Abstract
Catecholaminergic activation of myocardial β-adrenergic receptors (βAR) is the principle mechanism regulating cardiac function. Agonists desensitize βAR through G protein-coupled receptor kinase-mediated uncoupling and β-arrestin-mediated internalization. Although inhibition of myocardial G protein-coupled receptor kinase-2 enhances cardiac function and reverses heart failure, pathophysiological effects of modulated βAR internalization/recycling are unknown. We used mutation and transgenic expression of Rab4, which regulates vesicular transport of heptahelical receptors to plasma membranes, to interrogate in vivo βAR trafficking and cardiac function. Expression of constitutively active Rab4 Q72L had no effects on cardiac structure or function, but dominant inhibitor Rab4 S27N impaired responsiveness to endogenous and exogenous catecholamines. To relate βAR trafficking to diminished cardiac function, Rab4 mutant mice were crossbred with mice overexpressing human β2AR. In unstimulated β2AR overexpressors, β2AR localized to heavier endosomes and translocated to lighter, caveolin-rich fractions after isoproterenol stimulation. Coexpression of β2AR with activated Rab4 Q72L caused loss of receptors from heavier endosomes while retaining normal inotropy. In contrast, coexpression of β2AR with inhibitory Rab4 S27N mimicked isoproterenol-induced receptor redistribution to caveolae, with diminished cardiac inotropy. Rab4 inhibition alone prevented resensitization after isoproterenol-induced in vivo adrenergic desensitization. Confocal and ultrastructural analyses revealed bizarre vesicular structures and abnormal accumulation of β2AR in the sarcoplasm and subsarcollema of Rab4 S27N, but not Q72L, mice. These data provide evidence for constant bidirectional sarcollemal–vesicular βAR trafficking in the in vivo heart and show that Rab4-mediated recycling of internalized βAR is necessary for normal cardiac catecholamine responsiveness and resensitization after agonist exposure.
Acritical regulator of minute-by-minute cardiac function is the β-adrenergic receptor (βAR). In response to acute physical or emotional stress, stimulation of myocardial βAR by circulating epinephrine or locally released norepinephrine increases cardiac contractility, heart rate, and blood flow to vital organs (1). In chronic heart failure, activation of this pathway to compensate for cardiac insufficiency is defective, resulting in further increased sympathetic outflow and myocardial catecholamine toxicity (2, 3). Specific βAR abnormalities that have been identified in heart failure include receptor down-regulation and uncoupling from signaling effectors (4, 5). The therapeutic efficacy of pharmacological βAR blockade in heart failure has been linked to reversal of these abnormalities (6).
Agonist-mediated uncoupling of βAR signaling involves G-protein receptor kinase (GRK)-mediated phosphorylation events (7, 8), whereas down-regulation of receptors is a β-arrestin-dependent process of transvesicular receptor internalization and trafficking (9). βAR move from plasma membranes into endosomal structures where they are either targeted for lysosomal degradation or recycled as fully functional receptors by vesicular trafficking back to plasma membranes (resensitization) (10). Although the roles for GRK-mediated βAR receptor–effector uncoupling in cardiac health and disease have been established (11–14), the pathophysiological roles of modulated βAR receptor internalization/recycling have not been defined, largely because suitable in vivo experimental models do not exist. Herein, we used Rab-GTPases, regulators of inter-vesicular membrane transport (15), to define effects of modulated βAR trafficking in the in vivo heart. These studies indicate that bidirectional sarcolemmal–vesicular βAR trafficking occurs continuously in the healthy heart and is necessary for normal baseline adrenergic responsiveness and resensitization after catecholamine exposure.
Methods
Generation of Mutant Rab4 Transgenic Mice. Transgenic mice used the α-myosin heavy chain (MHC) promoter to express constitutively active (GTPase deficient) Q72L or dominant inhibitory (non-GTP-binding) S27N Rab4 mutants (16), identified by genomic Southern analysis of tail clip DNA. Human β2AR (h-β2AR) transgenic mice were created by using an attenuated α-MHC promoter (17) as previously described (18). Animals were treated in accordance with approved University of Cincinnati Institutional Animal Care and Use Committee protocols.
Immunoblot Analysis. Analysis of h-β2AR content and subcellular trafficking were performed by using Santa Cruz H-20 anti-β2AR. Proteins were visualized by using enhanced chemifluorescence and quantitated by using a Storm PhosphorImager. Anti-FLAG was from Sigma. Antibodies recognizing Rab1b, Rab3, Rab4, Rab5, Rab6, Gαs, Gαq/11, Gαo, Gβ, GRK2, and clathrin were from Santa Cruz. Anti-caveolin-3 was from Transduction Laboratories (Lexington, KY). Anti Gαi-1/Gαi-2 was from Perkin–Elmer.
Buoyant density fractionation of myocardial endosomes was modified from Condorelli and coworkers (19). For each preparation, three frozen mouse ventricles were pulverized on liquid nitrogen. The powder was added to 1.5 ml of Mes-buffered saline (MBS; 0.15 M NaCl/25 mM Mes, pH 6.5) and homogenized. Sucrose was added to 40% final concentration, and the sample was placed in an ultracentrifuge tube and sequentially overlaid with 2.25 ml of MBS/30% sucrose/250 mM Na2CO3; and 2.25 ml of MBS/5% sucrose/250 mM Na2CO3. After centrifugation in a Sorvall TH641 swinging bucket rotor at 100,000 × g for 16 h, ten sequential 0.75-ml fractions were collected by aspiration, and 20 μl of each was size-separated on 10% SDS/PAGE gels.
Functional Assessments. Two-dimensional guided M-mode echocardiography of unsedated mice measured left ventricular diastolic and systolic dimensions, from which fractional shortening was derived. Pulsed wave Doppler was used to measure aortic ejection time and calculate velocity of circumferential shortening (Vcf). Invasive hemodynamic studies were performed on anesthetized, spontaneously breathing 8- to 12-week-old transgenic mice and their NTG littermate controls (17, 18).
Histopathology, Confocal Immunohistochemistry, and Ultrastructural Studies. Histopathological examination was performed on Masson's trichrome stained sections. For immunofluorescence, deparafinized sections underwent antigen retrieval by heating and were immunolabeled with anti-β2AR, goat anti-rabbit IgG/Alexa Fluor 488, and phalloidin/Alexa Fluor 568 (Molecular Probes). Ultrastructural examination was as described (20).
Statistical Analysis. Results are mean ± SE. Experimental groups were compared by using Student's t test or one-way ANOVA. A Bonferroni test was used for post hoc comparisons, with P < 0.05 significant.
Results
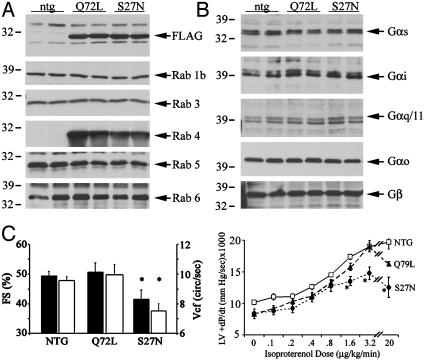
In vivo models for gain-and-loss of myocardial Rab4 function were created by cardiomyocyte-specific transgenic expression of activated (Q72L) and inhibitory (S27N) Rab4 mutants (15, 16). Multiple lines of both transgenes were grossly normal for 1 year. In contrast to the cardiomyopathy of Rab1 overexpression (20), Rab4 mutants had minimal cardiac effects. Heart weights were similar between NTG (134 ± 9 mg, n = 7), Rab4 Q72L (151 ± 4 mg, n = 10), and Rab4 S27N (143 ± 9 mg, n = 13). Northern analysis showed no abnormalities of hypertrophy-associated genes, and histological appearances were normal (data not shown). For the current studies, Rab4 mutants were expressed at approximately the same levels (Fig. 1A) and did not affect myocardial expression of other Rabs (Fig. 1 A), G proteins (Fig. 1B), or GRK2 (data not shown).
Fig. 1.
(A) Myocardial Rab expression in NTG Rab4 Q72L and S27N transgenic models. Mutant Rab4 was epitope-tagged with FLAG. (B) Myocardial G protein expression. (C) Effects of mutant Rab expression on cardiac function assessed as left ventricular fractional shortening (FS; left axis, black bars) and Vcf (right axis; white bars) (Left) and +dP/dt as a function of isoproterenol dose (Right). n = 6–13; *, P < 0.05 versus NTG.
Functional assays revealed a phenotype specific for Rab4 inhibition. Dominant inhibition of Rab4 (S27N mice) diminished echocardiographic left ventricular ejection fraction by 16% and Vcf by 22%, compared with NTG or Q72L mice (Fig. 1C and Table 1). Given that conscious echocardiography induces a hyperadrenergic state, the consequences of infusing the nonselective βAR agonist isoproterenol were assessed by invasive hemodynamics. Left ventricular systolic function (peak + dP/dt) of both Rab4 mutant mice was normal at baseline but was diminished by ≈25% at high isoproterenol doses in S27N mice (Fig. 1C and Table 1). There was no difference in [125I]-cyanopindolol-binding capacity in Rab4 transgenics (S27N = 21 ± 2 fmol/mg, Q72L = 20 ± 3 fmol/mg; n = 3 for each) compared with NTG (23 ± 2 fmol/mg), suggesting that Rab4 inhibition depressed inotropic responsiveness to endogenous and exogenous βAR agonists without altering the number of βAR.
Table 1. Functional characteristics of Rab4 Q72L and S27N transgenic mice.
| ECHO
|
CATH (basal)
|
CATH (+Iso)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mice | LVEDD, mm | LVESD, mm | FS, % | HR, bpm | Vcf, cps | LVP, mmHg | HR, bpm | +dp/dt, mmHg/sec | LVP, mmHg | HR, bpm | +dp/dt, mmHg/sec |
| NTG | 3.2 ± 0.07 | 1.6 ± 0.07 | 50 ± 1 | 500 ± 16 | 9.6 ± 0.4 | 91 ± 1 | 460 ± 2 | 9,396 ± 93 | 109 ± 5 | 628 ± 5 | 19,031 ± 233 |
| Q72L | 3.6 ± 0.14 | 1.8 ± 0.17 | 51 ± 3 | 492 ± 28 | 9.9 ± 0.9 | 87 ± 1 | 456 ± 5 | 8,568 ± 164 | 103 ± 1 | 592 ± 7 | 19,060 ± 399 |
| S27N | 3.4 ± 0.11 | 2.0 ± 0.16 | 42 ± 3* | 486 ± 20 | 7.5 ± 0.8* | 85 ± 1 | 437 ± 5 | 8,357 ± 73 | 100 ± 3 | 607 ± 11 | 14,788 ± 379* |
ECHO, echocardiogram; CATH (basal), baseline catheterization-derived hemodynamics; CATH (+Iso), catheterization-derived hemodynamics at peak isoproterenol stimulation; LVEDD, left ventricular diastolic dimensions; LVESD, left ventricular systolic dimensions; FS, fractional shortening. HR, heart rate; LVP, peak left ventricular systolic pressure. bpm, beats per minute. cps, circumferences per second. n = 6—13.
P < 0.05 versus NTG by ANOVA
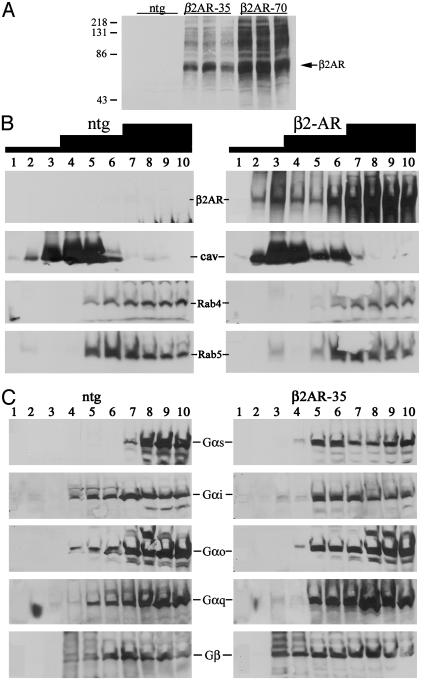
Because Rab4 regulates endosomal sorting and recycling of transferrin receptors (21, 22), we tested whether diminished βAR sensitivity of Rab4 S27N hearts was from disruption of βAR trafficking and impaired accessibility to signaling effectors. βAR subcellular distribution was examined in Rab4 mutant mouse hearts by crossbreeding with transgenic mice overexpressing myocardial h-β2AR as a marker. Because massive overexpression of h-β2AR can cause a fibrotic cardiomyopathy (23, 24), attenuated mutant α-MHC promoters (17) were used to achieve a low level of h-β2AR expression (18). Immunoblotting of h-β2AR transgenic myocardia detected h-β2AR as a broad ≈70-kDa band accompanied by higher molecular weight species previously identified as glycosylated forms (25) (Fig. 2A). [125I]-cyanopindolol binding capacity was 760 ± 111 fmol/mg in the lower expressors (≈35-fold NTG and 1,350 ± 57 fmol/mg in the higher expressors (≈70-fold NTG). Compartmentation was determined by buoyancy density fractionation over discontinuous (5%/30%/40%) sucrose gradients into light, medium, or heavy endosomes. Caveolin-3, a muscle-specific marker for lipid-rich plasma membrane invaginations (26), was enriched at the interface between the 5% and 30% gradients (Fig. 2B, fractions 3 and 4), whereas alkaline phosphatase (plasma membranes) was at the 30–40% interface and clathrin was enriched in the pellet (not shown). Markers for Golgi, endoplasmic reticulum, and lysosomes were in fractions 7–10 (data not shown). h-β2AR were located primarily in the heavier 40% gradient fractions (fractions 7–10), although some also copurified with caveolin (Fig. 2B). Endogenous Rab4, a marker for recycling vesicles (16, 22), localized to fractions 7–10, whereas Rab5 (Fig. 2B) and transferrin receptor (not shown) marked early endosomes (27, 28) at the interface between intermediate and heavy density gradients (fractions 6 and 7). As with h-β2AR, Gβ and the Gαs subunits of heterotrimeric G proteins were in heavier endosomal fractions of NTG mice (Fig. 2C). Gαs had a broader distribution in h-β2AR overexpressing hearts, most likely because of its association with overexpressed receptors (Fig. 2C).
Fig. 2.
(A) Immunoblot analysis of myocardial h-β2AR expression in NTG and 35- and 70-fold h-β2AR overexpressors. Each lane is an individual mouse heart. (B and C) Subcellular fractionation of myocardial membranes with discontinuous 5%/30%/40% sucrose gradients (black staircase). (B) Immunoblot comparison of h-β2AR, Rab4, and Rab5 between NTG (Left) and h-β2AR (Right). Cav, caveolin. (C) Analogous comparisons of myocardial G proteins.
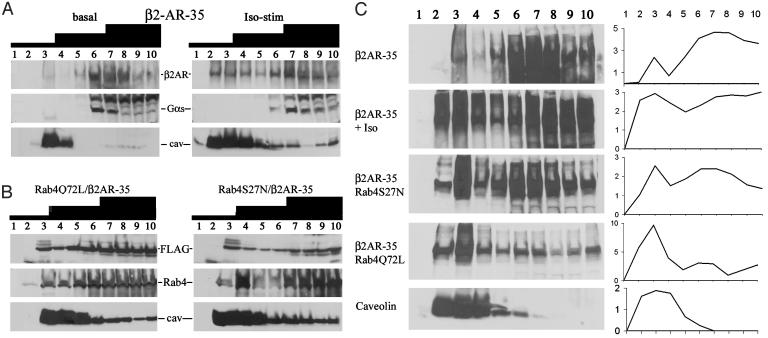
The effects of agonists on subcellular distribution of h-β2AR and G protein effectors were assessed before and after 5-min infusion of high-dose isoproterenol. Agonist stimulation provoked receptor translocation from heavier to lighter, caveolin-enriched fractions (Fig. 3A). In contrast, Gαs remained in the heavier fractions, consistent with a previously postulated role for caveolar uncoupling of β2AR from effectors (29). Rab-specific effects on β2AR trafficking were assessed in Q72L and S27N Rab4 transgenic mice crossbred with h-β2AR overexpressors and compared with receptor translocation by isoproterenol (Fig. 3C, β2AR-35 and β2AR-35 + Iso). Neither Rab mutant affected the level of h-β2AR overexpression (data not shown). However, dominant inhibition of Rab4 (S27N) recapitulated agonist-induced subcellular redistribution (Fig. 3C, β2AR-35/Rab4S27N), and Rab4 activation (Q72L) resulted in accumulation of h-β2AR in the lighter sucrose fractions (Fig. 3C, β2AR-35/Rab4Q72L).
Fig. 3.
(A) h-β2AR immunoblots of subcellular fractions in h-β2AR overexpressing hearts before (Left) and after (Right) in vivo stimulation with 20 μg/kg isoproterenol per minute. (B) Rab4 subcellular fractioning (FLAG and Rab4) in compound Rab4/β2AR mice. (C) Subcellular redistribution of h-β2AR as a function of stimulation with 20 μg/kg isoproterenol per minute and coexpression of mutant Rabs. Graphs depict quantitative data (arbitrary densitometric units; representative of three experiments). Iso, isoproterenol. Cav, caveolin.
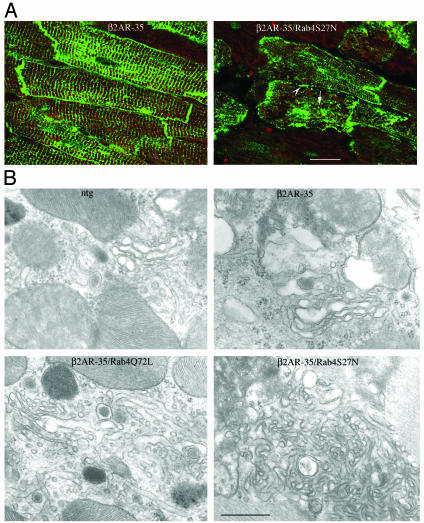
Functional consequences of Rab4-mediated βAR compartmentation were assessed. As previously reported (18, 23, 30), h-β2AR overexpression enhanced basal left ventricular systolic function (percent of fractional shortening and +dP/dt), compared with NTG (compare Tables 1 and 2). Constitutive activation of Rab4 with the Q72L mutant had no effect on these parameters. In contrast, expression of inhibitory S27N Rab4 significantly depressed both inotropic indices (Table 2). Together, these data show that inhibition of Rab4 diminishes β2AR-mediated cardiac contractile function through redistribution of βAR in a manner analogous to that occurring after agonist exposure. Indeed, evidence of altered βAR trafficking was visualized by confocal microscopy. In h-β2AR-overexpressing mice representing the normal pattern of β2AR compartmentation, there was highly defined labeling of sarcolemma and transverse tubular structures (Fig. 4A), consistent with sarcolemmal localization (31). In contrast, Rab4 S27N/h-β2AR cardiomyocytes exhibited granular cytoplasmic staining and coarse labeling of sarcolemma and transverse tubules, suggesting subsarcolemmal vesicular localization of receptors (Fig. 4A). Consistent with this notion, ultrastructural examination revealed abnormal cytoplasmic and subsarcolemmal vesicular structures in compound Rab4 S27N/h-β2AR (Fig. 4B) and Rab4 S37N (data not shown) hearts, compared to normal-appearing structures in NTG, h-β2AR, Rab4 Q72L/h-β2AR (Fig. 4B), and Rab4 Q72L (data not shown) hearts.
Table 2. Functional characteristics of 35-fold h-β2AR overexpressing mice.
| ECHO
|
CATH
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Mice | LVEDD, mm | LVESD, mm | FS, % | HR, bpm | Vcf, cps | LVP, mmHg | HR, bpm | +dp/dt, mmHg/sec |
| β2AR-35 | 3.2 ± 0.04 | 1.4 ± 0.04 | 57 ± 1 | 572 ± 14 | 12.3 ± 1 | 102 ± 1 | 486 ± 5 | 15,254 ± 64 |
| Q72L/β2—35 | 3.5 ± 0.12 | 1.6 ± 0.09 | 55 ± 1.4 | 534 ± 29 | 11.4 ± 1 | 112 ± 1 | 418 ± 8 | 14,483 ± 228 |
| S27N/β2—35 | 3.2 ± 0.06 | 1.5 ± 0.06 | 49 ± 3* | 549 ± 21 | 10.8 ± 0.3 | 101 ± 1 | 433 ± 5 | 12,852 ± 99* |
Mice (n = 4—9) expressed h-βAR alone (β2AR-35) and with Rab4 activation (Q72L) or inhibition (S27N). ECHO, echocardiogram. CATH, catheterization-derived hemodynamics; LVEDD, left ventricular diastolic dimensions; LVESD, left ventricular systolic dimensions; FS, fractional shortening; HR, heart rate; LVP, peak left ventricular systolic pressure. bpm, beats per minute. cps, circumferences per second.
P < 0.05 versus h-β2AR-35 by ANOVA
Fig. 4.
(A) Distribution of h-β2AR (green fluorescence) in h-β2AR and h-β2AR/Rab4 S27N compound transgenic myocardia. Arrow indicates granular cytoplasmic staining; arrowhead shows the coarse labeling of the sarcolemma. Red fluorescence is f-actin. Scale bar, 20 μm (Magnification, ×600.) (B) Ultrastructural features: h-β2AR/Rab4 S27N vesicular structures are intensely convoluted and the membranous profiles abnormally fused. Magnification is the same for all panels. (Scale bar, 0.5 μm.) (Magnification, ×41,000.)
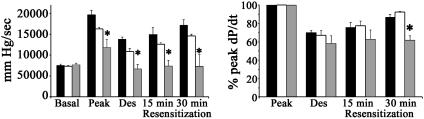
Given that Rab4 regulates receptor insertion into membranes (21, 22) and in vivo myocardial Rab4 inhibition resulted in βAR insensitivity, we hypothesized that myocardial Rab4 mediates βAR recycling after agonist-mediated internalization (resensitization). Therefore, we assayed in vivo cardiac desensitization after 20 min of high-dose isoproterenol, and measured time-dependent functional recovery. As in Fig. 1B, Rab4 S27N mice had depressed responsiveness to isoproterenol, compared with NTG or Rab4 Q72L (Fig. 5). Corrected for this basal abnormality, desensitization (percentage of initial agonist response) was 49% and 62% in NTG and Q72L mice, respectively, but was complete (i.e., no agonist response) in S27N. Additionally, there was no significant resensitization (normalization of agonist response) in S27N mice, versus functional recovery after 30 min of 57% and 65% in NTG and Q72L mice, respectively. Thus, the phenotype of myocardial Rab4 activation is β2AR translocation with normal function, whereas that of Rab4 inhibition is β2AR insensitivity because of impaired receptor resensitization through defective receptor recycling.
Fig. 5.
Desensitization and resensitization of inotropic (+dP/dt) response to 20 μg/kg isoproterenol per minute for 20 min. (Left) Absolute +dP/dt (mmHg/sec; 1 mmHg = 133 Pa). (Right) +dP/dt indexed to initial “peak” inotropic response. Black bars represent NTG, white bars represent Rab4 Q72L, and gray bars represent Rab4 S27N. *, P < 0.01 versus NTG and Q72L
Discussion
These studies establish a necessary role for Rab4-mediated βAR sarcolemmal targeting in cardiac adrenergic responsiveness and resensitization. Depressed inotropic indices with Rab4 inhibition in the conscious, unsedated mouse suggest that βAR internalization and reinsertion occur continuously under physiological in vivo conditions. The combination of GRK-mediated receptor-effector uncoupling, β-arrestin-stimulated receptor internalization, and Rab4-mediated resensitization therefore constitute a highly regulated mechanism for modulating myocardial adrenergic responsiveness through adjustments of adrenergic receptor homeostasis.
To interrogate βAR trafficking in the in vivo heart, we modified a Rab small G protein thought to regulate receptor movement from endosomes to plasma membranes (21, 22). Emerging data indicate that individual Rab proteins control distinct vesicular transport events in a highly specific manner by binding to donor vesicles, facilitating transport, and modulating docking and fusion to targets (15, 32). Expression in cultured fibroblasts has shown that Rab5 regulates protein internalization into early endosomes, whereas Rab4 regulates vesicular transport from endosomes to plasma membranes (27, 28). Although Rab1 transgenesis caused cardiomyopathy associated with disordered Golgi (20) (consistent with Rab1 regulation of transGolgi transport; see ref. 33), mutant Rab4 mice showed no evidence of cardiac pathology, only diminished catecholamine-stimulated myocardial function with Rab4 inhibition.
To define βAR trafficking events that could account for impaired cardiac function of Rab4 S27N, we used antibodybased techniques and a low-expressing transgenic h-β2AR mouse (18). An advantage of combining the αMHC-driven Rab4 and h-β2AR transgenic models is that receptors and Rab4 mutants were located exclusively in cardiac myocytes, which eliminates potentially confounding effects of other myocardial cell types. Also, endogenous mouse β1AR and β2AR were not visualized by using the human receptor-specific antibody. Compound h-β2AR/Rab4 S27N mice revealed an association between functional inhibition and translocation of β2AR to caveolin-rich endosomal fractions. This pattern of receptor trafficking recapitulated redistribution of β2AR with agonist, but was associated with loss of agonist responsiveness and blunting of the hallmark intrinsic cardiac activation of β2AR overexpression (23, 24). In contrast, “recycling activated” Rab4 Q72L/h-β2AR compound transgenic, exhibited normal β2AR responsiveness, and appropriate inotropic enhancement with h-β2AR overexpression.
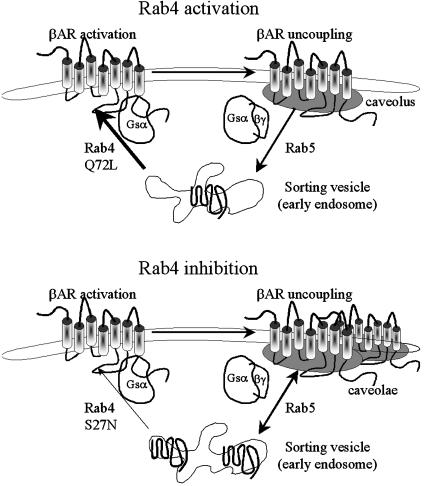
Fig. 6 depicts our concept of Rab4-mediated βAR trafficking in context of these phenotypes. Although our use of overexpressed receptors to track Rab4-mediated trafficking events has the potential to exaggerate uncoupled, or “spare” receptor effects, we suggest that activation of Rab4 (Fig. 6 Upper, Q72L) accelerates βAR reinsertion into plasma membranes where it functions normally. Agonist stimulation causes normal caveolar translocation and desensitization, but because internalized receptors are rapidly reinserted, there is also rapid resensitization. In contrast, inhibition of Rab4 (Fig. 6 Lower, S27N) prevents βAR recycling, causing receptor accumulation in caveolae and early endosomes, where receptors cannot interact with G protein effectors, and adrenergic insensitivity. Agonist-mediated translocation of receptors to caveolae increases endosomal accumulation of receptors and reinforces the phenotype by preventing resensitization.
Fig. 6.
βAR trafficking with Rab4 activation with Q72L (Upper) and inhibition with S27N (Lower). In both panels, activated sarcolemmal βAR are coupled to Gαs but translocate to caveolae where they disassociate from G proteins. Receptor internalization, presumably mediated by Rab5 (28), brings βAR to sorting vesicles (Upper), where Rab4 promotes their reinsertion into sarcolemma. Rab4 activation accelerates this process, constantly renewing the supply of functional receptors (Upper). Rab4 inhibition (Lower) impairs recycling, causing accumulation of uncoupled receptors in vesicles and caveolae.
Independent of Rab4 activity, our findings in h-β2AR overexpressing hearts indicate that in vivo cardiac β2AR [like β1AR in cultured cardiac myocytes (29)] colocalize with Gαs, Rab4, and Rab5 in plasma membranes and endosomes but translocate to fractions enriched in caveolae within minutes of agonist simulation. This finding is consistent with previously described agonist-induced caveolar targeting of m2 muscarinic and kinin B1 receptors (34, 35) but differs from prior descriptions of cardiomyocyte β2AR as being located entirely in the caveolar-rich fractions of cultured neonatal rat cardiomyocytes (29, 31). Although the h-β2AR transgenic model may, like any overexpression or transfection system, generate nonphysiological results, other possible explanations for these differences include species differences between rodent and human β2AR, developmental differences between neonatal and adult cardiomyocytes (25), the total absence of intrinsic catecholamine stimulation and contractile activity in quiescent tissue culture, and altered β2AR localization after enzymatic cardiomyocyte isolation (25). Reports of apparent caveolar egress of β2AR after agonist stimulation in cultured cardiomyocytes (29), compared with our observation of caveolar targeting of β2AR after isoproterenol stimulation in intact hearts, are more readily explained. The prolonged (30-min) period of isoproterenol exposure in the tissue culture study likely induced receptor internalization (36), explaining the loss of receptors from caveolae.
In summary, these studies reveal a critical function for trafficking of β2AR in maintaining normal myocardial responsiveness to catecholamines and in promoting adrenergic resensitization after sustained adrenergic stimulation. Our data suggest that βAR internalization and reexpression occur continuously to maintain proper adrenergic responsiveness in the in vivo heart. Given reports of regulated Rab protein expression in heart failure (20) and the recently described existence of polymorphic human βAR exhibiting abnormal receptor internalization or trafficking (β1AR Gly 49 and β2AR Glu 27 and Arg 16 variants) (37), these data support a contributory role for altered βAR recycling in cardiomyopathic syndromes.
Acknowledgments
This work was supported by National Institutes of Health Grants P01HL69779 and R01HL58010.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: βAR, β-adrenergic receptor; h-βAR, human βAR; GRK, G protein-coupled receptor kinase; NTG, nontransgenic; Vcf, velocity of circumferential shortening.
References
- 1.Rockman, H. A., Koch, W. J. & Lefkowitz, R. J. (2002) Nature 415, 206-212. [DOI] [PubMed] [Google Scholar]
- 2.Brodde, O. E. (1993) Pharmacol. Ther. 60, 405-430. [DOI] [PubMed] [Google Scholar]
- 3.Bristow, M. R., Ginsburg, R., Minobe, W., Cubicciotti, R. S., Sageman, W. S., Lurie, K., Billingham, M. E., Harrison, D. C. & Stinson, E. B. (1982) N. Engl. J. Med. 307, 205-211. [DOI] [PubMed] [Google Scholar]
- 4.Ungerer, M., Bohm, M., Elce, J. S., Erdmann, E. & Lohse, M. J. (1993) Circulation 87, 454-463. [DOI] [PubMed] [Google Scholar]
- 5.Ungerer, M., Parruti, G., Bohm, M., Puzicha, M., DeBlasi, A., Erdmann, E. & Lohse, M. J. (1994) Circ. Res. 74, 206-213. [DOI] [PubMed] [Google Scholar]
- 6.Bristow, M. R. (2000) Circulation 101, 558-569. [DOI] [PubMed] [Google Scholar]
- 7.Pitcher, J. A., Freedman, N. J. & Lefkowitz, R. J. (1998) Annu. Rev. Biochem. 67, 653-692. [DOI] [PubMed] [Google Scholar]
- 8.Choi, D. J., Koch, W. J., Hunter, J. J. & Rockman, H. A. (1997) J. Biol. Chem. 272, 17223-17229. [DOI] [PubMed] [Google Scholar]
- 9.Lohse, M. J., Benovic, J. L., Codina, J., Caron, M. G. & Lefkowitz, R. J. (1990) Science 248, 1547-1550. [DOI] [PubMed] [Google Scholar]
- 10.Lohse, M. J. (1993) Biochim. Biophys. Acta 1179, 171-188. [DOI] [PubMed] [Google Scholar]
- 11.Koch, W. J., Rockman, H. A., Samama, P., Hamilton, R. A., Bond, R. A., Milano, C. A. & Lefkowitz, R. J. (1995) Science 268, 1350-1353. [DOI] [PubMed] [Google Scholar]
- 12.Rockman, H. A., Chien, K. R., Choi, D. J., Iaccarino, G., Hunter, J. J., Ross, J., Jr., Lefkowitz, R. J. & Koch, W. J. (1998) Proc. Natl. Acad. Sci. USA 95, 7000-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding, V. B., Jones, L. R., Lefkowitz, R. J., Koch, W. J. & Rockman, H. A. (2001) Proc. Natl. Acad. Sci. USA 98, 5809-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhter, S. A., Skaer, C. A., Kypson, A. P., McDonald, P. H., Peppel, K. C., Glower, D. D., Lefkowitz, R. J. & Koch, W. J. (1997) Proc. Natl. Acad. Sci. USA 94, 12100-12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somsel, R. J. & Wandinger-Ness, A. (2000) J. Cell Sci. 113, 183-192. [DOI] [PubMed] [Google Scholar]
- 16.Mohrmann, K. & van der Sluijs, P. (1999) Mol. Membr. Biol. 16, 81-87. [DOI] [PubMed] [Google Scholar]
- 17.Hahn, H. S., Yussman, M. G., Toyokawa, T., Marreez, Y., Barrett, T. J., Hilty, K. C., Osinska, H., Robbins, J. & Dorn, G. W. (2002) Circ. Res. 91, 741-748. [DOI] [PubMed] [Google Scholar]
- 18.Hahn, H. S., Marreez, Y., Odley, A., Sterbling, A., Yussman, M. G., Hilty, K. C., Bodi, I., Liggett, S. B., Schwartz, A. & Dorn, G. W. (2003) Circ. Res. 93, 1111-1119. [DOI] [PubMed] [Google Scholar]
- 19.De Luca, A., Sargiacomo, M., Puca, A., Sgaramella, G., De Paolis, P., Frati, G., Morisco, C., Trimarco, B., Volpe, M. & Condorelli, G. (2000) J. Cell Biochem. 77, 529-539. [DOI] [PubMed] [Google Scholar]
- 20.Wu, G., Yussman, M. G., Barrett, T. J., Hahn, H. S., Osinska, H., Hilliard, G. M., Wang, X., Toyokawa, T., Yatani, A., Lynch, R. A., et al. (2001) Circ. Res. 89, 1130-1137. [DOI] [PubMed] [Google Scholar]
- 21.van der Sluijs, P., Hull, M., Webster, P., Male, P., Goud, B. & Mellman, I. (1992) Cell 70, 729-740. [DOI] [PubMed] [Google Scholar]
- 22.Daro, E., van der Sluijs, P., Galli, T. & Mellman, I. (1996) Proc. Natl. Acad. Sci. USA 93, 9559-9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liggett, S. B., Tepe, N. M., Lorenz, J. N., Canning, A. M., Jantz, T. D., Mitarai, S., Yatani, A. & Dorn, G. W. (2000) Circulation 101, 1707-1714. [DOI] [PubMed] [Google Scholar]
- 24.Milano, C. A., Allen, L. F., Rockman, H. A., Dolber, P. C., McMinn, T. R., Chien, K. R., Johnson, T. D., Bond, R. A. & Lefkowitz, R. J. (1994) Science 264, 582-586. [DOI] [PubMed] [Google Scholar]
- 25.Rybin, V. O., Pak, E., Alcott, S. & Steinberg, S. F. (2003) Mol. Pharmacol. 63, 1338-1348. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto, T., Schlegel, A., Scherer, P. E. & Lisanti, M. P. (1998) J. Biol. Chem. 273, 5419-5422. [DOI] [PubMed] [Google Scholar]
- 27.Gorvel, J. P., Chavrier, P., Zerial, M. & Gruenberg, J. (1991) Cell 64, 915-925. [DOI] [PubMed] [Google Scholar]
- 28.Bucci, C., Parton, R. G., Mather, I. H., Stunnenberg, H., Simons, K., Hoflack, B. & Zerial, M. (1992) Cell 70, 715-728. [DOI] [PubMed] [Google Scholar]
- 29.Rybin, V. O., Xu, X., Lisanti, M. P. & Steinberg, S. F. (2000) J. Biol. Chem. 275, 41447-41457. [DOI] [PubMed] [Google Scholar]
- 30.Dorn, G. W., Tepe, N. M., Lorenz, J. N., Koch, W. J. & Liggett, S. B. (1999) Proc. Natl. Acad. Sci. USA 96, 6400-6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang, Y., Rybin, V. O., Steinberg, S. F. & Kobilka, B. (2002) J. Biol. Chem. 277, 34280-34286. [DOI] [PubMed] [Google Scholar]
- 32.Martinez, O. & Goud, B. (1998) Biochim. Biophys. Acta 1404, 101-112. [DOI] [PubMed] [Google Scholar]
- 33.Tisdale, E. J., Bourne, J. R., Khosravi-Far, R., Der, C. J. & Balch, W. E. (1992) J. Cell Biol. 119, 749-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feron, O., Smith, T. W., Michel, T. & Kelly, R. A. (1997) J. Biol. Chem. 272, 17744-17748. [DOI] [PubMed] [Google Scholar]
- 35.Sabourin, T., Bastien, L., Bachvarov, D. R. & Marceau, F. (2002) Mol. Pharmacol. 61, 546-553. [DOI] [PubMed] [Google Scholar]
- 36.Seachrist, J. L., Anborgh, P. H. & Ferguson, S. S. (2000) J. Biol. Chem. 275, 27221-27228. [DOI] [PubMed] [Google Scholar]
- 37.Small, K. M., McGraw, D. W. & Liggett, S. B. (2003) Annu. Rev. Pharmacol. Toxicol. 43, 381-411. [DOI] [PubMed] [Google Scholar]