SUMMARY
Selectins play a central role in leukocyte trafficking by mediating tethering and rolling on vascular surfaces. Here we have reported that T cell immunoglobulin and mucin domain 1 (TIM-1) is a P-selectin ligand. We have shown that human and murine TIM-1 binds to P-selectin, and that TIM-1 mediates tethering and rolling of T helper-1 (Th1) and Th17, but not Th2 and regulatory T cells on P-selectin. Th1 and Th17 cells lacking the TIM-1 mucin domain showed reduced rolling in thrombin-activated mesenteric venules and inflamed brain microcirculation. Inhibition of TIM-1 had no effect on naive T cell homing, but reduced T cell recruitment in a skin hypersensitivity model and blocked experimental autoimmune encephalomyelitis. Uniquely, the TIM-1 IgV domain was also required for P-selectin binding. Our data demonstrate that TIM-1 is a major P-selectin ligand with a specialized role in T cell trafficking during inflammatory responses and the induction of autoimmune disease.
INTRODUCTION
The leukocyte adhesion cascade is a central paradigm of inflammation and immunity, involving a multistep process including tethering, rolling, activation, arrest, crawling and transmigration (Butcher, 1991; Springer, 1994; Luster et al., 2005; Ley et al., 2007). Selectins mediate the first step (tethering and rolling), allowing circulating leukocytes to sense activating signals on the endothelium and hence adhere to vessel walls under blood flow (Ley et al., 2007; Ley and Kansas, 2004; McEver and Zhu, 2010; Zarbock et al., 2011). The inhibition of selectin-dependent rolling strongly reduces such inflammatory responses, so the investigation of molecular mechanisms controlling leukocyte trafficking, particularly primary adhesion to endothelial cells, has both biological and clinical relevance (Luster et al., 2005).
The selectin family of cell adhesion molecules has three members: L-selectin (CD62L), which is constitutively expressed by most leukocytes, E-selectin (CD62E), which is upregulated on endothelial cells following cytokine stimulation, and P-selectin (CD62P), which is stored in endothelial Weibel-Palade bodies and platelet α-granules and is rapidly expressed by activated endothelium and platelets and by platelet-derived microparticles (Ley and Kansas, 2004; McEver and Zhu, 2010). The principal leukocyte ligand for P-selectin is the mucin P-selectin glycoprotein ligand 1 (PSGL-1), which can act as a ligand for all three selectins (Ley and Kansas, 2004; Zarbock et al., 2011). The majority of research on selectin ligands has focused on myeloid cells, and PSGL-1-independent rolling on P-selectin has been observed for T cells suggesting that the repertoire of physiological ligands that interact with endothelial selectins is still incompletely understood (Ley and Kansas, 2004; Zarbock et al., 2011).
The T cell immunoglobulin and mucin domain (TIM) gene family encodes glycoproteins involved in a variety of immunity-related processes including T cell proliferation and survival, tissue inflammation and atopy (Rodriguez-Manzanet et al., 2009). In mice, eight TIM genes encode the proteins TIM-1 to TIM-8, whereas only three TIM genes are found in humans encoding TIM-1, TIM-3 and TIM-4 (Rodriguez-Manzanet et al., 2009). TIM-1 expression has been observed on activated T cells, B cells, natural killer T (NKT) cells and dendritic cells (Rennert, 2011). Several lines of evidence suggest that TIM-1 regulates T cell activity in vivo through responses mediated by T helper 1 (Th1), Th2, Th17 and regulatory T (Treg) cells (Umetsu et al., 2005; Meyers et al., 2005; Xiao et al., 2007; Degauque et al., 2008). TIM-1 is recruited to the T cell receptor signaling complex and has a co-stimulatory role (Rodriguez-Manzanet et al., 2009).
Members of the TIM family share common structural motifs, such as immunoglobulin variable (IgV)-like and mucin-like domains on the extracellular portion, a single transmembrane region, and a cytoplasmic tail that generally contains tyrosine phosphorylation motifs (Kuchroo et al., 2003). Here we have demonstrated that TIM-1 is a major selectin ligand that mediates leukocyte rolling on P-selectin in vivo. We also have provided compelling evidence that TIM-1 controls T cell trafficking during inflammation and autoimmune disease. Our data have shown that TIM-1 can be included in the restricted group of major adhesion receptors involved in leukocyte tethering and rolling under inflammatory conditions (Zarbock et al., 2011).
RESULTS
TIM-1 binds selectins in vitro in a cell-free system
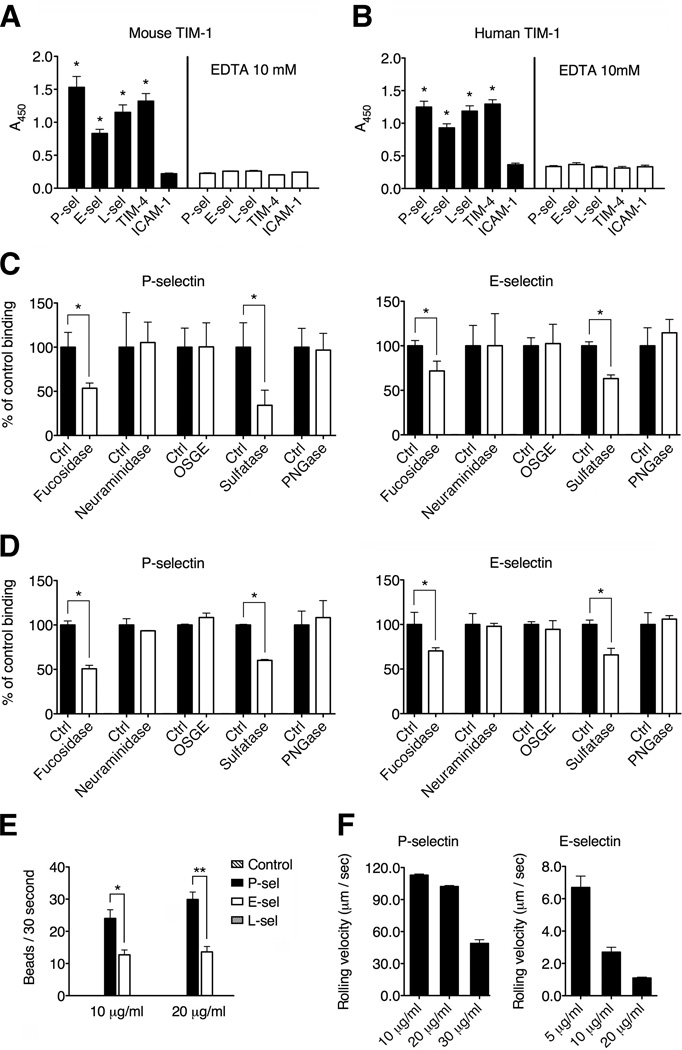
The TIM-1 mucin domain is rich in threonine, serine and proline residues, providing 56 predicted O-glycosylation sites in humans and 32 in mice (Kuchroo et al., 2003). We hypothesized that TIM-1 may present carbohydrate moieties to selectins and we therefore carried out microtiter plate binding assays to check for direct binding between TIM-1 and the three known selectins. We found that both mouse and human TIM-1 were able to bind all three selectins in cell-free assays, with higher binding to P-selectin than E- or L-selectin (Figures 1A and 1B). The calcium chelator EDTA, completely blocked the binding of both mouse and human TIM-1 to all three selectins (Figures 1A and 1B), suggesting that the TIM-1 mucin domain is necessary for selectin binding. We tested other members of the TIM family and found that murine TIM-2 and TIM-3 were unable to bind selectins, suggesting that TIM-1 may be the sole candidate selectin receptor (Figure S1).
Figure 1. TIM-1 interacts with selectins in vitro requiring a specific glycosylation profile and mediates tethering and rolling on endothelial selectins under flow conditions.
(A and B) Microtiter plates were coated with 5 µg/ml murine or human P-selectin, E-selectin or L-selectin, TIM-4 (positive control) (Meyers et al., 2005) or ICAM-1 (negative control) (Santiago et al., 2007B), and tested for their ability to bind recombinant mouse or human TIM-1 respectively. In some experiments, 10 mM EDTA was added to chelate divalent cations. Both murine (A) and human (B) TIM-1 bound to all three selectins, and binding was dependent on the presence of divalent cations (*P < 0.0001 compared to ICAM-1 binding). (C and D) Microtiter plate assays show the binding of recombinant mouse TIM-1 protein from CHO (shown in C) and 293T cells (shown in D) to P- and E-selectin after treatment with α1,(3,4)-fucosidase, tyrosine sulfatase, PNGase, OSGE and neuraminidase treatment (*P < 0.001). (E) Protein A-covered microspheres were coated with a murine TIM-1 Fc-chimera (Sizing et al., 2007) or a control mouse IgG Fc-chimera (control beads) and were infused into glass capillary tubes pre-coated with P-selectin, E-selectin or L-selectin, under physiological shear stress conditions (2 dyne/cm2) (*P < 0.002, **P < 0.0001). See also Movies S1 and S2. (F) The rolling velocity of TIM-1-covered beads declines as the concentration of P-selectin and E-selectin increases under physiological flow conditions. Data represent the means ± standard error of the mean (SEM) of three independent experiments. In (A), (B), (C) and (D), data represent means ± SEM of at least three independent experiments performed in triplicate for each condition.
TIM-1 requires α1-(3,4)-fucosylation and tyrosine sulfation for efficient binding to P- and E-selectin
We next sought to identify the carbohydrate moieties required for high-affinity binding between TIM-1 and endothelial selectins. By treating recombinant mouse TIM-1 proteins derived from Chinese hamster ovary (CHO) or human embryonic kidney (HEK) 293T cells with different glycosidases, we found that α1-(3,4)-fucosylation and tyrosine sulfation were the post-translational modifications necessary for binding between TIM-1 and selectins (Figures 1C and 1D). The effect of the sulfatase pretreatment on TIM-1-P-selectin interactions was more pronounced than on TIM-1-E-selectin. Treatment with Peptide:N-glycosidase F (PNGaseF) did not inhibit TIM-1 binding, suggesting that N-linked glycans have no role in the interaction with selectins (Figures 1C and 1D). Treating TIM-1 with neuraminidase or O-sialoglyco-endopeptidase did not inhibit TIM-1 binding either (Figures 1C and 1D), suggesting that sialic acid moieties are dispensable for the interaction with selectins. These data suggest that TIM-1 presents a specific glycosylation profile necessary for selectin binding, but that the profile differs from that of PSGL-1, particularly in the requirement for sialylated carbohydrates (Zarbock et al., 2011).
TIM-1 mediates capture and rolling on P-selectin and E-selectin under flow conditions in vitro
The capture and rolling of leukocytes on vascular endothelium mediated by L-selectin and P-selectin requires shear stress conditions (Zarbock et al., 2011). Therefore we carried out TIM-1-selectin binding assays under flow conditions in glass capillary tubes using 10-µm protein A polystyrene microspheres covered with a murine TIM-1 Fc-chimera (Sizing et al., 2007). The TIM-1-covered beads were infused into capillary tubes pre-coated with each of the three murine selectins under shear stress conditions of 2 dyne/cm2 (Deban et al., 2010). The TIM-1-covered microspheres were efficiently captured by P-selectin (Figure 1E and Movie S1) and E-selectin (Figure 1E and Movie S2) under these conditions. Notably, TIM-1-mediated tethering and rolling was more efficient on P-selectin than E-selectin, suggesting that TIM-1 may preferentially bind P-selectin under flow conditions. TIM-1 also mediated the transition from capture to rolling interactions, and the rolling velocity was dependent on the quantity of each selectin used to coat the capillary tubes (Figure 1F). The rolling of beads on E-selectin was slow (Ley et al., 2007), and slowed further as the quantity of E-selectin in the coating increased (Figure 1F). In contrast, TIM-1 did not bind L-selectin under these flow conditions, suggesting that it may interact preferentially with selectins expressed on endothelial cells (Figure 1E). These results demonstrate that TIM-1 can mediate capture and rolling on P-selectin and E-selectin under flow conditions and may represent an adhesion mechanism that facilitates efficient lymphocyte-endothelium interactions in vivo.
The TIM-1 mucin domain is required for Th1 and Th17 cell rolling on P-selectin in vitro
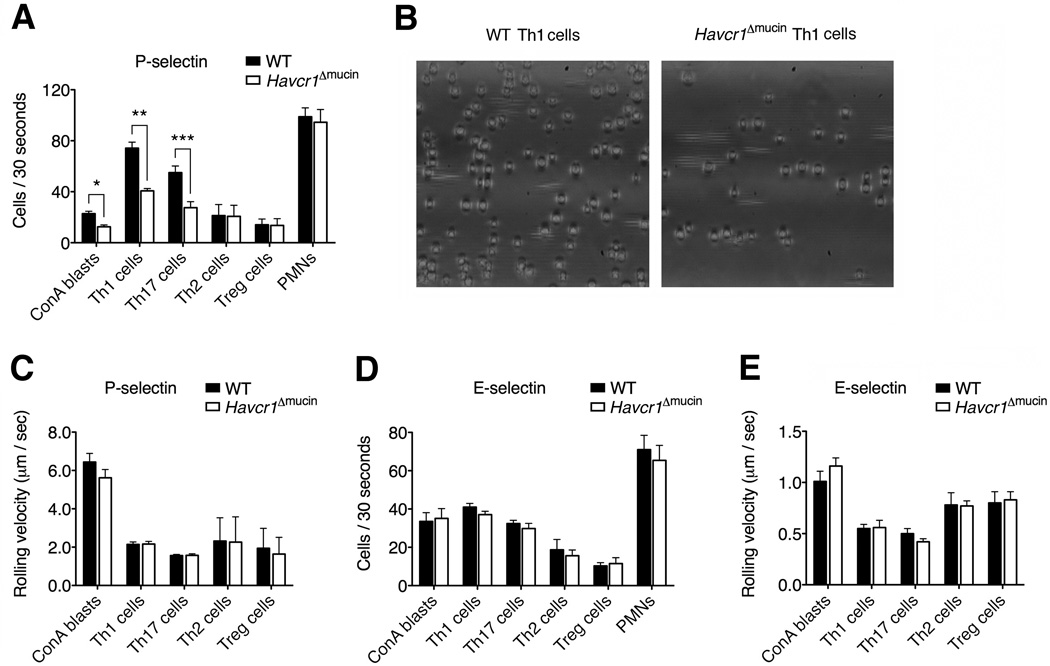
To determine the relevance of the TIM mucin domain in selectin binding, we tested the behavior of T cells derived from Havcr1Δmucin mice, which selectively lack the O-glycosylated TIM-1 mucin domain encoded by exon 3 of the Havcr1 gene (Xiao et al., 2012). First we tested several T cell populations for TIM-1 expression, and found that Th1 and Th17 cells expressed the highest amount of TIM-1 on the cell membrane and intracellularly, compared to naïve CD4+ T cells and Treg cells, whereas TIM-1 was preferentially expressed intracellularly in Th2 cells (Figures S2A and S2B). Notably, TIM-1 was expressed at higher amounts on the surface of Th1 cells, just beneath the plasma membrane and partially in endosomal pools, but TIM-1 mRNA amount was lower in Th1 cells than in naïve T cells, in which TIM-1 was exclusively intracellular (Figures S2C–S2G). Also, as previously described (Xiao et al., 2012), we found no differences in the expression of TIM-1 between wild-type and Havcr1Δmucin T cells (data not shown).
We carried out further in vitro assays in capillary tubes pre-coated with recombinant P-selectin or E-selectin and found that Havcr1Δmucin T cells activated with concanavalin A (ConA blasts) were consistently deficient in their ability to tether and roll on P-selectin under flow conditions (Figure 2A), providing more evidence that the TIM-1 mucin domain is necessary for interactions with P-selectin. Furthermore, Th1 and Th17 cells lacking the mucin domain also showed a significant reduction in P-selectin-mediated tethering and rolling (Figures 2A and 2B and Movies S3 and S4), suggesting that despite the presence of PSGL-1 on these cells, TIM-1 is nevertheless required for effective P-selectin-dependent tethering and rolling. Importantly, activated Th1 and Th17 cells from wild-type and Havcr1Δmucin mice expressed comparable amounts of several adhesion molecules and activation markers (Figure S3), suggesting there were no potential adhesion defects associated with the Havcr1Δmucin mutation.
Figure 2. The TIM-1 mucin domain is required for the capture and rolling of Th1 and Th17 cells on P-selectin under physiological flow conditions in vitro.
(A–E) Treg cells or CD4+ T cells stimulated with concanavalin A (ConA) or polarized toward Th1, Th17 or Th2 phenotypes were infused into capillary tubes pre-coated with 5 µg/ml P-selectin or E-selectin under shear stress conditions (2 dyne/cm2) (*P < 0.002; **P < 0.0003; ***P < 0.0001). Bone marrow-derived PMNs, which do not express TIM-1, were used as negative controls. (B) Representative images of wild-type and Havcr1Δmucin Th1 cells rolling on P-selectin, showing a consistently lower number of rolling Havcr1Δmucin Th1 cells. See also Movies S3 and S4. (C and E) Rolling velocities of wild-type and Havcr1Δmucin ConA blasts, Th1, Th17, Th2 and Treg cells on P- and E-selectin. Data represent means ± SEM of four independent experiments. For rolling velocities, data represent means ± SEM of at least 100 cells per condition.
Although the loss of PSGL-1 impaired not only Th1, but also Th2 and Treg cell rolling on P-selectin (Figures S4A, S4E and S4F), both Havcr1Δmucin Th2 and Treg cell subsets displayed no defects in their rolling ability on P-selectin (Figures 2A and 2C), suggesting that TIM-1-P-selectin adhesive interactions are a prerogative of Th1 and Th17 cells. Also Havcr1Δmucin bone marrow-derived polymorphonuclear neutrophils (PMNs), which do not express TIM-1, displayed normal rolling activity (Figure 2A), suggesting that the adhesion defect in Havcr1Δmucin mice is specific to T cells. No differences in rolling velocity were observed between wild-type and Havcr1Δmucin T cells (Figure 2C), suggesting that TIM-1 does not influence the quality of rolling interactions when functional PSGL-1 is highly expressed.
Because TIM-1 also mediated rolling on E-selectin in vitro, we investigated whether the TIM-1 mucin domain is also required for E-selectin-dependent rolling. In contrast to the P-selectin experiments, we found that Havcr1Δmucin ConA blasts, Th1, Th17, Th2 and Treg cells showed no defects in their interactions with E-selectin in vitro under flow conditions when compared to wild-type cells (Figure 2D). There was also no change in rolling velocity when Havcr1Δmucin cells were tested on E-selectin (Figure 2E), suggesting that the TIM-1 mucin domain plays a specific role in P-selectin-dependent rolling.
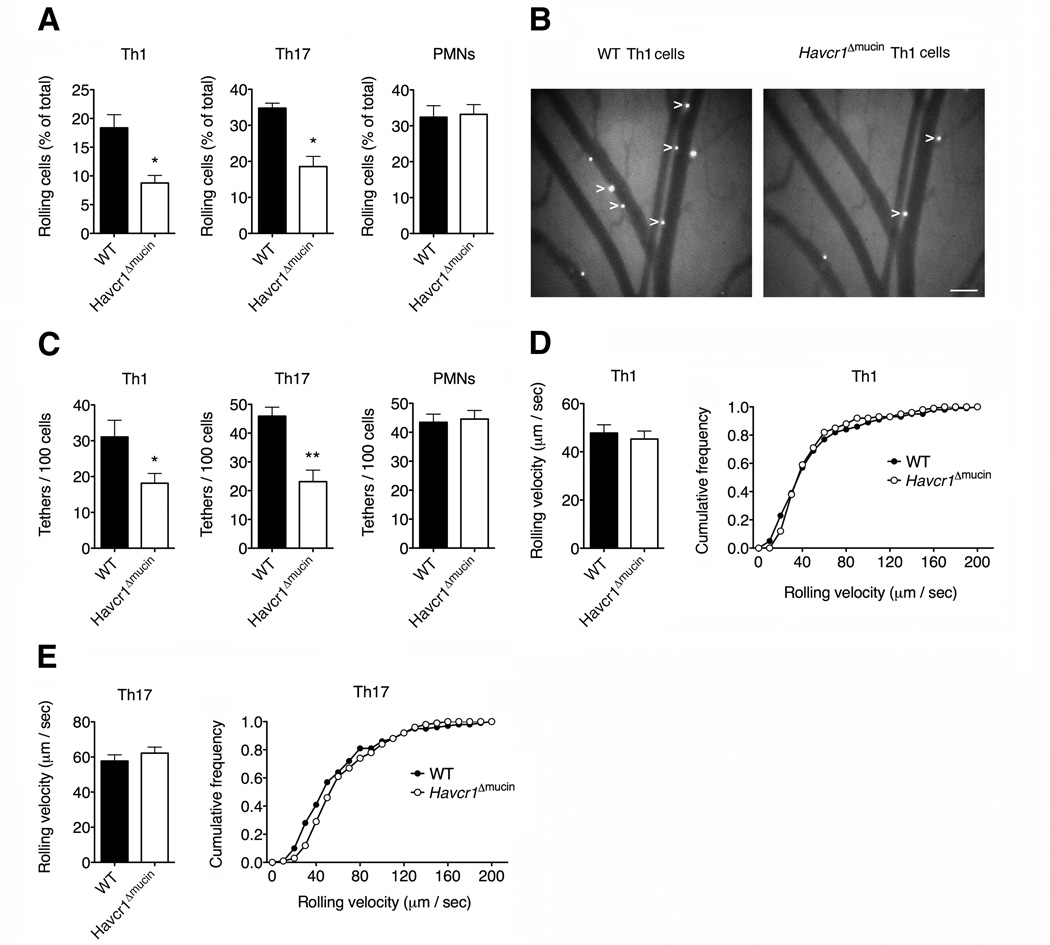
TIM-1 mediates Th1 and Th17 cell capture and rolling in vivo
To establish the in vivo relevance of TIM-1 in leukocyte adhesion interactions, we carried out intravital microscopy experiments in exposed mesenteric venules pre-treated with thrombin, which rapidly induces the expression of endothelial P-selectin (Deban et al., 2010), focusing on pro-inflammatory Th1 and Th17 cells that displayed defective P-selectin-dependent rolling in vitro (Figure 2A). Th1 and Th17 cells from Havcr1Δmucin mice showed a significant reduction in the ability to roll on P-selectin compared to wild-type cells (Figures 3A and 3B and Movie S5). Importantly, the number of total tethers was also reduced for Th1 and Th17 cells (Figure 3C), suggesting that TIM-1 can also mediate T cell tethering in vivo. As expected, Havcr1Δmucin PMNs displayed no defects in their ability to interact with P-selectin in vivo (Figures 3A and 3C), indicating that the defect in rolling is lymphocyte-specific. As shown in vitro, we found no differences in rolling velocities between wild-type and Havcr1Δmucin cells, suggesting that TIM-1 does not influence the quality of activated T cell rolling on P-selectin in vivo (Figures 3D and 3E).
Figure 3. TIM-1 mediates tethering and rolling of Th1 and Th17 cells in vivo.
Th1 and Th17 cells were generated from murine wild-type and Havcr1Δmucin CD4+ cells. Bone marrow-derived PMNs were used as negative controls. Cells were injected intravenously into the exposed mesenteric vessels of mice pre-treated with bovine thrombin to upregulate P-selectin on the vascular endothelium. (A) Havcr1Δmucin T cells have a significantly reduced ability to roll on P-selectin in vivo (*P < 0.002 compared to wild-type cells). See also Movie S5. (B) Representative images of Th1 cells rolling in mesenteric vessels. Cells are the white spots inside the venules (arrow tips). Scale bar: 100 µm. (C) The number of tethers (each new interaction with the vessel wall) are shown. (*P < 0.03 and **P < 0.004 compared to the corresponding wild-type cells). Data in (A) and (C) represent means ± SEM of 13-15 independent experiments for a total of 15-20 total venules/condition. (D and E) Havcr1Δmucin Th1 (D) and Th17 (E) cells display no defects in their rolling velocity in mesenteric venules, compared to wild-type cells. Rolling velocities represent the mean ± SEM of at least 100 cells per condition (left panel). The distribution of leukocyte rolling velocities is also shown (right panel).
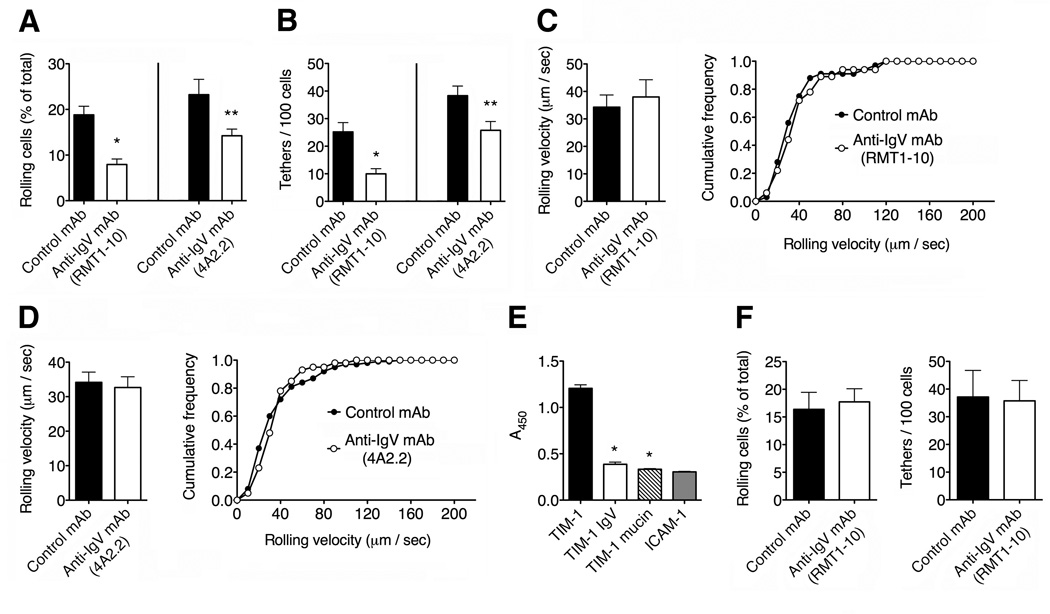
The TIM-1 IgV domain is also required for T cell rolling on P-selectin
We next investigated the potential role of the TIM-1 IgV domain in P-selectin binding by testing cells in the presence of two antibodies that specifically block this domain: RMT1-10 and 4A2.2 (Xiao et al., 2007; Sizing et al., 2007). We carried out intravital microscopy experiments in exposed mesenteric venules pre-treated with thrombin. We found that inhibiting the IgV domain with antibodies led to a significant reduction in the ability of the cells to roll on P-selectin in vivo, compared to cells treated with a control IgG antibody, suggesting that the TIM-1 IgV domain is required for interactions with P-selectin (Figure 4A). In addition, the total number of tethers was significantly reduced when the IgV domain was blocked, suggesting that the TIM-1 IgV domain is required for tether formation under flow conditions (Figure 4B). As shown for the mucin domain, there was no difference in rolling velocity between cells treated with anti-IgV antibodies and the control antibody (Figures 4C and 4D), providing further evidence that TIM-1 does not affect the quality of rolling interactions in the presence of functional PSGL-1. The IgV domain has not previously been shown to play a role in tethering or rolling on other selectin ligands and our results suggest that TIM-1 therefore uses a regulatory mechanism that is unique within the family of selectin receptors.
Figure 4. The TIM-1 IgV domain is required for P-selectin-dependent rolling in vivo.
(A–D) Wild-type Th1 cells were treated with control rat IgG or the blocking anti-TIM-1 antibodies RMT1-10 or 4A2.2, which recognize epitopes in the TIM-1 IgV domain (Xiao et al., 2007; Sizing et al., 2007). (A) Cells treated with RMT1-10 and 4A2.2 showed a strongly reduced ability to roll on P-selectin (*P < 0.005 and **P < 0.04 compared to cells treated with rat IgG). (B) RMT1-10 and 4A2.2 treatments also reduced the number of total tethers (*P < 0.008 and **P < 0.03 compared to control cells). Data in (A) and (B) represent means ± SEM of 3-4 independent experiments for a total of 6-8 total venules per condition. (C and D) Cells treated with both RMT1-10 (C) and 4A2.2 (D) showed no defects in their rolling velocity in mesenteric venules. Rolling velocities represent the mean ± SEM of at least 50 cells per condition. The distribution of leukocyte rolling velocities is also shown (right panels). (E) Microtiter plate assays showing the binding of full-length versus truncated TIM-1 proteins to P-selectin (*P < 0.001 compared to entire TIM-1 protein). ICAM-1 was used a negative control for TIM-1 binding. (F) Effect of RMT1-10 antibody on Havcr1Δmucin Th1 cell tethering and rolling in thrombin-treated mesenteric venules. Data represent means ± SEM of two experiments for a total of 5–6 venules per condition.
To investigate the role of the TIM-1 mucin and IgV domains in more detail, we carried out in vitro binding assays between P-selectin and truncated TIM-1 proteins. We found that removing the mucin or IgV domain almost completely inhibited the binding between TIM-1 and P-selectin (Figure 4E). Furthermore, treatment of Havcr1Δmucin Th1 cells with the TIM-1 IgV domain-blocking antibodies RMT1-10 or 4A2.2 did not reduce their in vivo ability to roll and tether on P-selectin any further in thrombin-treated mesenteric venules (Figure 4F and data not shown). Taken together, these data demonstrate that the IgV and mucin domains of TIM-1 are both pivotal for the adhesive interaction between TIM-1 and P-selectin.
TIM-1 mediates T cell recruitment in inflamed skin
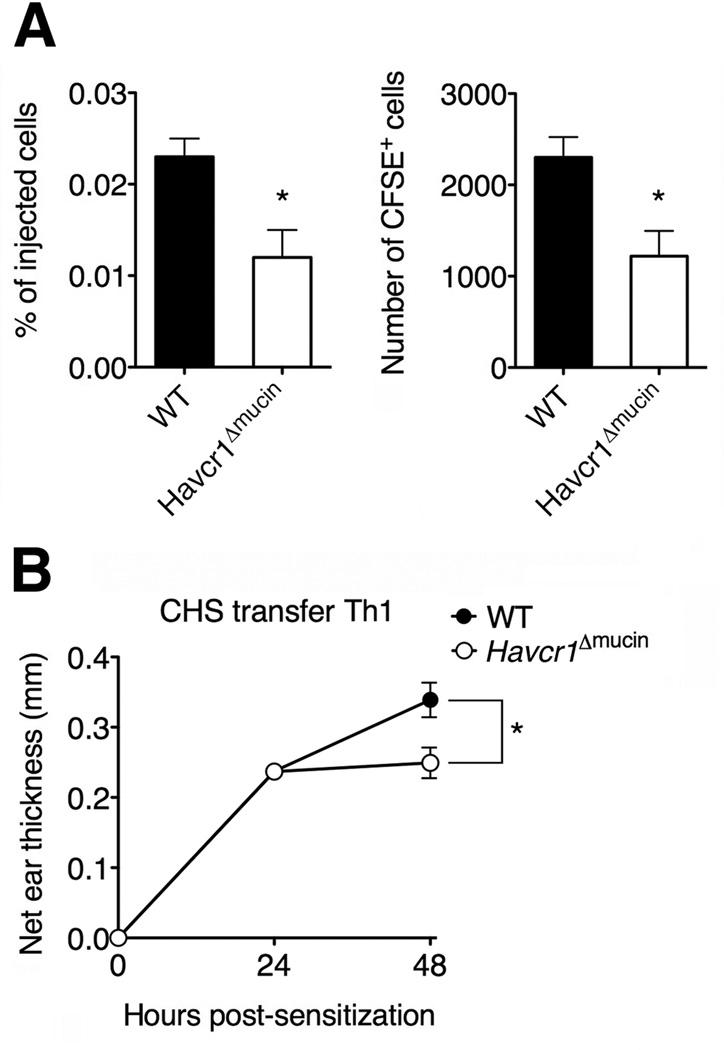
We next evaluated the potential pathophysiological role of the interaction between TIM-1 and P-selectin in leukocyte trafficking in vivo during inflammation, using a contact hypersensitivity model (CHS) in which P-selectin on the skin endothelium is necessary for activated T cell recruitment (Austrup et al., 1997). To evaluate the ability of wild-type and Havcr1Δmucin Th1 cells to migrate in the inflamed ear pinnae, wild-type mice were sensitized with DNFB and challenged 5 days later on the right ear. After 24 h, Th1 cells were labeled with CFSE dye and injected into the CHS mice. We found that Havcr1Δmucin Th1 cells showed a 48% reduction in their ability to migrate in the inflamed skin compared to wild-type cells (Figure 5A), suggesting that TIM-1 plays a role in activated T cell migration in the inflamed skin. In addition, the Havcr1Δmucin Th1 cells failed to amplify the inflammatory response in the challenged ear by increasing ear thickness compared to wild-type Th1 cells (Figure 5B), clearly supporting a pathological role for TIM-1-dependent T cell trafficking.
Figure 5. TIM-1 mediates T cell recruitment in the inflamed skin.
Mice were sensitized with 1-fluoro-2,4-dinitrobenzene (DNFB) to induce cutaneous hypersensitivity (CHS) and were challenged again 5 days later on each side of the right ear pinnae with DNFB. The ear thickness was measured 0, 24 and 48 h after challenge. (A) 10 × 106 CFSE-labeled Th1 cells from wild-type and Havcr1Δmucin mice were transferred to CHS mice 24 h after the ear pinnae were painted, and Th1 accumulation was evaluated 24 h later by flow cytometry (*P < 0.02). (B) The injection of wild-type but not Havcr1Δmucin Th1 cells increased the inflammation response in the challenged ear (*P<0.01). In both A and B, results represent the mean ± SEM of 9-11 mice per condition.
TIM-1 controls activated T cell interactions with inflamed pial vessels
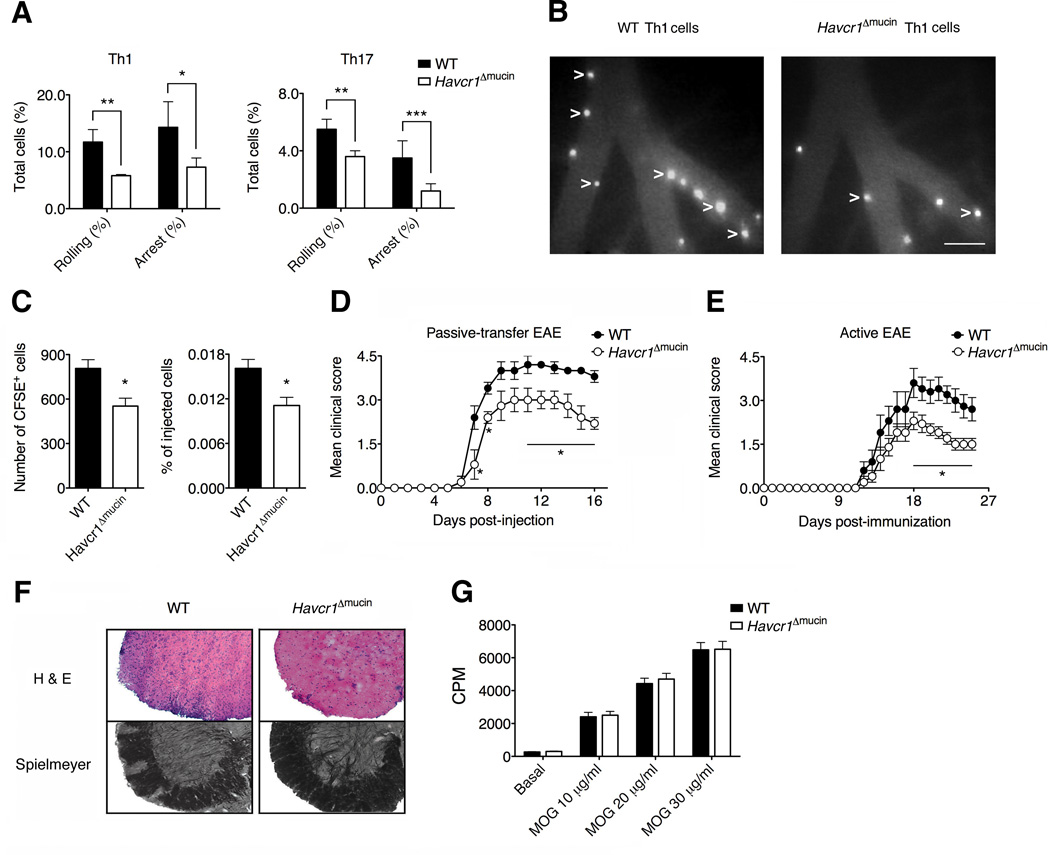
We investigated the impact of interactions between TIM-1 and P-selectin during inflammatory responses in more detail by studying the role of TIM-1 in T cell adhesion within the inflamed venules of the central nervous system (CNS). We carried out intravital microscopy experiments in the inflamed brain pial vessels, a key point for T cell ingress into the CNS during experimental autoimmune encephalomyelitis (EAE). We therefore used an experimental model of LPS-induced vasculitis mimicking early CNS vascular inflammation during EAE, in which the inhibition of P-selectin almost completely abolishes T cell rolling (Piccio et al., 2002). Our results showed that the Havcr1Δmucin mutation caused a significant reduction in the ability of Th1 and Th17 cells to roll, and their capacity to maintain firm adhesion in the inflamed brain pial venules, compared to wild-type cells (Figures 6A and 6B). Thus, as shown in mesenteric venules, TIM-1 is also necessary to control adhesion interactions in the inflamed brain microcirculation.
Figure 6. TIM-1 controls T cell trafficking in the inflamed CNS and the induction of EAE.
(A) Havcr1Δmucin Th1 and Th17 cells ability to roll and adhere firmly in LPS-inflamed brain pial vessels, in intravital microscopy experiments (**P < 0.008; *P < 0.01; ***P < 0.007). Data are mean ± SEM of three experiments for a total of 8-9 venules per condition. (B) Representative images of brain venules showing adhered Th1 cells as white spots inside the vessels (arrow tips). Scale bar = 100 µm. (C) 5 × 106 MOG35-55-specific Th1 cells were labeled with CFSE and injected into C57BL/6J wild-type mice treated 5 h previously with 20 ng pertussis toxin (PTX). The accumulation of CSFE+ cells in the CNS was evaluated 60 h after cell transfer. Data represent means ± SEM of 12 mice per condition from two experiments (*P < 0.006). (D) 5 × 106 MOG35-55-specific Th1 cells were transferred in C57BL/6J wild-type mice. Data represent the mean ± SEM of 10 mice per condition from two experiments (*P < 0.05). (E) EAE was actively induced by immunization with the MOG35-55 peptide. Data represent the mean ± SEM of 10 mice per condition from two independent experiments with similar results (*P < 0.05). (F) Hematoxylin & eosin and Spielmeyer stainings are shown at disease peak. (G) CD4+ T cells were isolated from the draining lymph nodes 7 days post-immunization with the MOG35-55 peptide and the proliferative response was determined. Data are shown as counts per minute (CPM) of [3H]-thymidine radioactivity and represent the mean ± SD of five mice per condition.
TIM-1 controls T cell accumulation in the inflamed CNS and the induction of autoimmune disease
The pathological role of TIM-1 in T-cell trafficking was investigated by studying the ability of TIM-1 to control T cell recruitment in the CNS during EAE. We therefore generated in vitro MOG35-55-specific Th1 cells from wild-type and Havcr1Δmucin mice (Lees et al., 2008) and evaluated their migration in the CNS using a model of EAE (Kerfoot et al., 2004). We found no differences in interferon-γ (IFN-γ) production and adhesion molecule expression between wild-type and Havcr1Δmucin MOG35-55-specific Th1 cells (Figure S5). However, Havcr1Δmucin Th1 cells showed a significant reduction in the ability to migrate compared to wild-type cells (Figure 6C), suggesting that TIM-1 plays a role in activated T cell migration in the inflamed CNS. Notably we also found that MOG35-55-specific Th1 cells obtained from Havcr1Δmucin mice induced a significantly less severe form of EAE compared to wild-type cells (Figure 6D). This clearly demonstrates that TIM-1 expression plays a pivotal role in the trafficking of T cells into the CNS and consequently in the induction of EAE.
We also investigated the induction of EAE in C57BL/6J wild-type and Havcr1Δmucin mice by immunization with the MOG35-55 peptide. The disease was significantly less severe in the Havcr1Δmucin mice (Figure 6E), with reduced demyelination and inflammatory cell infiltration in the CNS parenchyma (Figure 6F), indicating a key role for TIM-1 in EAE pathogenesis. Notably CD4+ T cells isolated from draining lymph nodes of the wild-type and Havcr1Δmucin EAE mice 7 days post-immunization had a similar capacity to proliferate (Figure 6G), suggesting that the inhibition of EAE in Havcr1Δmucin mice reflects a defect in T cell trafficking, rather than antigen-dependent T cell activation.
TIM-1 and PSGL-1 cooperate to mediate tethering and rolling in vitro and in vivo
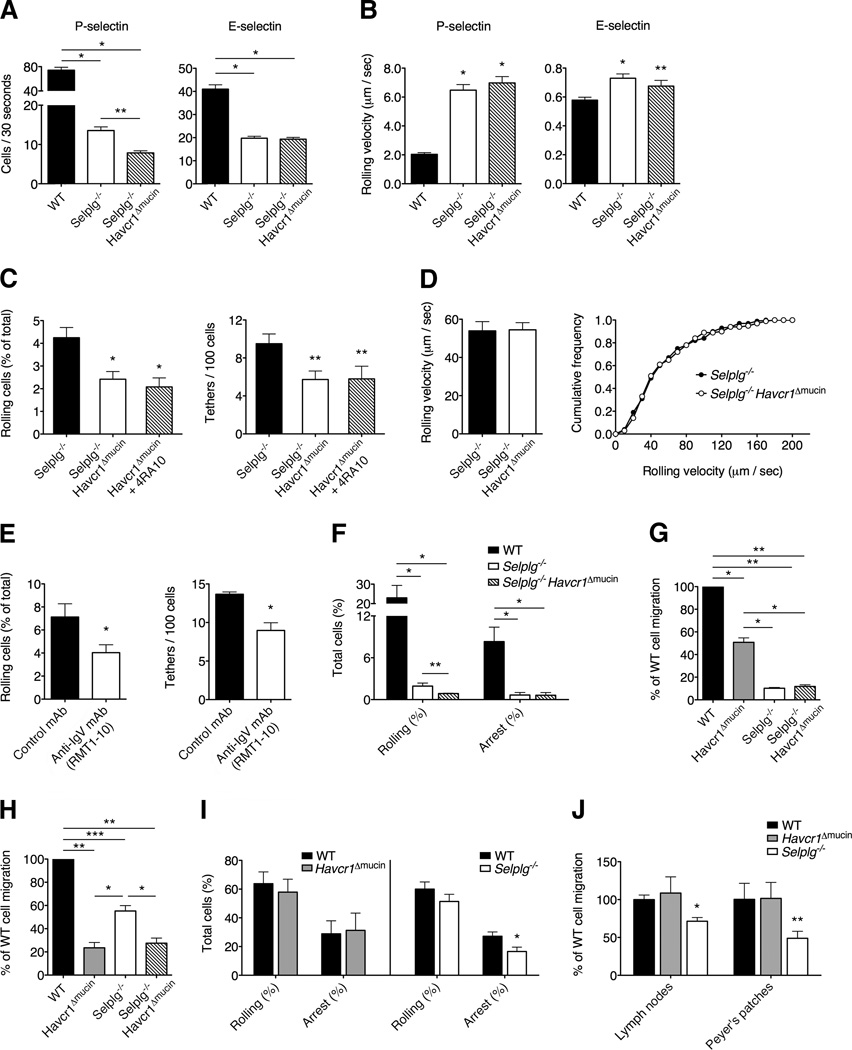
PSGL-1 is currently regarded as the major P-selectin ligand expressed on activated T cells (Ley and Kansas, 2004; Zarbock et al., 2011). Selplg−/− Th1 cells showed a reduced ability to interact with endothelial selectins under flow conditions in vitro, but residual interactions with P-selectin still occur in PSGL-1-deficient T cells in vitro and in vivo (Figure S4). We therefore investigated whether the residual rolling observed in Selplg−/− cells is mediated by TIM-1-dependent interactions. We generated Selplg−/− Havcr1 Δmucin mice and found that the absence of the TIM-1 mucin domain in Selplg−/− Havcr1 Δmucin Th1 cells inhibited PSGL-1-independent residual rolling in capillary tubes coated with P-selectin, whereas rolling on E-selectin was not affected (Figure 7A).
Figure 7. TIM-1 and PSGL-1 concur to control activated T cell rolling on P-selectin and T cell trafficking in inflamed tissues.
(A) Th1 cells were infused into capillary tubes pre-coated with P-selectin or E-selectin (*P < 0.00001; **P<0.0003) Data represent the mean ± SEM of four experiments. (B) Rolling velocities in capillary tubes coated with P-selectin or E-selectin are shown (*P < 0.001 and **P < 0.008 compared to WT cells). Rolling velocities represent the mean ± SEM of at least 50 cells per condition. (C) Shown is the ability to tether and roll in thrombin-treated mesenteric venules. Data obtained by treating Havcr1Δmucin cells with antibody 4RA10 are also shown (*P < 0.001 and **P < 0.02 compared to WT cells). Data represent the mean ± SEM of 10 independent experiments for a total of 16-17 total venules per condition. (D) Rolling velocities are shown in mesenteric venules. Rolling velocities represent the mean ± SEM of at least 50 cells per condition. The distribution of leukocyte rolling velocities is also shown in vivo (right panel). (E) Selplg−/− Th1 cells treated with RMT1-10 showed a reduced ability to tether ad roll on P-selectin in vivo, compared to cells treated with the control rat IgG (*P < 0.05, left panel; *P < 0.002, right panel). Data represent the mean ± SEM of three experiments for a total of seven venules per condition. (F) Rolling and arrest interactions are shown in intravital microscopy experiments in LPS-treated cerebral vessels (*P < 0.001; **P < 0.04). (G and H) Accumulation of wild-type, Havcr1ΔmucinSelplg−/− and Selplg−/− Havcr1Δmucin Th1 cells in the inflamed ear pinnae of CHS mice (G) and in the CNS of pertussis toxin-treated mice (H) (*P < 0.01, **P < 0.001 and ***P < 0.03). Data represent the means ± SEM of five mice per condition from one representative experiment from a series of two with similar results. (I) Rolling and arrest of naïve CD4+ T cells in Peyer’s patches HEVs (*P < 0.03 compared to WT cell arrest). Data represent the mean ± SEM of four mice per condition for a total of nine venules per condition. (J) Accumulation of exogenous naïve CD4+ T cells in lymphoid organs of wild-type resting mice at 2h after transfer (*P < 0.003 and **P < 0.04 compared to WT cells). Data represent the mean ± SEM of six mice per condition from one representative experiment from a series of three with similar results.
As shown for Havcr1 Δmucin cells (Figure 3), Selplg−/− Th1 cells also displayed a reduced ability to roll in thrombin-treated mesenteric venules, demonstrating that TIM-1 and PSGL-1 cooperate to mediate primary adhesion (Figure S4C) (D’Ambrosio et al., 2004). In agreement with our in vitro data, we found that Selplg−/− Havcr1Δmucin Th1 cells showed a significant reduction in their ability to tether and roll on P-selectin compared to Selplg−/− cells also in mesenteric venules (Figure 7C), and comparable inhibition was also observed by blocking PSGL-1 on Havcr1 Δmucin Th1 cells with antibody 4RA10 (Figure 7C), suggesting that TIM-1 mediates a significant proportion of the residual tethering and rolling interactions observed in Selplg−/− cells. Accordingly, the TIM-1 IgV domain-blocking antibody RMT1-10 significantly inhibited both residual tethering and rolling interactions in Selplg−/− cells (Figure 7E). As expected, no differences in rolling velocities were observed between Selplg−/− and Selplg−/− Havcr1Δmucin double mutant Th1 cells in vitro and in vivo (Figure 7B, D). Furthermore, intravital microscopy in the inflamed brain microcirculation showed similar results to those described in the mesenteric venules, further supporting the hypothesis that PSGL-1 and TIM-1 concur to achieve efficient rolling interactions during inflammation and that TIM-1 is involved in PSGL-1-independent residual rolling (Figure 7F).
TIM-1 and PSGL-1 cooperate to control activated but not naïve T cell trafficking
To evaluate the concurrency of TIM-1 and PSGL-1 in T cell trafficking under inflammatory conditions in more detail, we analyzed the accumulation of Havcr1 Δmucin, Selplg−/− and Selplg−/−Havcr1 Δmucin Th1 cells in the CHS and inflamed CNS. As expected, the lack of either PSGL-1 or TIM-1 in the CHS model led to a significant inhibition of T cell accumulation, with PSGL-1 deficiency having a greater impact, probably due to the additional role of PSGL-1 as a rolling receptor for E-selectin in this model (Figure 7G) (Austrup et al., 1997). There was no further reduction in accumulation when we used double mutant Selplg−/− Havcr1 Δmucin T cells in the CHS experiments, potentially reflecting the very strong inhibition due to the PSGL-1 deficiency in this model. When we studied non-antigen specific Th1 cell accumulation in the inflamed CNS in an animal model with relevance to EAE in which cerebral vessels express P-selectin (Keerfoot et al., 2004), there was a significantly higher decrease in the accumulation of Havcr1Δmucin T cells compared to Selplg−/− T cells (Figure 7H). These data suggest that TIM-1 better compensates PSGL-1 deficiency playing a more prevalent role in Th1 cell accumulation in the inflamed brain. There was no additional reduction in T cell accumulation when we used double mutant Selplg−/−Havcr1 Δmucin Th1 cells compared to Havcr1 Δmucin Th1 cells, further supporting a prevalent role for TIM-1 in the accumulation of T cells in the CNS. Taken together, these results suggest that TIM-1 and PSGL-1 cooperate to mediate activated T cell trafficking during inflammatory diseases, with TIM-1 playing the more important role in activated T cell trafficking in the CNS and PSGL-1 having the more prevalent role in the inflamed skin.
Finally we compared the roles of PSGL-1 and TIM-1 in the homing of naïve T cells by using intravital microscopy in high endothelial venules (HEVs) of Peyer’s patches and T-cell accumulation assays in secondary lymphoid organs in naïve recipient mice. We found that naïve CD4+ T cells lacking PSGL-1 were less capable of firm adhesion in HEVs and accumulation in Peyer's patches and lymph nodes (Figures 7I and 7J), presumably because they were less able to bind chemokines exposed on the surface of HEVs (Zarbock et al., 2011). Notably, Havcr1 Δmucin naïve T cells had no defects in their rolling and arrest in HEVs or accumulation in lymphoid organs, compared to wild-type cells (Figure 7I and 7J), suggesting that TIM-1 has a more specialized role in activated T cell recruitment to sites of inflammation. Thus, TIM-1 is a major P-selectin ligand and a key trafficking mechanism for Th1 and Th17 cells during inflammatory and autoimmune diseases.
DISCUSSION
In this study we have provided direct evidence that TIM-1 is a ligand for endothelial selectins and that it controls the tethering and rolling of activated T cells in the inflamed microcirculation and the accumulation of T cells at inflammation sites. We have demonstrated that TIM-1 is a major P-selectin ligand and our results fulfill all criteria for the definitive assignment of TIM-1 as a selectin ligand, namely: (i) microspheres covered with TIM-1 can roll on selectins under flow conditions; (ii) monoclonal antibodies against TIM-1 block selectin-dependent T cell rolling in vitro and in vivo; and (iii) the mutation of the Havcr1 gene impairs selectin-mediated T cell functions on intact cells in vitro and in experimental models in vivo (Zarbock et al., 2011).
The existence of P-selectin ligands other than PSGL-1 on T cells (and their ability to mediate rolling interactions) has been predicted, but the identity and precise role of these ligands in leukocyte trafficking has been unclear (Ley and Kansas, 2004). Although PSGL-1 has been shown to mediate the rolling of neutrophils, monocytes and T cells, our data show that TIM-1 plays a more specialized role in the trafficking of T cells. Furthermore, whereas PSGL-1 is involved in naïve T cell homing to lymphoid organs as well leukocyte trafficking during inflammation, TIM-1 has a more specialized role in activated T cell recruitment to sites of inflammation, suggesting a level of diversity between PSGL-1 and TIM-1.
The significant reduction in the ability of Havcr1Δmucin Th1 and Th17 cells to roll on P-selectin was observed in the presence of high amounts of functional PSGL-1 on their surface. Indeed, our studies showed that the inhibition of TIM-1 in Th1 and Th17 cells strongly reduced rolling on P-selectin in vitro and in vivo, and PSGL-1-independent residual rolling was inhibited by TIM-1 inactivation, clearly indicating that TIM-1 is a major P-selectin ligand on T cells. Our data show that both TIM-1-P-selectin and PSGL-P-selectin pro-adhesive mechanisms are necessary to achieve “fully efficient” recruitment and reveal that the TIM-1-P-selectin molecular pathway is concurrently involved in T cell trafficking at the regulatory level of cell tethering and rolling. (D’Ambrosio et al., 2004). Notably, TIM-1 does not appear to affect Th2 and Treg cell rolling, suggesting that TIM-1 differentially regulates Th1 and Th17 versus Th2 and Treg cell trafficking, playing a restricted role in the control of Th1 and Th17 cell trafficking.
TIM-1 requires post-translational fucosylation and tyrosine sulfation for efficient binding to both P- and E-selectin, whereas PSGL-1 does not require tyrosine sulfation for binding to E-selectin (Zarbock et al., 2011). Moreover, TIM-1-selectin binding does not require sialylated glycans, which are instead critical for the binding of other receptors such as PSGL-1 and CD44 suggesting that TIM-1 presents a specific glycosylation profile necessary for selectin binding, with clear differences to PSGL-1 and other ligands, particularly in the requirement of sialylated carbohydrates (Zarbock et al., 2011). Inhibiting the mucin domain of TIM-1 in activated T cells causes rolling defects on P-selectin but not E-selectin, suggesting that the interaction between TIM-1 and P-selectin has a more significant role in T cell trafficking and/or that E-selectin-dependent rolling mediated by TIM-1 could be masked by the functional redundancy of other selectin ligands.
We also sought to determine the role of individual TIM-1 domains in selectin binding. We showed that the mucin domain is not required for T cell activation in the presence of an antigen, but it is selectively involved in the interaction with P-selectin in vitro and in vivo. Our results revealed that IgV domain-blocking antibodies also inhibit rolling on P-selectin in vivo and that both the mucin and IgV domains are necessary for TIM-1-selectin binding. Notably, the IgV domain has not previously been shown to be required for selectin binding. The IgV domain of TIM proteins was shown to share functional characteristics with Ca2+-dependent (C-type) lectins and SIGLEC sialic acid-binding proteins, which also play a role in leukocyte trafficking (Wilker et al., 2007). We hypothesize that the IgV domain may be engaged via an intramolecular association with the TIM mucin domain, which may open up under shear conditions.
In addition to its presence on the cell surface, TIM-1 was previously found in large intracellular pools in endosomes, the Golgi apparatus and lysosomal compartments (Santiago et al., 2007A; Balasubramanian et al., 2012; Santiago et al., 2007B). In naïve, Th2 and Treg cells we found substantial intracellular pools of endogenous TIM-1, but very low TIM-1 expression on the surface. In contrast, TIM-1 expression was upregulated on the surface of Th1 and Th17 cells, but was found predominantly just beneath the plasma membrane. These results suggest that TIM-1 is poised to be exposed on the cell surface after activation under specific conditions and that TIM-1 plays a role in lymphocyte extravasation as an activation-dependent primary adhesion molecule. Moreover recent data suggest that cells transfected with TIM-1 expose the mucin domain on the cell surface following intracellular calcium release (Santiago et al., 2007B) and a "flip-flop" model was proposed in which the TIM-1 extracellular domain resides on the cytosolic side of the membrane, with the IgV domain interacting with phosphatidylserine (Santiago et al., 2007A). However, TIM-1 molecule in Th2 and Treg cells may also not be properly glycosylated, compared to Th1 and Th17 cells, and its differential role as P-selectin rolling receptor may reflect both distribution and glycosylation differences between T cell subsets.
Our findings show that TIM-1 mediates T cell trafficking in three models of inflammatory conditions: thrombin-activated mesenteric vessels, the inflamed brain endothelium, and CHS in the skin. Moreover, we showed that TIM-1 plays a prevalent role in Th1 cell accumulation in the CNS when compared to inflamed skin, suggesting that TIM-1 is important to achieve tissue specificity in leukocyte trafficking. We also show that TIM-1 is required for the recruitment of Th1 and Th17 cells, which are potent inducers of inflammation and autoimmunity, suggesting that interference with TIM-1 activity may provide a therapeutic approach in T cell-mediated diseases. Considering that Th1 and Th17 cells facilitate pathogen clearance and promote anti-tumor immunity, we hypothesize that the TIM-1-P-selectin interaction may also play a role during infection and cancer (Ruffell et al., 2010; Zhu et al., 2010).
In conclusion, our findings collectively indicate that TIM-1 is a major P-selectin ligand and a pivotal trafficking mechanism for Th1 and Th17 cells during inflammation. Our results refine the paradigm of the leukocyte adhesion cascade and show that the primary adhesion of T cells to P-selectin in vivo is no longer exclusively dependent on PSGL-1, but also requires TIM-1, thereby providing a form of concurrency in leukocyte trafficking between these two critical components of the immune system.
EXPERIMENTAL PROCEDURES
Mice
Selplg−/− mice and C57BL/6J age-matched controls were obtained from Jackson Laboratories. Havcr1Δmucin mice were obtained as previously described (Xiao et al., 2012). All mice were housed and used in according to the current European Community rules.
Antibodies and fluorescence-activated cell sorting (FACS) analysis
The following rat-anti mouse monoclonal antibodies were used: anti-PSGL-1 (clone 4RA10; BD Bioscience), PE-conjugated anti-CD25 (eBioscience), purified anti-α4 integrin (clone PS-2), anti-LFA-1 (clone TIB-213), anti-L-selectin (clone Mel-14) and anti-CD44 (clone MJ64). Rat-anti mouse TIM-1 RMT1-10, 4A2.2 and 5F12 clones (Xiao et al., 2007; Sizing et al., 2007; Xiao et al., 2012). Samples were collected with the MACSQuant Analyzer (Miltenyi Biotec) and analyzed with the FlowJo software (Tree Star Inc.). Intracellular cytokines were evaluated as described at Supplemental Information.
Real time PCR for TIM-1 expression to is described at Supplemental Information.
Microtiter plate binding assay
The assay was carried out as previously described (Deban et al., 2010). A brief description including treatment with glycosidases is provided in the Supplemental Information.
Under flow assays in capillary tubes
Microcap glass capillary tubes (100 jal; Sigma-Aldrich) were coated with different concentrations of mouse P-selectin or E-selectin Fc-chimeras. Mean site density of selectins in our experiments were 1560 ± 74 molecules/µm2 for 5 µg/ml selectin, 2130 ± 145 molecules/µm2 for 10 µg/ml selectin, 2770 ± 150 molecules/µm2 for 20 µg/ml selectin and 3321 ± 94 molecules/µm2 for 30 µg/ml selectin (see Supplementary Information for more details).
For the experiments with TIM-1-covered microspheres, 9.7-µm protein A-covered non-deformable polystyrene microspheres (Bangs Laboratories) were washed and incubated for 45 min at room temperature with 10 µg/ml human PSGL-1 Fc chimera (AbD Serotec) or 10 µg/ml murine TIM-1 Fc-chimera (Sizing et al., 2007), following the manufacturer’s instructions. Mean site density on TIM-1-coated microspheres was 2410 sites/µm2. Beads covered with mouse IgG Fc-chimera (R&D Systems) were used as a negative control. In separate experiments, T cell populations were fluxed into the tubes at a density of 106 cells/ml. T cells or beads were fluxed at a shear stress of 2 dyne/cm2 in a buffer containing 1 mM Ca2+ and Mg2+, 10% vol/vol FBS in PBS. Adhesion interactions were recorded and analyzed as previously described (Deban et al., 2010).
CD4+ T cell isolation and Th1, Th17, Th2 and Treg cell production
These procedures are described in the Supplemental Information.
Generation of MOG35–55-specific Th1 cells
MOG35–55-specific Th1 cells were generated as previously described (Lees et al., 2008). A brief description is provided in the Supplemental Information.
PMN isolation from mouse bone marrow
Immature mouse bone marrow PMNs were isolated from the femurs and tibias of wild-type and Havcr1Δmucin mice as previously described (Deban et al., 2010).
Intravital microscopy in mesenteric vessels
Intravital microscopy experiments were carried out as previously described (Deban et al., 2010). A brief description is provided in the Supplemental Information.
Intravital microscopy in Peyer’s patches microvasculature and naïve T cell accumulation assays in secondary lymphoid organs are described in the Supplemental Information.
Cutaneous hypersensitivity (CHS)
Mice were sensitized by applying to the shaved abdomen 30 µl of acetone/olive oil (4:1; Sigma-Aldrich) plus 0.5% (v/v) 1-fluoro-2,4-dinitrobenzene (DNFB; Sigma-Aldrich). Five days later, sensitized mice were challenged with 7 µl of acetone/olive oil (4:1) plus 0.3% DNFB on each side of the right ear pinnae. For the control condition the left ear was painted with an identical amount of vehicle. The ear thickness was measured at times 0 and 24 and 48 h after challenge with a dial thickness gauge (Swiss Precision Instruments). In Th1 transfer experiments, 10 × 106 Th1 cells labeled with green carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) were injected intravenously 24 h after ear pinnae painting, and 24 h later, ears were processed as previously described to isolate migrated leukocytes (DeKrey et al., 1999).
Intravital microscopy in brain vessels
Intravital microscopy experiments were carried out in inflamed brain microcirculation in an experimental model mimicking early inflammation during EAE were performed as previously described (Piccio et al., 2002). A brief description is provided in the Supplemental Information.
Active and transfer EAE induction
For active EAE induction, 6–8-week-old C57Bl/6J wild-type and Havcr1Δmucin mice were immunized subcutaneously in the flanks and at tail base with 150 µg of MOG35–55 peptide in 200 µl emulsion consisting of equal volumes of PBS and CFA supplemented with 0.8 mg/mouse Mycobacterium tuberculosis (strain H37Ra; Becton-Dickinson). Mice received 20 ng of pertussis toxin (PTX; List Biological Laboratories) intravenously at the time of immunization and 48 h later. For passive-transfer EAE induction, C57Bl/6J wild-type mice were injected with 20 ng PTX 4 days and 1 day before cell transfer. The day after the second PTX injection, 5 × 106 wild-type or Havcr1Δmucin MOG35–55-specific Th1 cells were injected intravenously. The EAE clinical course was recorded daily as previously described (Piccio et al., 2002).
Proliferation assays from EAE mice
CD4+ T cells were isolated from wild-type and Havcr1Δmucin EAE mice 7 days post-immunization with MOG35–55 peptide. Cells were re-stimulated in vitro in the presence of antigen and pulsed for 18 h with 1 µCi/well of [3H]-thymidine. Cells were then collected and radioactivity was detected using a β-counter (Perkin-Elmer).
Neuropathology
Mice were sacrificed at the disease peak, the spinal cords were collected and frozen, and 10-µm sections were analyzed using hematoxylin and eosin staining for the detection of inflammatory infiltrates and Spielmeyer coloration for myelin.
Migration of Th1 cells in the brain
C57Bl/6J wild-type mice were treated intravenously with 30 ng PTX as previously described (Kerfoot et al., 2004). After 5 h, 5 × 106 MOG35–55-specific CFSE-labeled Th1 cells or CFSE-labeled non-antigen specific Th1 cells were transferred into mice. CFSE+ cells were detected after 60 h from brain homogenates by flow cytometry analysis.
Immunofluorescence staining for confocal microscopy
The procedure is described in the Supplemental Information.
Statistics
Quantitative data are shown as mean values ± standar deviation (SD) or standard error of the mean (SEM). A two-tailed Student’s t test was used for the statistical comparison of two samples. Multiple comparisons were performed by one-way ANOVA followed by Dunnett’s test for multiple comparisons. A P-value <0.05 was considered statistically significant.
Supplementary Material
HIGHLIGHTS.
TIM-1 mediates Th1 and Th17 cell capture and rolling on P-selectin in vitro.
TIM-1 is a major P-selectin ligand controlling T cell adhesion in inflamed vessels.
Both mucin and IgV domains of TIM-1 are required for the interaction with P-selectin.
TIM-1-mediated adhesion controls autoimmune and inflammatory disease development.
ACKNOWLEDGEMENTS
This work was supported in part by grants from the European Research Council grant 261079-NEUROTRAFFICKING (G.C); Fondazione Italiana Sclerosi Multipla (FISM) (G.C.); National Multiple Sclerosis Society (NMSS), New York, NY, USA (G.C.); PRIN 2009 grant from the Ministry of Education and Research (MIUR) (C.L., G.C.), Fondazione Cariverona (C.L., G.C.) and National Institutes of Health, grant 2P01AI054456-06A1 (J.M.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution: G.C. and S.A designed the experiments and analyzed the data. S.A., T.D., B.R., S.B., E.Z., E.P., S.D., L.T., G.P. and V.D.B. performed the experiments. C.L. provided expertise in in vitro under flow assays. V.K.K and S.X. provided mice lacking the TIM-1 mucin domain and anti-TIM-1 antibodies. J.M.C. provided full-length and truncated TIM-1 proteins. P.R. provided TIM-1 and TIM-2 fusion proteins and anti-TIM-1 antibodies. S.A. and G.C. wrote the paper.
REFERENCES
- Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Kota SK, Kuchroo VK, Humphreys BD, Strom TB. TIM family proteins promote the lysosomal degradation of the nuclear receptor NUR77. Sci. Signal. 2012;5:ra90. doi: 10.1126/scisignal.2003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio D, Lecca P, Constantin G, Priami C, Laudanna C. Concurrency in leukocyte vascular recognition: developing the tools for a predictive computer model. Trends Immunol. 2004;8:411–416. doi: 10.1016/j.it.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, Alexopoulos S, Oukka M, Umetsu DT, DeKruyff RH, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J. Clin. Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKrey GK, Titus RG. A method for the isolation and analysis of leucocytic cells from Leishmanial ear lesions in mice. J. Immunol. Methods. 1999;228:1–11. doi: 10.1016/s0022-1759(99)00085-x. [DOI] [PubMed] [Google Scholar]
- Kerfoot SM, Long EM, Hickey MJ, Andonegui G, Lapointe BM, Zanardo RC, Bonder C, James WG, Robbins SM, Kubes P. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J. Immunol. 2004;173:7070–7077. doi: 10.4049/jimmunol.173.11.7070. [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: emerging roles in immunity and disease. Nat. Rev. Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- Lees JR, Iwakura Y, Russell JH. Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines. J. Immunol. 2008;180:8066–8072. doi: 10.4049/jimmunol.180.12.8066. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- McEver RP, Zhu C. Rolling cell adhesion. Annu. Rev. Cell. Dev. Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers HJ, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat. Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- Piccio L, Rossi B, Scarpini E, Laudanna C, Giagulli C, Issekutz AC, Vestweber D, Butcher EC, Constantin G. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J. Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- Rennert PD. Novel roles for TIM-1 in immunity and infection. Immunol. Lett. 2011;141:28–35. doi: 10.1016/j.imlet.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol. Rev. 2009;229:259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;1:3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, Ballesteros A, Martínez-Muñoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007A;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, Ballesteros A, Tami C, Martínez-Muñoz L, Kaplan GG, Casasnovas JM. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007B;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizing ID, Bailly V, McCoon P, Chang W, Rao S, Pablo L, Rennard R, Walsh M, Li Z, Zafari M, et al. Epitope-dependent effect of anti-murine TIM-1 monoclonal antibodies on T cell activity and lung immune responses. J. Immunol. 2007;178:2249–2261. doi: 10.4049/jimmunol.178.4.2249. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- Wilker PR, Sedy JR, Grigura V, Murphy TL, Murphy KM. Evidence for carbohydrate recognition and homotypic and heterotypic binding by the TIM family. Int. Immunol. 2007;19:763–773. doi: 10.1093/intimm/dxm044. [DOI] [PubMed] [Google Scholar]
- Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, Korn T, Anderson AC, Zhang Z, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J. Exp. Med. 2007;201:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, Petecka S, Yeste A, Quintana FJ, Ichimura T, Sobel RA, et al. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proc. Natl. Acad. Sci. U S A. 2012;109:12105–12110. doi: 10.1073/pnas.1120914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.