Abstract
Estrogens are the primary female sex hormones and play important roles in both reproductive and non-reproductive systems. Estrogens can be synthesized in non-reproductive tissues such as liver, heart, muscle, bone and the brain. During the past decade, increasing evidence suggests that brain estrogen can not only be synthesized by neurons, but also by astrocytes. Brain estrogen also works locally at the site of synthesis in paracrine and/or intracrine fashion to maintain important tissue-specific functions. Here, we will focus on the biology of brain estrogen and its impact on cognitive function and Alzheimer’s disease. This comprehensive review provides new insights into brain estrogens by presenting a better understanding of the tissue-specific estrogen effects and their roles in healthy ageing and cognitive function.
Keywords: brain estrogen, cognition, Alzheimer’s disease
Introduction
The sex differences in brain structure and activities are confirmed by both human and animal studies from early developmental stages throughout their life spans. In addition, many neurological diseases exhibit sex differences, including risk factors [1], incidence [2], and severity of neuropathology [3]. In both genders, epidemiological studies show increased risk of Alzheimer’s disease (AD) with the age-related loss of sex steroid hormones, while AD is more prevalent in postmenopausal women than in age-matched men [4]. The sharp decline of ovarian estrogens and progestogens after menopause has been presumed to account for the increased female susceptibility to AD, but recent studies in both animals and humans suggest that depletion of brain-derived, rather than circulating, estrogen is a more direct and significant risk factor [5]. For example, a reduction of brain estrogen levels in postmenopausal women may associate with a higher risk of developing AD compared to age-matched men, while lower incidences of Parkinson’s diseases were found in reproductive females [6; 7; 8; 9; 10; 11]. However, the effects of hormone replacement therapy on cognitive function and risk of AD have been controversial. Although estrogen has many effects in the brain, such as neuroprotective [12; 13], neurotrophic, neurogenerative, antioxidative [14; 15] and anti-inflammatory [16; 17; 18], pro-inflammatory and other potential neurotoxic actions of estrogen have also been reported with various timing and context [19; 20; 21; 22]. Here, we will be focusing on brain estrogen in cognitive functioning during ageing and AD, with an emphasis on sex differences.
Brain estrogen synthesis
Estrogens are the primary female sex hormones and play important roles in female sexual development and regulation of the menstrual cycle. In addition to the well-established functions of estrogens in female reproduction, studies demonstrated significant non-reproductive functions of estrogens in the regulation of lipid and carbohydrate metabolism, skeletal homeostasis, the cardiovascular system, and the central nervous system (CNS) in both males and females [23; 24; 25; 26; 27; 28; 29].
Estrogen can be synthesized locally in the brain, which provides the unique importance of brain estrogen function. Studies showed that the brain is able to synthesize estrogen de novo from cholesterol [30]. Indeed, all enzymes required for the synthesis and metabolism of estrogen, and critical intermediate metabolites, were reported to exist in various regions of the human brain. In particular, aromatase, a key enzyme for the last step in the synthesis process, is widely expressed in the brain with a regional-specific manner in humans, rodents and primates [31; 32; 33; 34; 35]. For example, aromatase mRNA was first time reported in the rat hippocampus by Abdelgadir in 1994 [36]. Since then, more studies have showed that this enzyme is in fact released from hippocampal neurons and is functional in this part of the brain in both human and animals [37; 38; 39; 40; 41; 42]. Recent studies using positron emission tomography (PET) imaging with radiolabeled aromatase inhibitors have allowed a general map of the human brain aromatase distribution to be drawn. The highest level of aromatase was found in the thalamus, followed by the amygdala, preoptic area and medulla oblongata. Significant levels of aromatase were also observed in the temporal and occipital cortices, hippocampus, basal ganglia, cerebellum, pons and white matter [43; 44; 45; 46; 47; 48]. With respect to brain cell type specificity, aromatase in the human brain is mainly produced by neurons, but there is also an astrocyte subpopulation that constitutively expresses the enzyme [49; 50; 51]. As shown in figure 1, estradiol can either be produced from circulating testosterone by the local estrogen synthase aromatase, or be synthesized de novo from cholesterol, by neurons or astrocytes, but not by microglia and oligodendrocytes [41; 51; 52; 53; 54; 55; 56; 57]. It is important to notice that the neuron is the major site for brain estradiol synthesis. In the normal physiological condition, only a few astrocytes in the brain express aromatase while aromatase expression is increased in reactive astrocytes due to various brain injuries [58]. Although tissue-specific promoters as well as first exons have been reported in human aromatase gene [59; 60], it is unknown whether the usage of specific promoters of the aromatase gene can be translated into the level of brain region-specific expression. Such knowledge is important to develop selective aromatase modulators to regulate brain region-specific estrogen synthesis including in the human brain. Studies have shown that the cell type and brain region-specific aromatase expression may directly alter the local estradiol production and specific brain functions [61]. For example, estrogen locally produced in the synapse is reported to regulate synaptogenesis [40], neurotransmission [62] and synaptic plasticity [63; 64]. Furthermore, the synthesis, physiological roles, and regulations of brain estradiol are more or less distinct from ovarian estrogen. For example, mice with depletion of aromatase showed increased brain damage in an ischemic model compared to the wild type littermates or ovariectomized mice [65]. Depletion of aromatase in an animal model for AD caused early and more severe neuropathology than the ovariectomized control mice [5] and responded better to estrogen replacement treatment than in ovariectomized control mice [66]. In addition, studies showed that the fast effect of estradiol on neuronal spin density and synaptic transmission is more mediated through local synthesized estradiol in the developing and adult rat hippocampus [67].
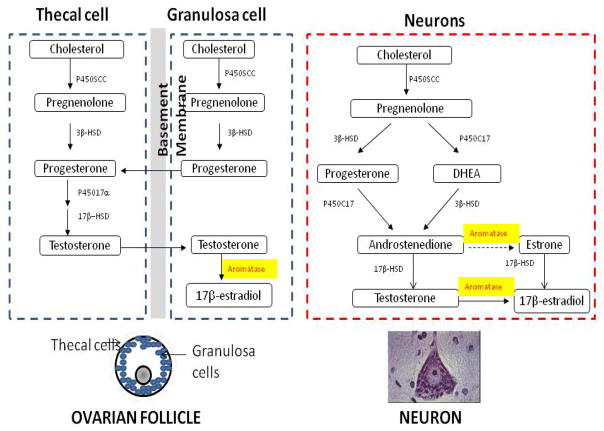
Fig. 1. Estrogen synthesis in the ovary and brain.
(A) Cell-specific estrogen synthesis in the ovary. Production of estrogens starts with the synthesis of pregnenolone from cholesterol, catalyzed by the cytochrome P450 side-chain cleavage enzyme (P450scc). Pregnenolone is then converted to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD) in both thecal and granulosa cells. Progesterone is converted to androgens via cytochrome P450 17α-hydroxylase (P45017α) and 17β-hydroxysteroid dehydrogenase (17β-HSD) in thecal cells during the follicular phase. The conversion of E2 is catalyzed by aromatase (P450Arom) in granulosa cells. (B) Neurons express all enzymes required for estrogen synthesis to produce brain estrogen.
Taken together, the brain produces its own estrogen while circulating estrogen and C19 steroid precursors (substrate for estrogen synthesis) can also penetrate through the blood-brain barrier into the brain and provide the essential substrates for estrogen synthesis in the CNS [68]. In the case of the human brain, three C19 androgen precursors, such as 16α-OH-DHEA, androstenedione and testosterone, have been identified [69; 70; 71]. Therefore, the level of brain estrogen might not be the same level as the blood estrogen which is often used as diagnostic marker in clinic [72; 73; 74; 75]. It is worth to point out that brain estrogen local synthesis and activity becomes more independent from circulating estrogen in non-reproductive females, such as surgical or natural menopausal women. For example, in an earlier study we showed that women with AD express lower activity of aromatase in the brain than that in age-matched women without AD [5]. Therefore, maintaining brain estrogen synthesis during ageing might be critically important for normal cognitive function, as well as for reduction of risk for AD in females
Brain estrogen biology and cognition
A lot of evidence has documented profound effects of estrogens on learning, memory, mood as well as neurodevelopmental processes [12; 76; 77; 78]. Although estrogen is a female hormone, increasing studies have shown that estrogen also plays important roles in the male brain partially due to the converting of testosterone to estrogen by brain aromatase [45; 46; 47; 79]. However, it is well documented that men and women have different types of cognitive function, and the sex differences in cognition are associated with brain estrogen and testosterone in the regions important for cognition, memory and mood such as the cortex, hippocampus and amygdala [80; 81; 82]. In addition, as reported by extensive studies, most adulthood mental disorders begin in childhood and adolescence [83]. Therefore, understanding the effect of sex hormones on early neurological development, especially on the cognition related brain regional development, would be important for developing further strategies for preventing sex-related neurodegenerative disease, such as AD.
As early as prenatal period development, once the Y chromosome (SRY) gene forms the fetal testes, sexual differentiation of the brain is a sex hormone dependent process [84; 85]. Studies in rats showed that circulating levels of sex hormones (mainly testosterone) during a short critical developmental window (between embryonic day 18 and 10 days after birth) caused permanent sex-dependent changes in brain morphology and functions [86]. Interestingly, the roles of testosterone in sex differentiation during brain development might be mediated by its metabolite estradiol, a conversion process catalyzed by neuronal aromatase [87]. This suggests that the vertebrate brain is organized in a sex-dependent fashion under the control of perinatal gonadal steroid hormones, such as estrogen or testosterone [88]. While sex hormones are present during early development and are well known to have permanent effects on sex-related behaviors in human and various animals, recent work has renewed interest in puberty as another organizational period for the effects of sex hormones. For example, late maturing boys and girls had higher spatial abilities than their early maturing counterparts [89]. Furthermore, studies reported that a longer reproductive period was associated with better cognition in later life, including verbal fluency in females [90]. The sex hormone-dependency of brain regional development has been further supported by the study in girls with partial or complete loss of their X-chromosome, which showed disproportionately reduced hippocampal volume, and increased amygdala volume relative to age-matched controls [91]. The estrogen-related brain regional volume reduction in females with endogenous estrogen deficiency is further evidenced by the impairment of executive performance on the task as reported by other groups of researchers [92]. In animal studies, female mice exposed to stress in early adolescence showed more disturbed sexual behaviors than those exposed to stress in late adolescence, emphasizing the importance of timing of exposure even within adolescence [93]. Finally, gender differences in cognitive function have been well demonstrated in adulthood as well as ageing. For example, men demonstrated larger amygdala and thalamus volumes compared to women [94; 95; 96], whereas the size of the hippocampus is larger in females compared to males [94; 97]. It is also worth noticing that there are a relatively higher number of androgen receptors in the amygdala [98] and a relatively higher number of estrogen receptors in the hippocampus [99].
The sex differences in regional brain structure might be responsible for the sex differences in specific cognitive and behavioral tasks [80; 81; 100]. For example, males usually outperform females on visuospatial, quantitative, and targeted motor tasks, whereas females perform better on verbal and perceptual tasks [101; 102]. Furthermore, both human and animal studies showed that administration of androgens to females may induce male-typical cognition and behavior, and the male-type cognition disappearing when the treatment was withdrawn [80; 103]. For example, one recent cross-sectional human study showed that women with polycystic ovary syndrome characterized by elevated endogenous testosterone performed significantly better at three-dimensional mental rotation, a male-favored cognitive behavior, than a female control group [104]. Another study also demonstrated that a single administration of testosterone improves 3D mental rotation abilities in young women [105]. The sex hormone-specific action on cognition is also demonstrated by animal studies, such as testosterone treatment in aged females mice, which enhanced spatial retention in water mazes (male-typical cognition). However, there was no significant improvement in object recognition (female-typical cognition) compared to the placebo treated group [106].
In congruence with brain structure studies, the estrogen action in cognition is also related to the brain’s regional activities. Recent animal studies showed that endogenous estrogen levels changed by reproductive experience in females are associated with enhanced hippocampus-dependent memory [107; 108]. Furthermore, sex steroids have been linked to many of the mechanisms thought to be associated with cognitive decline and several types of dementia. A recent review by Hogervorst [109] reported that women who underwent surgical menopause or had menopause before 47 years old without hormone treatments had an increased risk for global cognitive impairment and dementia in later life, suggesting that earlier menopause is associated with a higher risk for cognitive impairment. Moreover, a new study reported that 17β-estradiol increased neurogenesis in the hippocampus and increased activation of new neurons in response to spatial memory retrieval, while estrone decreased cell survival and had no significant effect on the activation of new neurons [110]. It is known that hippocampal neurogenesis is linked to spatial learning and memory. Estrogen not only modulates neurogenesis in the hippocampus, but also modulates hippocampus-dependent learning and memory across a variety of species from rodents to primates [111]. For instance, studies of object-in-place tasks combined with object recognition and object location showed that the ovariectomized female rats treated with estradiol and progesterone were able to discriminate between moved and unmoved objects while ovariectomized vehicle-treated females and gonadally intact males did not, suggesting that female rats outperform males only when levels of estrogen and progesterone are elevated [112]. However, the interaction between sex hormones and brain regional-driven cognition might be more complicated than previously thought. A longitudinal human study of 10 males and 7 females for 8 weeks showed that both genders showed significant sex hormone related cycle effects in mental rotation performance only at the beginning of the study, while at the end of the study, none of the hormones were significantly related to performance. Thus, the relationship between hormones and certain cognitive activity, such as mental rotation performance, disappeared with repeated testing [113]. Furthermore, a cross-sectional analysis of the association between sex hormones, metabolic parameters, and psychiatric diagnoses with verbal memory in healthy aged men showed that higher levels of serum sex hormone binding globulin (SHBG) were associated with a worse verbal memory [114], suggesting that levels of free testosterone influence male verbal memory.
Indeed, the concept that brain estrogen regulates cognitive function independent from circulating estrogen levels is also supported by more evidence. For instance, serum concentrations of estradiol are much lower than estradiol concentrations in the male rat hippocampus [46], suggesting a locally synthesized estradiol may contribute even stronger to the maintenance of structural synaptic plasticity than estradiol originating from the gonads [40]. The brain estrogen theory is also supported by cell culture studies which showed that axonal outgrowth in hippocampal neurons only occurs when estradiol concentrations are higher than physiological serum levels [42].
Brain estrogen and AD
AD is the most common cause of dementia in the elderly, with women being at a higher risk for AD even after controlling for an increased life span [115; 116]. Women not only have a higher risk of developing AD than age-matched men, supported by higher numbers of female AD patients than male patient with AD [4; 117; 118; 119], but also showed significantly age-related faster decline and greater deterioration of cognition than elderly male [120; 121; 122].
Several studies showed that AD patients face more problems in anterograde episodic memory than frontotemporal lobar degeneration (FTLD) patients [123; 124; 125; 126]. Others reported that both AD and FTLD dementia groups did not differ in their verbal and forgetting rates, besides a potentially worse visual memory in the AD group compared to the FTLD group [127]. Mild cognitive impairment (MCI) is a clinical condition often associated with early stages of AD. A study of 425 MCI patients showed that early onset MCI patients suffer more visuospatial memory impairment, while individuals with late onsets of MCI showed more verbal memory problems [128]. Moreover, other studies also reported no significant differences in the demographic characteristics of patients with mixed dementia and AD, where patients with mixed dementia were significantly more impaired than AD patients in global cognitive composite, attention and visuoconstruction [129]. Other cognitive measurements have also been used in MCI and AD. For example, an antisaccade task is a measurement for executive functions and is correlated with AD-sensitive cortical regional thickness in MCI subjects, but not in normal elderly patients as reported by Heuer [130]. A study using a new cognitive test in 97 mild AD/MCI and 200 control subjects demonstrated a significant detection of mild AD/MCI who “pass” the MMSE from controls [131]. Although females have a high prevalence of AD, there are no clear evidence showing that whether there are gender differences in MCI or FTLD. For example, one study showed that more men develop MCI than women [132], while other studies showed that the incidence or pathology of MCI did not differ by sex or education [133; 134]. While FTLD has a strong genetic basis, studies found equal heritability for both sexes regardless of parental gender [135], as well as no differences in sex distribution in FTLD in different countries [136; 137].
There are two major pathological lesions in AD brain: intracellular inclusions of tau protein in the form of neurofibrillary tangles, and extracellular plaque formation by accumulations of amyloid beta peptide (Aβ) derived from the β-amyloid precursor protein (APP). Accumulating Aβ, a proteolytic byproduct of amyloid precursor protein (APP) metabolism, is an important aspect of AD pathology. APP is processed by two competing pathways, the amyloidogenic pathway through β-secretase (BACE1) and γ-secretase, which produce β-APPs and Aβ40/Aβ42 peptides, and the predominant non-amyloidogenic pathway via α-secretase, which produces neuroprotective α-APPs and several non-amyloidogenic peptides [138]. The sex-specific neuropathology in AD has been reported by neuroimaging and postmortem human studies, such as men with AD have more pronounced pathology in the right hemisphere, whereas women with AD often have more manifested pathology than male AD patients [139; 140].
Compared to age-matched controls, reduced circulating levels of 17β-estradiol and testosterone are observed in female and male patients with AD, respectively [116; 141], suggesting a potential important role of sex hormones in the epidemiology and pathology of AD. With an increase in human longevity, women spend an average of one third of life without sufficient endogenous estrogens due to the significant reduction of endogenous estrogen after menopause. Estrogen is known to have a protective effect on the brain, and loss of estrogen during menopause could, in part, lead to the deficits seen in brain metabolism (mitochondrial impairment) in AD [142]. Estrogen also plays critical roles in neurogenesis. As reviewed by Galea [111], E2 can increase neurogenesis in various brain regions such as dentate gyrus of hippocampus, and these newly generated neurons in the hippocampus contribute to region-specific learning and memory. Studies showed hippocampal atrophy in females with MCI, suggesting an important role for estrogen in the association between cognitive function and hippocampus [143]. The brain region-specific effects of estrogen have also been confirmed by recent studies, which further highlighted the potent effects of estrogens on neuronal morphology and plasticity in area CA1 of hippocampus [63; 67; 144; 145; 146; 147]. As reviewed by Brinton ([148] and references therein), the beneficial effects of estrogen on neural plasticity occur at cellular, morphological, and synaptic levels. E2 can increase neurogenesis in various brain regions such as dentate gyrus of hippocampus, and these newly generated neurons in the hippocampus contribute to region-specific learning and memory. In addition, E2 can rapidly increase dendritic spine numbers or dendritic spine contacts in the hippocampus, the medial amygdala and hypothalamus, thus enhancing hippocampal-dependent memory in monkeys. E2 is also a potent and efficacious mediator of synaptic transmission in hippocampal system. Together, E2 promotes neurogenesis and neuronal plasticity to maintain healthy cognitive function and protect against cognitive decline in females during aging.
Another important neuroprotective role of estrogens in AD is that estrogen could reduce Aβ levels, or prevent them from rising, in the presence of pathological triggers [149]. Estrogens can also reduce Aβ production by favoring the non-amyloidogenic pathway through MARK/ERK activation, reducing BACE1 levels, and promoting Aβ clearance by stimulating microglial phagocytosis and degradation and regulating the levels of major enzymes involved in Aβ degradation [150]. Estrogens can also be protective by regulating the Bcl-2 protein family, increasing the expression of antiapoptotic Bcl-xL and Bcl-w and suppressing expression of proapoptotic Bim to prevent neuronal loss from Aβ-mediated toxicity [151]. Decreased levels of hyperphosphorylated tau (a major component of neurofibrillary tangles) can also be mediated by estrogens through kinases and phosphatases, such as the GSK-3β, Wnt, or PKA pathways [152].
Although the precipitous loss of ovarian estrogens at menopause has been presumed to account for the increased female susceptibility to AD, the role of this peripheral estrogen pool is still disputed [153; 154; 155; 156]. The brain is a sex hormone-responsive tissue and is affected by age related loss of estrogen [5; 66]. As shown in table 1, studies of brain aromatase expression and polymorphisms as well as direct measurement of estradiol levels in the brain of AD suggest that brain estrogen deficiency may increase risk of AD. The hypothesis of brain estrogen deficiency increases risk of AD is further supported by animals studies which showed that genetic eliminating aromatase in the female AD transgenic mice caused early and more severe AD pathology then control mice [5]. Based on analyses of brain estrogens, particularly E2, in the postmortem brain samples from female AD patients and age-matched healthy subjects, studies demonstrated a great reduction of estrogen levels and estrogen biosynthesis in AD brains while same serum estrogen levels was found in both AD and controls, suggesting that brain estrogen deficiency, not circulating E2, is associated with AD pathology [5]. Furthermore, one study also reported a reduction of estradiol in the CSF from AD patients and provided further confirmation of important role of brain estrogen in AD [157]. The differences in brain estrogen level between female AD and health individuals provide some level of explanation on why only 13–15% aged females developed AD, even though every woman lost sufficient endogenous estrogen after menopause in advanced age. In accordance with the downregulation of brain estrogen level, the changes of sex steroid (i.e. estrogens, androgens and progesterone) biosynthesis pathways in human prefrontal cortex have also been reported during the course of AD. For example, brain aromatase is expressed in AD-relevant regions and downregulated in AD [50; 158; 159]. The brain estrogen deficiency in AD is also confirmed by several studies which showed a downregulation of brain aromatase expression in the AD hippocampus [158; 159; 160]. However, in the prefrontal or temporal cortex of AD patients, the expression of aromatase mRNA was found elevated in the astrocyte [50] and no changes in the neurons [161], suggesting a cell type and regional specificity of brain estrogen synthesis. A reduction of brain aromatase immunoreactivity is also identified in the hippocampus of AD patients [159], but not in patients affected by epilepsy [57]. In contrast, during normal ageing, a clear increase in aromatase immunoreactivity has been reported in the basal forebrain [158], suggesting an age-related elevation of brain specific estrogen synthesis as a compensation of circulating estrogen reduction in normal postmenopausal women. In concert to the theory that maintaining healthy brain estrogen level is critical for preventing AD in females, studies reported that APP transgenic mice with low brain estrogen developed earlier, and with more severe AD pathology than ovariectomized APP mice [5]. Furthermore, a greater and better neuroprotective response from estrogen treatment was found in APP mice with brain estrogen deficiency compared to in the ovariectomized APP mice [66]. Together, evidences suggest an important role of brain estrogen in female risk of developing AD.
Table 1.
| Authors | year | Sample type | Sample size | Findings |
|---|---|---|---|---|
| Brain aromatase expression in AD | ||||
| Ishunina et al. [158] | 2005 | Human hypothalamus and basal brain | AD = 25 Control = 25 |
Lower aromatase immunoactivity in AD vs controls, except in NBM |
| Ishunina et al. [159] | 2007 | Human hippocampus | AD = 6 Control = 7 |
Lower aromatase mRNA expression in AD vs controls |
| Bulter et al. [160] | 2010 | Human hippocampus | AD = 207 MCI = 23 Control = 233 |
Lower aromatase mRNA expression in AD vs controls |
| Luchetti et al. [50] | 2011 | Human prefrontal cortex | AD = 14 MCI = 14 Control = 21 |
Higher astrocyte aromatase mRNA expression in AD vs. control |
| Wozniak et al. [161] | 1998 | Human frontal and temporal cortex | AD = 18 Control = 13 |
No differences in aromatase activity between AD and control |
| Aromatase polymorphisms in AD | ||||
| Livonen et al. [167] | 2004 | human | AD = 394 Control = 469 |
3 aromatase SNPs are associated with AD in women only |
| Medway et al. [168] | 2013 | human | AD =1757 Control = 6294 |
1 aromatase SNP is associated with AD in women only |
| Janicki et al. [169] | 2013 | human | AD + control = 1686 | 6 aromatase SNPs are associated with AD in women |
| Brain estrogen in AD | ||||
| Schonknecht et al. [157] | 2001 | Human CSF | AD = 30 Control = 11 |
Lower estradiol levels in AD vs controls |
| Yue et al. [5] | 2005 | Human brain | AD = 9 Control = 10 |
Lower estradiol level in AD vs controls |
| Rosario et al. [170] | 2011 | Human brain | AD = 32 Control = 12 |
Lower estradiol level in AD vs controls |
The concept of brain estrogen regulating cognitive function is also partially supported by clinical studies. For example, aromatase inhibitors (AIs) such as exemestane, anastrozole or letrozole, have been used to treat breast cancer. Studies showed that anastrozole treatment impaired processing speed and verbal memory in postmenopausal women with breast cancer in comparison with healthy women without the anastrozole treatment, or breast cancer patients treated with non AIs [162; 163]. However, AI users showed no significant decline of cognitive function compared to non-AI users in breast cancer patients were reported from a number of other studies, including a later study of anastrozole showing no impairment to cognitive performance [164; 165]. It is worth to note that selective estrogen receptor modulators (SERMs) are also frequently used as an endocrinal therapy to suppress estrogen for breast cancer patients. While the clinical data on the roles of SERMs in cognitive function are not conclusive, some studies suggest that tamoxifen administration may form a risk for cognitive functioning particularly in older women [166]. Since SERMs interfere with ER signaling, one could hypothesize that estrogen receptors are potentially involved in the SERMs-induced cognitive impairment.
Conclusion
Although the roles of estrogens in gonadal organs are well understood, recent studies have begun to demonstrate that localized estrogen production plays tissue-specific roles, with or without dependency on circulating estrogen. Although it is still unclear whether estrogen replacement therapy is beneficial for cognitive function and preventing AD in postmenopausal women, we believe that estrogens, especially brain estradiol, are no longer just sex hormones, but also important therapeutic targets for preventing brain disorders and neurodegeneration.
Highlights.
Neurobiology of sex-type spatial memory from young to old
Sex differences in prevalence of Alzheimer’s disease
Potential protective role of sex hormones in Alzheimer’s disease
Acknowledgments
This work was supported by the American Health Assistance Foundation (G2006-118), and the National Institutes of Health (R01AG032441-01 and R01AG025888). We also thank Juliet Shen for editing and proofreading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azad NA, Al Bugami M, Loy-English I. Gender differences in dementia risk factors. Gend Med. 2007;4:120–9. doi: 10.1016/s1550-8579(07)80026-x. [DOI] [PubMed] [Google Scholar]
- 2.Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177–83. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–98. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vina J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis. 2010;20(Suppl 2):S527–33. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- 5.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–7. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Amaducci LA, Schoenberg BS. Epidemiology of clinically diagnosed Alzheimer’s disease. Ann Neurol. 1986;19:415–24. doi: 10.1002/ana.410190502. [DOI] [PubMed] [Google Scholar]
- 8.Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MM. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22:575–80. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 9.Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 2000;55:1358–63. doi: 10.1212/wnl.55.9.1358. [DOI] [PubMed] [Google Scholar]
- 10.Marder K, Tang MX, Mejia H, Alfaro B, Cote L, Louis E, Groves J, Mayeux R. Risk of Parkinson’s disease among first-degree relatives: A community-based study. Neurology. 1996;47:155–60. doi: 10.1212/wnl.47.1.155. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–22. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 12.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiocchetti M, Ascenzi P, Marino M. Neuroprotective effects of 17beta-estradiol rely on estrogen receptor membrane initiated signals. Front Physiol. 2012;3:73. doi: 10.3389/fphys.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta. 2010;1800:1113–20. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Numakawa T, Matsumoto T, Numakawa Y, Richards M, Yamawaki S, Kunugi H. Protective Action of Neurotrophic Factors and Estrogen against Oxidative Stress-Mediated Neurodegeneration. J Toxicol. 2011;2011:405194. doi: 10.1155/2011/405194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004;50:1967–75. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 17.Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC. Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. J Immunol. 2008;180:630–6. doi: 10.4049/jimmunol.180.1.630. [DOI] [PubMed] [Google Scholar]
- 18.Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol. 2010;184:5029–37. doi: 10.4049/jimmunol.0903463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104:1404–10. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 20.Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab. 2011;22:467–73. doi: 10.1016/j.tem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2010;31:1618–28. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30:6852–61. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Zhang Y, Brockman DA, Hahn W, Bernlohr DA, Chen X. Lipocalin 2 deficiency alters estradiol production and estrogen receptor signaling in female mice. Endocrinology. 2012;153:1183–93. doi: 10.1210/en.2011-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saengsirisuwan V, Pongseeda S, Prasannarong M, Vichaiwong K, Toskulkao C. Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism. 2009;58:38–47. doi: 10.1016/j.metabol.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol. 2008;252:68–80. doi: 10.1016/j.cellimm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Stice JP, Lee JS, Pechenino AS, Knowlton AA. Estrogen, aging and the cardiovascular system. Future Cardiol. 2009;5:93–103. doi: 10.2217/14796678.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohlsson C, Vandenput L. The role of estrogens for male bone health. Eur J Endocrinol. 2009;160:883–9. doi: 10.1530/EJE-09-0118. [DOI] [PubMed] [Google Scholar]
- 28.Varea O, Garrido JJ, Dopazo A, Mendez P, Garcia-Segura LM, Wandosell F. Estradiol activates beta-catenin dependent transcription in neurons. PLoS One. 2009;4:e5153. doi: 10.1371/journal.pone.0005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci. 2008;268:40–7. doi: 10.1016/j.jns.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Callard GV, Petro Z, Ryan KJ. Identification of aromatase in the reptilian brain. Endocrinology. 1977;100:1214–8. doi: 10.1210/endo-100-4-1214. [DOI] [PubMed] [Google Scholar]
- 32.Callard GV, Petro Z, Ryan KJ. Phylogenetic distribution of aromatase and other androgenconverting enzymes in the central nervous system. Endocrinology. 1978;103:2283–90. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- 33.Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK. Aromatization and 5alpha-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology. 1977;101:841–8. doi: 10.1210/endo-101-3-841. [DOI] [PubMed] [Google Scholar]
- 34.Steimer T, Hutchison JB. Aromatization of testosterone within a discrete hypothalamic area associated with the behavioral action of androgen in the male dove. Brain Res. 1980;192:586–91. doi: 10.1016/0006-8993(80)90912-9. [DOI] [PubMed] [Google Scholar]
- 35.MacLusky NJ, Naftolin F, Goldman-Rakic PS. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc Natl Acad Sci U S A. 1986;83:513–6. doi: 10.1073/pnas.83.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 37.Montelli S, Peruffo A, Zambenedetti P, Rossipal E, Giacomello M, Zatta P, Cozzi B. Expression of aromatase P450(AROM) in the human fetal and early postnatal cerebral cortex. Brain Res. 2012;1475:11–8. doi: 10.1016/j.brainres.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Anthoni H, Sucheston LE, Lewis BA, Tapia-Paez I, Fan X, Zucchelli M, Taipale M, Stein CM, Hokkanen ME, Castren E, Pennington BF, Smith SD, Olson RK, Tomblin JB, Schulte-Korne G, Nothen M, Schumacher J, Muller-Myhsok B, Hoffmann P, Gilger JW, Hynd GW, Nopola-Hemmi J, Leppanen PH, Lyytinen H, Schoumans J, Nordenskjold M, Spencer J, Stanic D, Boon WC, Simpson E, Makela S, Gustafsson JA, Peyrard-Janvid M, Iyengar S, Kere J. The aromatase gene CYP19A1: several genetic and functional lines of evidence supporting a role in reading, speech and language. Behav Genet. 2012;42:509–27. doi: 10.1007/s10519-012-9532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fester L, Ribeiro-Gouveia V, Prange-Kiel J, von Schassen C, Bottner M, Jarry H, Rune GM. Proliferation and apoptosis of hippocampal granule cells require local oestrogen synthesis. J Neurochem. 2006;97:1136–44. doi: 10.1111/j.1471-4159.2006.03809.x. [DOI] [PubMed] [Google Scholar]
- 40.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–21. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–34. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- 42.von Schassen C, Fester L, Prange-Kiel J, Lohse C, Huber C, Bottner M, Rune GM. Oestrogen synthesis in the hippocampus: role in axon outgrowth. J Neuroendocrinol. 2006;18:847–56. doi: 10.1111/j.1365-2826.2006.01484.x. [DOI] [PubMed] [Google Scholar]
- 43.Abdelgadir SE, Roselli CE, Choate JV, Resko JA. Distribution of aromatase cytochrome P450 messenger ribonucleic acid in adult rhesus monkey brains. Biol Reprod. 1997;57:772–7. doi: 10.1095/biolreprod57.4.772. [DOI] [PubMed] [Google Scholar]
- 44.Sasano H, Takashashi K, Satoh F, Nagura H, Harada N. Aromatase in the human central nervous system. Clin Endocrinol (Oxf) 1998;48:325–9. doi: 10.1046/j.1365-2265.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- 45.Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem Mol Biol. 1999;70:237–41. doi: 10.1016/s0960-0760(99)00114-4. [DOI] [PubMed] [Google Scholar]
- 46.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27:207–17. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, Hubbard B, King P, Logan J, Muench L, Pareto D, Schlyer D, Shea C, Telang F, Wang GJ, Xu Y, Fowler JS. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64:801–7. doi: 10.1002/syn.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138:389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 50.Luchetti S, Bossers K, Van de Bilt S, Agrapart V, Morales RR, Frajese GV, Swaab DF. Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer’s disease. Neurobiol Aging. 2011;32:1964–76. doi: 10.1016/j.neurobiolaging.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- 52.Simpson E, Rubin G, Clyne C, Robertson K, O’Donnell L, Davis S, Jones M. Local estrogen biosynthesis in males and females. Endocr Relat Cancer. 1999;6:131–7. doi: 10.1677/erc.0.0060131. [DOI] [PubMed] [Google Scholar]
- 53.Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475:261–9. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- 54.Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106–18. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–78. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- 56.Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata) J Neurobiol. 2005;64:192–201. doi: 10.1002/neu.20147. [DOI] [PubMed] [Google Scholar]
- 57.Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. doi: 10.1016/j.brainres.2009.09.111. [DOI] [PubMed] [Google Scholar]
- 58.Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol. 2005;96:89–91. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. 2010;181:209–32. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- 60.Demura M, Reierstad S, Innes JE, Bulun SE. Novel promoter I.8 and promoter usage in the CYP19 (aromatase) gene. Reprod Sci. 2008;15:1044–53. doi: 10.1177/1933719108322441. [DOI] [PubMed] [Google Scholar]
- 61.Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–47. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, Hatanaka Y, Ogiue-Ikeda M. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim Biophys Acta. 2010;1800:1030–44. doi: 10.1016/j.bbagen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151:1153–60. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]
- 65.McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–5. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li R, He P, Cui J, Staufenbiel M, Harada N, Shen Y. Brain endogenous estrogen levels determine responses to estrogen replacement therapy via regulation of BACE1 and NEP in female Alzheimer’s transgenic mice. Mol Neurobiol. 2013;47:857–67. doi: 10.1007/s12035-012-8377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-Ikeda M, Mukai H, Kimoto T. Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity. Front Endocrinol (Lausanne) 2011;2:43. doi: 10.3389/fendo.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kancheva R, Hill M, Novak Z, Chrastina J, Kancheva L, Starka L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience. 2011;191:22–7. doi: 10.1016/j.neuroscience.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 69.Schindler AE. Metabolism of androstenedione and testosterone in human fetal brain. Prog Brain Res. 1975;42:330. doi: 10.1016/s0079-6123(08)63689-4. [DOI] [PubMed] [Google Scholar]
- 70.Hammond GL, Hirvonen J, Vihko R. Progesterone, androstenedione, testosterone, 5 alphadihydrotestosterone and androsterone concentrations in specific regions of the human brain. J Steroid Biochem. 1983;18:185–9. doi: 10.1016/0022-4731(83)90086-9. [DOI] [PubMed] [Google Scholar]
- 71.Milewich L, MacDonald PC, Carr BR. Estrogen 16 alpha-hydroxylase activity in human fetal tissues. J Clin Endocrinol Metab. 1986;63:404–6. doi: 10.1210/jcem-63-2-404. [DOI] [PubMed] [Google Scholar]
- 72.Matsuba C, Uolevi J, Palo LKS, Merila J. Evidence for multiple retroposition events and gene evolution in the ADP/ATP translocase gene family in Ranid frogs. J Hered. 2007;98:300–10. doi: 10.1093/jhered/esm038. [DOI] [PubMed] [Google Scholar]
- 73.Caruso D, D’Intino G, Giatti S, Maschi O, Pesaresi M, Calabrese D, Garcia-Segura LM, Calza L, Melcangi RC. Sex-dimorphic changes in neuroactive steroid levels after chronic experimental autoimmune encephalomyelitis. J Neurochem. 2010;114:921–32. doi: 10.1111/j.1471-4159.2010.06825.x. [DOI] [PubMed] [Google Scholar]
- 74.Caruso D, Scurati S, Maschi O, De Angelis L, Roglio I, Giatti S, Garcia-Segura LM, Melcangi RC. Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem Int. 2008;52:560–8. doi: 10.1016/j.neuint.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Caruso D, Melcangi RC. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm Behav. 2010;57:46–55. doi: 10.1016/j.yhbeh.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 76.Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Murphy DG. Physiological variation in estradiol and brain function: a functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Horm Behav. 2008;53:503–8. doi: 10.1016/j.yhbeh.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Craig MC, Murphy DG. Estrogen: effects on normal brain function and neuropsychiatric disorders. Climacteric. 2007;10(Suppl 2):97–104. doi: 10.1080/13697130701598746. [DOI] [PubMed] [Google Scholar]
- 78.Craig MC, Murphy DG. Oestrogen, cognition and the maturing female brain. J Neuroendocrinol. 2007;19:1–6. doi: 10.1111/j.1365-2826.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 79.Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–27. doi: 10.1016/j.brainres.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 81.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction. 2007;133:331–59. doi: 10.1530/REP-06-0078. [DOI] [PubMed] [Google Scholar]
- 83.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 84.Simerly RB. Wired on hormones: endocrine regulation of hypothalamic development. Curr Opin Neurobiol. 2005;15:81–5. doi: 10.1016/j.conb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 85.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davis EC, Shryne JE, Gorski RA. A revised critical period for the sexual differentiation of the sexually dimorphic nucleus of the preoptic area in the rat. Neuroendocrinology. 1995;62:579–85. doi: 10.1159/000127053. [DOI] [PubMed] [Google Scholar]
- 87.Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–26. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bao AM, Swaab DF. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol. 2011;32:214–26. doi: 10.1016/j.yfrne.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Waber DP. Neuropsychological aspects of Turner’s syndrome. Dev Med Child Neurol. 1979;21:58–70. doi: 10.1111/j.1469-8749.1979.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 90.Ryan J, Carriere I, Scali J, Ritchie K, Ancelin ML. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34:287–98. doi: 10.1016/j.psyneuen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 91.Kesler SR, Garrett A, Bender B, Yankowitz J, Zeng SM, Reiss AL. Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologia. 2004;42:1971–8. doi: 10.1016/j.neuropsychologia.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hart SJ, Davenport ML, Hooper SR, Belger A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 2006;129:1125–36. doi: 10.1093/brain/awl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm Behav. 2011;59:565–71. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19:464–73. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- 95.Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21:636–46. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koolschijn PC, Crone EA. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 2013;5:106–18. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 98.Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123:932–40. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- 99.Morse JK, Scheff SW, DeKosky ST. Gonadal steroids influence axon sprouting in the hippocampal dentate gyrus: a sexually dimorphic response. Exp Neurol. 1986;94:649–58. doi: 10.1016/0014-4886(86)90244-x. [DOI] [PubMed] [Google Scholar]
- 100.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–8. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 101.Sherwin BB. Steroid hormones and cognitive functioning in aging men: a mini-review. J Mol Neurosci. 2003;20:385–93. doi: 10.1385/JMN:20:3:385. [DOI] [PubMed] [Google Scholar]
- 102.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav. 2006;50:612–22. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 104.Barry JA, Parekh HS, Hardiman PJ. Visual-spatial cognition in women with polycystic ovarian syndrome: the role of androgens. Hum Reprod. 2013;28:2832–7. doi: 10.1093/humrep/det335. [DOI] [PubMed] [Google Scholar]
- 105.Aleman A, Bronk E, Kessels RP, Koppeschaar HP, van Honk J. A single administration of testosterone improves visuospatial ability in young women. Psychoneuroendocrinology. 2004;29:612–7. doi: 10.1016/S0306-4530(03)00089-1. [DOI] [PubMed] [Google Scholar]
- 106.Benice TS, Raber J. Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learn Mem. 2009;16:479–85. doi: 10.1101/lm.1428209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li R, Cui J, Jothishankar B, Shen J, He P, Shen Y. Early reproductive experiences in females make differences in cognitive function later in life. J Alzheimers Dis. 2013;34:589–94. doi: 10.3233/JAD-122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glynn P. Neuronal phospholipid deacylation is essential for axonal and synaptic integrity. Biochim Biophys Acta. 2013;1831:633–41. doi: 10.1016/j.bbalip.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 109.Hogervorst E. Effects Of Gonadal Hormones On Cognitive Behavior In Elderly Men And Women. J Neuroendocrinol. 2013 doi: 10.1111/jne.12080. [DOI] [PubMed] [Google Scholar]
- 110.McClure RE, Barha CK, Galea LA. 17beta-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav. 2013;63:144–57. doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 111.Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones, and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013 doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- 112.Cost KT, Williams-Yee ZN, Fustok JN, Dohanich GP. Sex differences in object-in-place memory of adult rats. Behav Neurosci. 2012;126:457–64. doi: 10.1037/a0028363. [DOI] [PubMed] [Google Scholar]
- 113.Courvoisier DS, Renaud O, Geiser C, Paschke K, Gaudy K, Jordan K. Sex hormones and mental rotation: an intensive longitudinal investigation. Horm Behav. 2013;63:345–51. doi: 10.1016/j.yhbeh.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 114.Takayanagi Y, Spira AP, McIntyre RS, Eaton WW. Sex Hormone Binding Globulin and Verbal Memory in Older Men. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dye RV, Miller KJ, Singer EJ, Levine AJ. Hormone replacement therapy and risk for neurodegenerative diseases. Int J Alzheimers Dis. 2012;2012:258454. doi: 10.1155/2012/258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–7. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aronson MK, Ooi WL, Morgenstern H, Hafner A, Masur D, Crystal H, Frishman WH, Fisher D, Katzman R. Women, myocardial infarction, and dementia in the very old. Neurology. 1990;40:1102–6. doi: 10.1212/wnl.40.7.1102. [DOI] [PubMed] [Google Scholar]
- 118.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–15. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 119.Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–79. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 120.Proust-Lima C, Amieva H, Letenneur L, Orgogozo JM, Jacqmin-Gadda H, Dartigues JF. Gender and education impact on brain aging: a general cognitive factor approach. Psychol Aging. 2008;23:608–20. doi: 10.1037/a0012838. [DOI] [PubMed] [Google Scholar]
- 121.Read S, Pedersen NL, Gatz M, Berg S, Vuoksimaa E, Malmberg B, Johansson B, McClearn GE. Sex differences after all those years? Heritability of cognitive abilities in old age. J Gerontol B Psychol Sci Soc Sci. 2006;61:137–43. doi: 10.1093/geronb/61.3.p137. [DOI] [PubMed] [Google Scholar]
- 122.Henderson VW, Buckwalter JG. Cognitive deficits of men and women with Alzheimer’s disease. Neurology. 1994;44:90–6. doi: 10.1212/wnl.44.1.90. [DOI] [PubMed] [Google Scholar]
- 123.Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology. 2000;55:1613–20. doi: 10.1212/01.wnl.0000434309.85312.19. [DOI] [PubMed] [Google Scholar]
- 124.Perri R, Koch G, Carlesimo GA, Serra L, Fadda L, Pasqualetti P, Pettenati C, Caltagirone C. Alzheimer’s disease and frontal variant of frontotemporal dementia-- a very brief battery for cognitive and behavioural distinction. J Neurol. 2005;252:1238–44. doi: 10.1007/s00415-005-0849-1. [DOI] [PubMed] [Google Scholar]
- 125.Giovagnoli AR, Erbetta A, Reati F, Bugiani O. Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer’s disease in a study of diagnostic concordance. Neuropsychologia. 2008;46:1495–504. doi: 10.1016/j.neuropsychologia.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 126.Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, Cross K, Grossman M. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–75. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- 127.Frisch S, Dukart J, Vogt B, Horstmann A, Becker G, Villringer A, Barthel H, Sabri O, Muller K, Schroeter ML. Dissociating memory networks in early Alzheimer’s disease and frontotemporal lobar degeneration - a combined study of hypometabolism and atrophy. PLoS One. 2013;8:e55251. doi: 10.1371/journal.pone.0055251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ye BS, Seo SW, Lee Y, Kim SY, Choi SH, Lee YM, Kim do H, Han HJ, Na DL, Kim EJ. Neuropsychological performance and conversion to Alzheimer’s disease in early- compared to late-onset amnestic mild cognitive impairment: CREDOS study. Dement Geriatr Cogn Disord. 2012;34:156–66. doi: 10.1159/000342973. [DOI] [PubMed] [Google Scholar]
- 129.Dong Y, Gan DZ, Tay SZ, Koay WI, Collinson SL, Hilal S, Venketasubramanian N, Chen C. Patterns of neuropsychological impairment in Alzheimer’s disease and mixed dementia. J Neurol Sci. 2013;333:5–8. doi: 10.1016/j.jns.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 130.Heuer HW, Mirsky JB, Kong EL, Dickerson BC, Miller BL, Kramer JH, Boxer AL. Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology. 2013;81:1235–43. doi: 10.1212/WNL.0b013e3182a6cbfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brown JM, Wiggins J, Dong H, Harvey R, Richardson F, Hunter K, Dawson K, Parker RA. The hard Test Your Memory. Evaluation of a short cognitive test to detect mild Alzheimer’s disease and amnestic mild cognitive impairment. Int J Geriatr Psychiatry. 2013 doi: 10.1002/gps.4005. [DOI] [PubMed] [Google Scholar]
- 132.Lipnicki DM, Sachdev PS, Crawford J, Reppermund S, Kochan NA, Trollor JN, Draper B, Slavin MJ, Kang K, Lux O, Mather KA, Brodaty H. Risk factors for late-life cognitive decline and variation with age and sex in the Sydney Memory and Ageing Study. PLoS One. 2013;8:e65841. doi: 10.1371/journal.pone.0065841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ganguli M, Fu B, Snitz BE, Hughes TF, Chang CC. Mild cognitive impairment: incidence and vascular risk factors in a population-based cohort. Neurology. 2013;80:2112–20. doi: 10.1212/WNL.0b013e318295d776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheng X, He P, Lee T, Yao H, Li R, Shen Y. High Activities of BACE1 in Brains with Mild Cognitive Impairment. Am J Pathol. 2014;184:141–7. doi: 10.1016/j.ajpath.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Borroni B, Grassi M, Bianchi M, Bruni AC, Maletta RG, Anfossi M, Pepe D, Cagnin A, Caffarra P, Cappa S, Clerici F, Daniele A, Frisoni GB, Galimberti D, Parnetti L, Perri R, Rainero I, Tremolizzo L, Turla M, Zanetti O, Padovani A. Estimating the Inheritance of Frontotemporal Lobar Degeneration in the Italian Population. J Alzheimers Dis. 2013 doi: 10.3233/JAD-130128. [DOI] [PubMed] [Google Scholar]
- 136.Borroni B, Alberici A, Grassi M, Rozzini L, Turla M, Zanetti O, Bianchetti A, Gilberti N, Bonvicini C, Volta GD, Rozzini R, Padovani A. Prevalence and demographic features of early-onset neurodegenerative dementia in Brescia County, Italy. Alzheimer Dis Assoc Disord. 2011;25:341–4. doi: 10.1097/WAD.0b013e3182147f80. [DOI] [PubMed] [Google Scholar]
- 137.Ren RJ, Huang Y, Xu G, Li CB, Cheng Q, Chen SD, Wang G. History, present, and progress of frontotemporal dementia in china: a systematic review. Int J Alzheimers Dis. 2012;2012:587215. doi: 10.1155/2012/587215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Selkoe DJ, Yamazaki T, Citron M, Podlisny MB, Koo EH, Teplow DB, Haass C. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- 139.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–50. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 140.Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord. 2010;30:43–50. doi: 10.1159/000314681. [DOI] [PubMed] [Google Scholar]
- 141.Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:976–97. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Long J, He P, Shen Y, Li R. New evidence of mitochondria dysfunction in the female Alzheimer’s disease brain: deficiency of estrogen receptor-beta. J Alzheimers Dis. 2012;30:545–58. doi: 10.3233/JAD-2012-120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fleisher A, Grundman M, Jack CR, Jr, Petersen RC, Taylor C, Kim HT, Schiller DH, Bagwell V, Sencakova D, Weiner MF, DeCarli C, DeKosky ST, van Dyck CH, Thal LJ. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953–7. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- 144.Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol. 2012;131:24–9. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 145.Kramar EA, Chen LY, Lauterborn JC, Simmons DA, Gall CM, Lynch G. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging. 2012;33:708–19. doi: 10.1016/j.neurobiolaging.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, Komatsuzaki Y, Ogiue-Ikeda M, Kimoto T, Mukai H. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol. 2012;131:37–51. doi: 10.1016/j.jsbmb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 147.Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–46. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–22. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anastasio TJ. Exploring the contribution of estrogen to amyloid-Beta regulation: a novel multifactorial computational modeling approach. Front Pharmacol. 2013;4:16. doi: 10.3389/fphar.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–88. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem. 1999;72:1552–63. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 152.Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28:8430–41. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hogervorst E, Bandelow S. The controversy over levels of sex steroids in cases with Alzheimer’s disease. J Neuroendocrinol. 2004;16:93–4. doi: 10.1111/j.0953-8194.2004.01134.x. [DOI] [PubMed] [Google Scholar]
- 154.Gleason CE, Cholerton B, Carlsson CM, Johnson SC, Asthana S. Neuroprotective effects of female sex steroids in humans: current controversies and future directions. Cell Mol Life Sci. 2005;62:299–312. doi: 10.1007/s00018-004-4385-z. [DOI] [PubMed] [Google Scholar]
- 155.Rune GM, Lohse C, Prange-Kiel J, Fester L, Frotscher M. Synaptic plasticity in the hippocampus: effects of estrogen from the gonads or hippocampus? Neurochem Res. 2006;31:145–55. doi: 10.1007/s11064-005-9004-8. [DOI] [PubMed] [Google Scholar]
- 156.Correia SC, Santos RX, Cardoso S, Carvalho C, Santos MS, Oliveira CR, Moreira PI. Effects of estrogen in the brain: is it a neuroprotective agent in Alzheimer’s disease? Curr Aging Sci. 2010;3:113–26. doi: 10.2174/1874609811003020113. [DOI] [PubMed] [Google Scholar]
- 157.Schonknecht P, Pantel J, Klinga K, Jensen M, Hartmann T, Salbach B, Schroder J. Reduced cerebrospinal fluid estradiol levels are associated with increased beta-amyloid levels in female patients with Alzheimer’s disease. Neurosci Lett. 2001;307:122–4. doi: 10.1016/s0304-3940(01)01896-1. [DOI] [PubMed] [Google Scholar]
- 158.Ishunina TA, van Beurden D, van der Meulen G, Unmehopa UA, Hol EM, Huitinga I, Swaab DF. Diminished aromatase immunoreactivity in the hypothalamus, but not in the basal forebrain nuclei in Alzheimer’s disease. Neurobiol Aging. 2005;26:173–94. doi: 10.1016/j.neurobiolaging.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 159.Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer’s disease. Neurobiol Aging. 2007;28:1670–81. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 160.Butler HT, Warden DR, Hogervorst E, Ragoussis J, Smith AD, Lehmann DJ. Association of the aromatase gene with Alzheimer’s disease in women. Neurosci Lett. 2010;468:202–6. doi: 10.1016/j.neulet.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 161.Wozniak A, Hutchison RE, Morris CM, Hutchison JB. Neuroblastoma and Alzheimer’s disease brain cells contain aromatase activity. Steroids. 1998;63:263–7. doi: 10.1016/s0039-128x(98)00029-4. [DOI] [PubMed] [Google Scholar]
- 162.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology. 2004;13:61–6. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- 163.Bender CM, Sereika SM, Brufsky AM, Ryan CM, Vogel VG, Rastogi P, Cohen SM, Casillo FE, Berga SL. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause. 2007;14:995–8. doi: 10.1097/gme.0b013e318148b28b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jenkins VA, Ambroisine LM, Atkins L, Cuzick J, Howell A, Fallowfield LJ. Effects of anastrozole on cognitive performance in postmenopausal women: a randomised, double-blind chemoprevention trial (IBIS II) Lancet Oncol. 2008;9:953–61. doi: 10.1016/S1470-2045(08)70207-9. [DOI] [PubMed] [Google Scholar]
- 165.Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28:1294–300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 166.Phillips KA, Ribi K, Sun Z, Stephens A, Thompson A, Harvey V, Thurlimann B, Cardoso F, Pagani O, Coates AS, Goldhirsch A, Price KN, Gelber RD, Bernhard J. Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1–98 randomized trial. Breast. 2010;19:388–95. doi: 10.1016/j.breast.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Iivonen S, Corder E, Lehtovirta M, Helisalmi S, Mannermaa A, Vepsalainen S, Hanninen T, Soininen H, Hiltunen M. Polymorphisms in the CYP19 gene confer increased risk for Alzheimer disease. Neurology. 2004;62:1170–6. doi: 10.1212/01.wnl.0000118208.16939.60. [DOI] [PubMed] [Google Scholar]
- 168.Medway C, Combarros O, Cortina-Borja M, Butler HT, Ibrahim-Verbaas CA, de Bruijn RF, Koudstaal PJ, van Duijn CM, Ikram MA, Mateo I, Sanchez-Juan P, Lehmann MG, Heun R, Kolsch H, Deloukas P, Hammond N, Coto E, Alvarez V, Kehoe PG, Barber R, Wilcock GK, Brown K, Belbin O, Warden DR, Smith AD, Morgan K, Lehmann DJ. The sex-specific associations of the aromatase gene with Alzheimer’s disease and its interaction with IL10 in the Epistasis Project. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Janicki SC, Park N, Cheng R, Schupf N, Clark LN, Lee JH. Aromatase variants modify risk for Alzheimer’s disease in a multiethnic female cohort. Dement Geriatr Cogn Disord. 2013;35:340–6. doi: 10.1159/000343074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32:604–13. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]