Abstract
Background
Reductions in skeletal muscle mass and increased adiposity are key elements in the aging process and in the pathophysiology of several chronic diseases. Systemic low grade inflammation associated with obesity has been shown to accelerate the age-related decline in skeletal muscle. The aim of this investigation was to determine the effects of 12 months of progressive resistance training (PRT) on systemic inflammation, and whether reductions in systemic inflammation were associated with changes in body composition. We hypothesized that reductions in systemic inflammation following 12 months of PRT in older adults with type 2 diabetes would be associated with reductions in adiposity and increases in skeletal muscle mass.
Methods
Participants (n = 103) were randomized to receive either PRT or sham-exercise, 3 days a week for 12 months. C-reactive protein (CRP) was used to assess systemic inflammation. Skeletal muscle mass and total fat mass were determined using bioelectrical impedance.
Results
Twelve months of PRT tended to reduce CRP compared to sham exercise (β = −0.25, p = 0.087). Using linear mixed-effects models, the hypothesized relationships between body composition adaptations and CRP changes were significantly stronger for skeletal muscle mass (p = 0.04) and tended to be stronger for total fat mass (p = 0.07) following PRT when compared to sham-exercise. Using univariate regression models, stratified by group allocation, reductions in CRP were associated with increases in skeletal muscle mass (p = 0.01) and reductions in total fat mass (p = 0.02) in the PRT group, but not in the sham-exercise group (p = 0.87 and p = 0.32, respectively).
Conclusions
We have shown for the first time that reductions in systemic inflammation in older adults with type 2 diabetes following PRT were associated with increases in skeletal muscle mass. Furthermore, reductions in CRP were associated with reductions in adiposity, but only when associated with PRT. Lifestyle interventions aimed at reducing systemic inflammation in older adults with type 2 diabetes should therefore incorporate anabolic exercise such as PRT to optimize the anti-inflammatory benefits of favorable body composition adaptations.
Keywords: PRT, Inflammation, Body composition, Skeletal muscle, Adiposity
Introduction
Type 2 diabetes mellitus is a chronic condition characterized by the presence of insulin resistance, systemic low-grade inflammation, and an increased risk of cardiovascular disease. Increasing evidence suggests that the presence of inflammation is a key factor in the development and progression of insulin resistance, as well as other cardiovascular disease co-morbidities such as atherosclerosis [1, 2]. C-reactive protein (CRP) is a biomarker commonly used as an index of systemic inflammation and has been shown to be an independent predictor of cardiovascular disease mortality [3, 4].
Excess adiposity, particularly visceral adipose tissue (VAT), is central to increased levels of circulating CRP [5, 6], while CRP is known to accelerate age-related loss of skeletal muscle mass (SMM) [7], highlighting CRP’s dual association with body composition. PRT is a unique mode of exercise that can reduce adiposity whilst concomitantly increasing SMM, thus potentially addressing the two body composition parameters associated with increased CRP. The aim of this investigation was therefore to determine the effect of 12 months of high intensity PRT on circulating CRP in older adults with type 2 diabetes, and whether improvements in body composition (increases in SMM and reductions in adiposity) were related to reductions in CRP. Our hypotheses were as follows:
CRP would be significantly associated with indices of adiposity and SMM at baseline.
PRT would significantly reduce CRP compared to the sham-exercise control group.
Reductions in CRP would be associated with favorable changes in body composition variables, namely increased SMM and mid-thigh muscle area, and decreased FM and VAT.
Materials and Methods
The GREAT2DO study is an ongoing clinical trial investigating the efficacy of 6 years of PRT on insulin resistance and glucose homeostasis in older adults with type 2 diabetes. The first year was a randomized, double-blind, sham-exercise controlled trial. After the initial year, participants were asked to continue training, with participants in the sham-exercise control group crossed over to high intensity PRT. The data presented in this report are from the RCT phase. Between July 2006 and November 2009, 103 participants were randomized to receive 12 months of high intensity, high velocity, PRT, or sham-exercise (low intensity, non-progressive exercise), in addition to usual care. Inclusion criteria were ≥60 years, sedentary (no progressive resistance training; structured exercise ≤1/week; less than 150 min/week low or moderate intensity walking or other unstructured exercise) and type 2 diabetes and metabolic syndrome. Participants could be treated with diet alone, oral medications, insulin, or combination at the time of enrolment, without recent changes in medication (<3 months). Randomization was performed using a investigator not involved in the study and was stratified by sex and insulin usage in blocks of 4. Exclusion criteria for the study were the presence of unstable chronic diseases or any contraindications to PRT, or being unwilling to commit to a 12-month exercise training program, 3 times per week. Written informed consent was obtained from all participants, and the protocol was approved by the Sydney South West Area Health Service and the University of Sydney Human Research Ethics Committees (Australian New Zealand Clinical Trial Registry number 12606000436572).
Training Protocol
The PRT group trained 3 days per week under supervision using pneumatic resistance equipment (Keiser Sports Health Ltd., Fresno, CA), at two sites. A version of PRT known as power training was employed, in which the concentric contraction (lifting) was completed as quickly as possible, whilst the eccentric contraction (lowering) was completed over 4 s. The exercises targeted large symmetrical muscle groups of the arms, legs, and trunk: seated row, chest press, leg press, knee extension, hip flexion, hip extension, and hip abduction. For each exercise, participants performed 3 sets of 8 repetitions (2 sets of 8 on each leg for hip flexion, hip extension, and hip abduction). The intensity was set at 80 % of the most recently determined 1 repetition maximum (1RM), which was re-assessed every 4 weeks. When 1RM testing was not feasible, resistances were increased by targeting a Borg scale rating of perceived exertion between 15 and 18.
The sham-exercise group trained on the same equipment, 3 times a week, under supervision from the same trainers at different times of the day so as to remain blinded to the investigators’ hypotheses, with both interventions offered as potentially beneficial. The resistance was set as low as possible and not progressed, and participants were instructed to perform concentric and eccentric contractions slowly.
Assessments
Blinded outcome assessments were conducted at 0 and 12 months at the University of Sydney. Participants were required to withhold food, liquids, and medication for 12 h prior to metabolic testing. A 24-h food recall was performed on the day of the baseline assessment, and participants were asked to follow the same diet prior to subsequent assessments at 12 months. Assessments at 12 months were performed 72 h after each participant’s final training session.
Demographics and health status
Participants were asked routine questions to obtain demographic information, as well as their current health status relating to prescribed medications, smoking status (current or past), and the presence of other chronic diseases.
Measure of systemic inflammation
High-sensitivity CRP was measured in duplicate by enzyme-linked immunosorbent assay (ELISA; eBIOSCIENCE, Camarillo, CA), with the average value used in statistical analyses. The lowest detectable concentration was 0.01 mg/L, and the intra-assay coefficient of variation was 6.3 %. To remove the potential confounding of acute inflammation, participants with concentrations of CRP >10 mg/L were excluded from analyses [4].
Anthropometric measurements
Morning fasting stretch stature (wall-mounted Holtain stadiometer, Holtain Limited, Crymych Pembs., UK) and naked weight [weight in gown (kg) − weight of gown (kg)] were measured in triplicate to the nearest 0.1 cm and 0.01 kg, respectively.
Measures of body composition
SMM and total fat mass (FM) were determined using bioelectrical impedance analysis (BIA; RJL Systems, Inc., Clinton, MI, USA) [8, 9]. All participants were fasting and in a state of euhydration, and BIA was performed in the morning, at a similar time of day for all participants. BIA was not available in 3 subjects due to presence of a pacemaker. SMM was determined using the equation from Janssen et al [8]. Skeletal muscle mass was determined using the following equation [8]:
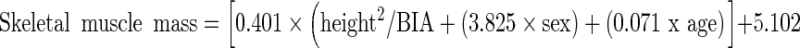 |
with height in cm, BIA resistance in ohms (average of 3 measures), sex coded 1 for men and 0 for women, and age in years.
FM was determined using BIA, by subtracting lean body mass from total mass to determine FM. Lean body mass was determined using the equation from Lukaski et al [9].
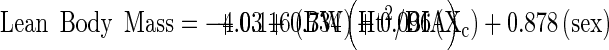 |
with height (Ht) in cm, BIA resistance in ohms (average of 3 measures), naked body weight (BW) in kg, Xc reactance in ohms, and sex coded 1 for men and 0 for women
Computed tomography (CT; GE High Speed CTI Scanner, MIL, USA at the Royal Prince Alfred Hospital, Sydney) was used to quantify VAT (cm2), mid-thigh muscle cross-sectional area (cm2; CSA), and mid-thigh muscle attenuation.
For CT scans of the abdomen, a 1-mm-thick slice was taken at the mid-point between the iliac crest and lowest rib (determined with the participant supine). This scan location is concordant with the waist circumference measurement site used by the International Diabetes Federation and is a criterion used by the IDF to classify metabolic syndrome [10]. Settings were kV = 100 and mA = 170 with a displayed field of view (DFOV) 45–48, depending on subject size.
For CT scans of the mid-thigh, a 1-mm slice was performed at the mid-point between the inguinal crease and the proximal pole of the patella measured with the subject supine and knee flexed. The non-dominant leg was used. Settings were kV = 100 and mA = 170 with a (DFOV) 25. Scan images were analysed according to optical density by a blinded investigator using NIH Image software (Version 1.63, National Institutes of Health) programmed with specific macros to quantify cross-sectional adipose tissue. To determine VAT, macros were programmed to select the outer perimeter extending from the paraspinal muscles to the anterior abdominal muscles. The program calculated this measure by summing the area within the selected perimeter occupied by pixels with optical density in the range of 140 to 240. Thigh muscle density was calculated according to a specific optical density range (10–113) chosen to best discriminate muscle from fat and bone. Co-efficient of repeatability (CR), determined using a Bland–Altman plot on a subset of 10 scans, was found to be excellent at 0.49 for VAT, 0.44 and for mid-thigh CSA [11].
Statistical analyses
All data were assessed for normality using histograms and descriptive statistics. Normally distributed data are presented as mean ± SD; otherwise, data are presented as median (range) or frequencies as appropriate. CRP data required square root transformation to achieve normal distribution for use with parametric statistics. Comparisons of variables between groups were performed using a one-way ANOVA. Linear regressions were performed to determine the relationship between continuous variables at baseline. A linear mixed-effects model with repeated measures was used to determine changes over time as this method allows for all available data to be used without imputation for missing values. CRP was entered as the dependent variable with group, time, and group × time interaction entered as fixed effects and an unstructured covariance matrix. To determine if there was any association between group assignment and changes in measures of body composition on reductions in CRP, sequential linear mixed models were constructed with CRP as the dependent variable and full factorial interaction between group, time and the change in the body composition variable of interest. Any variables found to be associated with CRP using mixed-effects models (p < 0.10) were then explored using linear regression models stratified by group allocation. A p value <0.05 indicated statistical significance as all hypotheses were specified a priori. IBM SPSS version 21 (SPSS Inc. Chicago, IL, USA) was used for all analyses.
Results
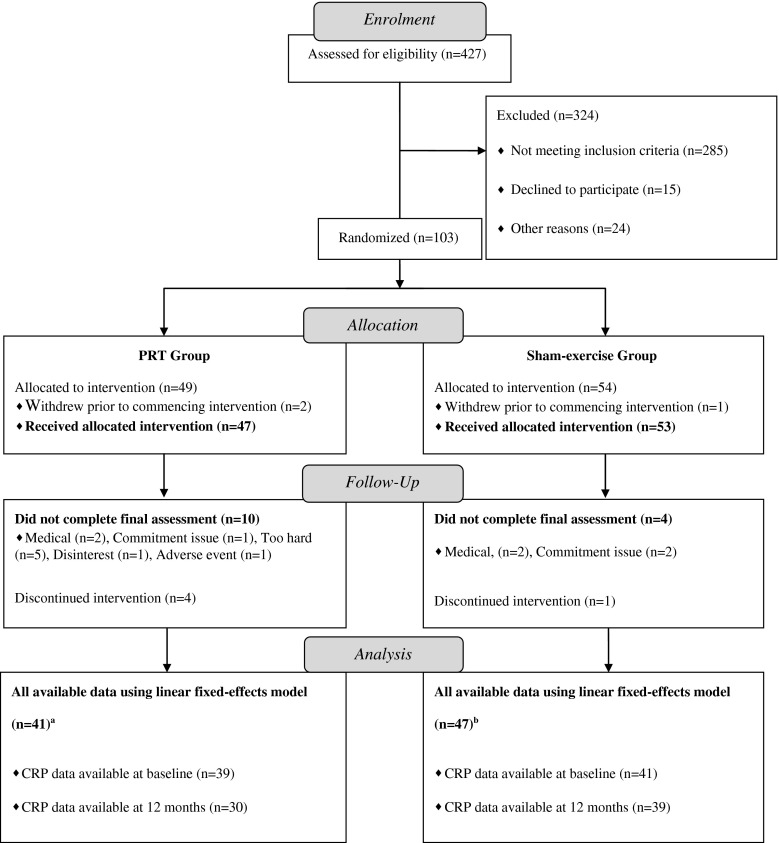
Participant flow relevant to this report is shown in Fig. 1. Participant characteristics are presented in Table 1. Three participants withdrew prior to commencing the intervention and were thus excluded from the analyses. Among the remaining 100 participants, CRP data were available in 91 at either baseline or 12-month time points. However, 4 participants had a baseline CRP > 10 mg/L, 2 of whom had a follow-up CRP > 10 mg/L, while the other 2 subsequently dropped out and thus provided no follow-up sample, resulting in all CRP data from these 4 participants to be removed from analysis as recommended here [4]. Thus, baseline data from the remaining 88 participants are shown in Table 1. At baseline, no difference was observed in CRP between participants in the PRT group and the sham-exercise group (p = 0.69). Participants in the sham-exercise group had a significantly higher HbA1c (p = 0.03) and tended to have a longer duration of diabetes (p = 0.09) at baseline, but did not differ in any other demographic, body composition, or metabolic health parameters (p = 0.10-0.96). Median compliance to the intervention was 82 % (range; 87 %), with the median attendance rate of 77 % (range; 86 %) in the PRT group non-significantly lower than the median attendance of 83 % (range; 84 %) in the sham-exercise group (p = 0.28).
Fig. 1.
CONSORT participant flow chart. PRT progressive resistance training. a Forty-one participants were derived from 11 participants with baseline data only, 2 participants with 12-month data only, and 28 participants with both baseline and 12-month data. b Forty-one participants were derived from 8 participants with baseline data only, 5 participants with 12-month data only, and 34 participants with both baseline and 12-month data
Table 1.
Baseline participant characteristics
| Variable | Total, n = 88 | PRT, n = 41 | SHAM, n = 47 | p value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 68.2 ± 5.7 | 67.2 ± 4.9 | 69.2 ± 6.3 | 0.10 |
| Sex (men/women) | 46/42 | 22/19 | 24/23 | 0.81 |
| Duration of diabetes (years) | 7 (28) | 6 (27) | 8 (28) | 0.06 |
| Number of chronic diseases (n) | 5.1 ± 1.9 | 5.1 ± 1.9 | 5.0 ± 1.8 | 0.80 |
| History of smoking (yes/no)a | 51/31 | 24/14 | 27/17 | 0.87 |
| Current smoker (yes/no) | 6/81 | 3/39 | 3/42 | 0.86 |
| Number of medications (n) | 5.7 ± 3.0 | 5.2 ± 2.8 | 6.1 ± 3.1 | 0.15 |
| Number on insulin (yes/no) | 15/73 | 7/34 | 8/39 | 0.78 |
| Metformin users (yes/no) | 64/24 | 30/11 | 34/13 | 0.93 |
| Metformin dosage (mg/day) | 1541 ± 666 | 1548 ± 646 | 1534 ± 693 | 0.93 |
| Metabolic health | ||||
| CRP | 2.1 (9.9) | 2.4 (9.9) | 2.1 (9.4) | 0.69 |
| HOMA2-IRb | 2.7 ± 1.1 | 2.7 ± 1.0 | 2.8 ± 1.2 | 0.55 |
| HbA1c (%) | 7.1 ± 1.1 | 6.8 ± 0.8 | 7.4 ± 1.3 | 0.03 |
| HbA1c (mmol/mol) | 54 ± 12 | 51 ± 9 | 57 ± 14 | 0.03 |
| Body composition | ||||
| Body weight (kg) | 89.0 ± 16.3 | 89.8 ± 16.0 | 88.3 ± 16.7 | 0.66 |
| BMI (kg/m2) | 31.3 ± 5.1 | 31.1 ± 4.8 | 31.4 ± 5.4 | 0.80 |
| Skeletal muscle mass (kg) | 30.6 ± 4.0 | 31.0 ± 4.5 | 30.3 ± 3.6 | 0.43 |
| Total fat mass (kg) | 31.1 ± 10.8 | 31.4 ± 10.4 | 30.9 ± 11.3 | 0.81 |
| VAT (cm2) | 214.6 ± 90.0 | 221.0 ± 88.3 | 209.0 ± 92.0 | 0.54 |
| Mid-thigh CSA (cm2) | 110.5 ± 24.0 | 111.6 ± 26.4 | 109.6 ± 21.9 | 0.70 |
| Mid-thigh muscle attenuation | 84.3 ± 2.3 | 84.3 ± 2.3 | 84.2 ± 2.4 | 0.79 |
Normally distributed data presented as mean ± SD. Non-normally distributed data presented as median (range). Difference between groups was assessed via one-way ANOVA. Duration of diabetes data were log-transformed before use with parametric statistics. CRP data required square-root transformation before use with parametric statistics. Categorical variables were assessed using a chi-square. Mid-thigh muscle attenuation is a measure of intramuscular lipid accumulation. Higher mid-thigh muscle attenuation index (unitless measure based on optical density gradient from image analysis of CT scans) indicates greater intramuscular lipid
PRT progressive resistance training group, SHAM sham-exercise training group, CRP C-reactive protein, HOMA2-IR Homeostatic Model of Assessment of Insulin Resistance 2, HbA1c glycated hemoglobin, BMI body mass index, CSA cross-sectional area, VAT visceral adipose tissue area
aThis excludes current smokers
b15 participants (7 PRT and 8 SHAM) were excluded from HOMA2-IR analyses due to insulin therapy
Associations between body composition and CRP at baseline
Results are presented in Table 2. As hypothesized, CRP was directly associated with body weight (r = 0.53, p < 0.0001) and BMI (r = 0.52, p < 0.0001) and FM (r = 0.59, p < 0.0001); but unexpectedly, only a trend was observed with VAT (r = 0.21, p = 0.10). SMM was directly associated with CRP (r = 0.33, p < 0.01); however, mid-thigh CSA was not associated with CRP (r = 0.03, p = 0.84).
Table 2.
Changes in CRP
| PRT group | SHAM group | f | β | p GxT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||||||
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | ||||
| CRP (mg/L) | 39 | 2.37 (9.88) | 30 | 1.60 (6.90) | 41 | 2.07 (9.43) | 39 | 1.84 (9.77) | 3.03 | −0.25 | 0.087 |
Data presented as median (range) of raw values. Difference between groups was assessed via linear mixed-effects modeling with an unstructured covariance matrix. Data required square-root transformation before use with parametric statistics
GxT group × time interaction, CRP C-reactive protein, PRT progressive resistance training, SHAM sham exercise control
A stepwise multiple regression model was then constructed with CRP entered as the dependent variable, and SMM and BMI entered as the independent variables, since both variables were related to CRP, and did not display colinearity (r = 0.51). In this model, only BMI was associated with CRP (r = 0.50, p < 0.0001), while SMM no longer showed any independent association (r = 0.08, p = 0.53). Body weight was unable to be used as this displayed colinearity with SMM (r > 0.7). Similarly, FM displayed colinearity with body weight and BMI (r > 0.7), and thus precluded the use of this variable in the model.
Changes in body composition
Data for the full cohort of participants has been previously reported here [12]. In line with our previously reported data, there was also no group × time interaction for changes in whole body SMM or FM within this subset with CRP data (data not shown). However, in contrast to the whole cohort, a significant group × time interaction was found within this subset for changes in mid-thigh CSA (β = 5.49, p = 0.03) and VAT (β = −19.14, p = 0.03) in favor of the PRT group.
Changes in CRP
Results are presented in Table 2. Using a linear mixed-effects model, a trend for a group × time interaction was present, with greater reductions in CRP in the PRT group relative to sham-exercise, as hypothesized (β = −0.25, p = 0.087).
Association between changes in body composition and changes in CRP
Results are presented in Table 3. Changes in body composition variables were entered as covariates, with a full factorial interaction with group and time to compare the effects of changes in body composition on CRP between the PRT and sham groups. In these models, increases in SMM in the PRT group were significantly associated with reductions in CRP compared to increases in SMM in the sham group (β = −0.23, p = 0.04). Similarly, reductions in FM tended to be significantly associated with reductions in CRP in the PRT group compared to reductions in FM in the sham group (β = 0.09, p = 0.07). No differences between the PRT and sham groups in terms of the relationship with CRP were observed with changes in body weight (β > −0.01, p = 0.94), BMI (f < 0.01, β = 0.01, p = 0.95), VAT (β < 0.01, p = 0.56), mid-thigh CSA (β = 0.01, p = 0.70), or mid-thigh muscle attenuation (β = −0.03, p = 0.73).
Table 3.
Changes in body composition variables and changes in CRP
| PRT group | SHAM group | SHAM-PRT group | ||||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Body weight (kg) | 0.01 | 0.82 | 0.01 | 0.70 | 0.00 | 0.94 |
| BMI (kg/m2) | 0.03 | 0.72 | 0.03 | 0.75 | 0.01 | 0.95 |
| Skeletal muscle mass (kg) | −0.16 | 0.06 | 0.07 | 0.34 | −0.23 | 0.04 |
| Total fat mass (kg) | 0.06 | 0.12 | −0.03 | 0.31 | 0.09 | 0.07 |
| VAT (cm2) | 0.00 | 0.71 | 0.00 | 0.64 | 0.00 | 0.56 |
| Mid-thigh CSA (cm2) | 0.00 | 0.90 | −0.01 | 0.63 | 0.01 | 0.70 |
| Mid-thigh muscle attenuation | 0.06 | 0.36 | 0.09 | 0.09 | −0.03 | 0.73 |
Mid-thigh muscle attenuation is a measure of intramuscular lipid accumulation. Higher mid-thigh muscle attenuation index (unitless measure based on optical density gradient from image analysis of CT scans) indicates greater intramuscular lipid
CRP C-reactive protein, BMI body mass index, CSA cross-sectional area, VAT visceral adipose tissue area
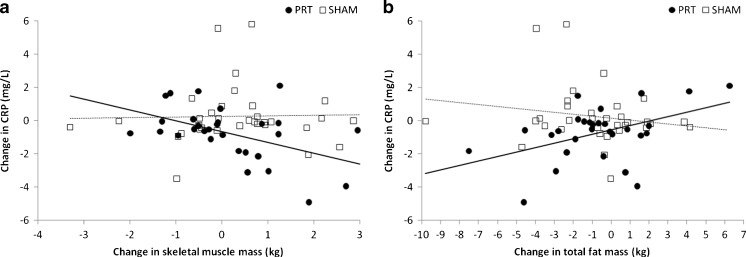
Due to the significant interaction noted above of group, time, and body composition change, we stratified by group assignment to explore the relationships within each group. Using univariate regression models, stratified by group allocation, a significant inverse association was found between changes in CRP and changes in SMM in the PRT group (r = −0.48, p = 0.01), but this was not seen in the sham-exercise group (r = 0.03, p = 0.87; Fig. 2a). Similarly, changes in CRP were directly related to changes in FM within the PRT group (r = 0.45, p = 0.02), but not in the sham group (r = −0.18, p = 0.32; Fig. 2b).
Fig. 2.
Changes in body composition vs. changes in CRP. CRP C-reactive protein. a Reductions in CRP were related to increases in skeletal muscle mass in the PRT group (closed circles, solid line; r = −0.48, p = 0.01) but not in the sham-exercise group (open squares, dashed line; r = 0.03, p = 0.87). Linear mixed-effects models showed that the relationship within the PRT group was significantly different to the relationship in sham-exercise group (β = −0.23, p = 0.04). b Reductions in CRP were directly related to reductions in total fat mass in the PRT group (closed circles, solid line; r = 0.45, p = 0.02) but not in the SHAM group (open squares, dashed line; r = −0.18, p = 0.32). Our linear mixed-effects models showed that the relationship within the PRT group tended to be significantly different to the relationship in sham-exercise group (β = 0.09, p = 0.07)
Discussion
We have shown that 12 months of PRT tended to reduce CRP compared to sham-exercise in older adults with type 2 diabetes. There is conflicting evidence from previous literature regarding the ability of PRT to reduce systemic inflammation [13], including recent trials in individuals with type 2 diabetes [14, 15]. However, for the first time, we have shown that reductions in CRP were mediated, in part, by increases in skeletal muscle following high intensity PRT. Similarly, reductions in FM tended to mediate the reduction in CRP in the PRT group. These results were further confirmed by our linear regression models stratified by group allocation (Fig. 2a, b), with significant associations only seen in the PRT group and not the sham group. Previously, we showed similar associations between increases in SMM and reductions in FM with insulin resistance and glucose homeostasis within the same cohort [12], and so the present study provides further evidence that PRT should be incorporated into treatment strategies for individuals with type 2 diabetes in order to improve their body composition, systemic inflammation, and their metabolic health.
The data in this study highlight the association between body composition and systemic inflammation in older adults with type 2 diabetes, and suggest that both increases in SMM and reductions in adiposity be targeted with lifestyle interventions within this cohort. This concept is further supported by findings that changes in CRP were not associated with changes in either body weight or BMI. This is in agreement with Balducci et al [15] who reported that 12 months of either aerobic training, or a combination of aerobic and PRT resulted in reductions in CRP independent of weight loss within individuals with type 2 diabetes. Concomitant increases in SMM and reductions in adiposity may result in minimal changes in body weight; however, given their dual positive effects on reducing CRP, reductions in systemic inflammation may be observed that are not associated with overall weight loss. This is also true for other metabolic outcomes within individuals with type 2 diabetes [16].
Association between skeletal muscle and CRP
The direct association between SMM and CRP at baseline within our cohort is in agreement with previous investigations reporting the cross-sectional relationship between systemic inflammation and SMM or lean body mass in other cohorts [17, 18]. The authors in prior studies concluded that lean tissue might in fact contribute to systemic inflammation. However, our analyses show that after adjusting for BMI, SMM is no longer associated with CRP. This is likely due to the direct relationship between SMM and BMI; with over nutrition and excess adiposity resulting in a higher quantity of SMM. Furthermore, our longitudinal data, in agreement with some but not all previous investigations [13] indicate that anabolic exercise such as PRT can reduce systemic inflammation. Furthermore, our linear mixed-effects models and linear regression models indicate that increases in SMM following PRT were associated with reductions in CRP, indicating that increasing lean tissue with anabolic exercise may potentially mediate reductions in systemic inflammation.
Given that CRP has been shown to predict the loss of SMM in older adults [7], the observed association between reductions in CRP with increases in SMM is of great significance. Previous data within a similar cohort showed no relationship between changes in lean body mass and changes in CRP following 4 months of PRT [19]. To our knowledge, ours is the only other study to investigate the relationship between changes in markers of systemic inflammation, and changes in lean tissue in a cohort of individuals with type 2 diabetes. Therefore, our results provide a significant and novel advance in knowledge in this field. A notable difference between our study and that of Brooks et al [19] is that in the latter, this relationship was explored between changes in CRP and changes in quadriceps muscle type I fiber cross-sectional area only, as opposed to whole body SMM within our investigation. It is possible that changes in whole body SMM are more reflective of changes in systemic inflammation. In agreement with this, increases in regional indices of SMM (CT scan) within our cohort showed no relationship with reductions in CRP. Further studies utilizing more precise whole body composition techniques such as dual-energy X-ray absorptiometry or multi-slice MRI scanning are required to further explore this relationship.
Association between adiposity and CRP
Figure 2b illustrates that reductions in CRP following PRT were related to reductions in fat mass. This is important, given the predictive value of CRP in CVD. Until recently, CRP was believed to be produced exclusively by hepatocytes, namely in response to circulating interleukin-6 (IL-6) [20]. However, production of CRP within subcutaneous and visceral adipose tissue has also been reported [21–23]. Thus, reductions in circulating CRP can be influenced both directly, and indirectly, by reductions in adipose tissue. The finding that CRP is produced by both subcutaneous and visceral adipose tissue supports our finding that reductions in FM (inclusive of both depots) predicted reductions in CRP, rather than VAT in isolation. In addition to adipose tissue, macrophages and smooth muscle cells from atherosclerotic plaques [24–26] and inflamed renal epithelial cells [27] have been shown to contribute to circulating levels of CRP. Adipose tissue is a major contributor to circulating cytokines, which could initiate or mediate local inflammatory responses (including CRP production) within these tissues [28]. Thus, reduced adiposity after PRT could have both directly and indirectly reduced circulating CRP.
The role of inflammation in adaptations to PRT
Given the known catabolic effects of systemic inflammation, and its role in anabolic resistance [29], our data suggest that reductions in CRP may allow more robust increases in SMM in response to PRT. However, our study does not prove that reductions in inflammation secondary to reduced adiposity preceded the skeletal muscle hypertrophy, and thus further investigations are needed to delineate the time course of these adaptations to PRT. An alternative pathway to CRP reduction after PRT is also possible. Skeletal muscle is known to secrete IL-6 in response to muscle contraction, which can bring about an anti-inflammatory environment by inducing the production of the anti-inflammatory cytokines IL-1ra and IL-10 [30]. Importantly, the magnitude of IL-6 production is dependent on the amount of skeletal muscle recruited [30, 31], which would be optimized by training with high speeds/volumes/intensities/numbers of muscle groups (such as our power training group). These circulating anti-inflammatory myokines may also potentially mediate the effects of PRT by suppressing CRP production within adipose and non-adipose tissues, thus contributing to reduced inflammation. These pathways to lower CRP (reduced fat mass and increased muscle contractile activity) may operate simultaneously and require further study to determine their relevance and importance in type 2 diabetes and other cohorts characterized by chronic systemic inflammation.
Anti-inflammatory efficacy of body composition changes may depend on how they are achieved
Reductions in FM following PRT were significantly associated with reductions in CRP, while reductions in FM following sham-exercise were not (Fig. 2b), despite similar reductions in FM between groups, as previously reported [12]. This suggests that not only is reduction in adipose tissue necessary for reduction in systemic inflammation, but the intervention chosen to mediate this body composition shift may play an important role. Other strategies to reduce adipose tissue, such as dietary changes, would not incorporate any potential therapeutic effect of skeletal muscle contraction. In addition, dietary restriction by itself may cause losses of muscle tissue [32] as well as adipose tissue. Thus, the benefits of fat loss could be attenuated or eliminated by the adverse effects of muscle loss and reduced contractile activity on overall inflammatory milieu.
It is also possible that combined strategies to reduce inflammation may be even more beneficial than any single intervention such as anabolic exercise. While our data suggest that reductions in CRP can be achieved through PRT-mediated reductions in adiposity, other interventions to reduce systemic inflammation, such as non-steroidal anti-inflammatory drugs [33], poly-unsaturated fatty acids [34, 35], or antioxidants could potentially enhance the anabolic response to PRT in older adults with type 2 diabetes.
Future directions, limitations, and conclusions
Most importantly, further prospective studies are needed to determine the nature of the relationship between CRP and SMM, which is potentially bi-directional. Elevated CRP may attenuate a robust response to anabolic stimuli, thus both leading to sarcopenia and impeding recovery from it. Alternatively, sarcopenia/dynapenia and the reduced contractile activity accompanying this condition could reduce anti-inflammatory myokine release into the circulation and thus contribute to an overall enhancement of systemic inflammatory profile. Understanding of these relationships may improve targeting of individuals likely to require augmentation of standard PRT regimens to enhance body composition and metabolic outcomes in clinical cohorts.
In conclusion, we have demonstrated that 12 months of PRT reduced systemic inflammation in older adults with type 2 diabetes, mediated through improvements in body composition, relationships which were not seen with sham exercise. The significant associations between changes in CRP and changes in FM and SMM after PRT indicate that both body composition compartments should be targeted in evidence-based lifestyle interventions aimed at maximally reducing systemic inflammation in older adults with type 2 diabetes. It is time that the insufficient goal of “weight loss” be replaced with “muscle gain and fat loss” in overweight/obese cohorts if optimal metabolic health is to be achieved.
Acknowledgements
We would like to thank Freshwater Health and Fitness for the use of their gym facilities, Keiser Sports Health, Ltd. for donations of resistance training equipment, and participants for their generosity of time and spirit. The Graded Resistance Exercise and Type 2 Diabetes in Older adults (GREAT2DO) study was funded by project grant #512381 from the National Health and Medical Research Council (NHMRC), and grants from The Australian Diabetes Society and Diabetes Australia. Y. Mavros was supported by the Australian Postgraduate Award Scholarship. Y. Wang was supported by University of Sydney International Postgraduate Research Scholarship. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
Conflict of interest
Yorgi Mavros, Shelley Kay, Kylie A Simpson, Michael K Baker, Yi Wang, Ren Ru Zhao, Jacinda Meiklejohn, Mike Climstein, Anthony J O’Sullivan, Nathan de Vos, Bernhard T Baune, Steven N Blair, David Simar, Kieron Rooney, Nalin A Singh, and Maria A Fiatarone Singh declare that they have no conflict of interest.
Author contribution statement
Y.M. assisted with data collection, participant training, primarily analysed the data, and wrote the manuscript. S.K, R.Z, J.M, and N.DV, assisted with participant training. K.R, D.S, M.K.B, A.J.O’S, N.A.S and B.T.B assisted with data analysis, contributed to the discussion and edited the manuscript. K.A.S and Y.W assisted data collection. M.C and S.N.B reviewed and edited the manuscript. M.A.F S supervised data analyses, supervised and assisted data collection, contributed to the discussion and reviewed, and edited the manuscript. Y.M and M.A.F S are the guarantors of this work and, and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD). All authors had final approval of the version to be published.
Author agreement and consent
All authors have read and consent the publication of this manuscript. The manuscript has not been submitted for publication elsewhere.
Abbreviations
- SMM
Skeletal muscle mass
- FM
Total fat mass
- BMI
Body mass index
- VAT
Visceral adipose tissue area
- CSA
Cross-sectional area
- CRP
C-reactive protein
- PRT
Progressive resistance training
- SHAM
Sham-exercise training group
- BIA
Bioelectrical impedance assessment
- CT
Computed tomography
- RCT
Randomized controlled trial
- HbA1c
Glycated hemoglobin
Footnotes
Australian New Zealand Clinical Trials Registry Number
ANZCTRN 12606000436572 www.anzctr.org.au
References
- 1.Festa A, D’Agostino R, Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.CIR.102.1.42. [DOI] [PubMed] [Google Scholar]
- 2.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four U.K. prospective cohort studies. Diabetes Care. 2012;35:396–403. doi: 10.2337/dc11-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.CIR.0000053730.47739.3C. [DOI] [PubMed] [Google Scholar]
- 5.Lapice E, Maione S, Patti L, Cipriano P, Rivellese AA, Riccardi G, et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care. 2009;32:1734–1736. doi: 10.2337/dc09-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.ATV.19.4.972. [DOI] [PubMed] [Google Scholar]
- 7.Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (Sarcopenia) and strength. Am J Med. 2006;119:526.e9-e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 9.Lukaski HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol. 1986;60:1327–1332. doi: 10.1152/jappl.1986.60.4.1327. [DOI] [PubMed] [Google Scholar]
- 10.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 11.Martin Bland J, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 12.Mavros Y, Kay S, Anderberg KA, Baker MK, Wang Y, Zhao R, et al. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with Type 2 Diabetes: interim outcomes from the GREAT2DO Trial. Diabetes Care. 2013 doi: 10.2337/dc12-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Salles BF, Simao R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E. Effects of resistance training on cytokines. Int J Sports Med. 2010;31:441–450. doi: 10.1055/s-0030-1251994. [DOI] [PubMed] [Google Scholar]
- 14.Jorge MLMP, de Oliveira VN, Resende NM, Paraiso LF, Calixto A, Diniz ALD, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60:1244–1252. doi: 10.1016/j.metabol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608–617. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Boulé N, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 17.You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89:5517–5522. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 18.Brochu M, Mathieu ME, Karelis AD, Doucet É, Lavoie ME, Garrel D, et al. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: a Monet study. Obesity. 2008;16:1085–1093. doi: 10.1038/oby.2008.23. [DOI] [PubMed] [Google Scholar]
- 19.Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4:19–27. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bataille RÉG, Klein B. C‐reactive protein levels as a direct indicator of interleukin‐6 levels in humans in vivo. Arthritis Rheum. 2005;35:982–983. doi: 10.1002/art.1780350824. [DOI] [PubMed] [Google Scholar]
- 21.Calabro P, Chang DW, Willerson JT, Yeh ETH. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112–1113. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Anty R, Bekri S, Luciani N, Saint-Paul MC, Dahman M, Iannelli A, et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am J Gastroenterol. 2006;101:1824–1833. doi: 10.1111/j.1572-0241.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.CIR.0000055188.83694.B3. [DOI] [PubMed] [Google Scholar]
- 24.Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158:1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabs WJ, Theissing E, Nitschke M, Bechtel JFM, Duchrow M, Mohamed S, et al. Local generation of C-reactive protein in diseased coronary artery venous bypass grafts and normal vascular tissue. Circulation. 2003;108:1428–1431. doi: 10.1161/01.CIR.0000092184.43176.91. [DOI] [PubMed] [Google Scholar]
- 26.Calabró P, Willerson JT, Yeh ETH. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–1932. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 27.Jabs WJ, Lögering BA, Gerke P, Kreft B, Wolber EM, Klinger MHF, et al. The kidney as a second site of human C‐reactive protein formation in vivo. Eur J Immunol. 2002;33:152–161. doi: 10.1002/immu.200390018. [DOI] [PubMed] [Google Scholar]
- 28.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 29.Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the ‘anabolic resistance’of ageing. Nutr Metab. 2011;8:68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen B. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25:811–816. doi: 10.1016/j.bbi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Morley JE, Chahla E, AlKaade S. Antiaging, longevity and calorie restriction. Curr Opin Clin Nutr Metab Care. 2010;13:40–45. doi: 10.1097/MCO.0b013e3283331384. [DOI] [PubMed] [Google Scholar]
- 33.Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–5492. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fetterman JW, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009;66:1169–1179. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 35.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, et al. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci. 2011;121:267–278. doi: 10.1042/CS20100597. [DOI] [PMC free article] [PubMed] [Google Scholar]