Background: The BH3-only protein Noxa is an unstable protein degraded by the proteasome.
Results: C-terminal tail of Noxa contains a degradation signal for the ubiquitin-independent Noxa degradation, and mutation of this sequence stabilizes Mcl-1.
Conclusion: The C-terminal tail of Noxa regulates the stability of Noxa and Mcl-1.
Significance: These results suggest new mechanisms of regulation on the apoptosis regulators Noxa and Mcl-1 through protein stability modulation.
Keywords: Bcl-2 Family Proteins, Mitochondrial Apoptosis, Protein Complex, Protein Stability, Ubiquitylation (Ubiquitination), BH3-only Proteins, Mcl-1, Noxa
Abstract
The BH3-only protein Noxa is a critical mediator of apoptosis and functions primarily by sequestering/inactivating the antiapoptotic Bcl-2 family protein Mcl-1. Although Noxa is a highly labile protein, recent studies suggested that it is degraded by the proteasome in a ubiquitylation-independent manner. In the present study, we investigated the mechanism of Noxa degradation and its ability to regulate the stability of Mcl-1. We found that the ubiquitylation-independent degradation of Noxa does not require a physical association with Mcl-1. A short stretch of amino acid residues in the C-terminal tail was found to mediate the proteasome-dependent degradation of Noxa. Ectopic placement of this degron was able to render other proteins unstable. Surprisingly, mutation of this sequence not only attenuated the rapid degradation of Noxa, but also stabilized endogenous Mcl-1 through the BH3-mediated direct interaction. Together, these results suggest that the C-terminal tail of Noxa regulates the stability of both Noxa and Mcl-1.
Introduction
Apoptosis is a major form of cell death necessary for proper development and maintenance of tissue homeostasis in multicellular organisms (1, 2). The Bcl-2 protein family, which consists of both the antiapoptotic and the proapoptotic members, plays a critical role in the regulation and execution of mitochondria-dependent apoptosis (3). Within the Bcl-2 family, the antiapoptotic group includes Bcl-xL, Bcl-2, Mcl-1, etc., whereas the proapoptotic group is divided into the multidomain proteins, which include Bax, Bak, and Bok, and the BH3-only proteins, which include Bad, Bid, Puma, Bim, Noxa, Bik, etc. (4). The BH3-only proteins are believed to activate Bax and Bak, either directly or indirectly, by sequestering the antiapoptotic family proteins and/or physically binding to Bax/Bak (5, 6). Once activated, Bax and Bak form homo-oligomers and cause the formation of mitochondrial outer membrane pores, which allow the release of apoptogenic factors, such as cytochrome c, SMAC, and AIF (7, 8). In the cytoplasm, cytochrome c triggers the assembly of the apoptosome, which in turn activates caspases 3, 6, and 7 and leads to the demolition of the cell (9).
The BH3-only protein Noxa was originally identified as a phorbol 12-myristate 13-acetate-inducible protein (10) and later found to be a major transcriptional target of the tumor suppressor p53 (11). Since its discovery, Noxa has been shown to be critically involved in numerous apoptotic pathways, including DNA damage, endoplasmic reticulum stress, and proteasomal inhibition, through both p53-dependent and p53-independent pathways (12). Noxa preferentially binds to Mcl-1 and A1 and primarily functions to neutralize Mcl-1 during apoptosis (13). However, in the absence of functional Mcl-1, Noxa also has the capacity to bind to and inactivate Bcl-xL (11, 14).
Noxa has been shown to be a short-lived protein, which is degraded by the proteasome (15). It appears that Noxa is degraded in a ubiquitylation-independent fashion as lysine-free Noxa mutants are still degraded efficiently by the proteasome (16). However, because endogenous Noxa is complexed with Mcl-1 under normal conditions, it is not clear whether the degradation of Noxa is truly ubiquitylation-independent as ubiquitylation of Mcl-1 might target the Noxa-Mcl-1 complex for degradation. Nonetheless, the mechanism of the degradation of Noxa remains unclear.
Like Noxa, Mcl-1 was also found to be a short-lived protein degraded by the proteasome (17–19). Several E3 ligases and deubiquitinases have been identified as regulators of the ubiquitylation and proteasomal degradation of Mcl-1 (20). However, the mechanism of the degradation of Mcl-1 becomes more unclear as a recent study suggested that mouse Mcl-1 can be degraded by the proteasome in a ubiquitin-independent manner (21). Interestingly, it has been suggested that Noxa plays a positive role in the degradation of Mcl-1 as overexpression of Noxa was found to decrease the level of endogenous Mcl-1 (22). In this study, we investigated the mechanism of the degradation of Noxa and its ability to regulate the stability of Mcl-1. We identified a structural element in Noxa that is important for both.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Antibodies used in this study were anti-Noxa (Imgenex, IMG-349A), anti-multiubiquitin (StressGen Bioreagents Corp., SPA-205), anti-Mcl-1 (Santa Cruz, Sc-819), anti-GFP (Santa Cruz, Sc-9996), and anti-β-actin (Sigma, A5441). Cycloheximide (CHX,5 catalog number 357420010) was purchased from Acros Organics (Thermo Fisher Scientific). MG-132 (catalog number 81-5-15) was obtained from American Peptide (Sunnyvale, CA).
Cell Lines and Cell Culture
Regular and retrovirus-infected HeLa cells stably expressing various proteins were maintained in Dulbecco's modified Eagle's medium supplemented with antibiotics and 10% fetal bovine serum.
Plasmid Construction
To generate the transient expression plasmid of wild-type Noxa, the cDNA of human Noxa was PCR-amplified with the addition of a His6 tag to the N terminus. An XhoI site and an EcoRI site were engineered into the forward and reverse primers, respectively. Following digestion with XhoI and EcoRI, the PCR product was ligated into the XhoI- and EcoRI-digested pcDNA3.1(−) vector (Invitrogen). This construct was used as a template for the generation of Noxa BH3 mutant (L29E), different K/R mutants, and the compound mutants of BH3 and K/R by site-directed mutagenesis. The His6-tagged ubiquitin plasmid (pMT107) is a gift from Dr. Richard Baer and Dr. Dirk Bohmann. To construct the retroviral expression plasmid for Noxa and its mutants, the Noxa sequences were amplified by PCR, with the forward primer containing an XhoI site followed by a FLAG tag, and the reverse primer containing an EcoRI site. The PCR products were digested with XhoI and EcoRI and then cloned into the XhoI-EcoRI-digested pMSCV-IRES-GFP vector (pMIG, a gift from Dr. Robert Lewis). To construct the retroviral expression plasmid for GFP-Noxa fusion protein (pMIG-GFP-Noxa), human Noxa was first cloned into the pEGFPC3 vector (Clontech) by ligation of the PCR-amplified Noxa sequence into the XhoI and EcoRI sites. Subsequently, the XhoI site in pEGFPC3-Noxa, which is between GFP and Noxa sequence, was destroyed by site-directed mutagenesis. The resulting plasmid was then used as a template to amplify the sequence of GFP-Noxa, with XhoI in the forward primer and HindIII in the reverse primer. The resulting PCR product was then digested by XhoI and HindIII and ligated into the XhoI-HindIII cut pMIG vector. The XhoI-HindIII digestion deleted a large portion of the original GFP sequence within the pMIG vector. All the C-terminal truncation mutants of Noxa, including G-Noxa1–50, G-Noxa1–45, G-Noxa1–39, and G-Noxa1–30, and GFP were cloned into pMIG using the same strategy. N-terminal His-tagged G-Noxa1–50, G-Noxa1–45, G-Noxa1–39, and G-Noxa1–30 were also cloned into pMIG by ligation using XhoI and HindIII sites. The constructs for the N-terminal truncation mutants of Noxa, including pMIG-G-Noxa16–54, pMIG-G-Noxa35–54, pMIG-G-Noxa40–54, and pMIG-G-Noxa45-54, were made by site-directed mutagenesis with pMIG-G-Noxa as the template. The alanine-scanning constructs of Noxa, including pMIG-G-Noxa40–41A, pMIG-G-Noxa42–43A, pMIG-G-Noxa44–45A, pMIG-G-Noxa46–47A, pMIG-G-Noxa48–49A, pMIG-G-Noxa50–51A, and pMIG-G-Noxa52–54A, were made by site-directed mutagenesis based on pMIG-G-Noxa. pMIG-G-Bcl-xL was made by site-directed mutagenesis by replacing Noxa sequence with Bcl-xL sequence. pMIG-G-Bcl-xL/NoxaCT20 was made by site-directed mutagenesis by replacing the sequence of Bcl-xL209–233 with the sequence of Noxa35–54 (NoxaCT20) using pMIG-G-Bcl-xL as the template. pMIG-G-NoxaL29E and pMIG-His6-G-NoxaL29E were made by site-directed mutagenesis based on their wild-type Noxa constructs. pMIG-His6-G-Noxa42–43A was made by site-directed mutagenesis using pMIG-His6-G-Noxa as the template. pMIG-His6-G-NoxaL29E/42–43A was made by site-directed mutagenesis using pMIG-His6-G-NoxaL29E as the template. pMIG-G-NoxaCT42–43A was made by site-directed mutagenesis based on pMIG-G-Noxa40–54 (pMIG-G-NoxaCT). The constructs were verified by sequencing.
Transfections
HeLa cells were plated (at a density of 2.5 × 105 cells/35-mm plate) and cultured for 20–24 h before transfection with the Effectene reagents (Qiagen) according to the manufacturer's recommendation. For each transfection in a 35-mm plate, 50 ng of plasmid of interest were used and pcDNA3.1(+) was included in the mixture to maintain the total amount of DNA at 0.5 μg/transfection. Eighteen hours after transfection, cells were collected for Western blot.
Retrovirus Production and Stable Cell Line Generation
All the retroviruses were produced by transfecting the pMIG constructs into the virus packaging cell line 293GP. The details of retrovirus production and transduction into HeLa cells were described elsewhere (14).
Assessment of Protein Stability Using Cycloheximide Chase
HeLa cells or stable cell lines of HeLa cells expressing different proteins were plated in a 35-mm plate at a density of ∼3 × 105/plate 1 day before treatment. Cycloheximide (50 μm) was added for 0–4 h. At different time points, cells were harvested and then lysed in EBC buffer (0.5% Nonidet P-40, 120 mm Tris-HCl, pH 7.5, 120 mm NaCl, 1 mm EDTA) for Western blot.
Nickel Bead Pulldown Assay
HeLa cells with or without overexpression of various proteins were pelleted by centrifugation at 1000 ×g for 4 min, washed once with ice-cold PBS, and lysed in the appropriate volume of EBC buffer with protease inhibitors for 1 h at 4 °C with gentle rotation. The lysates from cell pellet of a 6-cm plate were cleared by centrifugation at 22,000 ×g for 20 min. The supernatant was incubated with 20 μl of nickel-nitrilotriacetic acid-agarose beads (Qiagen) by gentle rotation at 4 °C for 3 h. The beads were washed twice by EBC buffer containing 10 mm imidazole. SDS loading dye with 250 mm imidazole was used to elute proteins from nickel beads.
RESULTS
Degradation of Noxa Is Ubiquitylation-independent and Does Not Require the Association of Noxa with Mcl-1
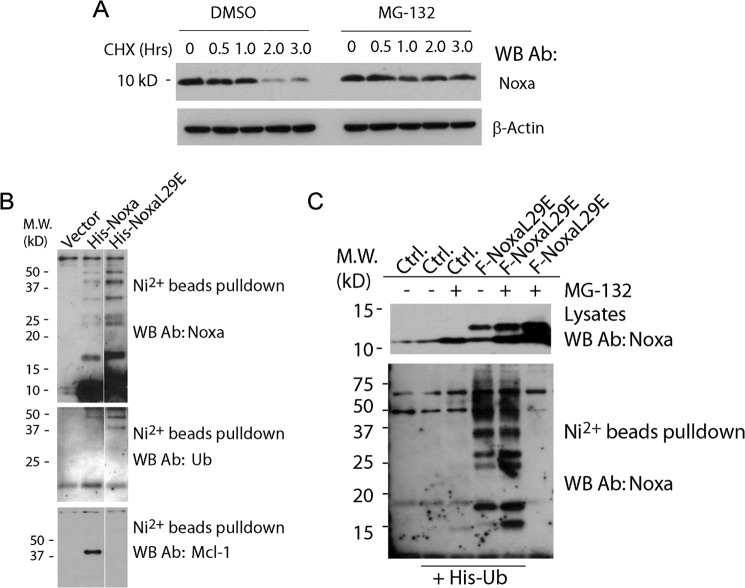
To study the regulation of the stability of Noxa, we first measured the half-life of Noxa by Western blot following the addition of cycloheximide, an inhibitor of protein synthesis, in HeLa cells. Consistent with earlier findings, we found that Noxa is short-lived with a half-life of 45–60 min, and the degradation of Noxa is completely blocked by the proteasome inhibitor MG-132 (Fig. 1A). Because most proteins are targeted to the proteasome for degradation through ubiquitylation, we tested for the ubiquitylation of Noxa. Cell lysates from HeLa cells transiently expressing polyhistidine-tagged Noxa (His6-Noxa) were subjected to nickel bead pulldown, and Western blots were probed with an antibody recognizing human Noxa. As shown in Fig. 1B, in addition to the monomeric Noxa, the Noxa antibody detected a ladder of larger proteins, reminiscent of ubiquitylated Noxa. Moreover, two protein bands within the 37–50-kDa range of that ladder were also detected by an antibody against ubiquitin, supporting the conclusion that Noxa is ubiquitylated (Fig. 1B).
FIGURE 1.
Noxa is short-lived and is ubiquitylated independently of Mcl-1 association. A, Western blot (WB) analysis showing the half-life of Noxa. HeLa cells were treated with CHX (50 μm) or the combination of CHX (50 μm) and proteasome inhibitor MG132 (1 μm). DMSO, dimethyl sulfoxide; Ab, antibody. B, HeLa cells were transfected with empty vector, plasmid expressing wild-type His6-tagged Noxa (His-Noxa), or a plasmid expressing a His6-tagged BH3 mutant of Noxa (His-NoxaL29E) in the presence of 20 μm z-VAD. Following an overnight incubation, cells were lysed in EBC buffer and subjected to nickel-nitrilotriacetic acid affinity capture. The resulting protein complexes were resolved on SDS-PAGE gels and blotted using antibodies against Noxa, polyubiquitin, and Mcl-1. M. W., molecular weight markers; Ub, ubiquitin. C, HeLa cells were transfected with empty vector or plasmid expressing FLAG-NoxaL29E alone or in combination with His-tagged ubiquitin (His-Ub). After overnight incubation, cells were treated with MG-132 (10 μm) in the presence of the caspase inhibitor z-VAD (20 μm) for 8 h, lysed, and subjected to nickel-nitrilotriacetic acid affinity capture. The proteins in the bound fraction were resolved on SDS-PAGE and blotted with a Noxa antibody. Ctrl., control.
As Mcl-1 is complexed with Noxa in the cell (13, 14), it is important to see whether the ubiquitylation of Noxa is dependent on its association with Mcl-1. A BH3 mutant of Noxa (FLAG-NoxaL29E), which lacks the ability to bind Mcl-1 (14), was transiently expressed in HeLa cells and tested for ubiquitylation. As shown in Fig. 1B (bottom panel), unlike wild-type Noxa, NoxaL29E was unable to bind endogenous Mcl-1 in the nickel bead pulldown assay. However, both wild-type Noxa and NoxaL29E displayed a similar pattern of protein species in Western blot using an anti-Noxa antibody. Further, the same two protein bands within the 37–50-kDa range were detected by the anti-ubiquitin antibody in the nickel bead pulldown for both wild-type Noxa and the NoxaL29E mutant, indicating that the ubiquitylation of Noxa is not dependent on its association with Mcl-1. As another test for ubiquitylation, we examined the presence of NoxaL29E among the ubiquitylated proteins. HeLa cells were co-transfected with two plasmids expressing His6-ubiquitin and FLAG-NoxaL29E, respectively, and were subjected to nickel bead pulldown. The bound fraction, presumably enriched for ubiquitylated proteins, was probed with an anti-Noxa antibody. As shown in Fig. 1C, multiple larger protein species with a pattern similar to that detected in nickel bead pulldown from His6-Noxa-transfected cells (Fig. 1B) were detected. Together, these results demonstrated that Noxa is efficiently ubiquitylated independently of Mcl-1 association.
We next sought to identify the ubiquitylation site(s) of Noxa through systematic mutagenesis, in which the lysine residues of the wild-type Noxa were changed into arginine in various combinations. His-tagged Noxa and its mutant proteins were expressed in HeLa cells, and the ubiquitylation was monitored by nickel bead pulldown. As shown in Fig. 2A, one Noxa mutant, termed C3KR, with Lys35, Lys41, and Lys48 simultaneously changed to arginine residues, showed a complete loss of ubiquitylation. On the other hand, the ubiquitylation of any other mutant remains, as long as one of the three aforementioned lysine residues is still intact, suggesting that Lys35, Lys41, and Lys48 are all ubiquitylated in vivo.
FIGURE 2.
Degradation of Noxa is ubiquitylation-independent and is not dependent on the association of Noxa with Mcl-1. A, HeLa cells were transfected with empty vector or different His-tagged constructs for Noxa. NoxaN3KR is a Noxa mutant with the three N-terminal lysine residues Lys4, Lys5, and Lys8 all mutated to arginine. NoxaC3KR is a Noxa mutant with the three C-terminal lysine residues Lys35, Lys41, and Lys48 all mutated to arginine. Noxa K35/41R is a Noxa mutant with both lysine residues Lys35 and Lys41 mutated to arginine. Following an overnight incubation, cells were treated with MG-132 (10 μm) for 8 h and lysed in EBC buffer. The lysates were resolved on SDS-PAGE and blotted with a Noxa antibody (Ab). M. W., molecular weight markers; WB, Western blot. B, HeLa cells were transiently transfected with various expression plasmids expressing FLAG-tagged Noxa or its mutants in the presence of 20 μm z-VAD. Sixteen hours after transfection, cells were lysed in EBC buffer. The lysates were subjected to immunoprecipitation using the M2 beads. The proteins in the total lysates and the bound fractions were resolved on SDS-PAGE and blotted with antibodies against Noxa and Mcl-1. C, HeLa cells stably expressing Bcl-2 and the FLAG-tagged Noxa or its mutants were treated with CHX (50 μm) or a combination of CHX (50 μm) and MG-132 (1 μm) and harvested at the indicated time points. The cells were lysed in EBC buffer and subjected to Western blot analysis with the Noxa antibody.
To investigate whether ubiquitylation and Mcl-1 association are necessary for the degradation of Noxa by the proteasome, Noxa mutants that are defective in ubiquitylation (F-NoxaC3KR) or binding to Mcl-1 (F-NoxaL29E), or both (F-NoxaL29E/C3KR), were cloned into transient expression plasmids as well as the retroviral vectors. As expected, in transient transfection assays, the wild-type Noxa and the C3KR mutant both bind endogenous Mcl-1, whereas the NoxaL29E and NoxaL29E/C3KR mutants both lost the ability to bind Mcl-1 (Fig. 2B). The retroviral plasmids allowed us to establish HeLa cell lines stably expressing these proteins at levels similar to that of the endogenous Noxa. As a precaution against the proapoptotic activity of Noxa due to its overexpression, Noxa or its mutant-expressing cell lines in Fig. 2C were established in the presence of Bcl-2 overexpression. However, we did not detect apoptosis in the stable cell lines of Noxa or its mutants in the absence of Bcl-2 overexpression in subsequent experiments. Because the addition of the FLAG tag causes the protein to migrate slower on the SDS-PAGE, we were able to detect both the exogenous and the endogenous Noxa, with the latter serving as an internal control, using an antibody against Noxa in Western blot. The half-lives of these proteins were measured by cycloheximide-induced degradation. As shown in Fig. 2C, all four FLAG-tagged Noxa proteins showed half-lives of less than an hour, similar to that of endogenous Noxa. These results indicate that the degradation of Noxa is independent of ubiquitylation and is not dependent on its association with Mcl-1.
The C-terminal Tail Is Responsible for the Degradation of Noxa
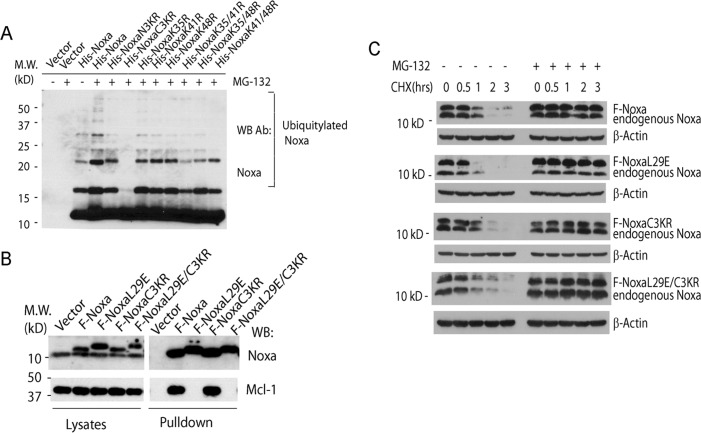
To investigate the mechanism of the ubiquitylation-independent degradation of Noxa, we carried out a serial truncation analysis of the Noxa protein. As human Noxa contains only 54 a. a., it is difficult to detect the expression of all the truncation mutants. We therefore resorted to GFP-Noxa fusion proteins, which enabled us to detect all the Noxa truncation mutants with an antibody against GFP. Importantly, GFP-Noxa displayed a short half-life similar to that of the endogenous Noxa (Fig. 3A). Most mutants with truncations at the N terminus generated short-lived proteins. In particular, mutants G-Noxa35–54 and G-Noxa40–54, containing only the last 20 and 15 amino acid residues, respectively, even showed a half-life less than 30 min, suggesting a loss of sequences inhibitory to the degradation of Noxa. However, a further truncation (G-Noxa 45–54) resulted in a stable protein, with a half-life longer than 2 h, suggesting that the sequence element critical for the degradation of Noxa is disrupted in this mutant.
FIGURE 3.
The C-terminal tail is responsible for the degradation of Noxa. A, expression and half-lives of Noxa N-terminal truncation mutants. HeLa cells stably expressing Bcl-2 and GFP fusions of wild-type Noxa or its N-terminal truncation mutants (schematically shown in the middle panel) were treated with CHX (50 μm) for 0–4 h, harvested, and lysed in EBC buffer. The cell lysates were subjected to Western blot (WB) with an antibody (Ab) against GFP. * indicates the nonspecific protein recognized by the GFP antibody. # G-Noxa40–54 is named GFP-NoxaCT in Fig. 5B. B, expression and half-lives of Noxa C-terminal truncation mutants. HeLa cells stably overexpressing Bcl-2 and the wild-type Noxa or its C-terminal truncation mutants (schematically shown in the middle panel) were treated with CHX (50 μm) for 0–4 h, harvested, and lysed in EBC buffer. The cell lysates were subjected to Western blot with an antibody against GFP.
We next generated a series of C-terminal truncation mutants. Surprisingly, all the truncation mutants with deletions in the C terminus, including a mutant that has a deletion of only the last 5 amino acid residues, became stable proteins with half-lives longer than 4 h (Fig. 3B). For unknown reasons, the G-Noxa1–45 mutant appears moderately less stable than G-Noxa 1–50 and G-Noxa 1–39. Nonetheless, these results, combined with those from the N-terminal truncation mutants, strongly suggest that the last 15 amino acid residues (40–54 a. a.) may carry a degradation signal (degron) for Noxa.
To map the amino acid residues critical for the degradation of Noxa, an alanine-scanning mutagenesis was performed on the last 15 amino acid residues on the background of full-length G-Noxa. As shown in Fig. 4A, one mutant, G-Noxa42–43A, showed a significantly higher basal level as compared with WT Noxa, suggesting that this mutant became more stable. Indeed, G-Noxa43–43A showed a significantly longer half-life than that of the WT G-Noxa (Fig. 4B). Similar results were obtained when G-Noxa42–43A was expressed in the colorectal cancer HCT116 cells (Fig. 4C). Of note, when Leu42 or Leu43 was singly mutated to Ala, the L42A mutant showed a modest increase in half-life, whereas the L43A mutant became much more stable, indicating that although both residues contribute to the degradation, Leu43 plays a more critical role in mediating the degradation of Noxa (Fig. 4D).
FIGURE 4.
Leu42 and Leu43 are required for the degradation of Noxa. A, expression of Noxa and its C-terminal tail alanine-scanning mutants. HeLa cells stably expressing GFP-Noxa and the indicated mutants were lysed in EBC buffer. Cell lysates were subjected to SDS-PAGE and Western blot (WB) with an anti-GFP antibody (Ab). B, half-lives of Noxa and its C-terminal tail mutant Noxa42–43A. HeLa cells stably expressing G-Noxa or G-Noxa42–43A were treated with CHX for 0–4 h. The cells were lysed in EBC buffer and subjected to SDS-PAGE and Western blot with an anti-GFP antibody. * indicates the nonspecific protein recognized by the GFP antibody. C, the indicated stable pools of HCT116 cells were treated with CHX (50 μm) for 0–4 h, harvested, and lysed in EBC buffer. The cell lysates were subjected to Western blot analysis. D, the half-lives of the indicated proteins were measured by cycloheximide treatment as described in B. E, HeLa and HCT116 cell pools stably expressing the indicated GFP fusions of wild-type and point mutants of Noxa were lysed in EBC buffer and subjected to Western blot analysis. F and G, cells from HeLa (F) or HCT116 (G) stable pools were treated with 0.5 or 1 μm ABT737 for 6 h before they were harvested and lysed in EBC buffer. The cell lysates were subjected to Western blot analysis. H, HeLa cell stably expressing GFP-Noxa or GFP-Noxa42–43A were stained with MitoTracker and subjected to fluorescence microscopy. PARP, poly(ADP-ribose) polymerase-1. The results from B, C, D, F, and G are representatives of two or more independent experiments.
To examine the effect of the C-terminal tail mutation on the apoptotic activity of Noxa, we generated stable pools expressing G-Noxa42–43A, G-Noxa, or the BH3-mutant G-NoxaL29E in both HeLa and HCT116 cells by retroviral infection (Fig. 4E). The wild-type Noxa (G-Noxa) and the G-Noxa42–43A mutant displayed similar abilities to cooperate with the Bcl-2/Bcl-xL inhibitor ABT-737 to induce apoptosis in HeLa and HCT116 stable pools (Fig. 4, F and G). In addition, both WT and the G-Noxa42–43A mutant partially localized to the mitochondria (Fig. 4H). Thus, the Leu to Ala mutations on Leu42 and Leu43 diminished the degradation of Noxa without affecting the mitochondria localization and function of the protein, suggesting that these two residues in the Noxa C-terminal tail play a critical role in the degradation of Noxa.
Noxa Contains a Degron Sequence
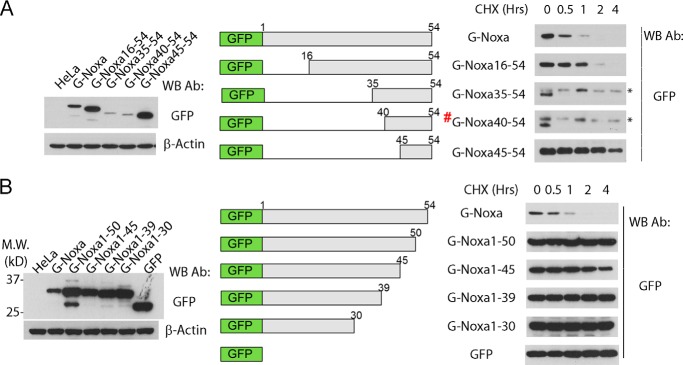
To examine the ability of the C-terminal region of Noxa to serve as a degron, we constructed a Bcl-xL mutant protein, replacing the C-terminal tail of Bcl-xL with that of Noxa (last 20 amino acid residues of Noxa). Although GFP-Bcl-xL showed a long half-life, the GFP-Bcl-xL-NoxaCT chimeric protein became unstable with a half-life of ∼1 h, indicating that the C-terminal region of Noxa contains a functional degron responsible for the short half-life of Noxa (Fig. 5A).
FIGURE 5.
Noxa C-terminal tail contains a degradation signal. A, C-terminal tail of Noxa converts Bcl-xL into an unstable protein. HeLa cells stably expressing the GFP fusions of Bcl-xL and Bcl-xL/Noxa chimera were subjected to CHX (50 nm) treatment for 0–4 h. The cells were harvested at the indicated time points. The cell lysates were loaded onto an SDS-PAGE and probed with an anti-GFP antibody (Ab). A diagram of Bcl-xL/Noxa chimera is shown on the top panel. Bcl-xL/NoxaCT20 was made by replacing Bcl-xL C-terminal tail (a. a. 209–233) with the Noxa C-terminal tail (a. a. 35–54). WB, Western blot. B, HeLa cells stably expressing GFP-NoxaCT or GFP-NoxaCT42–43A were treated with either vehicle or MG-132 (2 μm) for 4 h and harvested for Western blot analysis with an anti-GFP antibody. G-NoxaCT# was also named G-Noxa40–54 in Fig. 3A. C, Western blot with anti-GFP antibody showing the levels of the indicated proteins following treatment with CHX (50 μm).
To further examine the role of the C-terminal tail (CT) as the degron of Noxa, we generated a GFP fusion protein (G-NoxaCT) consisting of GFP and the CT of Noxa (the last 15 amino acid residues). When stably expressed in HeLa cells, G-NoxaCT exists at an extremely low level (Fig. 5B). However, this level was greatly elevated following the addition of MG-132, suggesting that, similar to the full-length Noxa, Noxa CT is a labile protein degraded by the proteasome (Fig. 5B). Indeed, this fusion protein displayed a very short half-life of less than 30 min. (Fig. 5C). To test the role of Leu42 and Leu43 in the degron function of the CT, these two residues were mutated to two alanine residues to generate G-NoxaCT42–43A. This mutant showed a significantly higher basal level than the wild-type Noxa CT and is less sensitive to stabilization by MG-132, suggesting that the degradation of the C-terminal tail is primarily mediated through leucine residues 42 and 43 (Fig. 5B). Not surprisingly, G-NoxaCT42–43A exhibited significantly longer half-lives than that of the wild-type CT (G-NoxaCT, Fig. 5C). These results strongly support the notion that Noxa C-terminal tail is a bona fide degron sequence.
The C-terminal Tail of Noxa Is Involved in the Regulation of Mcl-1 Stability
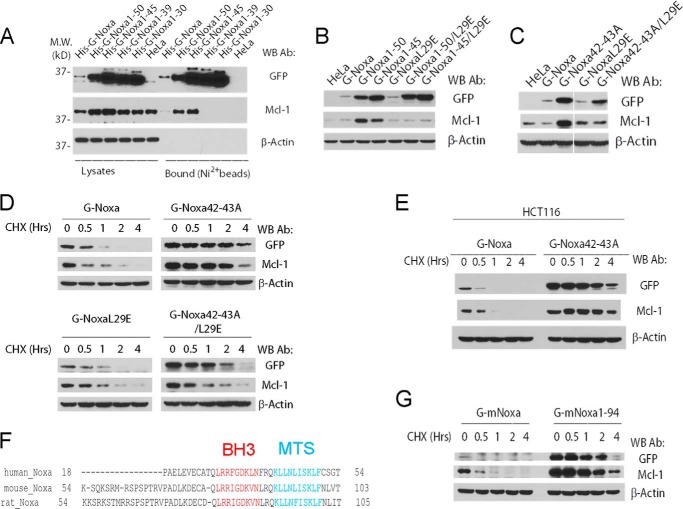
Because Mcl-1 is the major binding partner of Noxa in the cell, we examined the effect of the C-terminal tail mutations of Noxa on the level of endogenous Mcl-1 in the cells stably expressing the wild-type Noxa and its truncation mutants. As shown in Fig. 6A, whereas expression of full-length Noxa (G-Noxa) had little effect on Mcl-1, the expression of either G-Noxa1–50 or G-Noxa1–45 elevated the level of endogenous Mcl-1. This result suggested that the loss of an intact C-terminal tail caused the up-regulation of endogenous Mcl-1. However, the expression of Noxa1–39 or Noxa1–30, which both lost the C-terminal tail, failed to elevate the level of Mcl-1. Nickel bead pulldown of the His-tagged Noxa and its C-terminal truncation mutants revealed that full-length Noxa, Noxa1–50, and Noxa 1–45, but not Noxa1–39 and Noxa1–30, were able to bind endogenous Mcl-1. This correlation suggests that the elevation of endogenous Mcl-1 is dependent on the direct association with Mcl-1. Indeed, the BH3 mutation (L29E) completely abolished the interaction with Mcl-1 as well as the ability of full-length Noxa, Noxa1–50, and Noxa1–45 to up-regulate endogenous Mcl-1 (Fig. 6B).
FIGURE 6.
Noxa C-terminal tail regulates Mcl-1 stability through direct interaction. A, shown is a nickel bead pulldown analysis of HeLa stable cell lines expressing N-terminal His9-tagged GFP fusions of Noxa and its C-terminal truncation mutants. Cells from HeLa stable cell lines expressing the indicated proteins were harvested and lysed in EBC buffer. Cell lysates were subjected to nickel bead pulldown as described under “Experimental Procedures.” The bound proteins were eluted with imidazole and analyzed by Western blot (WB). M. W., molecular weight markers; Ab, antibody. B, HeLa cells stably expressing the indicated GFP fusions of wild type and truncation/point mutants of Noxa were lysed and subjected to Western blot analysis. C, HeLa cells stably expressing the indicated GFP fusions of wild type and point mutants of Noxa were lysed and subjected to Western blot analysis. D, HeLa cells stably expressing wild-type and point mutants of Noxa were treated with cycloheximide and harvested at the indicated times. Cell lysates were generated and subjected to Western blot analysis with the indicated antibodies. E, HCT116 cell pools stably expressing the indicated GFP fusions of wild-type and point mutants of Noxa were treated with cycloheximide (50 μm) for 0–4 h, harvested, and lysed in EBC buffer. The cell lysates were subjected to Western blot. F, sequence comparison of the C-terminal region of Noxa from various species. MTS, mitochondrial targeting sequence. G, HeLa cells stably expressing Bcl-2 and wild-type or C-terminal truncation mutant of mouse Noxa (G mNoxa) were treated with cycloheximide and harvested at the indicated time. Cell lysates were generated in EBC buffer and subjected to Western blot analysis with the indicated antibodies. The Western blots in D, E, and G are representatives of two or more independent experiments.
To further test the hypothesis that loss of an intact C-terminal tail is responsible for up-regulating endogenous Mcl-1, we examined the Mcl-1 level of cells stably expressing G-Noxa42–43A. Similar to the truncations (Noxa1–50 and Noxa1–45), the point mutation of the C-terminal tail elevated the level of endogenous Mcl-1 (Fig. 6C). As expected, this effect was completely abolished when the BH3 mutation (L29E) was introduced into this mutant, indicating that the up-regulation of Mcl-1 by the Noxa C-terminal tail mutants requires a physical binding to Mcl-1 (Fig. 6C).
To investigate whether up-regulation of Mcl-1 is through protein stabilization, we measured the half-life of Mcl-1 in the cell lines stably expressing Noxa and its C-terminal tail mutants. As shown in Fig. 6D, whereas Mcl-1 in regular HeLa or G-Noxa-expressing HeLa cells is short-lived with a half-life of less than an hour, its half-life in both G-Noxa42–43A-expressing and G-Noxa42–43D-expressing cells is significantly longer (>2 h). Not surprisingly, when either mutation is combined with the BH3 mutation (Noxa42–43A/L29E or Noxa42–43D/L29E), the half-life of the compound mutants remained long. However, this mutant completely failed to prolong the half-life of endogenous Mcl-1, indicating that the physical interaction between the Noxa C-terminal tail mutant and Mcl-1 is required for the stabilization of Mcl-1 (Fig. 6D). The stabilization of endogenous Mcl-1 was also observed in HCT116 cells stably expressing G-Noxa42–43A (Fig. 6E). Taken together, these results suggest that the Noxa C-terminal tail not only plays a critical role in the degradation of Noxa, but also serves as a determinant of the stability of Mcl-1 because Noxa C-terminal tail mutants stabilize endogenous Mcl-1 through a direct interaction with Mcl-1.
Although mouse Noxa (102 a. a.) and human Noxa (54 a. a.) are different in size, they share a highly conserved C-terminal tail. We therefore examined the role of the C-terminal tail in the stability of full-length mouse Noxa in HeLa cells. Similar to the situation of human Noxa, the removal of the C-terminal tail greatly stabilized mouse Noxa as well as the endogenous Mcl-1 (Fig. 6E), suggesting that the stability of mouse Noxa, similar to that of human Noxa, is controlled by its C-terminal tail. Further, like its human counterpart, the C-terminal tail of mouse Noxa has the capacity to regulate the stability of Mcl-1.
DISCUSSION
Noxa is an important modulator of apoptosis in both p53-dependent and p53-independent pathways in human cancer cells (12). With its unusually small size (54 amino acid residues), lack of secondary structures except for a predicted α helical BH3 domain, and an efficient ubiquitylation, human Noxa is easily predicted to be an unstable protein. In conjunction with a recent study (15), our study presents some surprising findings on the regulation of the stability of Noxa. First, even when not complexed with its binding partner Mcl-1, Noxa is degraded in a ubiquitylation-independent manner. Second, degradation of Noxa is primarily controlled by the C-terminal tail, which contains a bona fide degradation signal. Third, endogenous Mcl-1 is greatly stabilized by Noxa mutants with defects in the C-terminal tail.
The Ubiquitylation-independent Degradation of Noxa
Consistent with results from a recent study (16), Noxa is found to be efficiently ubiquitylated in this study. Moreover, this ubiquitylation is independent from association with Mcl-1, which is also a ubiquitylated protein. Interestingly, all three lysine residues at positions 35, 41, and 48 seem to be ubiquitylated as the single mutations were unable to abolish the polyubiquitylation. With such an efficient polyubiquitylation, it is surprising that polyubiquitylation is not required for the degradation of Noxa. Noxa therefore joins the growing number of proteins that are degraded in a polyubiquitylation-independent fashion (23, 24). This observation raised two obvious questions. First, how is Noxa recognized by the proteasome? The answer to this question may lie in part in the C-terminal tail, which serves as the degradation signal for Noxa. In most cases of ubiquitin-independent degradation of proteins, an unstructured sequence is involved in the degradation. It is therefore reasonable to predict that the Noxa C-terminal tail is unstructured. Second, what role does polyubiquitylation play in the function and regulation of Noxa? Apparently, polyubiquitylation dramatically increases the size of Noxa as multiple ubiquitins (80 a. a.) are added to Noxa (54 a. a.) through covalent bonds. However, at present, we are not able to observe any noticeable differences between the wild-type Noxa and the ubiquitin-free Noxa mutant (NoxaC3KR) as both localized to the mitochondria and both showed similar binding to Mcl-1 (data not shown).
It is worth noting that Mcl-1 was also shown to be degraded in a ubiquitin-independent fashion (21). However, because endogenous Noxa is complexed with Mcl-1 in vivo, it remains unclear whether the Noxa-Mcl-1 complex can be degraded in a ubiquitylation-independent fashion. In other words, it is still possible that degradation of Noxa-Mcl-1 complex is through the ubiquitylation of Mcl-1. A definitive answer to this question may come from lysine-free mutants of both Noxa and Mcl-1 expressed in cells that are doubly deficient for Noxa and Mcl-1. Regardless, our results demonstrated that Mcl-1-free Noxa is degraded through a ubiquitylation-independent mechanism.
Regulation of Mcl-1 Stability through the Noxa C-terminal Tail
Is endogenous Noxa a regulator of Mcl-1 stability? A recent study found that Mcl-1 stability in Noxa-deficient mouse embryonic fibroblasts remains the same as that in wild-type cells, suggesting that endogenous Noxa is not involved in the regulation of Mcl-1 stability in mice (25). Consistent with this finding, we were unable to detect a significant increase of Mcl-1 level following siRNA knockdown of Noxa in HeLa cells (data not shown). In the current study, expression of Noxa mutants with defects in the C-terminal tail was found to stabilize Mcl-1. Because expression of wild-type Noxa did not show this effect, the mutations in the C-terminal tail are apparently gain-of-function mutations as they stabilized the tail-less Noxa mutants, which bind to Mcl-1 and inhibit the degradation of Mcl-1. Therefore, our results suggest that Noxa C-terminal tail regulates Mcl-1 stability indirectly by regulating the stability of Noxa.
The tail mutants of Noxa apparently stabilize Mcl-1 by direct association as the BH3 mutation of Noxa, which abolishes the interaction with Mcl-1, completely abolished the stabilizing effect. It is possible that this binding competes with the binding of an E3 ligase for Mcl-1. A good candidate for the E3 ligase is Mule, which was shown to bind Mcl-1 through a putative BH3 domain (19). However, in light of the recent observation that a lysine-free Mcl-1 displayed a similar half-life as the wild-type Mcl-1 (21), alternative mechanisms might exist. It is also possible that the C-terminal tail of Noxa serves as the degradation signal for the Noxa-Mcl-1 complex; therefore, the removal of the Noxa tail prolonged the half-life of the Noxa-Mcl-1 complex.
The Mitochondrial Protease Responsible for Noxa Degradation
What is the nature of the protease responsible for the degradation of Noxa? Although the 26 S proteasome is apparently a candidate, the answer becomes less clear when considering the fact that the C-terminal tail of Noxa also contains a mitochondrial targeting sequence (26). Is mitochondrial localization required for the degradation of Noxa? Several Noxa C-terminal truncation mutants that lost the entire mitochondrial targeting sequence become extremely stable. Thus, it appears that mitochondrial localization is required for the degradation of Noxa. However, the L42A/L43A Noxa mutant, which retains mitochondrial localization, becomes much more stable than the wild-type Noxa, indicating that mitochondrial localization per se is not sufficient for degradation. A recent study identified a stress-response system for the degradation of mitochondrial proteins (27). However, this system is dependent on the ubiquitylation of the target proteins on the mitochondria. It is therefore possible that the Noxa C-terminal tail is associated with a ubiquitylated protein, which serves as a mediator for the degradation of Noxa through this system. Nonetheless, the mechanism of Noxa degradation remains unclear. However, with the identification of the C-terminal tail as the degron, it is possible to identify C-terminal tail interacting proteins that may serve as the link between Noxa and the unidentified protease. Alternatively, it is possible that the C-terminal tail of Noxa directly interacts with one or several of the subunits of the proteasome, therefore serving as the direct recruiter of the proteasome.
In summary, we present evidence that the C-terminal tail serves as the degradation signal for Noxa and can function indirectly to regulate the stability of Mcl-1. These results provide new insights into the regulation of stability of the Bcl-2 family proteins, which play important roles in apoptosis and drug resistance in cancer cells.
This work was supported by pilot grants from the Nebraska Center for Cellular Signaling (National Institutes of Health Grant 8P20GM103489) and Nebraska Research Initiative funds (to X. L.).
- CHX
- cycloheximide
- a. a.
- amino acids
- CT
- C-terminal tail
- G-Noxa
- GFP-Noxa fusion protein
- z
- benzyloxycarbonyl.
REFERENCES
- 1. Vaux D. L., Korsmeyer S. J. (1999) Cell death in development. Cell 96, 245–254 [DOI] [PubMed] [Google Scholar]
- 2. Fuchs Y., Steller H. (2011) Programmed cell death in animal development and disease. Cell 147, 742–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt T., Körner K., Karsunky H., Korsmeyer S., Müller R., Möröy T. (1999) The activity of the murine Bax promoter is regulated by Sp1/3 and E-box binding proteins but not by p53. Cell Death Differ. 6, 873–882 [DOI] [PubMed] [Google Scholar]
- 4. Youle R. J., Strasser A. (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 5. Adams J. M., Cory S. (2007) Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr. Opin. Immunol. 19, 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chipuk J. E., Moldoveanu T., Llambi F., Parsons M. J., Green D. R. (2010) The BCL-2 family reunion. Mol. Cell 37, 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tait S. W., Green D. R. (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632 [DOI] [PubMed] [Google Scholar]
- 8. Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang X., Wang X. (2004) Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 73, 87–106 [DOI] [PubMed] [Google Scholar]
- 10. Hijikata M., Kato N., Sato T., Kagami Y., Shimotohno K. (1990) Molecular cloning and characterization of a cDNA for a novel phorbol-12-myristate-13-acetate-responsive gene that is highly expressed in an adult T-cell leukemia cell line. J. Virol. 64, 4632–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T., Tokino T., Taniguchi T., Tanaka N. (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 [DOI] [PubMed] [Google Scholar]
- 12. Ploner C., Kofler R., Villunger A. (2008) Noxa: at the tip of the balance between life and death. Oncogene 27, Suppl. 1, S84–S92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L., Willis S. N., Wei A., Smith B. J., Fletcher J. I., Hinds M. G., Colman P. M., Day C. L., Adams J. M., Huang D. C. (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393–403 [DOI] [PubMed] [Google Scholar]
- 14. Lopez H., Zhang L., George N. M., Liu X., Pang X., Evans J. J., Targy N. M., Luo X. (2010) Perturbation of the Bcl-2 network and an induced Noxa/Bcl-xL interaction trigger mitochondrial dysfunction after DNA damage. J. Biol. Chem. 285, 15016–15026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baou M., Kohlhaas S. L., Butterworth M., Vogler M., Dinsdale D., Walewska R., Majid A., Eldering E., Dyer M. J., Cohen G. M. (2010) Role of NOXA and its ubiquitination in proteasome inhibitor-induced apoptosis in chronic lymphocytic leukemia cells. Haematologica 95, 1510–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Craxton A., Butterworth M., Harper N., Fairall L., Schwabe J., Ciechanover A., Cohen G. M. (2012) NOXA, a sensor of proteasome integrity, is degraded by 26S proteasomes by an ubiquitin-independent pathway that is blocked by MCL-1. Cell Death Differ. 19, 1424–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon J. H., Werneburg N. W., Higuchi H., Canbay A. E., Kaufmann S. H., Akgul C., Edwards S. W., Gores G. J. (2002) Bile acids inhibit Mcl-1 protein turnover via an epidermal growth factor receptor/Raf-1-dependent mechanism. Cancer Res. 62, 6500–6505 [PubMed] [Google Scholar]
- 18. Zhang B., Gojo I., Fenton R. G. (2002) Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood 99, 1885–1893 [DOI] [PubMed] [Google Scholar]
- 19. Zhong Q., Gao W., Du F., Wang X. (2005) Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121, 1085–1095 [DOI] [PubMed] [Google Scholar]
- 20. Gores G. J., Kaufmann S. H. (2012) Selectively targeting Mcl-1 for the treatment of acute myelogenous leukemia and solid tumors. Genes Dev. 26, 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stewart D. P., Koss B., Bathina M., Perciavalle R. M., Bisanz K., Opferman J. T. (2010) Ubiquitin-independent degradation of antiapoptotic MCL-1. Mol. Cell. Biol. 30, 3099–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., Huang D.C. (2005) Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erales J., Coffino P. (2014) Ubiquitin-independent proteasomal degradation. Biochim. Biophys. Acta 1843, 216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hwang J., Winkler L., Kalejta R. F. (2011) Ubiquitin-independent proteasomal degradation during oncogenic viral infections. Biochim. Biophys. Acta 1816, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weber A., Ausländer D., Häcker G. (2013) Mouse Noxa uses only the C-terminal BH3-domain to inactivate Mcl-1. Apoptosis 18, 1093–1105 [DOI] [PubMed] [Google Scholar]
- 26. Seo Y. W., Shin J. N., Ko K. H., Cha J. H., Park J. Y., Lee B. R., Yun C. W., Kim Y. M., Seol D. W., Kim D. W., Yin X. M., Kim T. H. (2003) The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53-mediated cell death. J. Biol. Chem. 278, 48292–48299 [DOI] [PubMed] [Google Scholar]
- 27. Heo J. M., Livnat-Levanon N., Taylor E. B., Jones K. T., Dephoure N., Ring J., Xie J., Brodsky J. L., Madeo F., Gygi S. P., Ashrafi K., Glickman M. H., Rutter J. (2010) A stress-responsive system for mitochondrial protein degradation. Mol. Cell 40, 465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]