Abstract
We previously characterized a methionine aminopeptidase (EC 3.4.11.18; Met-AP1; also called peptidase M) in Saccharomyces cerevisiae, which differs from its prokaryotic homologues in that it (i) contains an N-terminal zinc-finger domain and (ii) does not produce lethality when disrupted, although it does slow growth dramatically; it is encoded by a gene called MAP1. Here we describe a second methionine aminopeptidase (Met-AP2) in S. cerevisiae, encoded by MAP2, which was cloned as a suppressor of the slow-growth phenotype of the map1 null strain. The DNA sequence of MAP2 encodes a protein of 421 amino acids that shows 22% identity with the sequence of yeast Met-AP1. Surprisingly, comparison with sequences in the GenBank data base showed that the product of MAP2 has even greater homology (55% identity) with rat p67, which was characterized as an initiation factor 2-associated protein but not yet shown to have Met-AP activity. Transformants of map1 null cells expressing MAP2 in a high-copy-number plasmid contained 3- to 12-fold increases in Met-AP activity on different peptide substrates. The epitope-tagged suppressor gene product was purified by immunoaffinity chromatography and shown to contain Met-AP activity. To evaluate the physiological significance of Met-AP2, the MAP2 gene was deleted from wild-type and map1 null yeast strains. The map2 null strain, like the map1 null strain, is viable but with a slower growth rate. The map1, map2 double-null strains are nonviable. Thus, removal of N-terminal methionine is an essential function in yeast, as in prokaryotes, but yeast require two methionine aminopeptidases to provide the essential function which can only be partially provided by Met-AP1 or Met-AP2 alone.
Full text
PDF


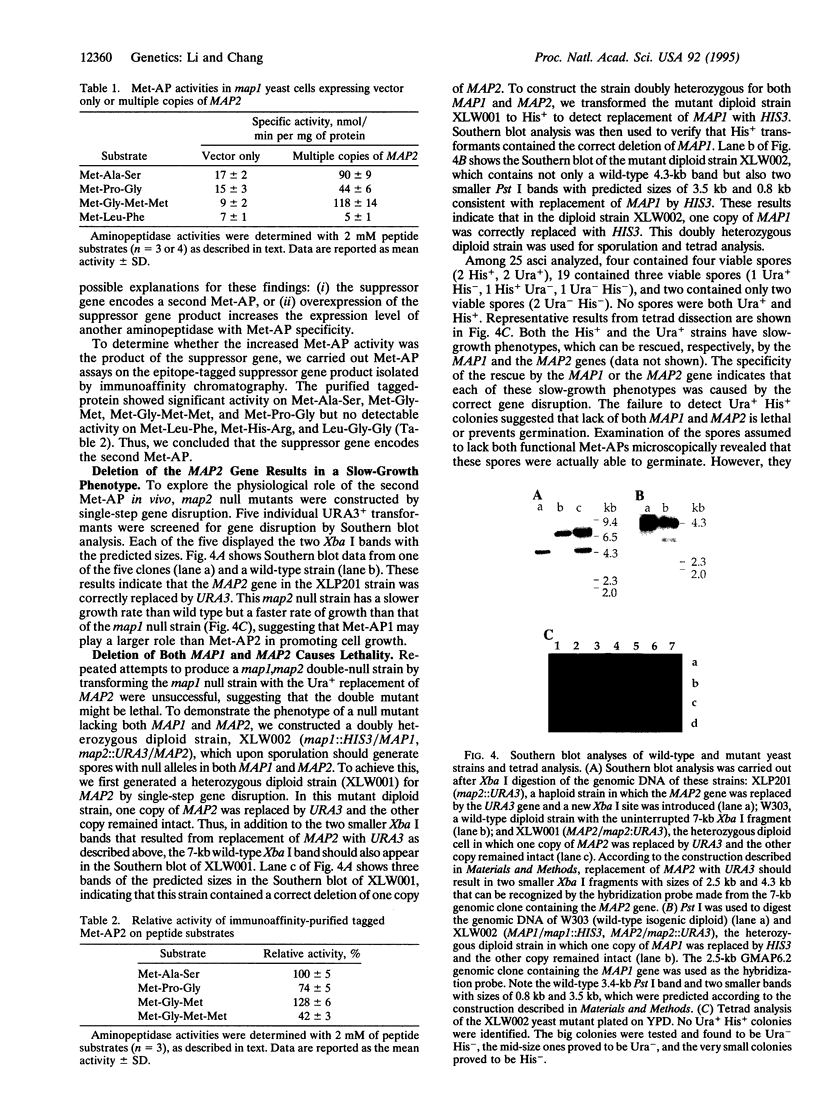

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Kendall R. L., Hall L., Weaver L. H., Stewart A. E., Matthews B. W., Bradshaw R. A. Eukaryotic methionyl aminopeptidases: two classes of cobalt-dependent enzymes. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7714–7718. doi: 10.1073/pnas.92.17.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F., Weaver L. H., Roderick S. L., Huber R., Matthews B. W. Sequence and structure comparison suggest that methionine aminopeptidase, prolidase, aminopeptidase P, and creatinase share a common fold. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2473–2477. doi: 10.1073/pnas.91.7.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Bassat A., Bauer K., Chang S. Y., Myambo K., Boosman A., Chang S. Processing of the initiation methionine from proteins: properties of the Escherichia coli methionine aminopeptidase and its gene structure. J Bacteriol. 1987 Feb;169(2):751–757. doi: 10.1128/jb.169.2.751-757.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang S. Y., McGary E. C., Chang S. Methionine aminopeptidase gene of Escherichia coli is essential for cell growth. J Bacteriol. 1989 Jul;171(7):4071–4072. doi: 10.1128/jb.171.7.4071-4072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. H., Labgold M. R., Richards J. H. Altering enzymatic activity: recruitment of carboxypeptidase activity into an RTEM beta-lactamase/penicillin-binding protein 5 chimera. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2823–2827. doi: 10.1073/pnas.87.7.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. H., Teichert U., Smith J. A. Molecular cloning, sequencing, deletion, and overexpression of a methionine aminopeptidase gene from Saccharomyces cerevisiae. J Biol Chem. 1992 Apr 25;267(12):8007–8011. [PubMed] [Google Scholar]
- Chang Y. H., Teichert U., Smith J. A. Purification and characterization of a methionine aminopeptidase from Saccharomyces cerevisiae. J Biol Chem. 1990 Nov 15;265(32):19892–19897. [PubMed] [Google Scholar]
- Donahue T. F., Cigan A. M., Pabich E. K., Valavicius B. C. Mutations at a Zn(II) finger motif in the yeast eIF-2 beta gene alter ribosomal start-site selection during the scanning process. Cell. 1988 Aug 26;54(5):621–632. doi: 10.1016/s0092-8674(88)80006-0. [DOI] [PubMed] [Google Scholar]
- Duronio R. J., Towler D. A., Heuckeroth R. O., Gordon J. I. Disruption of the yeast N-myristoyl transferase gene causes recessive lethality. Science. 1989 Feb 10;243(4892):796–800. doi: 10.1126/science.2644694. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Aigle M., Aljinovic G., André B., Baclet M. C., Barthe C., Baur A., Bécam A. M., Biteau N., Boles E. Complete DNA sequence of yeast chromosome II. EMBO J. 1994 Dec 15;13(24):5795–5809. doi: 10.1002/j.1460-2075.1994.tb06923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Elliott R. C., Liu P. S., Koduri R. K., Weickmann J. L., Lee J. H., Blair L. C., Ghosh-Dastidar P., Bradshaw R. A., Bryan K. M. Specificity of cotranslational amino-terminal processing of proteins in yeast. Biochemistry. 1987 Dec 15;26(25):8242–8246. doi: 10.1021/bi00399a033. [DOI] [PubMed] [Google Scholar]
- Kendall R. L., Bradshaw R. A. Isolation and characterization of the methionine aminopeptidase from porcine liver responsible for the co-translational processing of proteins. J Biol Chem. 1992 Oct 15;267(29):20667–20673. [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Li X., Chang Y. H. Molecular cloning of a human complementary DNA encoding an initiation factor 2-associated protein (p67). Biochim Biophys Acta. 1995 Feb 21;1260(3):333–336. doi: 10.1016/0167-4781(94)00227-t. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Kukral A. M., Miller J. L., Movva N. R. pepM is an essential gene in Salmonella typhimurium. J Bacteriol. 1989 Sep;171(9):5215–5217. doi: 10.1128/jb.171.9.5215-5217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Strauch K. L., Kukral A. M., Miller J. L., Wingfield P. T., Mazzei G. J., Werlen R. C., Graber P., Movva N. R. N-terminal methionine-specific peptidase in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1987 May;84(9):2718–2722. doi: 10.1073/pnas.84.9.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerschell R. P., Hosokawa Y., Tsunasawa S., Sherman F. The specificities of yeast methionine aminopeptidase and acetylation of amino-terminal methionine in vivo. Processing of altered iso-1-cytochromes c created by oligonucleotide transformation. J Biol Chem. 1990 Nov 15;265(32):19638–19643. [PubMed] [Google Scholar]
- Pathak V. K., Nielsen P. J., Trachsel H., Hershey J. W. Structure of the beta subunit of translational initiation factor eIF-2. Cell. 1988 Aug 26;54(5):633–639. doi: 10.1016/s0092-8674(88)80007-2. [DOI] [PubMed] [Google Scholar]
- Riles L., Olson M. V. Nonsense mutations in essential genes of Saccharomyces cerevisiae. Genetics. 1988 Apr;118(4):601–607. doi: 10.1093/genetics/118.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick S. L., Matthews B. W. Structure of the cobalt-dependent methionine aminopeptidase from Escherichia coli: a new type of proteolytic enzyme. Biochemistry. 1993 Apr 20;32(15):3907–3912. doi: 10.1021/bi00066a009. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tsunasawa S., Stewart J. W., Sherman F. Amino-terminal processing of mutant forms of yeast iso-1-cytochrome c. The specificities of methionine aminopeptidase and acetyltransferase. J Biol Chem. 1985 May 10;260(9):5382–5391. [PubMed] [Google Scholar]
- Vernet T., Dignard D., Thomas D. Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52(2-3):225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Wu S., Gupta S., Chatterjee N., Hileman R. E., Kinzy T. G., Denslow N. D., Merrick W. C., Chakrabarti D., Osterman J. C., Gupta N. K. Cloning and characterization of complementary DNA encoding the eukaryotic initiation factor 2-associated 67-kDa protein (p67). J Biol Chem. 1993 May 25;268(15):10796–10801. [PubMed] [Google Scholar]
- Zuo S., Guo Q., Chang Y. H. A protease assay via precolumn derivatization and high-performance liquid chromatography. Anal Biochem. 1994 Nov 1;222(2):514–516. doi: 10.1006/abio.1994.1529. [DOI] [PubMed] [Google Scholar]
- Zuo S., Guo Q., Ling C., Chang Y. H. Evidence that two zinc fingers in the methionine aminopeptidase from Saccharomyces cerevisiae are important for normal growth. Mol Gen Genet. 1995 Jan 20;246(2):247–253. doi: 10.1007/BF00294688. [DOI] [PubMed] [Google Scholar]