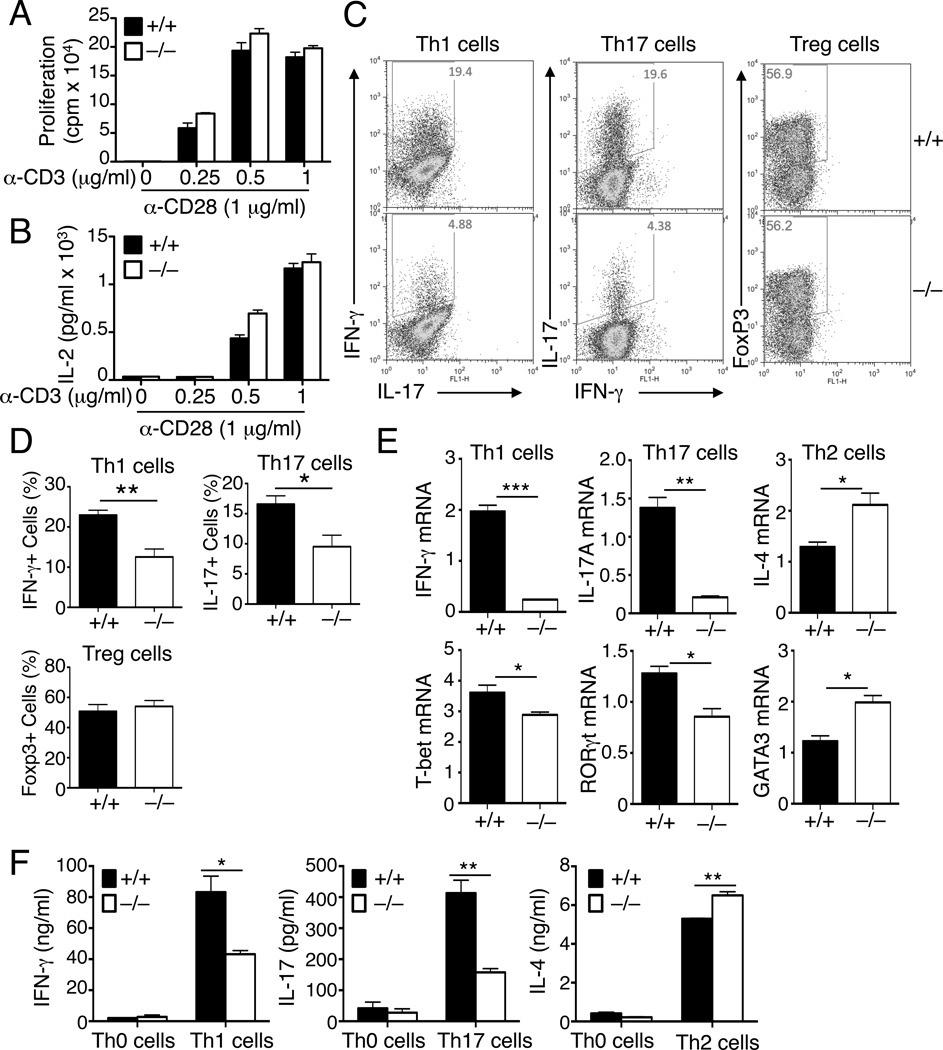
The reduction in memory T-cell population, particularly Th1 and Th17 cells, in the older Slc1a5−/− mice prompted us to examine the role of ASCT2 in the regulation of T-cell activation and differentiation. We purified naïve CD4+ T cells from young Slc1a5+/+ and Slc1a5−/− mice, since the young mutant mice had no obvious phenotype in T-cell homeostasis (Figures S2C and S2D). In response to stimulation by the TCR inducer anti-CD3 and the costimulator anti-CD28, the Slc1a5+/+ and Slc1a5−/− T cells displayed similar abilities to proliferate and produce the T-cell growth factor IL-2 (Figures 2A and 2B). However, in vitro CD4+ T-cell differentiation assays revealed a profound defect of the Slc1a5−/− naïve CD4+ T cells in the generation of the IFN-γ-producing Th1 and the IL-17-producing Th17 cells, although not in the generation of the Foxp3+ Treg cells, as determined by intracellular cytokine staining and flow cytometry assays (Figures 2C and 2D). This defect was due to the reduced percentage of Th1 and Th17 cells rather than alterations in gene expression on a single-cell basis, as shown by the comparable median florescence intensity (MFI) of IL-17 and IFN-γ on gated Slc1a5+/+ and Slc1a5−/− Th17 and Th1 cells (Figure S3A). Furthermore, the proliferation and survival ability of these different T cells was also similar (Figure S3B). The differentiation defect of the Slc1a5−/−naïve CD4+ T cells was also detected in parallel qPCR assays and ELISA, based on the detection of IFN-γ and IL-17 induction at the RNA and protein levels, respectively (Figures 2E and 2F). These latter assays also revealed attenuated induction of the genes encoding the Th1- and Th17 cell-lineage transcription factors, T-bet and RORγt (Figure 2F). In contrast to the defective production of Th1 and Th17 cells, the generation of Th2 cells was not reduced, but rather enhanced, in the Slc1a5−/− T cells (Figures 2E and 2F). Taken together with the reduced Th1 and Th17 cell frequencies in older Slc1a5−/− mice (Figure 1), these results suggest an important role for ASCT2 in the regulation of naïve CD4+ T-cell differentiation to the Th1 and Th17 cell subsets.
Figure 2. ASCT2 is dispensable for T-cell proliferation but is required for naïve CD4+ T-cell differentiation.
(A and B) Proliferation assay (A) and IL-2 ELISA (B) of naïve CD4+ T cells from age-and sex-matched wild-type (+/+) and Slc1a5−/− (−/−) mice (8 wk old), stimulated for 48 h with the indicated doses of plate-bound anti-CD3 plus 1µg/ml anti-CD28.
(C–F) Naïve CD4+ T cells from Slc1a5+/+ and Slc1a5−/− mice were stimulated under Th1, Th2, Th17, or Treg cell skewing conditions and analyzed, on day 4, for frequency of IFN-γ+ Th1, IL-17+ Th17, and Foxp3+ Treg cells by flow cytometry (C and D), relative mRNA amount of the indicated genes by QPCR (E), and the indicated secreted cytokines by ELISA (F). Data are representative of three (A, B, E and F) or four (C and D) independent experiments with three or more mice per group (mean ± s.d. in A, B, D, E and F). *P < 0.05 and **P < 0.01.
Also see Figure S3
We next examined whether ASCT2 deficiency affected the expression of other amino acid transporters. In Slc1a5+/+ naïve CD4+ T cells, TCR and CD28 signals induced the expression of ASCT2 as well as several other amino acid transporters, including SNAT1, SNAT2, Slc7a5, and CD98 (Figure S3C). ASCT2 mRNA was undetectable in the Slc1a5−/− T cells, confirming null phenotype at the RNA level (Figure S3B). Moreover, the loss of ASCT2 did not inhibit the induction of the other amino acid transporters, although it moderately reduced the basal level of CD98 mRNA (Figure S3C). To examine whether the effect of ASCT2 deficiency on CD4+ T-cell differentiation was direct, we transduced Slc1a5+/+ and Slc1a5−/− CD4+ T cells with a GFP-expressing retroviral vector or the same vector encoding ASCT2. Expression of exogenous ASCT2 moderately promoted Th1 and Th17 cell differentiation (Figure S3D). More importantly, reconstitution of Slc1a5−/− T cells with exogenous ASCT2 largely rescued their defect in Th1 and Th17 cell differentiation (Figure S3D). Parallel experiments revealed that transduction of SNAT1, another amino acid transporter implicated in glutamine uptake, failed to rescue the T-cell differentiation defect (Figure S3E). This result, along with the competent expression of SNAT1, in the Slc1a5−/− T cells (Figure S3C), suggest a non-redundant role for ASCT2 in mediating naïve CD4+ T-cell differentiation.
ASCT2 deficiency attenuates in vivo production of Th1 and Th17 cells
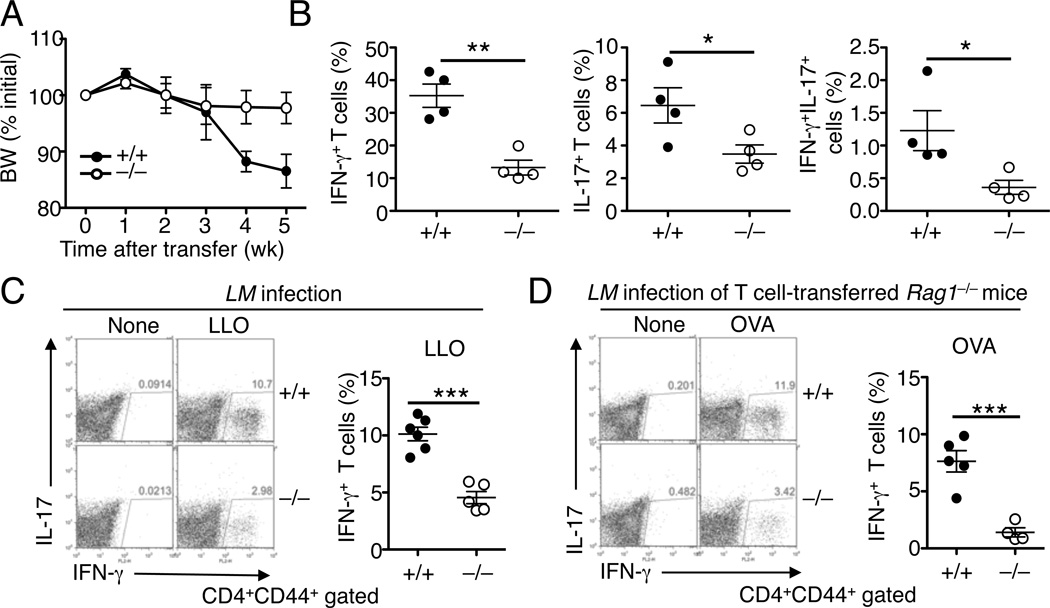
To study the in vivo function of ASCT2 in regulating CD4+ T-cell differentiation and proinflammatory T-cell responses, we employed a T-cell adoptive transfer of colitis model, involving the transfer of CD45RBhi naïve CD4+ T cells to Rag1−/− mice. In the absence of Treg cells, transferred naïve CD4+ T cells are known to differentiate into inflammatory Th1 and Th17 cells and mediate colonic inflammation and cause body weight loss(Morrissey et al., 1993). As expected, transfer of wild-type CD4+ naïve T cells to Rag1−/− mice induced severe body weight loss (Figure 3A). Importantly, this pathological phenotype was much milder in recipient mice transferred with ASCT2-deficient naïve CD4+ T cells (Figure 3A). Consistently, the transferred Slc1a5−/− T cells displayed a severe defect in the production of the IFN-γ+ Th1 and IL-17+ Th17 cells (Figure 3B). We also detected a population of IFN-γ+IL-17+ double-positive T cells, which was significantly reduced in the Slc1a5−/− T-cell recipients (Figure 3B). These results suggest a role for ASCT2 in regulating CD4+ T-cell differentiation in vivo under lymphopenic conditions.
Figure 3. ASCT2 regulates CD4+ T-cell differentiation in vivo.
(A and B) Body weight (BW) changes (percent of initial weight) (A) and frequency of the indicated effector T cells (percent of CD4+CD44+ cells) in the mesenteric lymph nodes (B) of RAG1-deficient mice (6 wk old) adoptively transferred with wild-type (+/+) or Slc1a5−/− (−/−) CD45RBhi CD4+ T cells (5 wk after adoptive transfer).
(C) Slc1a5+/+ or Slc1a5−/− mice were infected with L. monocytogenes for 7 days, and splenocytes were isolated and either not treated (None) or stimulated for 6 h with the LLO190–201 peptide (LLO) in the presence of momensin, followed by flow cytometry analysis of the frequency of CD4+ T cells producing IFN-γ (gated on CD4+CD44+ cells).
(D) RAG1-deficient mice were adoptively transferred with CD4+ T cells from Slc1a5+/+OT-II or Slc1a5−/− OT-II mice and infected with L. monocytogenes. On day 7 postinfection, splenocytes were isolated and either incubated with medium (None) or stimulated with OVA323–339 and then subjected to intracellular IFN-γ staining and flow cytometry analysis of CD4+ T cells producing IFN-γ (gated on CD4+CD44+ cells). Data are representative of three (A–C) or two (D) independent experiments with least four mice per group (mean ± s.d. in B–D). *P < 0.05, **P < 0.01, ***P<0.001.
Induction of the IFN-γ-producing Th1 cells is a hallmark of infections by intracellular bacterial pathogens, such as Listeria monocytogenes (L. monocytogenes) (Kaufmann, 1993). We employed the L. monocytogenes model to examine the role of ASCT2 in mediating Th1 cell responses against infections. Infection of the wild-type mice with L. monocytogenes induced a population of antigen-specific Th1 cells that produced IFN-γ upon re-stimulation with the Listerial antigen listeriolysin (LLO) (Figure 3C). Although the L. monocytogenes-challenged Slc1a5−/− mice also produced Th1 cells, this response was profoundly reduced (Figure 3C). To examine the T cell-intrinsic role of ASCT2, we adoptively transferred lymphocyte-deficient Rag1−/− mice with Slc1a5+/+ or Slc1a5−/− T cells expressing ovalbumin (OVA)-specific TCR (OT-II). Following infection with OVA-expressing recombinant L. monocytogenes, the recipient mice transferred with Slc1a5−/− OT-II T cells generated a reduced frequency of IFN-γ-producing T cells than those transferred with the Slc1a5+/+ OT-II T cells (Figure 3D). Thus, ASCT2 is required for CD4+ T-cell differentiation under both lymphopenic and antigen-stimulated conditions.
Slc1a5−/− mice are refractory to EAE induction
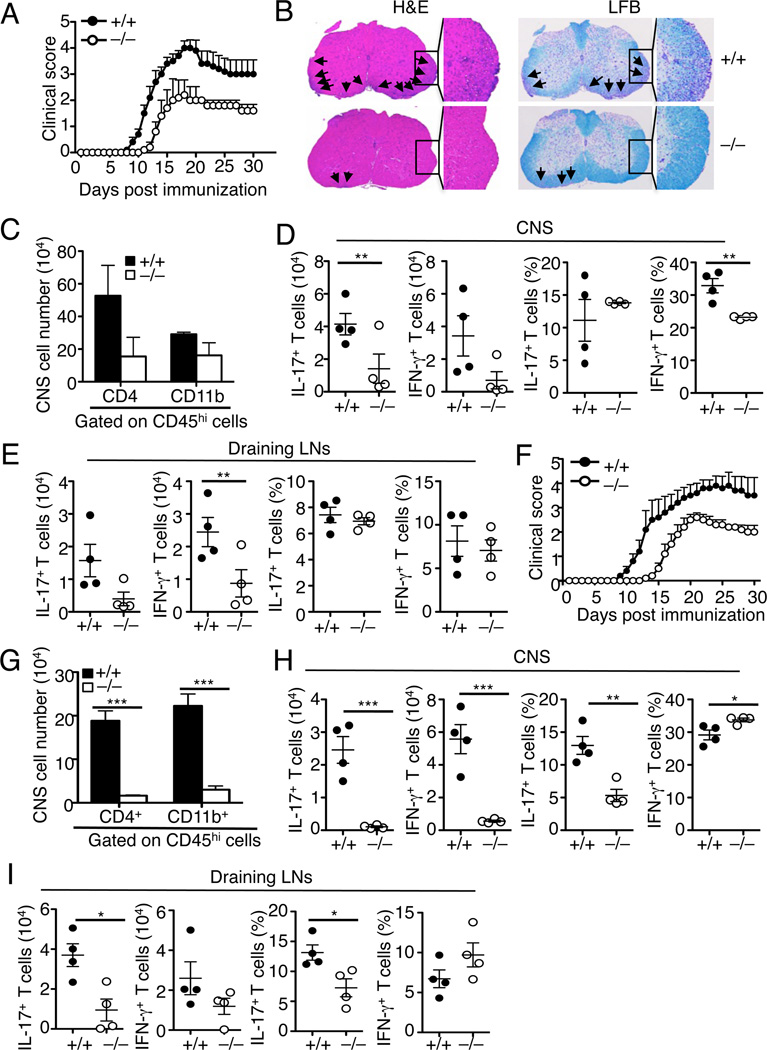
T cells have an important role in mediating the pathogenesis of experimental allergic encephalomyelitis (EAE), an animal model of multiple sclerosis (Miller et al., 2010). The finding that ASCT2 regulates T-cell homeostasis and differentiation prompted us to examine the role of ASCT2 in the regulation of EAE pathogenesis. Immunization of Slc1a5+/+ mice with a myelin oligodendrocyte glycoprotein (MOG) peptide (MOG35–55), along with injection with pertussis toxin, led to the induction of severe EAE clinical scores (Figure 4A). Under the same conditions, the Slc1a5−/− mice had much milder clinical scores (Figure 4A). Hematoxylin and eosin (H&E) and luxol fast blue (LFB) staining of the EAE-induced Slc1a5+/+ spinal cord sections revealed extensive immune cell infiltration and demyelination, which were profoundly weaker in the Slc1a5−/− spinal cord sections (Figure 4B). Parallel flow cytometry analyses revealed that the EAE-resistant phenotype of the Slc1a5−/− mice was associated with a substantial reduction in the number of central nervous system (CNS)-infiltrating cells, including both CD4+ T cells and CD11b+ myeloid cells, during the early stage (day 13) of EAE induction (Figure 4C). The number of the IL-17-producing Th17 and IFN-γ-producing Th1 cells was also reduced in the CNS (Figure 4D) as well as in the draining lymph nodes (Figure 4E) of the Slc1a5−/− mice.
Figure 4. ASCT2 has a T cell-intrinsic role in mediating EAE pathogenesis.
(A–E) EAE induction in Slc1a5+/+ and Slc1a5−/− mice (8 wk old). (A) Mean clinical scores. (B) H&E and Luxol Fast Blue (LFB) staining of spinal cord sections from MOG35–55-immunized EAE mice (day 30 post-immunization) for visualizing immune cell infiltration and demyelination, respectively (arrows). Original magnification, ×100. (C–E) Flow cytometry analyses of the CD4+ T-cell and the CD11b+ myeloid cell numbers in the CNS (C) and the absolute number and frequency of the IL-17+ Th17 cells and IFN-γ+ Th1 cells in the CNS (D) and draining lymph nodes (E) of day 13 EAE-induced Slc1a5+/+ and Slc1a5−/− mice.
(F–I) EAE induction in Rag1−/− mice (8 wk old) adoptively transferred with Slc1a5+/+ or Slc1a5−/− CD4+ T cells. (F) Mean clinical scores. (G–I) Flow cytometric analyses of the CD4+ T-cell and the CD11b+ myeloid cell numbers in CNS (G) and the absolute number and frequency of Th17 and Th1 cells in the CNS (H) and draining lymph nodes (I) of day 14 EAE-induced RAG1-deficient mice adoptively transferred with Slc1a5+/+ or Slc1a5−/−CD4+ T cells. *P < 0.05, **P < 0.01, ***P<0.001.
To examine the T cell-intrinsic role of ASCT2 in regulating EAE pathogenesis, we adoptively transferred Slc1a5+/+ or Slc1a5−/− CD4+ T cells into lymphocyte-deficient Rag1−/− mice followed by EAE induction in recipient mice. As expected, transfer of Slc1a5+/+ CD4+ T cells into the Rag1−/− mice rendered the recipient mice susceptible to EAE induction (Figure 4F). More importantly, mice transferred with Slc1a5−/− CD4+ T cells had a delayed onset and reduced clinical scores of EAE (Figure 4F). Consistently, these recipient mice also had a drastically reduced number of CNS infiltrating immune cells, including both CD4+ T cells and CD11b+ myeloid cells (Figure 4G). The number of both Th1 and Th17 cells was also low in the CNS of the Slc1a5−/− T cell recipients (Figure 4H). Furthermore, during the early stage of EAE induction (day 14 post-immunization), the draining lymph nodes of the mutant recipient mice contained significantly lower frequency and numbers of Th17 cells and moderately lower number of Th1 cells (Figure 4I). Considering their strikingly lower number of CNS T cells, these mutant T-cell recipient mice clearly had an overall reduction in both Th17 and Th1 cells. Collectively, these results suggest that ASCT2 has a T cell-intrinsic role in mediating EAE pathogenesis.
TCR signal stimulates glutamine uptake in an ASCT2-dependent manner
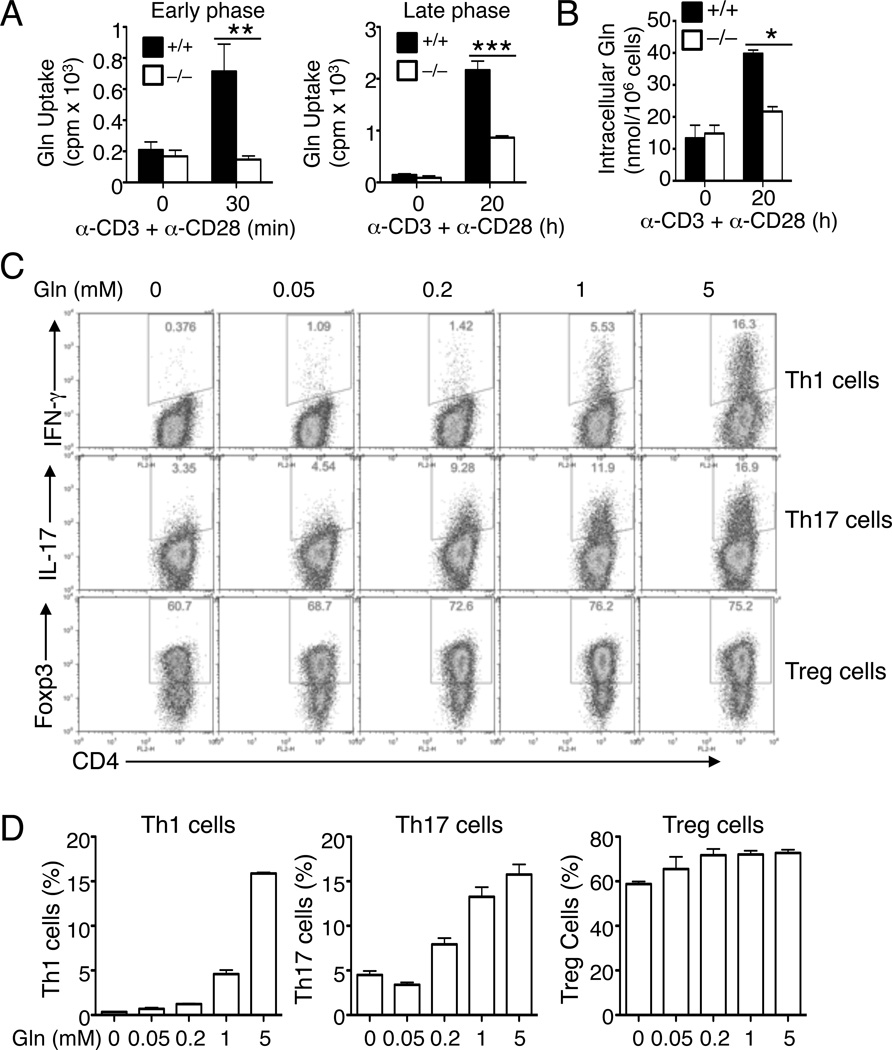
ASCT2 has been implicated as an amino acid transporter that mediates glutamine uptake in cell lines (Nicklin et al., 2009). T-cell activation triggers the induction of two glutamine transporters, SNAT1 and SNAT2, which is associated with enhanced glutamine uptake measured at 24 hr post-activation (Carr et al., 2010). It is unclear whether these glutamine transporters are required for TCR and CD28-stimulated glutamine uptake and T-cell functions. The role of ASCT2 in regulating naïve CD4+ T-cell differentiation raised the intriguing question of whether ASCT2 plays a role in glutamine uptake in naïve T cells and whether glutamine is required for CD4+ T-cell differentiation. We first examined whether glutamine uptake is altered during the early phase of T-cell activation. Stimulation of naïve T cells with anti-CD3 plus anti-CD28 rapidly promoted the uptake of glutamine (Figure 5A). Moreover, the anti-CD3- and anti-CD28-stimulated early phase glutamine uptake was completely blocked in the ASCT2-deficient T cells (Figure 5A). Prolonged T-cell activation further enhanced the glutamine uptake, and this late-phase induction of glutamine uptake was also critically dependent on ASCT2 (Figure 5A). The absolute concentration of intracellular glutamine was also stimulated by TCR and CD28 signals in a mechanism that was largely dependent on ASCT2 (Figure 5B). These findings suggest that naïve CD4+ T-cell activation is coupled with enhanced glutamine uptake, a molecular process that is largely dependent on ASCT2.
Figure 5. TCR and CD28-stimulated glutamine uptake requires ASCT2 and regulates CD4+ T-cell differentiation.
(A) Glutamine uptake analysis of Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells, stimulated for 30 min with anti-CD3 and anti-CD28 mixed with a crosslinking anti-hamster IgG (left) or for 20 h with plate-bound anti-CD3 and anti-CD28 (right).
(B) Intracellular glutamine analysis of Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells, stimulated for 20 h with plate-bound anti-CD3 and anti-CD28.
(C and D) Flow cytometry analysis of Th1, Th17, and Treg cells generated thorough in vitro differentiation of Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells (for 4 days) under the polarizing conditions described in Figure 2 in glutamine-free medium supplemented with the indicated amount of l-glutamine. Data are representative of three (A and B) or four (C and D) independent experiments with at least three mice per group (mean ± s.d. in A and C). *P < 0.05, **P < 0.01, ***P<0.001.
Also see Figure S4.
We next examined the role of glutamine in the regulation of naïve CD4+ T-cell differentiation. When activated in glutamine-free medium, naïve CD4+ T cells displayed a severe defect in the generation of both Th1 and Th17 cells (Figure 5C). In contrast, the production of Treg cells was normal in the absence of glutamine (Figure 5C). Furthermore, addition of glutamine into the cell culture led to the dose-dependent induction of Th1 and Th17 cells, but the glutamine only had a slight effect on the generation of Treg cells (Figures 5C and 5D). Glutamine also had a stimulatory effect on the proliferation and survival of T cells, although this function was detected with both the effector T cells and Treg cells (Figure S4).
ASCT2 mediates TCR-stimulated mTORC1 activation in naïve T cells
To understand the signaling function of ASCT2, we examined the TCR and CD28-mediated activation of three major families of transcription factors, NF-κB, AP-1, and NFAT in Slc1a5+/+ and Slc1a5−/− T cells. ASCT2 deficiency did not appreciably affect the activation of these transcription factors (Figure S5A). Consistently, the TCR and CD28-stimulated phosphorylation of several signaling factors, including the MAP kinases ERK, JNK, and p38, IKK, and the IKK target IκBα, was largely comparable in the Slc1a5+/+ and Slc1a5−/− T cells (Figure S5B). Therefore, ASCT2 is not required for the activation of several major signaling pathways involved in T-cell activation, which was in line with the dispensability of ASCT2 in the proliferation and IL-2 production.
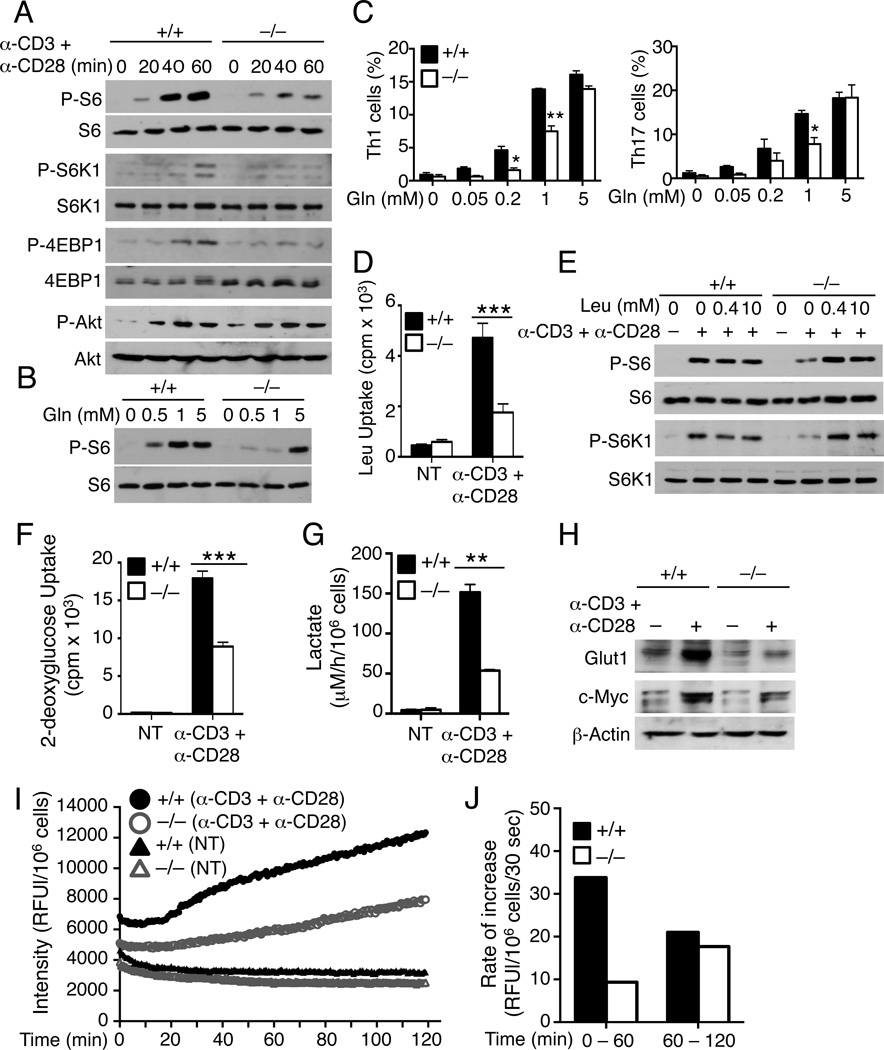
Amino acids have a crucial role in the activation of the mTOR signaling pathways (Jewell et al., 2013). Our finding that ASCT2 mediated TCR and CD28-stimulated glutamine uptake prompted us to examine whether ASCT2 is required for mTOR activation by the TCR and CD28 signals. Stimulation of T cells with anti-CD3 and anti-CD28 triggered the phosphorylation of ribosomal protein S6 (Figure 6A), a well-defined effector molecule of the mTORC1 signaling pathway (Ruvinsky and Meyuhas, 2006). The induction of S6 phsophorylation was severely attenuated in the ASCT2-deficient T cells (Figure 6A). Consistent with this result, TCR and CD28-stimulated phosphorylation of the S6 upstream kinase S6K1 was also defective in the ASCT2-deficient T cells (Figure 6A). Since S6K1 is a direct target of mTORC1 (Chi, 2012), this finding suggested the requirement of ASCT2 in TCR and CD28-mediated activation of mTORC1. Indeed, ASCT2 deficiency also abrogated the inducible phosphorylation of another known target of mTORC1, 4EBP1 (Figure 6A). Meanwhile ASCT2 was dispensable for TCR and CD28-stimulated phosphorylation of AKT (Figure 6A), a target of mTORC2 and PI3 kinase (Chi, 2012). Furthermore, attenuated mTORC1 activation was due to reduced glutamine uptake, since TCR and CD28-stimulated S6 phosphorylation could be restored in the presence of an excessive amount of glutamine (Figure 6B). Addition of excessive amounts of glutamine also rescued the defect of the Slc1a5−/− T cells in differentiation into Th1 and Th17 cells (Figures 6C and S5C).
Figure 6. ASCT2 mediates TCR and CD28-stimulated mTORC1 activation in naïve CD4+ T cells.
(A and B) IB analyses of the indicated phosphorylated (P-) and total proteins in whole-cell lysates of Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells, stimulated with anti-CD3 and anti-CD28 using the crosslinking method in regular RPMI 1640 medium (A) or glutamine-free medium supplemented with the indicated amount of l-glutamine (B).
(C) Flow cytometry analysis of Th1, Th17, and Treg cells generated thorough in vitro differentiation of Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells (for 4 days) under the Th1 or Th17 polarizing conditions in glutamine-free medium supplemented with the indicated amount of l-glutamine.
(D) Leucine uptake analysis of Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells, either not treated (NT) or stimulated for 20 h with plate-bound anti-CD3 and anti-CD28.
(E) IB analysis of the indicated proteins in whole-cell lysates of Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells that were either not (−) or stimulated (+) with anti-CD3 and anti-CD28 for 30 min.
(F) 2-deoxyglucose uptake analysis of the cells described in D.
(G) Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells were either not treated (NT) or stimulated with plate-bound anti-CD3 plus anti-CD28 for 20 h in a 96-well plate. Lactate concentration in the culture medium was measured and calculated as described in the Extended Experimental Procedures.
(H) IB analyses of Gult1 and c-Myc in whole-cell lysates of the Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells described in D.
(I and J) Oxygen consumption in Slc1a5+/+ or Slc1a5−/− naïve CD4+ T cells, either not treated (NT) or stimulated with plate-bound anti-CD3 plus anti-CD28 for 20 h in a 96-well plate. Fluorescence-probe intensity was measured for the indicated time periods and is presented as relative fluorescence unit (RFU) per 106 cells (I). The increase rate of oxygen consumption was calculated at early phase (0 – 60 min) and late phase (60 – 120 min) (J).
Data are representative of three independent experiments with at least three mice per group. *P < 0.05, **P < 0.01, ***P<0.001.
Also see Figures S5 and S6.
To examine whether ASCT2 also regulated mTORC1 activation by other inducers, we analyzed the effect of ASCT2 deficiency on TNF-α-stimulated mTORC1 activation. Like anti-CD3 and anti-CD28, TNF-α stimulated the phosphorylation of both S6 and the direct targets of mTORC1, S6K1 and 4EBP1 (Figure S5D). TNF-α-stimulated mTORC1 signaling was largely normal in the ASCT2-deficient T cells. This finding suggests that the function of ASCT2 in mediating mTORC1 activation is receptor specific. Recent studies suggest that T-cell activation induces the expression of the glutamine transporters SNAT1 and SNAT2 (Carr et al., 2010), raising the question of whether ASCT2 is still essential in effector T cells. We addressed this question using Th1 and T17 effector T cells generated through in vitro differentiation. ASCT2 was partially required for the induction of S6 phosphorylation and glutamine uptake in Th17 cells, but not in Th1 cells (Figure S5E). These results suggest that ASCT2 predominantly regulates glutamine uptake and mTORC1 signaling in naïve CD4+ T cells, although it also has a role in regulating these molecular events in the Th17 effector T cells.
ASCT2 is required for leucine uptake and metabolic activities
A recent study suggests that ASCT2-mediated glutamine uptake in cancer cells is required for the uptake of leucine by a System L amino acid transporter composed of CD98 (also called Slc3a2) and Slc7a5 (Nicklin et al., 2009). The Slc7a5-CD98 complex functions by mediating coupled glutamine efflux and leucine uptake, which is important for mTORC1 activation. Our finding that ASCT2 was a major glutamine transporter mediating TCR and CD28-stimulated glutamine uptake in naïve CD4+ T cells prompted us to test role of ASCT2 in leucine uptake under these conditions. Stimulation of naïve CD4+ T cells with anti-CD3 plus anti-CD28 strongly induced leucine uptake, and this molecular event indeed required ASCT2 (Figure 6D). The defect of the Slc1a5−/− T cells in leucine uptake was not due to reduced expression of Slc7a5 or CD98 (Figure S3C), thus supporting the previous finding that the ASCT2-mediated glutamine uptake might be required for leucine import by Slc7a5-CD98. Consistently, addition of excessive amounts of exogenous leucine rescued the defect of the ASCT2-deficient T cells in mTORC1 activation (Figure 6E). Exogenous leucine also rescued the defect of the ASCT2-deficient T cells in Th17 cell differentiation, albeit at a high concentration (Figure S6B). However, leucine had an inhibitory effect on Th1 cell differentiation in both the Slc1a5+/+ and Slc1a5−/− T cells (Figure S6B). Since exogenous leucine efficiently rescued the mTORC1 signaling in ASCT2-deficient T cells (Figure 6E), these data suggest that leucine may regulate T-cell differentiation via activation of mTORC1 and additional mechanisms.
Glutamine has an important role in the regulation of glucose uptake and anaplerosis (Kaadige et al., 2010; Kaadige et al., 2009). We found that activation of naïve CD4+ T cells by anti-CD3 and anti-CD28 led to induction of glucose uptake (Figure 6F). Furthermore, the TCR and CD28-stimualted glucose uptake was significantly inhibited, although not completely blocked, in the ASCT2-deficient T cells. The TCR and CD28-stimulated glucose uptake is known to be associated with increased rate of glycolysis (Frauwirth et al., 2002). ASCT2 deficiency also led to a reduction in lactate secretion by Slc1a5−/− T cells as compared to Slc1a5+/+ T cells, consistent with a reduction in glycolysis (Figure 6G). Furthermore, exogenous glutamine and leucine were able to rescue the defect of ASCT2-deficient T cells in glycolysis (Figure S6C). We also found that TCR and CD28 signals stimulated the expression of glucose transporter 1 (Glut1), which was severely attenuated in the Slc1a5−/− T cells (Figure 6H). This finding was in agreement with the defect of the Slc1a5−/− T cells in the activation of mTORC1 signaling, which is known to mediate induction of Glut1 expression (Buller et al., 2008; Taha et al., 1999; Taha et al., 1995). The mTORC1 signaling is also required for the expression of c-Myc, a transcription factor that in turn is important for the cell growth and various metabolic activities (Babcock et al., 2013; Kaadige et al., 2010; Wall et al., 2008). ASCT2 deficiency partially inhibited the TCR and CD28-stimulated expression of c-Myc (Figure 6H).
We next examined the effect of ASCT2 deficiency on oxidative phosphorylation (OXPHOS), since this is one of the major metabolic events regulated by glutamine (Kaadige et al., 2010). OXPHOS is stimulated by TCR and CD28 signals and is required for naïve T-cell activation (Chang et al., 2013). We assessed the induction of OXPHOS by measuring oxygen consumption rate (OCR), an indicator of OXPHOS (Chang et al., 2013). Stimulation of naïve CD4+ T cells with anti-CD3 plus anti-CD28 led to a rapid increase in the OCR, followed by a steady rate (Figures 6 I and 6J). Moreover, ASCT2 deficiency attenuated the initial burst of oxygen consumption, although the OCR was similar in Slc1a5+/+ and Slc1a5−/− T cells after longer time periods of stimulation (Figures 6 I and 6J). Collectively, these results indicate a role for ASCT2 in the regulation of metabolic changes along with T-cell activation.
The CBM complex is required for TCR-stimulated glutamine uptake and mTORC1 activation
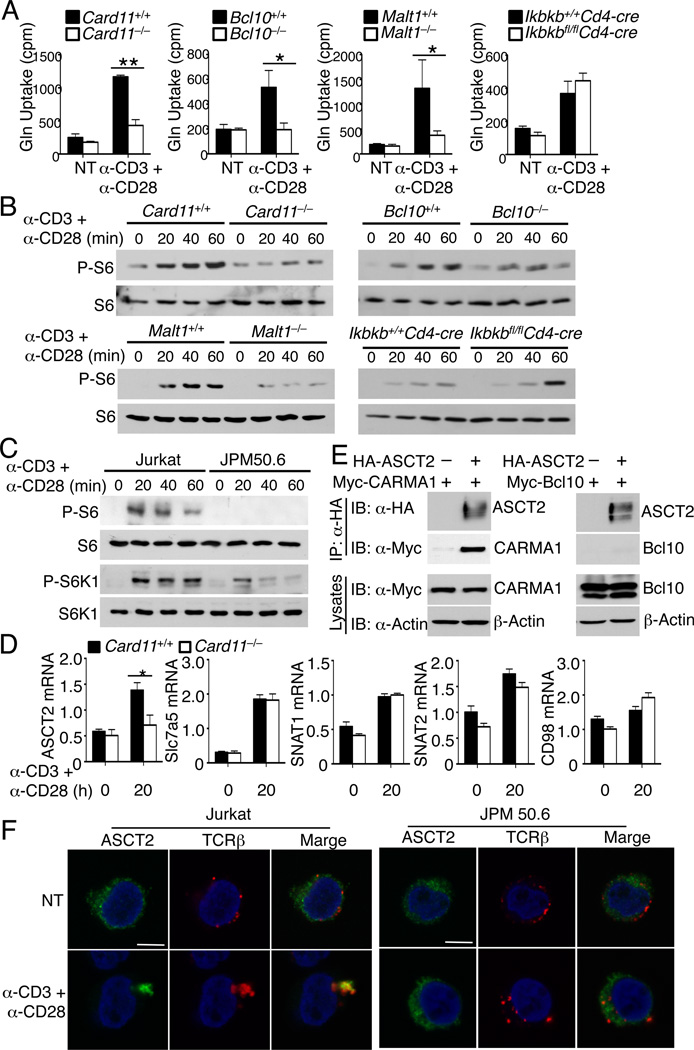
The results presented above suggested an ASCT2-dependent mechanism that couples the TCR and CD28 signals to glutamine uptake and mTORC1 activation. These findings also raised the question of which of the TCR signaling pathways are involved in this function. In this regard, the CBM complex, composed of CARMA1, BCL10, and MALT1, is known to link the TCR and CD28 proximal signals to downstream events, particularly activation of IKK and NF-κB (Blonska and Lin, 2009). We examined the role of this signaling complex in the induction of glutamine uptake and mTORC1 activation by TCR and CD28. Genetic deficiency in CARMA1, BCL10, or MALT1 severely attenuated the induction of glutamine uptake by anti-CD3 plus anti-CD28 (Figure 7A). Meanwhile, loss of the major CBM target, IKKβ, had no effect on the induction of glutamine uptake (Figure 7A). Consistent with these results, genetic deficiency in any of the CBM components, but not IKKβ, attenuated the induction of S6 phosphorylation by anti-CD3 plus anti-CD28 (Figure 7B). The role of CARMA1 in regulating mTORC1 activation was also demonstrated using a human Jurkat T-cell variant, JPM50.6 (Wang et al., 2002), deficient in CARMA1 expression (Figure 7C). These data suggested a function of the CBM complex that connects the TCR and CD28 signals to the induction of glutamine uptake and mTORC1 activation independently of the downstream IKKβ.
Figure 7. CBM complex is required for TCR and CD28-stimulated glutamine uptake and mTORC1 activation.
(A) Glutamine uptake analysis of naïve CD4+ T cells derived from the indicated genetic ablation and their wild-type control mice, either not treated (NT) or stimulated for 30 min with anti-CD3 and anti-CD28 using the crosslinking method.
(B) IB analysis of phosphorylated (P-) and total S6 in whole-cell lysates of the indicated genetic ablation and wild-type control naïve CD4+ T cells stimulated with anti-CD3 and anti-CD28 as in A.
(C) IB analyses of phosphorylated (P-) and total S6 and S6K in whole-cell lysates of parental Jurkat cells and the CARMA1-deficient JPM50.6 cells stimulated with anti-CD3 and anti-CD28.
(D) QPCR analysis of the relative mRNA amount of the indicated genes in Card11+/+ or Carma1−/− naïve CD4+ T cells, stimulated for 20 h with plate-bound anti-CD3 and anti-CD28.
(E) HEK293 cells were transfected with Myc-tagged CARMA1 or Myc-tagged Bcl10 in the absence (−) or presence (+) of HA-tagged ASCT2. ASCT2 was isolated by IP using anti-HA, and its associated CARMA1 and Bcl10 were detected by anti-Myc IB (upper). The expression of ASCT2, CARMA1, and Bcl10 was monitored by direct IB (lower).
(F) Jurkat cells and JPM50.6 cells were stimulated for 15 min with anti-CD3 and anti-CD28 and were stained with rabbit anti-ASCT2 or mouse anti-TCRβ, followed by incubation with Alexa-labeled goat anti-rabbit or goat anti-mouse secondary antibodies. The subcellular localization of ASCT2 (green), TCRβ (red) and nucleus (blue) were visualized by confocal fluorescent microscopy. Scale bar, 5 µm.
Data are representative of three independent experiments with three or more mice per group (mean ± s.d. in A and D). *P < 0.05, **P < 0.01.
Also see Figure S7.
To further confirm that the CBM regulates mTORC1 activation via the induction of glutamine uptake, we examined whether excessive leucine could rescue the mTORC1 signaling defect of the CARMA1-deficient T cells, as seen in ASCT2-deficient T cells. Indeed, the exogenous leucine was able to restore TCR and CD28-stimulated phosphorylation of S6 and its kinase S6K1 (Figure S7A). Similar to ASCT2-deficient T cells, CARMA1- and Bcl10-deficient T cells displayed a defect in TCR and CD28-stimulated glucose uptake (Figure S7B), further emphasizing the role of the CBM complex in mediating the induction of metabolic activities during T-cell activation.
To define the mechanism by which the CBM complex regulates glutamine uptake and mTORC1 activation, we examined the role of CARMA1 in regulating ASCT2 expression. CARMA1 deficiency only moderately reduced the basal level of ASCT2 mRNA; however, the loss of CARMA1 dramatically inhibited the TCR and CD28-stimulated ASCT2 mRNA expression (Figure 7D). The CARMA1 deficiency did not significantly affect the induction of other amino acid transporters, including SNAT1, SNAT2, Slc7a5, and CD98 (Figure 7D). These results explain the defect of the CARMA1-deficient T cells in the late-phase induction of glutamine uptake. In addressing the mechanism by which CBM regulates the early-phase glutamine uptake, we found that CARMA1 physically interacted with ASCT2 (Figures 7E and S7C). In the absence of CARMA1, Bcl10 and MALT1 did not bind ASCT2 (Figure 7E and data not shown). In response to TCR and CD28 stimulation, ASCT2 rapidly aggregated and colocalized with the TCR complex (Figure 7F). This molecular event was dependent on CARMA1, since it did not occur in the CARMA1-deficient cells. Although precisely how the CBM complex activates ASCT2-mediated glutamine uptake requires additional studies, we found that the protease activity of MALT1 was required, since a MALT1 inhibitor, z-VRPR-fmk (Rebeaud et al., 2008) blocked the TCR and CD28-stimulated glutamine uptake (Figure S7D) and the phosphorylation of S6 and S6K1 (Figure S7E). These data provide additional clues to the mechanism by which CARMA1 regulates ASCT2 function and glutamine uptake.
DISCUSSION
Glutamine has been implicated as a nutrient with immunomodulatory functions, but the role of glutamine in regulating T-cell function and the mechanism by which glutamine uptake is coupled to TCR signaling have been unclear. The existence of several potential glutamine transporters makes this system particularly complex. Here we have obtained genetic evidence that ASCT2 is crucial for mediating TCR-stimulated glutamine uptake in naïve T cells. We found that ASCT2 was required for TCR and CD28-stimulated activation of mTORC1 signaling, although it was dispensable for the activation of several other T-cell activation pathways, including the MAP kinase pathways and the IKK pathway. Consistently, the ASCT2 deficiency did not compromise the initial T-cell activation processes, including proliferation and IL-2 production. Meanwhile, loss of ASCT2 attenuated the differentiation of naïve CD4+ T cells to Th1 and Th17 cells and influenced the in vivo T-cell responses. In contrast, ASCT2 was completely dispensable for the generation of Treg cells from naïve CD4+ T cells. Treg cell differentiation also occurred normally under glutamine-free conditions. These results are in agreement with the previous finding that mTORC1 is required for the generation of Th1 and Th17 cells, but not Treg cells(Delgoffe et al., 2009).
Our data revealed that ASCT2 was particularly important for glutamine uptake and mTORC1 activation in naïve T cells. In effector T cells, ASCT2 was either completely or partially dispensable for these cellular events. Since T-cell activation is associated with the transcriptional induction of several other glutamine transporters, including SNAT1 and SNAT2 (Carr et al., 2010), it is possible that ASCT2 is functionally redundant with other glutamine transporters in effector T cells. Consistent with this hypothesis, we found that the ASCT2 deficiency did not affect the induction of SNAT1 or SNAT2. The ASCT2 ablation also did not significantly affect the TCR and CD28-stimulated expression of Slc7a5 and CD98, components of a major leucine transporter in T cells (Sinclair et al., 2013). However, the TCR and CD28-stimulated leucine uptake was attenuated in the ASCT2-deficient T cells, a finding that is consistent with a prior report that the ASCT2-mediated glutamine uptake in cancer cells is required for the uptake of leucine by the Slc7a5-CD98 amino acid transporter (Nicklin et al., 2009). Compared to glutamine, leucine was more efficient in rescuing mTORC1-activation defect of ASCT2-deficient T cells, supporting the idea that glutamine may regulate mTORC1 signaling via promoting leucine uptake (Nicklin et al., 2009). Previous studies suggest that amino acids activate mTORC1 by inducing its translocation to the lysosomal membrane (Sancak et al., 2010) and that glutamine and leucine may cooperate in this pathway of mTORC1 activation (Duran et al., 2012). Future studies will examine whether ASCT2-mediated glutamine uptake in T cells both promotes leucine uptake and synergizes with leucine in the activation of mTORC1.
Activation of mTORC1 by TCR and CD28 signals is known to involve AKT, which is activated by both mTORC2 and the PI3 kinase-activated kinase PDK1(Chi, 2012; Waickman and Powell, 2012). We found that the ASCT2 deficiency attenuated the TCR and CD28-stimulated mTORC1 activation without inhibiting AKT activation. This result suggests the requirement of two coordinated signals, the AKT signal and the amino acid (leucine and glutamine) signal, in the activation of mTORC1 by the TCR and CD28 stimuli. In further support of this idea, we found that the TCR and CD28 ligation stimulated rapid glutamine uptake as well as leucine uptake in naïve T cells in an ASCT2-dependent manner. While leucine uptake may be indirectly regulated by the ASCT2-mediated glutamine uptake (Nicklin et al., 2009), how the TCR and CD28 signals stimulate the rapid glutamine uptake via a ASCT2-dependent manner is still not quite clear. Nevertheless, our data suggests a crucial role for the CBM signaling complex. Ablation of any of the three CBM components severely attenuated TCR and CD28-stimulated glutamine uptake and mTORC1 activation. To date, the best-known function of the CBM complex is to mediate TCR and CD28-stimulated activation of IKK and its downstream transcription factor NF-κB(Thome et al., 2010). IKK was dispensable for the induction of glutamine uptake and mTORC1 activation in T cells. It is thus likely that a different mechanism mediates the function of the CBM complex in the regulation of mTORC1 activation. Our data suggested that the CBM complex is required for TCR and CD28-stimulated expression of ASCT2. In addition, CARMA1 physically interacted with ASCT2. In response to TCR and CD28 crosslinking, ASCT2 formed aggregates that are colocalized with the TCR complex. Moreover, these molecular events were dependent on CARMA1. Although precisely how the CBM complex mediates activation of ASCT2-mediated glutamine uptake remains to be further studied, we found that the protease activity of MALT1 is required for TCR and CD28-stimulated glutamine uptake and mTORC1 signaling. These findings provide important clues to the mechanism by which the TCR and CD28 signals stimulate ASCT2-dependent glutamine uptake.
A hallmark of T-cell activation is the association with metabolic changes (Maciver et al., 2013). We found that stimulation of naïve CD4+ T cells with the anti-CD3 and anti-CD28 antibodies triggered a number of metabolic activities, including the induction of glucose uptake and glycolysis, upregulation of Glut1 and c-Myc expression, as well as increased OXPHOS. Consistent with the important role of glutamine in promoting these metabolic activities, these activities were all compromised, although not completely blocked, in the Slc1a5−/− T cells. However, how ASCT2 regulates metabolic activities and whether these functions are attributed to the glutamine and leucine uptake warrant further studies. Nevertheless, it is likely that some of these metabolic events may involve the ASCT2-dependent activation of mTORC1. For example, mTORC1 is known to mediate induction of both Glut1 and c-Myc, which in turn are important for various other metabolic activities (Babcock et al., 2013; Buller et al., 2008; Kaadige et al., 2010; Taha et al., 1999; Taha et al., 1995). However, glutamine and leucine may also regulate mTORC1-independent functions in T cells. We found that addition of excessive leucine into the culture medium efficiently rescued the defect of the Slc1a5−/− T cells in mTORC1 signaling; however, it required a much higher concentration of leucine to rescue the differentiation of CD4+ T cells. Future studies will further investigate how glutamine regulates the differentiation and effector functions of T cells.
Our finding that ASCT2 deficiency had no obvious effect on TCR and CD28-stimulated IL-2 production and proliferation was seemingly surprising. Meanwhile, previous gene targeting studies have also revealed that mTORC1 is dispensable for IL-2 induction and partially dispensable for T-cell proliferation (Delgoffe et al., 2011). Thymocyte development and T-cell homeostasis are also normal in the absence of mTORC1 (Delgoffe et al., 2009). Like mTORC1, ASCT2 was particularly important for the differentiation of CD4+ T cells to Th1 and Th17 cells. The lack of a notable effect of ASCT2 deficiency on TCR and CD28-stimulated naïve T-cell proliferation may also be due to the partial inhibition, instead of complete blockade, of mTORC1 activation and metabolic activities in the ASCT2-deficient T cells. Although ASCT2 was crucial for TCR and CD28-stimulated initial glutamine uptake, it was partially dispensable for the uptake of both glutamine and leucine after longer periods of cell stimulation. TCR and CD28-stimulated glucose uptake, glycolysis, and Myc expression were also partially retained in the Slc1a5−/− T cells. Clearly, how precisely ASCT2 regulates T-cell functions requires further studies. Oxygen consumption, although not glycolysis, is important for naïve T-cell activation and proliferation (Chang et al., 2013). Our data suggest that ASCT2 deficiency attenuated the initial burst of the oxygen consumption but only had a minor effect on the sustained OCR. This result may also explain why the proliferation of T cells was not appreciably affected by the ASCT2 deficiency.
In summary, our data establish ASCT2 as a glutamine transporter that regulates CD4+ T-cell differentiation and T cell-mediated immunity against infections and self-antigens. Our findings also suggest a role for ASCT2 in coupling the TCR and CD28 signals to the induction of glutamine and leucine uptake and mTORC1 activation, thus providing insight into the dynamic interplay between the antigen-stimulated signal and the environmental signals in the control of T-cell function. Finally, we have identified a function of the CBM complex in mediating the induction of glutamine uptake and mTORC1 activation, which may involve activation of the glutamine transporter ASCT2.
EXPERIMENTAL PROCEDURES
Mice
Slc1a5−/− mice (in C57BL/6 × 129/sv genetic background) were generated by a conventional gene-targeting strategy, in which coding exons 2 and 4 were replaced with a lacZ–neomycin-resistance cassette (Taconic, Figure S1). Heterozygous Slc1a5+/– mice were bred to generate age- and sex-matched homozygous ASCT2-ablated (Slc1a5−/−) and wild-type (Slc1a5+/+) mice, which were used in the experiments at the indicated ages. Genotyping PCR and RT-PCR primers are listed in Supplementary Table1. The TCR transgenic OT-II mice and the lymphocyte-deficient Rag1−/− deficient mice were from Jackson Laboratory. Ikbkb-floxed mice, provided by Dr. Manolis Pasparakis (University of Cologne), were crossed with Cd4-cre mice (Jackson Laboratory) to produce T cell-conditional Ikbkb−/− mice. Card11−/− mice (C57BL/6 × 129/sv genetic background) were provided by Dr. Josef Penninger (Austria Academy of Sciences), Bcl10−/− mice were provided by Dr. Stephan Morris (St Jude Children’s Research Hospital), and the Malt1−/− mice were provided by Dr. Vishva Dixit (Genentech). Mice were maintained in specific pathogen-free facility of The University of Texas MD Anderson Cancer Center, and all animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Nutrient uptake
Glutamine, leucine, phenylalanine and glucose uptake were essentially as described (Carr et al., 2010; Sinclair et al., 2013). Following activation by anti-CD3 plus anti-CD28, the CD4+ T cells were resuspended (4 × 107 cells/ml) in a serum- and glutamine-free RPMI 1640 medium (for glutamine uptake), Hank’s balanced-salt solution (for leucine uptake), or glucose-free RPMI 1640 (for glucose uptake). 50 µl of the cell suspension (2 × 106 cells) was carefully added to the top layer of a 0.7-ml microfuge tube preloaded with 25 µl 8% sucrose and 20% perchloric acid (bottom layer), 200 µl 1-bromododecane (middle layer), and 50 µl uptake medium containing 2 μCi L −2,3,4-[3 H]glutamine, 1 μCi L -[3H]leucine, 1 μCi L-[3H]phenylalanine or 2 μCi [3H]2-deoxyglucose. Cells were allowed to take up the radiolabeled nutrient for 10 min at room temperature and then spun through the bromododecane into the sucrose-perchloric acid layer to stop the reaction and separate the cells from unincorporated 3H-labeled nutrient. The sucrose-perchloric acid layer, containing the T cells, was collected for liquid scintillation assay to quantify the radiolabeled nutrient taken up by the T cells.
Statistical analysis
A two-tailed unpaired t-test was done with the Prism software. P values of less than 0.05 were considered significant, and the level of significance was further defined at levels of *P<0.05, **P<0.01, ***P<0.001.
Supplementary Material
Highlights.
ASCT2 couples the TCR signal to the induction of glutamine and leucine uptake
ASCT2 mediates TCR-stimulated mTORC1 activation and metabolic activities
ASCT2 is required for Th1 and Th17 cell production and inflammatory T-cell responses
CARMA1 binds ASCT2 and regulates glutamine uptake and mTORC1 activation
ACKNOWLEDGEMENTS
We thank M Pasparakis, J Penninger, S Morris, V Dixit and Genentech for genetically ablated mouse strains. We also thank the personnel from the NIH/NCI-supported resources (flow cytometry, DNA analysis, histology, and animal facilities) under award number P30CA016672 at The MD Anderson Cancer Center. This study was supported by grants from the National Institutes of Health (AI057555, AI064639, GM84459, and AI104519).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes Extended Experimental Procedures, 7 figures, and 1 table.
AUTHOR CONTRIBUTIONS
M.N. designed and performed the research, prepared the Figures, and wrote part of the manuscript; J.-H.C., Y.X., X.Z., M.C., X.C., M.B. contributed experiments; X.L. contributed reagents and supervised M.B.; and S.-C.S. supervised the work and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Babcock JT, Nguyen HB, He Y, Hendricks JW, Wek RC, Quilliam LA. Mammalian target of rapamycin complex 1 (mTORC1) enhances bortezomib-induced death in tuberous sclerosis complex (TSC)-null cells by a c-MYC-dependent induction of the unfolded protein response. J. Biol. Chem. 2013;288:15687–15698. doi: 10.1074/jbc.M112.431056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonska M, Lin X. CARMA1-mediated NF-kappaB and JNK activation in lymphocytes. Immunol. Rev. 2009;228:199–211. doi: 10.1111/j.1600-065X.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, Inoki K, Guan KL, Brosius FC., 3rd A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am. J. Physiol. Cell. Physiol. 2008;295:C836–C843. doi: 10.1152/ajpcell.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, Frauwirth KA. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J. Biol. Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell. Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaadige MR, Elgort MG, Ayer DE. Coordination of glucose and glutamine utilization by an expanded Myc network. Transcription. 2010;1:36–40. doi: 10.4161/trns.1.1.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaadige MR, Looper RE, Kamalanaadhan S, Ayer DE. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc. Natl. Acad. Sci. U S A. 2009;106:14878–14883. doi: 10.1073/pnas.0901221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karinch AM, Pan M, Lin CM, Strange R, Souba WW. Glutamine metabolism in sepsis and infection. J. Nutr. 2001;131:2535S–2538S. doi: 10.1093/jn/131.9.2535S. discussion 2550S–2531S. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Immunity to intracellular bacteria. Annu. Rev. Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Lund P, Williamson DH. Inter-tissue nitrogen fluxes. Br. Med. Bull. 1985;41:251–256. doi: 10.1093/oxfordjournals.bmb.a072059. [DOI] [PubMed] [Google Scholar]
- Maciver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivan JD, Bungard CI. The transport of glutamine into mammalian cells. Frontiers in bioscience : a journal and virtual library. 2007;12:874–882. doi: 10.2741/2109. [DOI] [PubMed] [Google Scholar]
- Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse Chapter 15, Unit 15 11. Curr. Protoc. Immunol. 2010 doi: 10.1002/0471142735.im1501s88. [DOI] [PubMed] [Google Scholar]
- Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001;131:2515S–2522S. doi: 10.1093/jn/131.9.2515S. discussion 2523S–2514S. [DOI] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeaud F, Hailfinger S, Posevitz-Fejfar A, Tapernoux M, Moser R, Rueda D, Gaide O, Guzzardi M, Iancu EM, Rufer N, et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat. Immunol. 2008;9:272–281. doi: 10.1038/ni1568. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr. Opin. Cell. Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Simmons SB, Pierson ER, Lee SY, Goverman JM. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 2013 doi: 10.1016/j.it.2013.04.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha C, Liu Z, Jin J, Al-Hasani H, Sonenberg N, Klip A. Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin. Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J. Biol. Chem. 1999;274:33085–33091. doi: 10.1074/jbc.274.46.33085. [DOI] [PubMed] [Google Scholar]
- Taha C, Mitsumoto Y, Liu Z, Skolnik EY, Klip A. The insulin-dependent biosynthesis of GLUT1 and GLUT3 glucose transporters in L6 muscle cells is mediated by distinct pathways. Roles of p21ras and pp70 S6 kinase. J. Biol. Chem. 1995;270:24678–24681. doi: 10.1074/jbc.270.42.24678. [DOI] [PubMed] [Google Scholar]
- Thome M, Charton JE, Pelzer C, Hailfinger S. Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1. Cold Spring Harbor perspectives in biology. 2010;2:a003004. doi: 10.1101/cshperspect.a003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J. Biol. Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol. Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M, Poortinga G, Hannan KM, Pearson RB, Hannan RD, McArthur GA. Translational control of c-MYC by rapamycin promotes terminal myeloid differentiation. Blood. 2008;112:2305–2317. doi: 10.1182/blood-2007-09-111856. [DOI] [PubMed] [Google Scholar]
- Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS, Nunez G, Bertin J, Lin X. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nat. Immunol. 2002;3:830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- Yamane H, Paul WE. Cytokines of the gamma(c) family control CD4+ T cell differentiation and function. Nat. Immunol. 2012;13:1037–1044. doi: 10.1038/ni.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J. Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.