Abstract
The proteoglycan decorin, a key component of the tumor stroma, regulates the action of several tyrosine-kinase receptors, including the EGFR, Met and the IGF-IR. Notably, the action of decorin in regulating the IGF-I system differs between normal and transformed cells. In normal cells, decorin binds with high affinity to both the natural ligand IGF-I and the IGF-I receptor (IGF-IR) and positively regulates IGF-IR activation and downstream signaling. In contrast, in transformed cells, decorin negatively regulates ligand-induced IGF-IR activation, downstream signaling and IGF-IR-dependent biological responses. Whether decorin may bind another member of the IGF-I system, the insulin receptor A isoform (IR-A) and its cognate ligands, insulin, IGF-II and proinsulin, has not been established. Here we show that decorin bound with high affinity insulin and IGF-II and, to a lesser extent, proinsulin and IR-A. We utilized as a cell model system mouse embryonic fibroblasts homozygous for a targeted disruption of the Igf1r gene (designated R− cells) which were stably transfected with a human construct harboring the IR-A isoform of the receptor. Using these R−/IR-A cells, we demonstrate that decorin did not affect ligand-induced phosphorylation of the IR-A but enhanced IR-A downregulation after prolonged IGF-II stimulation without affecting insulin and proinsulin-dependent effects on IR-A stability. In addition, decorin significantly inhibited IGF-II-mediated activation of the Akt pathways, without affecting insulin and proinsulin-dependent signaling. Notably, decorin significantly inhibited IGF-II-mediated cell proliferation of R−/IR-A cells but affected neither insulin- nor proinsulin-dependent mitogenesis. Collectively, these results suggest that decorin differentially regulates the action of IR-A ligands. Decorin preferentially inhibits IGF-II-mediated biological responses but does not affect insulin- or proinsulin-dependent signaling. Thus, decorin loss may contribute to tumor initiation and progression in malignant neoplasms which depend on an IGF-II/IR-A autocrine loop.
Keywords: decorin, IR-A, signaling, proliferation
1. Introduction
Decorin, the prototype member of the small leucine-rich proteoglycans (SLRPs), modulates the biology of various cancer types by downregulating the activity of several tyrosine-kinase receptors important for cell growth, motility and survival (Iozzo, 1997; Schaefer and Iozzo, 2008). Decorin interacts with the epidermal growth factor receptor (EGFR) and the Met receptor and downregulates their activity and signaling (Buraschi et al., 2012; Csordas et al., 2000; Goldoni et al., 2009; Iozzo et al., 1999; Santra et al., 2000; Santra et al., 2002). In addition, decorin modulates the insulin-like growth factor (IGF-I) system by binding with high affinity both IGF-I and the insulin-like growth factor receptor 1 (IGF-IR) (Morrione et al., 2013). Notably, a recent in situ hybridization study encompassing a large cohort of human urothelial carcinomas has shown that decorin expression is totally absent in non-invasive and invasive bladder carcinomas (Sainio et al., 2013), suggesting that decorin loss might favor the malignant behavior of bladder cancer cells. Moreover, decorin has been implicated in a variety of pathologies including tendon, muscle, bone, cornea and various connective tissues where abnormal signaling and cell/matrix interactions may play an active pathogenetic role (Brandan and Gutierrez, 2013a, b; Chen et al., 2013; Dunkman et al., 2013; Jarvelainen et al., 2006; Nikitovic et al., 2012; Seidler, 2012). Recent evidence indicates that decorin antagonizes the vascular endothelial cell growth factor receptor 2 (VEGFR2) and suppresses angiogenesis (Neill et al., 2013a; Neill et al., 2012a) via induction of endothelial cell autophagy (Buraschi et al., 2013; Neill et al., 2013b).
The type I IGF receptor (IGF-IR) binds with high affinity both insulin-like growth factors I and II (IGF-I and IGF-II) and has a crucial role in the regulation of mammalian growth both in vitro (Scher et al., 1979; Stiles et al., 1979) and in vivo (Baker et al., 1993; Eggenschwiler et al., 1997; Liu et al., 1993). The IGF-IR and its ligands are frequently deregulated in cancer and may have an important role not only in the early phases of carcinogenesis but also in cancer progression and resistance to a variety of therapies (Baserga, 1995, 2000; Baserga et al., 1997; LeRoith and Roberts, 2003). IGF-II, and to a lesser extent IGF-I, binds to a second receptor tyrosine kinase (RTK), the isoform A of the insulin receptor (IR-A), which is highly homologous to the IGF-IR (Frasca et al., 1999; Krywicki and Yee, 1992) The IR-A is considered the fetal form of the IR and primarily mediates mitogenic effects upon IGF-II or insulin binding (Frasca et al., 1999; Morrione et al., 1997b; Pandini et al., 2002), and is also implicated in cancer (Belfiore, 2007; Belfiore et al., 2009). Proinsulin has been recently identified as another IR-A ligand and despite its lower affinity for the IR-A compared to insulin (similar to IGF-II), promotes IR-A phosphorylation and activation of downstream signaling (Malaguarnera et al., 2012).
The second IR isoform (IR-B) is involved in glucose metabolism of insulin-responsive organs (Belfiore, 2007; Frasca et al., 1999). Predominant expression of the IR-A over the IR-B has been detected in several cancer models and an autocrine proliferative loop between IGF-II and the IR-A has been demonstrated in malignant thyrocytes and breast cancer cells (Kalli et al., 2002; Sciacca et al., 1999; Sciacca et al., 2002; Vella et al., 2002).
Decorin regulates the IGF-I system at various levels but there is a surprising dichotomy in the mechanisms of decorin regulation of IGF-IR signaling, which differ between physiological and pathological cellular models (Morrione et al., 2013). In normal endothelial cells, decorin induces IGF-IR phosphorylation and IGF-IR-dependent Akt activation but it also modulates subsequent receptor downregulation (Schonherr et al., 2005). In addition, decorin induces IGF-IR-dependent endothelial cell adhesion and migration on collagen (Fiedler et al., 2008). In renal fibroblasts decorin regulates fibrillin-1 synthesis through an IGF-IR/mTOR/p70S6K signaling cascade (Schaefer et al., 2007). In extravillus trophoblasts, instead, decorin negatively regulates migration by promoting IGF-IR phosphorylation and activation in a dose-dependent manner but the anti-proliferative effect of decorin is IGF-IR-independent (Iacob et al., 2008).
In contrast, in urothelial cancer-derived cells decorin severely inhibits ligand-dependent IGF-IR activation and downstream activation of the Akt and MAPK pathways (Iozzo et al., 2011). In addition, prolonged exposure to decorin did not affect the stability of the IGF-IR in urothelial cancer cells either alone or in the presence of IGF-I but enhances degradation of IRS-1, one of the major downstream effectors of the IGF-IR signaling pathway (Rose et al., 1994; Sun et al., 1993). The effects on IGF-IR signaling led to decorin-evoked inhibition of IGF-I-mediated migration and invasion of urothelial cancer cells (Iozzo et al., 2011). Furthermore, decorin expression inversely correlated with IGF-IR expression in low- and high-grade bladder cancers suggesting that loss of decorin may contribute to bladder cancer initiation and progression of IGF-IR-dependent tumors (Iozzo et al., 2011).
In spite of increasing evidences for the role of the IR-A in cancer (Belfiore, 2007; Belfiore et al., 2009), it has not been established as of yet whether decorin directly binds IR-A ligands and regulate IR-A signaling. In this paper, using mouse embryo fibroblasts cells lacking the Igf1r (Sell et al., 1994) and expressing solely the human IR-A (R−/IR-A) (Miura et al., 1995; Morcavallo et al., 2012; Morrione et al., 1997b), we show that decorin binds IGF-II and insulin with high affinity and proinsulin and IR-A with a three-fold lower affinity. Although decorin did not affect ligand-induced IR-A phosphorylation, it attenuated IGF-II-induced Akt activation, enhanced IR-A downregulation after prolonged IGF-II stimulation and significantly reduced IGF-II-induced cell proliferation. Notably, decorin did not affect insulin or proinsulin-mediated signaling and biological responses downstream of the IR-A. These results indicate that decorin differentially regulates IR-A ligands and provide a plausible mechanism whereby decorin loss may contribute to tumor formation in cancer systems addicted to an IGF-II/IR-A autocrine loop.
2. Results
2.1. Decorin Binds the IR-A and its natural ligands, IGF-II, insulin and proinsulin
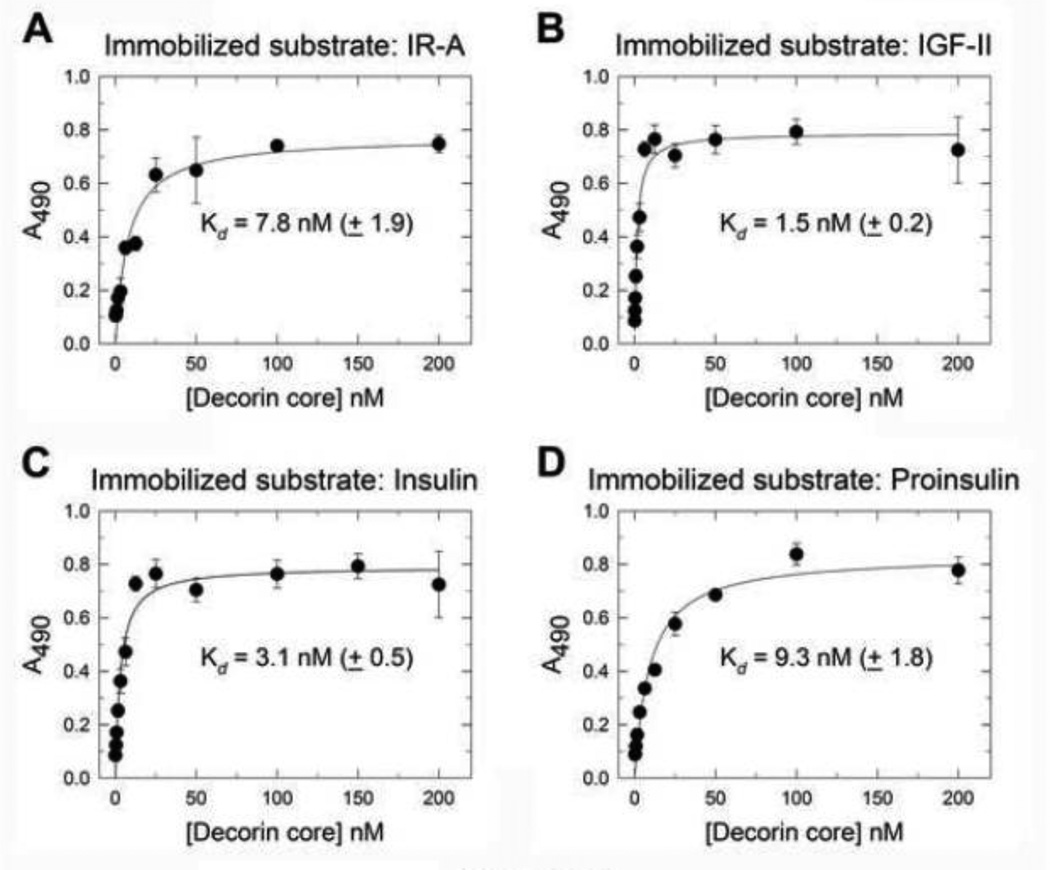
Decorin binds the IGF-IR and its cognate ligand IGF-I and regulates IGF-IR action in both physiological and pathological cell models (Morrione et al., 2013; Schonherr et al., 2005). However, whether decorin may bind the IR-A, another receptor member of the IGF-I system, and regulate its action has not yet been established. Thus, we first tested whether decorin core could bind either the IR-A or its ligands IGF-II, insulin and proinsulin in a cell-free system. Decorin protein core bound with high affinity (Kd = 7.8 nM ±1.9) to the IR-A as determined by ELISA assays using recombinant extracellular domain of human IR-A as the immobilized substrate (Fig. 1A). The decorin affinity for the IR-A was about 3-fold lower than the one we previously demonstrated for the IGF-IR using the same experimental approach (Iozzo et al., 2011). Decorin protein core also bound to immobilized IGF-II (Fig. 1B) and insulin (Fig. 1C) with very similar affinities (Kd = 1.5 nM ±0.2 for IGF-II; Kd = 3.1 nM ±0.5 for insulin) while the affinity for proinsulin (Fig. 1D) was lower (Kd = 9.3 nM ±1.8) and more similar to the decorin affinity for the IR-A. As previously shown for IGF-I (Iozzo et al., 2011), decorin proteoglycan bound insulin with high affinity (Kd 13.36 nM) even though the affinity was about 4-fold lower than decorin protein core (Data not shown).
Fig. 1.
Decorin protein core binds to the IR-A, IGF-II, insulin and proinsulin. [A–D] soluble decorin protein core binds to immobilized human recombinant IR-A and IR-A ligands, IGF-II, insulin and proinsulin in a saturable fashion. Solid phase ELISA assays were performed as described in details in Experimental Procedures. Values represent the mean ± SEM of three independent experiments run in triplicates.
These results indicate that decorin protein core binds IR-A and its ligands and may therefore regulate ligand-induced IR-A activity.
2.2. Decorin does not affect ligand-mediated IR-A phosphorylation
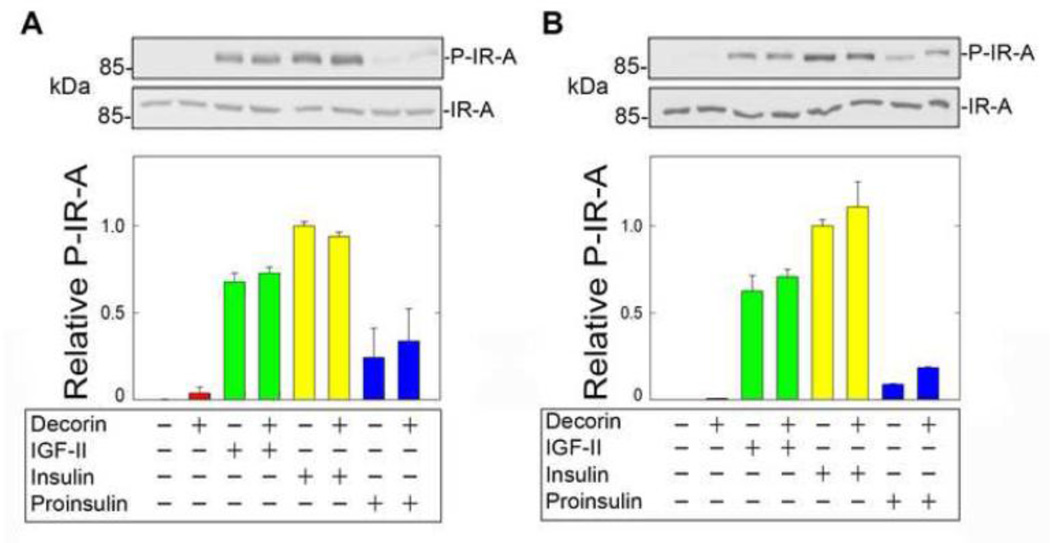
Given the concurrent binding of decorin core to both ligands and receptor in a cell-free system, we investigated whether decorin could play a role in regulating ligand-induced IR-A activation/phosphorylation. To bypass the possibility that decorin, given its high affinity binding to the IGF-IR (Iozzo et al., 2011), could interfere with its action on the IR-A, we used the unique model of R−/IR-A cells, which are mouse embryo fibroblasts cells lacking the Igf1r (Sell et al., 1994) and expressing solely the human IR-A (R−/IR-A) (Miura et al., 1995; Morcavallo et al., 2012; Morrione et al., 1997b). In addition, this well-defined genetic model allowed us to establish decorin modulation of IGF-II signaling exclusively through the IR-A. Thus, R−/IR-A cells were first preincubated for 1 h with decorin core (200 nM) and then stimulated for 10 min with physiological concentrations of IGF-II, insulin or proinsulin (10 nM each) (Fig. 2A). In addition, because decorin binds IGF-II, insulin and proinsulin, we performed the same experiments after pre-incubating all ligands with 200 nM decorin core for 1 h and then exposing the cells to the mixture of ligands for 10 min (Fig. 2B). In both experimental conditions, decorin protein core did not affect IR-A phosphorylation at Tyr1150/1151 induced by IGF-II or insulin, but slightly enhanced IR-A phosphorylation mediated by proinsulin (Fig. 2A and B). Decorin core alone had no effect on IR-A activation in both experimental approaches (Fig. 2A and B).
Fig. 2.
Decorin does not affect IR-A phosphorylation. [A] Serum-starved R−/IR-A cells were preincubated for 1h with decorin (200 nM) and then stimulated with decorin alone (200 nM), IGF-II, insulin or proinsulin (10 nM) or decorin supplemented with growth factors for 10 min. Phosphorylation of the IR-A was determined by immunoblot using anti-phospho-IGF-IRβ (Tyr1135/1136)/IRβ Tyr1150/1151) rabbit monoclonal antibodies (Cell signaling Technology). Total IR-A levels were detected using anti-IR polyclonal antibodies, which recognize the β subunit of the IR-A. [B] To test the effect of decorin core protein on ligands, IGF-II, insulin and proinsulin were preincubated with 200 nM decorin core and then supplemented to R−/IR-A cells. IR-A phosphorylation and total levels were determined as above. Blots are representative of three independent experiments. [C, D] Densitometric analysis was performed using the ImageJ program (rsbweb.nih.gov/ij/) and the values are reported as relative densitometric arbitrary units. Values represent the mean ±SD of three independent experiments run in duplicates.
These results suggest that decorin action on IR-A activation substantially differ from its activity on the IGF-IR, where decorin regulates IGF-IR phosphorylation either positively or negatively in non-transformed and transformed cellular models, respectively (Morrione et al., 2013).
2.3. Decorin preferentially affects IGF-II-modulated IR-A stability and signaling
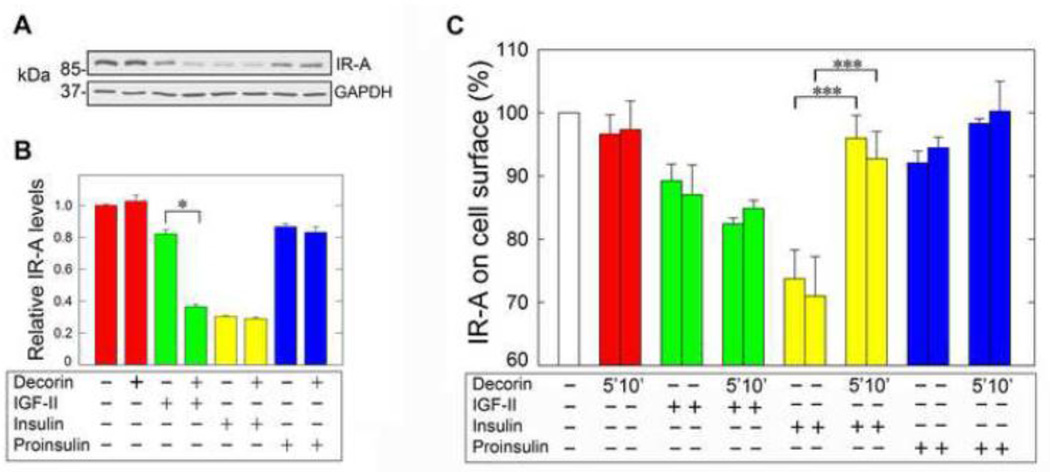
We have previously characterized IR-A endocytosis and trafficking and demonstrated that chronic insulin stimulation of R−/IR-A cells induces IR-A downregulation, which is instead only modestly affected after IGF-II or proinsulin stimulation (Malaguarnera et al., 2012; Morcavallo et al., 2012). To further investigate decorin’s mechanism of action, we tested by immunoblotting IR-A levels and determined whether decorin core could affect IR-A stability after prolonged stimulation with IGF-II, insulin or proinsulin. In agreement with our previous results, IR-A levels in R−/IR-A cells were considerably decreased after a 24-h stimulation with insulin (10 nM) but only modestly affected after incubation with equimolar concentrations of either IGF-II or proinsulin (Fig. 3A, B). Significantly, decorin core protein enhanced IR-A downregulation after prolonged IGF-II stimulation but had no effect on IR-A stability either alone or in combination with insulin or proinsulin (Fig.3A, B).
Fig. 3.
[A] Decorin enhances IGF-II-dependent IR-A downregulation. Serum-starved R−/IRA cells were incubated with either decorin alone (200 nM), IGF-II, insulin, proinsulin (10 nM) or ligands supplemented with decorin for 24 h and IR-A levels were detected by immunoblot using anti-IR polyclonal antibodies, which recognize the β subunit of the IR-A. Protein loading was monitored using anti-GAPDH monoclonal antibodies. [B] Densitometric analysis was performed using the ImageJ program (rsbweb.nih.gov/ij/) and the values are reported as relative densitometric arbitrary units. Values represent the mean ±SD of three independent experiments run in duplicates and we present the statistically significant differences between critical samples (*p<0.05). [C] Cell surface IR-A levels were measured by ELISA assay in unstimulated (basal cell surface level), decorin alone (200 nM) or ligand-stimulated cells (IGF-II, insulin and proinsulin at 10 mM) supplemented or not with decorin core (200 nM). Densitometric analysis was performed using the ImageJ program (rsbweb.nih.gov/ij/) and the values are reported as relative densitometric arbitrary units. Values represent the mean ±SD of three independent experiments run in duplicates (***p<0.001).
Because decorin enhanced IGF-II-induced IR-A downregulation we next determined whether decorin might affect IR-A levels at the cell surface and/or promote receptor endocytosis. Thus, we assessed ligand-induced IR-A internalization from the cell surface by ELISA, as described in previous work from our laboratories (Morcavallo et al., 2012). In agreement with our previous results (Morcavallo et al., 2012), insulin stimulation of R−/IR-A cells induced a clear reduction in the level of cell surface IR-A proteins compared to IGF-II while proinsulin promoted only a modest internalization of the IR-A which was not statistically significant (Fig. 3C). Decorin alone did not affect cell surface IR-A levels and did not modulate IGF-II- and proinsulin-induced IR-A internalization. On the contrary, decorin significantly reduced insulinmediated internalization of the IR-A (Fig. 3C). Collectively, these results demonstrate that decorin binds the IR-A at cell surface and may modulate cell surface IR-A levels.
One of the major downstream effectors of the IGF-IR signaling pathway is the docking protein insulin receptor substrate 1 (IRS-1), which is recruited in ligand-dependent manner to the IGF-IR thereby regulating the activation of the PI3K and Akt pathways. IRS-1 is critical for IGF-IR-dependent biological effects, including cell proliferation and transformation (Rose et al., 1994; Sun et al., 1993). Because we have recently shown that in urothelial cancer cells decorin enhanced IGF-I-mediated down-regulation of IRS-1 (Iozzo et al., 2011), we assessed in R−/IR-A cells IRS-1 protein levels after prolonged exposure to IGF-II, insulin, proinsulin and/or decorin. We discovered that decorin core had no appreciable effects in modulating IRS-1 stability either alone or in combination with all IR-A ligands (data not shown).
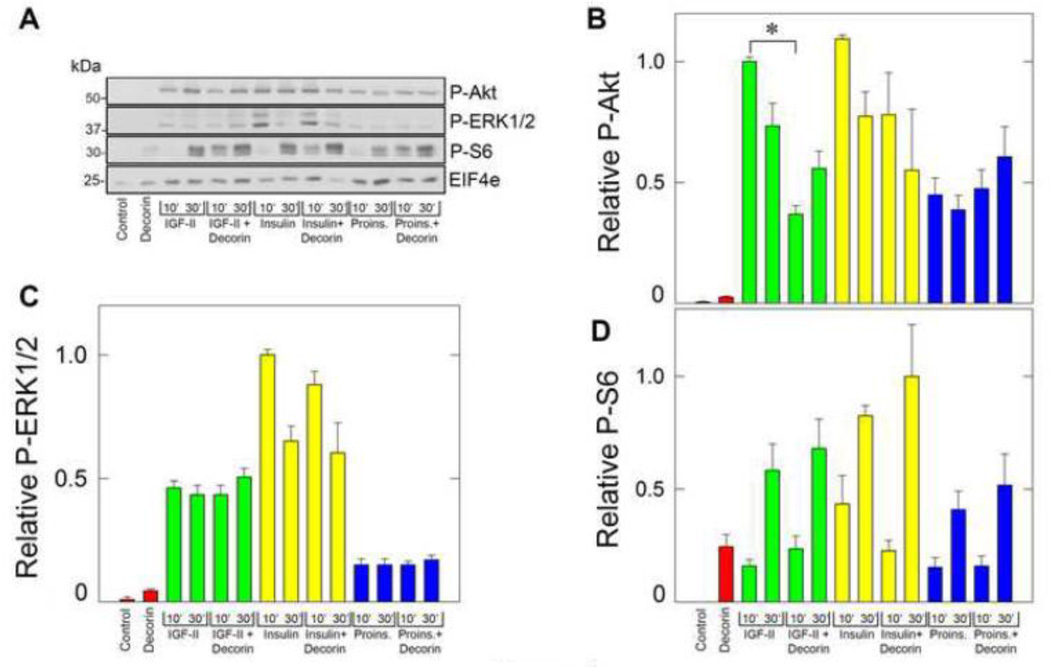
Next, we determined whether decorin could affect ligand-dependent IR-A signaling by analyzing the activation of the Akt and MAPK pathways, which are critical for IR-A-dependent cell proliferation (Frasca et al., 1999; Malaguarnera et al., 2012; Morrione et al., 1997b). To this end, we tested the activation of Akt and ERK1/2 kinases and downstream effectors S6 ribosomal protein using the PathScan Multiplex Western Cocktail I (Cell Signaling). This method allows testing the activation of multiple pathways in one single immunoblot, utilizing a mixture of well-defined phospho-specific antibodies targeting different activated proteins. Decorin core alone had no effect in activating both Akt and ERK1/2 but enhanced the activation of S6 ribosomal protein (Fig. 4A). In agreement with our previously published results, all biological ligands induced the activation of ERK1/2, Akt and S6 ribosomal protein. However, insulin and IGF-II were more effective than proinsulin in inducing Akt activation, while insulin was more potent than IGF-II and proinsulin in mediating ERKs activation (Frasca et al., 1999; Malaguarnera et al., 2012; Morcavallo et al., 2012; Morrione et al., 1997b). Importantly, decorin inhibited early time points of IGF-II-dependent activation of Akt without affecting either insulin or proinsulin-induced Akt signaling (Fig. 4A, B). In contrast, in three independent experiments decorin did not affected IGF-II-mediated ERK1/2 activation (Fig. 4C). While decorin core alone enhanced S6 activation at levels comparable to IGF-II and proinsulin, it did not significantly affect ligand-modulated S6 activity (Fig. 4D). Collectively, these results demonstrate that decorin specifically regulates IR-A stability and activation of downstream signaling effectors after IGF-II stimulation but has no effect on IR-A levels or signaling after insulin or proinsulin stimulation.
Fig. 4.
Decorin affects IGF-II-induced signaling. [A] Western immunoblotting of serum-starved R−/IR-A treated for 10 and 30 min with the various ligands as indicated at the bottom. The various bands were visualized using the PathScan Multiplex Western Cocktail I (Cell Signaling Technology). ElF4e protein provides a control to monitor protein loading. Blots are representative of three independent experiments. [B–D] Quantification of three independent experiments similar to that shown in panel A. Densitometric analysis was performed using the ImageJ program (rsbweb.nih.gov/ij/) and the values are reported as relative densitometric arbitrary units. Values represent the mean ±SD of three independent experiments run in duplicates and we present the statistically significant differences between critical samples (*p<0.05). The PathScan includes also antibodies for activated p90RSK, which was not detectable in these experimental conditions (data not shown).
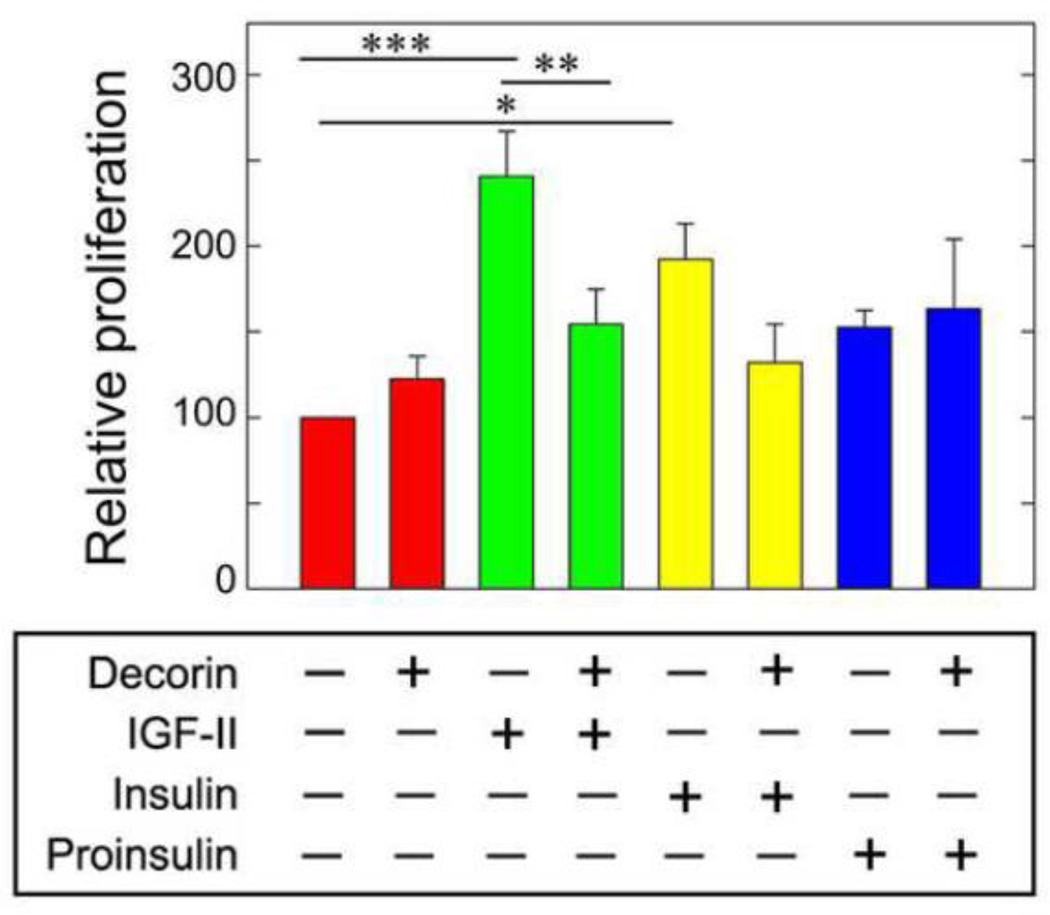
2.4. Decorin affects IGF-II-induced proliferation of R−/IR-A cells
Cell proliferation induced by IGF-II and insulin through the IR-A requires the simultaneous activation of the Akt and MAPK pathways (Frasca et al., 1999; Morrione et al., 1997b). Because decorin core protein preferentially enhanced IGF-II-mediated IR-A downregulation and inhibited IGF-II-induced IR-A signaling to Akt, we performed growth curves in R−/IR-A cells. The expected results should clarify whether decorin would differentially affect IR-A-dependent cell proliferation induced by IGF-II, insulin or proinsulin. While decorin alone (200 nM) had no effect on R−/IR-A cell proliferation, it significantly inhibited cell growth induced by IGF-II without significantly affecting insulin- or proinsulin-mediated mitogenesis (Fig. 5).
Fig. 5.
Decorin inhibits IGF-II-induced proliferation in R−/IR-A cells. Quantification of R−/IR-A cell growth was performed as previously described (Morcavallo et al., 2012) and detailed in Experimental Procedures. Cells were incubated with decorin core alone (200 nM), IGF-II, insulin, proinsulin (10 nM) or ligands supplemented with decorin core protein. Cells were counted after 48 h in a hemocytometer. Values represent the mean ±SD of three independent experiments run in triplicates (*p<0.05, **p< 0.01, ***p<0.001).
Altogether, these results provide the first evidence of a role for decorin in the regulation of ligand-dependent IR-A action and demonstrate that decorin specifically modulates IGF-II-dependent IR-A signaling and biological responses without affecting insulin or proinsulin-dependent IR-A function.
3. Discussion
Decorin is a stromal proteoglycan synthesized by fibroblasts, stressed vascular endothelial cells and smooth muscle cells (Neill et al., 2012b). The role of decorin in regulating the biology of various types of cancer is well-established as in fact decorin modulates the activity of several receptor tyrosine-kinases critical for cell growth and survival of malignant cells (Iozzo, 1997; Neill et al., 2012b; Schaefer and Iozzo, 2008). The mechanism of action has been partially elucidated and occurs via a transient activation of the EGFR and the Met receptor (Buraschi et al., 2010; Goldoni et al., 2009; Iozzo et al., 1999; Moscatello et al., 1998), which is associated to caveolin-mediated endocytosis, downregulation of the receptors in lysosomes and subsequent inhibition of cell growth (Csordas et al., 2000; Zhu et al., 2005). Decorin action on the IGF-I system, and in particular on the IGF-IR, is more complex as it differs between non-transformed (normal) and transformed cells (Morrione et al., 2013). Moreover, the insulin receptor A isoform (IR-A), together with autocrine production of its ligand IGF-II, is emerging as an important mechanism of normal and cancer cell expansion and is a key feature of several malignancies (Bartella et al., 2012; Belfiore and Malaguarnera, 2011).
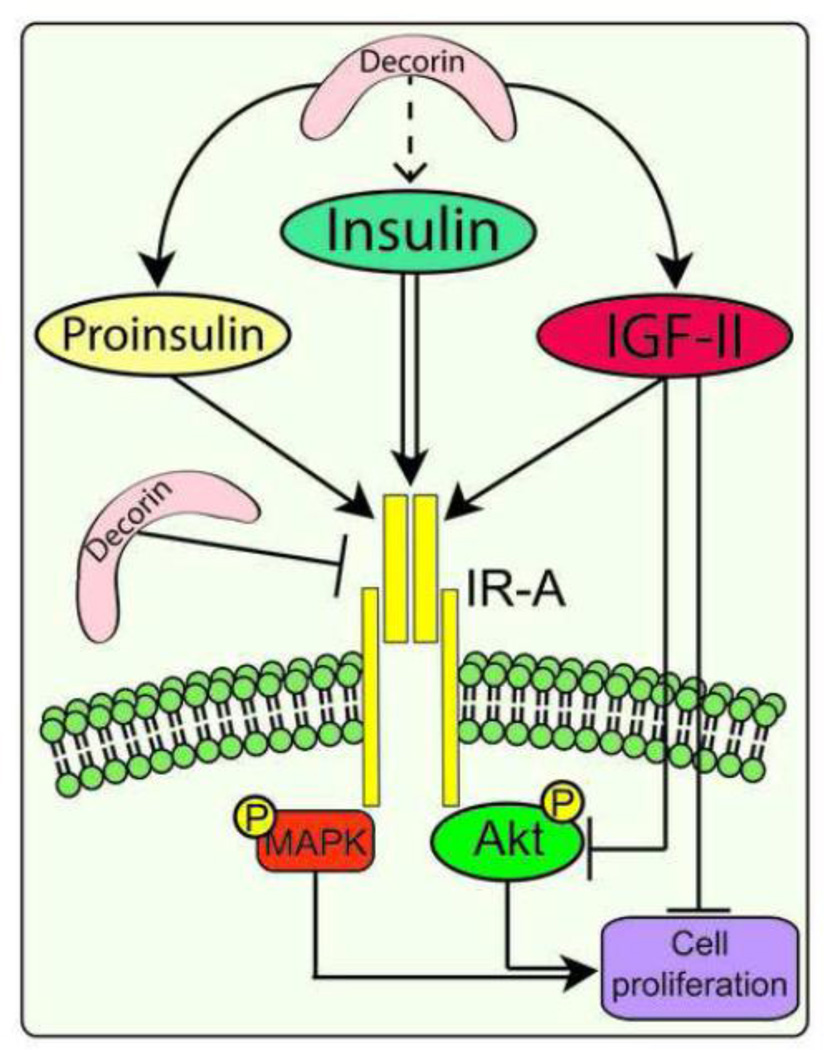
In this study we investigated whether decorin may regulate IR-A activity mediated by its ligands IGF-II, insulin and proinsulin using mouse embryo fibroblasts lacking the Igf1r and expressing exclusively the human IR-A (Miura et al., 1995). We found that: (i) Decorin binds with high affinity both the IR-A and IR-A ligands, although the affinity for the IR-A and proinsulin is three-fold lower than the affinity for IGF-II and insulin; (ii) Decorin does not affect ligand-mediated IR-A phosphorylation; (iii) Decorin enhances downregulation of the IR-A after prolonged IGF-II stimulation but does not affect IR-A stability after insulin or proinsulin stimulation; (iv) Decorin regulates cell surface IR-A levels by affecting insulin-dependent internalization; (v) Decorin inhibits IGF-II-mediated Akt activation without affecting insulin-and proinsulin-dependent signaling, and (vi) Decorin negatively regulates IGF-II-dependent mitogenesis but not cell proliferation induced by insulin or proinsulin. A schematic draw summarizing our results and decorin action on IR-A activity is shown in Fig. 6.
Fig. 6.
Schematic drawing summarizing decorin effects on the regulation of ligand-dependent IR-A activity. Decorin binds IGF-II, insulin, proinsulin and the IR-A and enhances IGF-II-induced IR-A downregulation, Akt activation and IGF-II-induced cell proliferation. In addition decorin may regulate IR-A levels at the cell surface. For more information consult the Discussion.
We have recently demonstrated that, in urothelial cancer cells, decorin binding to IGF-I and the IGF-IR inhibits receptor phosphorylation and downstream activation of the Akt and MAPK pathways, which is associated with a significant reduction in motility and invasion capabilities of these cells (Iozzo et al., 2011). Surprisingly, decorin did not affected IGF-IR stability (Iozzo et al., 2011) providing the first evidence in support of a very different mode of decorin action between transformed and non-transformed cells, where decorin promotes transient IGF-IR activation followed by IGF-IR degradation (Morrione et al., 2013). R−/IR-A mouse embryo fibroblast cells are able to grow in serum-free media supplemented solely with growth factors (Frasca et al., 1999; Morcavallo et al., 2012; Morrione et al., 1997b) but they are not fully transformed as in fact an overexpressed human IR-A in the absence of the Igf1r is not able to promote colony formation in soft-agar and anchorage-independent growth (Miura et al., 1995). Notably, our results suggest that decorin plays a role in regulating ligand-induced IR-A action in a way that mechanistically differs from both non-transformed and transformed cells. In fact decorin does not affect receptor phosphorylation but enhances IR-A downregulation and negatively modulates downstream signaling. More significantly, decorin specifically regulates IGF-II-mediated IR-A function and signaling without interfering with insulin and proinsulin-induced IR-A pathways in spite of the similar affinity of decorin for IGF-II, insulin and proinsulin.
IGF-binding proteins (IGF-BPs), which either positively or negatively modulate the IGF-I system, depending on cell and tissue models (Jogie-Brahim et al., 2009) bind IGF-I and IGF-II but not insulin or proinsulin (Forbes et al., 2012). We can speculate therefore that IGF-BPs may complex with decorin, potentiate decorin binding to IGF-II thereby promoting prolonged occupancy and enhancing decorin action on IGF-II but not on insulin and proinsulin. This different binding capacity would therefore differentially regulate IR-A signaling and biological responses. Whether decorin may complex IGF-II in the presence of IGF-BPs remains to be determined and further experiments are required to clarify this issue. However, there is ample evidence that decorin is synthesized and released in the circulation by activated macrophages during sterile and bacterial sepsis in both human and mouse blood (Merline et al., 2011). Thus, there is the possibility of a direct interaction between decorin and IGF-BP to modulate IGF system in peripheral tissues.
Our recent work (Iozzo et al., 2011) has shown an affinity of decorin for IGF-I very similar to the one we demonstrated in the present work for IGF-II and insulin, but while decorin strongly affects IGF-IR activation (Iozzo et al., 2011), it has no effect in modulating either IGF-II- or insulin-induced phosphorylation of the IR-A. However, our experiments on the IGF-IR were conducted in bladder cancer cells (Iozzo et al., 2011), which do not express decorin, while fibroblast cells used in these studies produce decorin as previously shown (Neill et al., 2012b).
We have recently demonstrated that insulin and IGF-II affect IR-A biological responses by differentially regulating IR-A trafficking (Morcavallo et al., 2012). Using R−/IR-A cells, we discovered that insulin significantly promotes IR-A internalization, a process only modestly affected by IGF-II. Notably, prolonged stimulation of R−/IR-A cells by insulin, but not by IGF-II, targets the receptor for degradation (Morcavallo et al., 2012). Decorin alone or in combination with either insulin or proinsulin has no effect on regulating IR-A stability but significantly enhances IGF-II-mediated IR-A downregulation. These results would suggest that decorin could play a role in regulating IR-A endocytosis by enhancing IGF-II-mediated IR-A internalization from the cell surface. However, decorin did not significantly affect IGF-II-mediated IR-A internalization from the cell surface indicating that decorin may play a more relevant role in regulating later stages of receptor endocytosis such as IGF-II-dependent sorting of the IR-A into lysosomal compartments. Significantly, decorine protein core reduced early time points of insulin-mediated IR-A internalization but it did not affect insulin-modulated IR-A stability. These results suggest the hypothesis that decorin may contribute to the regulation of cell surface IR-A levels by modulating receptor recycling to the membrane.
We also demonstrated that upon ligand stimulation the IR-A is internalized via both clathrin-dependent and -independent pathways (Morcavallo et al., 2012). However, clathrin-dependent endocytosis is absolutely necessary for ligand-induced IR-A degradation as siRNA-mediated depletion of endogenous clathrin severely reduced IR-A degradation, which was not affected instead by targeting caveolin-1 (Morcavallo et al., 2012). Decorin, therefore, may contribute to enhance the pool of IR-A internalized upon IGF-II stimulation through clathrin-dependent pathways thereby increasing the fraction of the IR-A sorted for degradation. Experiments are currently under way to determine the molecular details of decorin action on IR-A endocytosis and trafficking.
In conclusion, we have investigated the role of soluble decorin in modulating the bioactivity of various ligands for the insulin receptor isoforms, utilizing a well-defined genetic cell system lacking the endogenous Igf1r but expressing the insulin receptor isoform A (IR-A). We discovered that decorin binds with high affinity insulin and IGF-II and, to a lesser extent, proinsulin and IR-A, and that it does not affect ligand-induced IR-A phosphorylation. However, decorin evokes IR-A downregulation after prolonged IGF-II stimulation without affecting insulin- or proinsulin-dependent effects on IR-A stability. Moreover, soluble decorin attenuates IGF-II-mediated cell proliferation of R−/IR-A cells but affects neither insulin- nor proinsulin-dependent mitogenesis. These results suggest that decorin differentially regulates the action of IR-A ligands by preferentially inhibiting IGF-II-mediated biological responses without affecting insulin- or proinsulin-dependent signaling. Our findings corroborate the notion that decorin’s regulation of a wide network of tyrosine-kinase receptor signaling could play a critical role in the regulation of many aspects of mammalian biology in physiology and disease.
4. Experimental procedures
4.1. Cell lines and recombinant proteins
R−/IR-A cells are mouse embryo fibroblasts derived from Igf1r−/− mice (Sell et al., 1994) and expressing solely the human IR-A isoform (Miura et al., 1995). Cells are maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 µg/ml of puromycin. These cells were previously well characterized for their mitogenic response to insulin and IGF-II (Frasca et al., 1999; Morrione et al., 1997b). Serum-free medium (SFM) is DMEM supplemented with 0.1% bovine serum albumin and 50 µg/ml of transferrin (Sigma-Aldrich). The production of human recombinant decorin protein core has been previously described (Merline et al., 2011). Human recombinant IR-A was purchased from Sino Biological Inc. (11086-H08H) and consisted of the IR-A extracellular domain (Met 1-Lys 944) fused with a polyhistidine tag at the C-terminus. Recombinant human IGF-II was from Peprotech, insulin from porcine pancreas was from Sigma-Aldrich while human proinsulin was purchased from R&D System.
Decorin protein core binding to the IR-A was assessed by ELISA. Briefly, wells were coated with recombinant IR-A (100 ng/well) overnight at room temperature in the presence of carbonate buffer, pH 9.6. Plates were then washed with PBS and incubated for two h with serial dilutions of decorin core (up to 200 nM). Plates were then extensively washed with PBS, blocked with PBS/1% BSA, and incubated with primary antibody against the N-terminus of decorin and HRP-conjugated secondary antibody. Signal was detected using Sigma-Fast tablets (Sigma-Aldrich) and read at 450 nm OD. Decorin protein core binding to IGF-II, insulin or proinsulin was performed as described above but plates were coated with IGF-II, insulin or proinsulin (100 ng/well) instead. All experiments on IR-A phosphorylation, stability and downstream signaling were performed using decorin core protein at 200 nM, which is the optimal concentration previously established to affect IGF-IR activity and signaling (Iozzo et al., 2011).
4.2. Detection of IR-A activation and IR-A levels
Serum-starved R−/IR-A cells were preincubated with decorin (200 nM) for 1 h and then incubated with SFM or physiological concentration (10 nM) of IGF-II, insulin or proinsulin and decorin or decorin alone for 10 min. To test the effect of decorin binding to the ligand, IGF-II, insulin and proinsulin were preincubated with 200 nM decorin core and then supplemented to cells. IR-A phosphorylation was detected by immunoblot using anti-phospho-IGF-IRβ (Tyr1135/1136)/IRβ (Tyr1150/1151) rabbit monoclonal antibodies (Cell Signaling Technology). Total IR-A was assessed using anti-IR polyclonal antibodies, which recognize the β subunit of the IR-A (Santa Cruz Biotechnology). IR-A stability was assessed by testing IR-A levels by immunoblot after 24 h of IGF-II, insulin or proinsulin (10 nM) stimulation with or without 200 nM decorin. Protein loading was monitored using anti-GAPDH monoclonal antibodies (GeneTex). Blots are representative of three independent experiments.
4.3. Analysis of cell surface IR-A levels
The level of IR-A on the cell surface of R−/IR-A cells was determined by ELISA assays (Morcavallo et al., 2012) using a monoclonal antibody against the IR α-subunit (Novus Biologicals, diluted 1:1000), and goat anti-mouse alkaline phosphatase-conjugated antibody (diluted 1:1000; Sigma-Aldrich). Antibody binding was visualized by adding 0.25 ml of alkaline phosphatase substrate (Bio-Rad). The reaction was stopped by transferring 0.1 ml of the substrate to a 96-well microtiter plate containing 0.1 ml of 0.4 M NaOH. Plates were read at 405 nm in a microplate reader (Dynex Technologies) using Microplate Manager Software.
4.4. Detection of activated MAPK and Akt and cell growth experiments
Serum-starved R−/IR-A cells were preincubated with decorin for 1 h and then stimulated with IGF-II, insulin or proinsulin (10 nM), and decorin or decorin alone for 10 min. The activation of p90RSK, Akt, ERK1/2 and pS6 ribosomal protein was analyzed by Western immunoblot using the PathScan Multiplex Western Cocktail I (Cell Signaling Technology) (Monami et al., 2009; Monami et al., 2008; Monami et al., 2006). ElF4E protein is provided in the kit as control to monitor the loading of the samples. Densitometric analysis was performed using the ImageJ program (rsbweb.nih.gov/ij/).
Growth curves of R−/IR-A cells in the presence of either IGF-II, insulin or proinsulin alone (10 nM) or supplemented with decorin core (200 nM) were performed as previously described from our laboratories (Morcavallo et al., 2012; Morrione et al., 1997a; Morrione et al., 1997b). Briefly 3×104 cells were plated in serum-containing media and counted after 24 h (time 0). At that point cells were shifted to SFM or SFM supplemented with decorin alone, ligands alone or the combination of the two. Cells were counted after 48 h with a hemocytometer.
4.5. Statistical analysis
Experiments were carried out in triplicate and repeated at least three times. Results are expressed as mean ± SEM. All statistical analyses were carried out with SigmaStat for Windows version 3.10 (Systat Software, Inc., Port Richmond, CA). Results were compared using the two-sided Student’s t test. Differences were considered statistically significant at p < 0.05.
Highlights.
We tested decorin interaction with the IR-A and its ligands.
Decorin binds with high affinity the IR-A, IGF-II, insulin and proinsulin
Decorin does not affect ligand-induced IR-A phosphorylation
Decorin specifically modulates IGF-II-mediated IR-A downregulation
Decorin preferentially inhibits IR-A signaling induced by IGF-II
Acknowledgments
We thank Dr. Renato Baserga for the kind gift R−/IR-A cells. This work was supported in part by the Benjamin Perkins Bladder Cancer Fund and National Institutes of Health Grants RO1 CA164462 (A.M., R.V.I.), and RO1 CA39481 and RO1 CA47282 (R.V.I.), and grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) (grant n. 10625/12), AIRC project Calabria 2013 and Fondazione Cassa di Risparmio di Calabria e Lucania PON01_01078 (A.B.)
Abbreviations
- SLRP
small leucine-rich proteoglyvan
- RTK
receptor tyrosine-kinase
- R−
Igf1r−/− cells
- IR-A
insulin receptor isoform A
- IGF-IR
insulin-like growth factor receptor 1
- IGF-II
insulin-like growth factor II
- MAPK
mitogen-activated protein kinase
- IGF-BP
IGF-binding protein
- IRS-1
insulin receptor substrate 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bartella V, De Marco P, Malaguarnera R, Belfiore A, Maggiolini M. New advances on the functional cross-talk between insulin-like growth factor-I and estrogen signaling in cancer. Cell. Signal. 2012;24:1515–1521. doi: 10.1016/j.cellsig.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim. Biophys. Acta. 1997;1332:F105–F126. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr. Pharm. Des. 2007;13:671–686. doi: 10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125–R147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- Brandan E, Gutierrez J. Role of proteoglycans in the regulation of the skeletal muscle fibrotic response. FEBS J. 2013a;280:4109–4117. doi: 10.1111/febs.12278. [DOI] [PubMed] [Google Scholar]
- Brandan E, Gutierrez J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013b;32:289–297. doi: 10.1016/j.matbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E2582–E2591. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraschi S, Neill T, Owens RT, Iniguez LA, Purkins G, Vadigepalli R, Evans B, Schaefer L, Peiper SC, Wang ZX, Iozzo RV. Decorin protein core affects the global gene expression profile of the tumor microenvironment in a triple-negative orthotopic breast carcinoma xenograft model. PLoS One. 2012;7:e45559. doi: 10.1371/journal.pone.0045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes Met receptor activity and down-regulates β-catenin and Myc levels. J. Biol. Chem. 2010;285:42075–42085. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sun M, Iozzo RV, Kao WW, Birk DE. Intracellularly-retained decorin lacking the C-terminal ear repeat causes ER stress: a cell-based etiological mechanism for congenital stromal corneal dystrophy. Am J Pathol. 2013;183:247–256. doi: 10.1016/j.ajpath.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnoczky G, Iozzo RV. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler J, Ludwig T, Fisher P, Leighton PA, Tilghman SM, Efstratiadis A. Mouse mutant embryos overexpressing IGF-II exhibit phenotypic features of the Beckwith-Wiedemann and Simpson-Golabi-Behmel syndromes. Genes Dev. 1997;11:3128–3142. doi: 10.1101/gad.11.23.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler LR, Schonherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, Eble JA. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. J. Biol. Chem. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- Forbes BE, McCarthy P, Norton RS. Insulin-like growth factor binding proteins: a structural perspective. Frontiers in endocrinology. 2012;3:38. doi: 10.3389/fendo.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell. Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacob D, Cai J, Tsonis M, Babwah A, Chakraborty C, Bhattacharjee RN, Lala PK. Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different tyrosine kinase receptors. Endocrinology. 2008;149:6187–6197. doi: 10.1210/en.2008-0780. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit. Rev. Biochem. Mol. Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Buraschi S, Genua M, Xu SQ, Solomides CC, Peiper SC, Gomella LG, Owens RC, Morrione A. Decorin antagonizes IGF-IR function by interfering with IGF-IR activity and attenuating downstream signaling. J. Biol. Chem. 2011;286:34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- Jarvelainen H, Puolakkainen P, Pakkanen S, Brown EL, Hook M, Iozzo RV, Sage EH, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr. Rev. 2009;30:417–437. doi: 10.1210/er.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli KR, Falowo OI, Bale LK, Zschunke MA, Roche PC, Conover CA. Functional insulin receptors on human epithelial ovarian carcinoma cells: implications for IGF-II mitogenic signaling. Endocrinology. 2002;143:3259–3267. doi: 10.1210/en.2001-211408. [DOI] [PubMed] [Google Scholar]
- Krywicki RF, Yee D. The insulin-like growth factor family of ligands, receptors, and binding proteins. Breast Cancer Res. Treat. 1992;22:7–19. doi: 10.1007/BF01833329. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Malaguarnera R, Sacco A, Voci C, Pandini G, Vigneri R, Belfiore A. Proinsulin binds with high affinity the insulin receptor isoform A and predominantly activates the mitogenic pathway. Endocrinology. 2012;153:2152–2163. doi: 10.1210/en.2011-1843. [DOI] [PubMed] [Google Scholar]
- Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Surmacz E, Burgaud JL, Baserga R. Different effects on mitogenesis and transformation of a mutation at tyrosine 1251 of the insulin-like growth factor I receptor. J. Biol. Chem. 1995;270:22639–22644. doi: 10.1074/jbc.270.38.22639. [DOI] [PubMed] [Google Scholar]
- Monami G, Emiliozzi V, Bitto A, Lovat F, Xu SQ, Goldoni S, Fassan M, Serrero G, Gomella LG, Baffa R, Iozzo RV, Morrione A. Proepithelin regulates prostate cancer cell biology by promoting cell growth, migration, and anchorage-independent growth. Am. J. Pathol. 2009;174:1037–1047. doi: 10.2353/ajpath.2009.080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monami G, Emiliozzi V, Morrione A. Grb10/Nedd4-mediated multiubiquitination of the insulin-like growth factor receptor regulates receptor internalization. J. Cell. Physiol. 2008;216:426–437. doi: 10.1002/jcp.21405. [DOI] [PubMed] [Google Scholar]
- Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- Morcavallo A, Genua M, Palummo A, Kletvikova E, Jiracek J, Brzozowski AM, Iozzo RV, Belfiore A, Morrione A. Insulin and insulin-like growth factor II differentially regulate endocytic sorting and stability of insulin receptor isoform A. J. Biol. Chem. 2012;287:11422–11436. doi: 10.1074/jbc.M111.252478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrione A, Neill T, Iozzo RV. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J. 2013;280:2138–2149. doi: 10.1111/febs.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrione A, Valentinis B, Resnicoff M, Xu S, Baserga R. The role of mGrb10alpha in insulin-like growth factor I-mediated growth. J. Biol. Chem. 1997a;272:26382–26387. doi: 10.1074/jbc.272.42.26382. [DOI] [PubMed] [Google Scholar]
- Morrione A, Valentinis B, Xu SQ, Yumet G, Louvi A, Efstratiadis A, Baserga R. Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc. Natl. Acad. Sci. U. S. A. 1997b;94:3777–3782. doi: 10.1073/pnas.94.8.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J. Clin. Invest. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Jones HR, Crane-Smith Z, Owens RT, Schaefer L, Iozzo RV. Decorin induces rapid secretion of thrombospondin-1 in basal breast carcinoma cells via inhibition of Ras homolog gene family, member A/Rho-associated coiled-coil containing protein kinase 1. FEBS J. 2013a;280:2353–2368. doi: 10.1111/febs.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L, Iozzo RV. Decorin antagonizes the angiogenic network: concurrent inhibition of Met, hypoxia inducible factor 1alpha, vascular endothelial growth factor A, induction of thrombospondin-1 and TIMP3. J. Biol. Chem. 2012a;287:5492–5506. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am. J. Pathol. 2012b;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Torres A, Buraschi S, Iozzo RV. Decorin has an appetite for endothelial cell autophagy. Autophagy. 2013b;9 doi: 10.4161/auto.25881. [DOI] [PubMed] [Google Scholar]
- Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J. Biol. Chem. 2012;287:33926–33933. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J. Biol. Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- Rose DW, Saltiel AR, Majumdar M, Decker SJ, Olefsky JM. Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. Proc. Natl. Acad. Sci. U. S. A. 1994;91:797–801. doi: 10.1073/pnas.91.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainio A, Nyman M, Lund R, Vuorikoski S, Bostrom P, Laato M, Bostrom PJ, Jarvelainen H. Lack of decorin expression by human bladder cancer cells offers new tools in the therapy of urothelial malignancies. PLoS One. 2013;8:e76190. doi: 10.1371/journal.pone.0076190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J. Biol. Chem. 2000;275:35153–35161. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- Santra M, Reed CC, Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J. Biol. Chem. 2002;277:35671–35681. doi: 10.1074/jbc.M205317200. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J. Biol. Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L, Grone HJ, Reinhardt DP, Pfeilschifter J, Iozzo RV, Schaefer RM. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and Mammalian target of rapamycin. Am. J. Pathol. 2007;170:301–315. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Stone ME, Stiles CD. Platelet-derived growth factor prevents G0 growth arrest. Nature. 1979;281:390–392. doi: 10.1038/281390a0. [DOI] [PubMed] [Google Scholar]
- Schonherr E, Sunderkotter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J. Biol. Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18:2471–2479. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- Sciacca L, Mineo R, Pandini G, Murabito A, Vigneri R, Belfiore A. In IGF-I receptor-deficient leiomyosarcoma cells autocrine IGF-II induces cell invasion and protection from apoptosis via the insulin receptor isoform A. Oncogene. 2002;21:8240–8250. doi: 10.1038/sj.onc.1206058. [DOI] [PubMed] [Google Scholar]
- Seidler DG. The galactosaminoglycan-containing decorin and its impact on diseases. Curr. Opin. Struct. Biol. 2012;22:578–582. doi: 10.1016/j.sbi.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell. Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles CD, Isberg RR, Pledger WJ, Antoniades HN, Scher CD. Control of the Balb/c-3T3 cell cycle by nutrients and serum factors: analysis using platelet-derived growth factor and platelet-poor plasma. J. Cell. Physiol. 1979;99:395–405. doi: 10.1002/jcp.1040990314. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Crimmins DL, Myers MG, Jr, Miralpeix M, White MF. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol. Cell. Biol. 1993;13:7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella V, Pandini G, Sciacca L, Mineo R, Vigneri R, Pezzino V, Belfiore A. A novel autocrine loop involving IGF-II and the insulin receptor isoform-A stimulates growth of thyroid cancer. J. Clin. Endocrinol. Metab. 2002;87:245–254. doi: 10.1210/jcem.87.1.8142. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J. Biol. Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]