Summary
Herpesviruses, including human cytomegalovirus (HCMV), encode multiple microRNAs (miRNA) whose targets are just being uncovered. Moreover, miRNA function during the virus life cycle is relatively unknown. We find that HCMV miRs UL112-1, US5-1, and US5-2 target multiple components of the host secretory pathway, including VAMP3, RAB5C, RAB11A, SNAP23, and CDC42. A HCMV miR UL112-1, US5-1, and US5-2 triple mutant displayed aberrant morphogenesis of the virion assembly compartment (VAC), increased secretion of non-infectious particles, and increased IL-6 release from infected cells. Ectopic expression of UL112-1, US5-1, and US5-2 or siRNAs directed against RAB5C, RAB11A, SNAP23, and CDC42 caused the loss of Golgi stacks with reorganization into structures that resemble the VAC and a decrease in cytokine release. These observations indicate that multiple HCMV miRNAs coordinately regulate reorganization of the secretory pathway to control cytokine secretion and facilitate formation of the VAC for efficient infectious virus production.
Introduction
Human cytomegalovirus (HCMV) is a β-herpesvirus that encodes multiple microRNAs (miRNAs) (Grey et al., 2005, Pfeffer et al., 2004, Stark et al., 2012). miRNAs are small noncoding RNAs (19-22 nucleotides) that post-transcriptionally regulate gene expression. In general miRNAs target the 3′untranslated region (UTR) of mRNAs through the RNA-Induced Silencing Complex (RISC) that leads to translational repression and degradation of the targeted mRNA (Lim et al., 2003). The miRNA targets of herpesvirus miRNAs are slowly emerging and include both viral transcriptional activators and cellular genes involved in immune evasion, signaling, and apoptosis (Abend et al., 2010, Grey et al., 2007, Lei et al., 2010, Stern-Ginossar et al., 2007, Umbach et al., 2008, Samols et al., 2007, Ziegelbauer et al., 2009, Kim et al., 2012). HCMV miRNA targets include viral genes such as the virus major immediate-early gene product IE72 and US7 as well as cellular genes involved in immune defense such as the cellular major histocompatibility complex class I-related chain B (MICB), a stress induced ligand of the natural killer (NK) cell activating receptor NKG2D, and RANTES (Grey et al., 2007, Stern-Ginossar et al., 2007, Tirabassi et al., 2011, Kim et al., 2012).
miRNAs may converge on related pathways and even exhibit redundant roles in targeting genes such as cellular miR-1 and miR-133 regulating skeletal muscle proliferation and differentiation (Chen et al., 2006). The first indication that viral miRNAs target functionally related genes in a cellular pathway was the observation that the targets of HCMV miR-US25-1 regulate multiple genes involved in the cell cycle regulation, tumor progression, and chromatin remodeling (Grey et al., 2010). Additional evidence derives from the identification of the Kaposi's sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV) miRNA cellular targetomes using photoactivatable-ribonucleoside enhanced crosslinking (PAR-CLIP) with RISC immunoprecipitation biochemical analyses. In these studies KSHV and EBV miRNAs in latently infected transformed cells were observed to target multiple genes involved in transcriptional regulation, signal transduction, innate immunity, vesicular trafficking, and the regulation of cell cycle and apoptosis (Gottwein et al., 2011, Skalsky et al., 2012).
IL-6 and TNF-α are inflammatory cytokines that are induced by HCMV early in infection through the activation of Nuclear Factor-κB (NF-κB) (Kowalik et al., 1993, Yurochko et al., 1997a, Yurochko et al., 1997b). IL-6 and TNF-α play important roles in stimulating cellular innate immunity and HCMV has developed multiple mechanisms to block the antiviral effects of these inflammatory cytokines. These strategies include down-regulation of the TNFR1 from the plasma membrane, transcriptional repression of both IL-6 and TNF-α, and post-transcriptional repression of IL-6 through destabilization of mRNA (Baillie et al., 2003, Jarvis et al., 2006, Gealy et al., 2005). IL-6 and TNF-α are released from the cell through vesicles in the secretory pathway utilizing a number of endocytic proteins including VAMP-3, RAB11A, and SNAP23 during the process. Both EBV and KSHV encode miRNAs that regulate vesicular trafficking, thus regulating the cellular release of inflammatory cytokines through miRNA targeting of key endocytic proteins would provide an attractive mechanism of evading the innate immune response triggered by IL-6 and TNF-α.
The secretory pathway plays an essential role in HCMV assembly and egress from the cell. Following encapsidation and partial tegumentation of the HCMV genome in the nucleus, capsids are transported to the Virion Assembly Compartment (VAC) in the cytoplasm for additional tegumentation and final virion envelopment. Subsequently, enveloped viral particles egress from the cell using components of the secretory pathway although this process is poorly understood (Alwine, 2012, Das and Pellett, 2011, Das et al., 2007). The secretory pathway is composed of the Endoplasmic Reticulum (ER), Golgi Complex, and trafficking vesicles. These compartments are defined by their intracellular location, morphology, membrane protein components, and lipid composition. Although the site of virion envelopment is unknown, multiple secretory organelle markers for the Golgi, late and early endosomes and the endocytic recycling compartment (ERC) have been associated with the VAC (Buchkovich et al., 2009, Cepeda et al., 2010, Krzyzaniak et al., 2009). Additionally, the morphology of the Golgi appears altered in HCMV infected cells with the accumulation of Golgi, ER and endosomal proteins with viral glycoproteins and tegument proteins in the VAC adjacent to the nucleus (Alwine, 2012, Sanchez et al., 2000a, Sanchez et al., 2000b). The mechanism through which HCMV remodels the secretory compartment is unknown but has been attributed to viral proteins (Alwine, 2012).
In this report we show that multiple HCMV encoded miRNAs target several endocytic pathway genes that serves two purposes for the virus. The first is to interfere with the trafficking and release of pro-inflammatory cytokines providing the virus with a unique immune evasion strategy. The second is to restructure components of the secretory pathway, including the Golgi and endocytic compartment to form the VAC leading to increased efficiency of infectious particle production.
Results
HCMV miRNAs target multiple members of the endocytic pathway
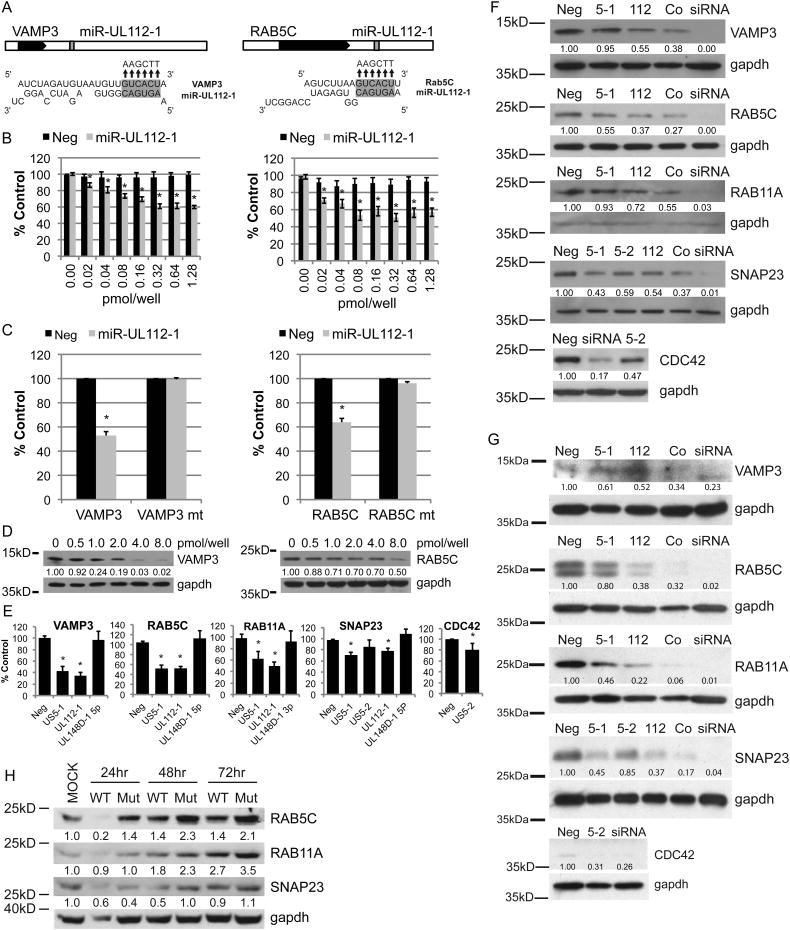
In order to identify cellular mRNA targets of HCMV miR-UL112-1 RISC immunoprecipitation followed by DNA microarray analysis (RIP-CHIP) was performed in HEK293T cells expressing myc-tagged Ago2 a component of the RISC complex (Grey et al., 2010, Karginov et al., 2007). Among the top ten mRNAs enriched in these experiments were vesicle-associated membrane protein 3 (VAMP3) and RAS-related protein 5C (RAB5C) that are essential components of the secretory/endocytic pathway (Table S1). RAB5C is a small GTPase that functions to ensure the fidelity of vesicle transport and docking to the acceptor compartment (Zerial and McBride, 2001). VAMP3 is the main component of a protein complex that includes synaptosomal-associated proteins that are involved in docking or fusion of vesicles with the presynaptic or plasma membrane (Bernstein and Whiteheart, 1999). These mRNAs were also enriched in RIP-CHIP experiments in human fibroblast cells infected with HCMV strain TR (Table S2). Analysis of the VAMP3 and RAB5C sequences indicated that both 3′ UTRs encoded potential target sites for miR-UL112-1 (Fig. 1A). Both of the VAMP3 and RAB5C miR-UL112-1 target sites were functional since transfection of HEK293T cells with increasing concentrations of a miR-UL112-1 double-stranded mimic reduced expression of luciferase reporters containing the VAMP3 and RAB5C 3′ UTRs (Fig. 1B). In addition mutation of the miR-UL112-1 target sites in the luciferase reporters restored wild-type activity (Fig. 1C). Lastly, both VAMP3 and RAB5C protein levels were reduced in HEK293T cells transfected with increasing concentrations of miR-UL112-1 (Fig. 1D). Together these data indicate that a single HCMV miRNA targets two important members of the secretory/endocytic pathway for reduced expression.
Fig. 1. HCMV miRs -US5-1, -US5-2, and -UL112-1 target components of the endocytic compartment.
(A) The 3′UTRs of VAMP3 and RAB5C each contain one potential target site for miR-UL112-1. The black boxes represent the open reading frames, white boxes the UTRs. The position of the target site within the 3′UTR is indicated (grey) as well as the predicted binding between miR-UL112-1 and target sites within each transcript. (B) Reporter constructs containing the 3′UTRs of VAMP3 (left panel) or RAB5C (right panel) cloned downstream of renilla were co-transfected into 293 cells with increasing concentrations of double-stranded miR-UL112-1 mimic or negative control (Neg). 16 hrs post-transfection, cell lysates were harvested and the relative renilla activity was determined by normalizing to firefly activity and then calculated as percentage of the negative control (% Control). (C) The predicted miR-UL112-1 target sites (A) were mutated by site-directed mutagenesis to the sequences indicated above the arrows and evaluated in luciferase assays. (D) 293 cells were transfected with the miR-UL112-1 concentrations indicated. VAMP3 and RAB5C proteins levels at 48 hrs post transfection were evaluated by western blot analysis. Relative band intensity was determined by dividing the intensity of the band by GAPDH followed by normalization to the untransfected control. (E) The 3′UTR reporter constructs were transfected into 293 cells with double-stranded mimics or negative control and relative renilla activity was determine 16 hours post-transfection by dual-luciferase assay and displayed at percent control (% Control). (F,G) Western blot analyses were performed on 293Ts (F) or NHDFs (G) transfected with the double stranded miRNA mimics or negative control (Neg) 48 hours post transfection. Relative band intensity was determined by dividing the intensity of the band by GAPDH followed by normalization to the negtransfected control. (H) NHDFs were infected with the AD169 wild type (WT) or the AD169 miR-US5-1, -US5-2, -UL112-1 mutant virus (Mut) and the levels of RAB5C, RAB11A, and SNAP23 were analyzed 24, 48, and 72 hpi by western blot analysis. Relative band intensity was determined by dividing the intensity of the band by GAPDH followed by normalization with the mock-infected control (MOCK). *P < 0.05 by two-tailed Student's t test. Data represent the mean +/- SD of a minimum of three experimental replicates. Figure 1, related to Figures S1 and S2.
To determine whether other HCMV miRNAs target the endocytic pathway, we examined whether the 3′ UTRs of VAMP3 and RAB5C as well as those of other pathway members contained HCMV miRNA seed target sites. We observed that in addition to miR-UL112-1 targets sites, VAMP3 and RAB5C also contained potential target sites for HCMV miR-US5-1 and that transfection of the miR-US5-1 with luciferase reporters containing the 3′ UTRs of these genes also reduced expression (Fig. S1A,B)(Fig. 1E). Mutation of the miR-US5-1 target sites in the luciferase reporters restored wild-type activity (Fig. S1A,B). Interestingly, both VAMP3 and RAB5C were coordinately down-regulated by miRs UL112-1 and US5-1 when co-transfected into HEK293Ts or NHDFs (Fig. 1F, G).
RAS-related protein 11A (RAB11A) and synaptosomal-associated protein 23 (SNAP23) are also members of the secretory pathway, while the cell division control protein 42 (CDC42) is critical for actin nucleation and retrograde transport of recycling endosomes within the secretory pathway. Examination of the 3′ UTRs of these genes revealed that each contained potential seed target sites for one or more HCMV miRNAs (Fig. S1C-E). These observations were confirmed using reporter assays combined with site-directed mutagenesis to confirm the target sites and indicated that miR-UL112-1 and miR-US5-1 down-regulated expression of the RAB11A and SNAP23, while miR-US5-2 reduced expression of the SNAP23 and CDC42 (Fig. 1E)(Fig.S1C-E). Similar to VAMP3 and RAB5C, co-transfection of the HCMV miRNAs that were predicted to target RAB11A or SNAP23, cooperatively down-regulated protein expression (Fig. 1F, G). Transfection of miR-US5-2 alone reduced CDC42 protein expression (Fig. 1F, G). These observations indicate that not only are the HCMV miRNAs cooperatively targeting several members of a single cellular pathway but also act together to down-regulate single genes within the pathway.
Next we generated a miR-UL112-1, miR-US5-1 and miR-US5-2 AD169 mutant virus to determine if the HCMV miRNAs down-regulate expression of endocytic proteins during viral infection. miR-UL112-1 is located directly antisense to the UL114 uracil DNA glycosylase (UDG) gene. To inactivate miR-UL112-1 function without affecting UDG, seven silent point mutations were introduced in UDG that disrupted the secondary structure of the miRNA (Fig.S2A, B). Analysis of the mutant demonstrated wild-type (WT) levels of expression of UDG, lack of miRUL112-1 expression and WT viral growth kinetics (Fig.S3A-C). To inactivate expression of miR-US5-1 and miR-US5-2 in the miR-UL112-1 mutant, a 190-nucleotide deletion was made in the noncoding region between US6 and US7 as previously described (Fig.S2C)(Tirabassi et al., 2011). HCMV with mutation of miR-US5-1 and miR-US5-2 alone as confirmed by stem-loop RT-PCR for miRNAs did not exhibit altered viral growth in cells (Fig.S3D,E). Sequence analysis of the HCMV miRNA triple mutant virus indicated that the only differences between the mutant and WT virus were the mutations introduced into the miRNAs and real-time PCR confirmed that miR-UL112-1, miR-US5-1, and miR-US5-2 were no longer expressed during infection (Fig.S4A). Western blot analysis of HCMV infected NHDFs revealed an increase in expression of RAB5C, RAB11A, and SNAP23 in cells infected with the HCMV miRNA triple mutant in comparison to cells infected with the WT virus (Fig. 1H). The increase in protein expression correlates with lack of HCMV miRNA expression and together with the above results indicates that these proteins are targets of miR-UL112-1, miR-US5-1, and miR-US5-2.
HCMV miRNA targeting of the secretory/endocytic pathway limits the release of TNF-α and IL-6
Since VAMP3, RAB5C, RAB11A, and SNAP23 play a critical role in the trafficking and release of TNF-α and IL-6 through the secretory pathway, we examined the effect of the HCMV miRNAs that target the endocytic pathway on cytokine release (Murray et al., 2005, Stow et al., 2006). THP-1 cells were used in these studies since neither lipopolysaccharide (LPS) nor HCMV infection induces significant production or release of TNF-α in fibroblasts. In these experiments THP-1 cells were transfected with miR-UL112-1, miR-US5-1, and miR-US5-2 or siRNAs that target VAMP3, RAB5C, RAB11A, and SNAP23 for 72hrs followed by treatment with or without LPS. The levels of TNF-α and IL-6 in the supernatants for each were assayed 8 hours post LPS treatment. Transfection of cells with either the HCMV miRNAs or siRNAs reduced secretion of TNF-α by 8-fold and IL-6 by approximately 2-fold (Fig 2A). Western analysis of transfected cells for SNAP23, RAB5C and RAB11A indicated a 3-5-fold reduction of protein (Fig 2B). Subsequently, we examined the ability of the HCMV miRNA triple mutant to induce secretion of IL-6 in virus infected HF cells. As shown in Fig. 2C mutation of the HCMV miRNAs increased cellular secretion of IL-6 by 6-10 fold in comparison to WT infection indicating that the viral miRNAs significantly inhibit cellular release of the inflammatory cytokine, possibly through altered function of the secretory pathway. The increased secretion of IL-6 induced by the HCMV triple miRNA mutant was reduced to WT levels by either transfection of miR-UL112-1, miR-US5-1, and US5-2 mimics or combinations of siRNAs targeting VAMP3, RAB5C, RAB11A, and SNAP23 (Fig 2D). Western analysis of transfected cells confirmed specific knockdown of these cellular proteins (Fig 2E). These observations indicate that HCMV has developed a unique mechanism to prevent cytokine release regardless of the virus induced innate immune activation events triggered in the cell.
Fig. 2. HCMV-encoded miRNAs downregulate VAMP3, RAB5C, RAB11A, and SNAP23 protein levels, limiting the release of pro-inflammatory cytokines TNFα and IL-6.
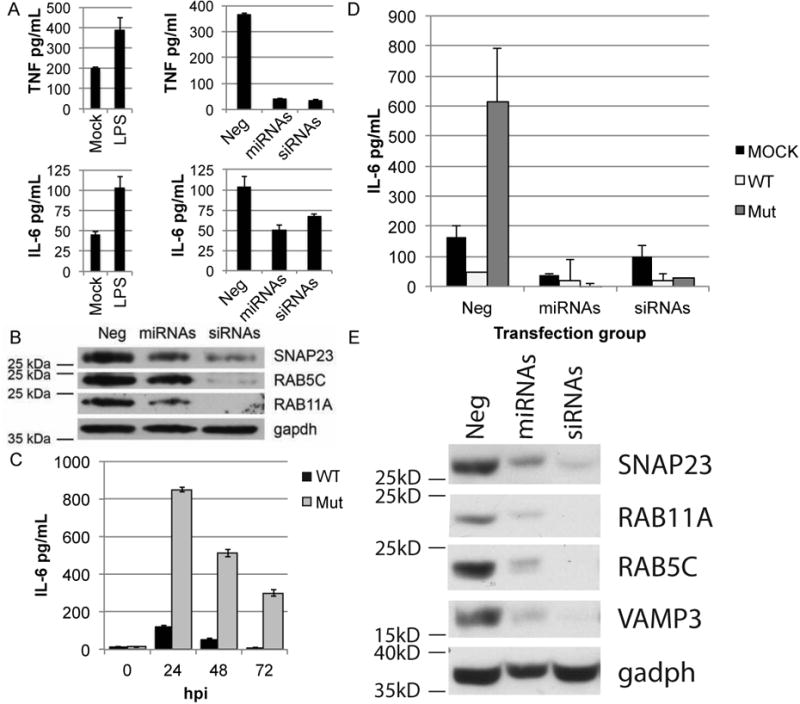
For each panel, one representative experiment is shown of at least three performed. (A-left panels) TPA-treated THP-1 cells transfected with a negative control siRNA were treated with LPS or mock treated to induce production and release of TNFα and IL-6. Supernatants were collected 8 hrs post-treatment, and analyzed for TNFα and IL-6 release by ELISA. (A-right panels) TPA-treated THP-1 cells were transfected with a combination of HCMV miRNAs that were shown to target components involved in cytokine release (miRNAs), a combination of siRNAs against those same transcripts (siRNAs), or a non-targeting negative control siRNA (Neg) (final concentration of RNA combined was 50nM). 72 hrs post-transfection cells were treated with LPS to induce production and release of TNFα and IL-6. Supernatants were collected 8 hrs post-treatment, and analyzed for TNFα and IL-6 release by ELISA. (B) Western blot analysis on TPA-treated THP-1 cells in (A-right panel) using the indicated antibodies. (C) NHDFs were infected with either the AD169 wild type virus (WT) or the AD169 miR-US5-1, -US5-2, -UL112-1 triple mutant virus (Mut) and the level of IL-6 present in the supernatant 24, 48, and 72 hpi was determined by ELISA. (D) NHDFs were transfected with HCMV miRNAs (miRs UL112-1, US5-1, and US5-2), siRNAs (VAMP3, RAB5C, RAB11A, and SNAP23), or negative control siRNA (Neg) (final concentration of RNA combined was 30nM) and 48 hpt were infected with the WT or Mut virus. Supernatants were collected at 24 hour intervals and tested for IL-6 release, shown is 48hpi. (E) Western blot analysis on uninfected NHDFs in (D) using the indicated antibodies. Data are represented as mean +/- SD.
HCMV miRNA targeting of the secretory/endocytic pathway mediates formation of the virion assembly compartment
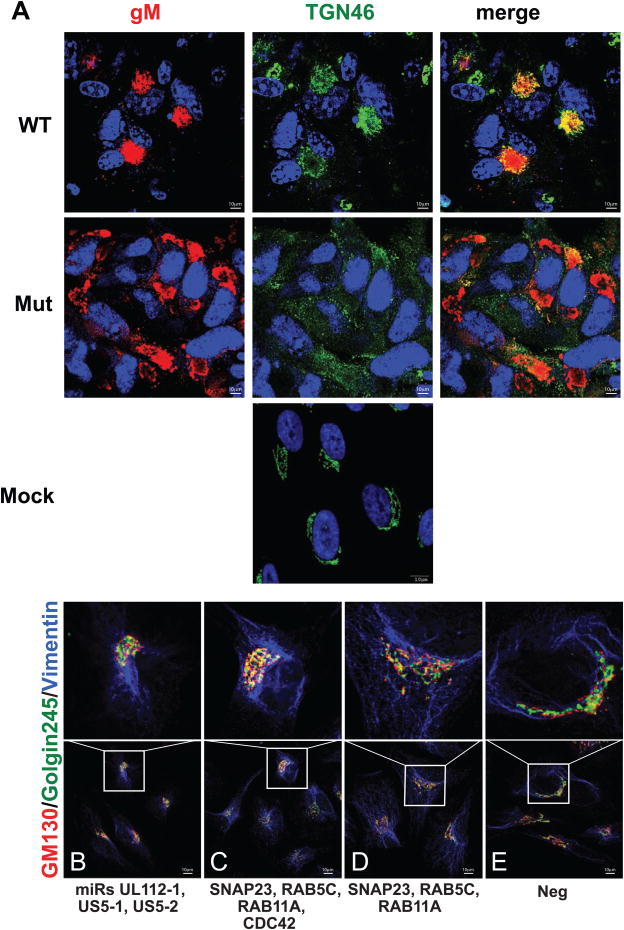
Formation of the VAC occurs during the late phase of HCMV infection when HCMV miRNAs are at the highest concentrations in the cell (Grey et al., 2005). Since miR-UL112-1, miR-US5-1, and miR-US5-2 target genes involved in vesicular transport and membrane fusion, we examined cells infected with the HCMV triple miRNA mutant to determine if viral protein localization in the VAC was altered during infection. Examination of HCMV WT infected cells revealed the characteristic accumulation of the viral glycoprotein gM in the VAC adjacent to the nucleus that co-stained with the Golgi marker TGN46 (Fig. 3A). However, the morphology of the ER was unaltered as determined by staining with an antibody to calreticulin (Fig. S5). In contrast infection of NHDFs with the HCMV miRNA triple mutant resulted in the disruption of the VAC into discrete structures staining with TGN46 and HCMV gM throughout the cell. In order to determine whether the HCMV miRNAs were sufficient to alter the morphology of the Golgi, cells were transfected with miR-UL112-1, miR-US5-1, and US5-2 mimics or combinations of siRNAs targeting RAB5C, RAB11A, SNAP23, and CDC42. As shown in Fig. 3B-D transfection of either the HCMV miRNAs or the pool of siRNAs disrupted the normal morphology of the Golgi ribbons and in some cells, resulted in the formation of spherically shaped juxtanuclear structures that were similar in the morphology of the Golgi during WT HCMV infection. This phenotype was in sharp contrast to the normal positioning and morphology of the Golgi in control-transfected cells (Fig. 3E). Transfection of a subset of the siRNAs to SNAP23, RAB11A, and RAB5C also resulted in altered morphology of the Golgi (Fig 3D).
Fig. 3. The HCMV miRNAs facilitate formation of the viral assembly compartment.
(A) NHDFs were infected with either the AD169 wild type (WT) or the AD169 miR-US5-1, -US5-2, -UL112-1 triple mutant virus (Mut) or mock-infected (Mock) and analyzed by immunofluorescence with the markers indicated as described in the Materials and Methods. (B-E) HeLa cells were transfected with a combination of HCMV miRs US5-1, US5-2, and UL112-1 (B), combinations of pathway specific siRNAs SNAP23, RAB5C, RAB11A, and CDC42 (C) or SNAP23, RAB5C, and RAB11A (D), or non-targeting negative control siRNA (E) and the Golgi complex was evaluated by immunofluorescence 4 days post-transfection for the cis-Golgi marker GM130 in red and the trans-Golgi marker Golgin245 in green as described in the Materials and Methods. Figure 3, related to Figure S5.
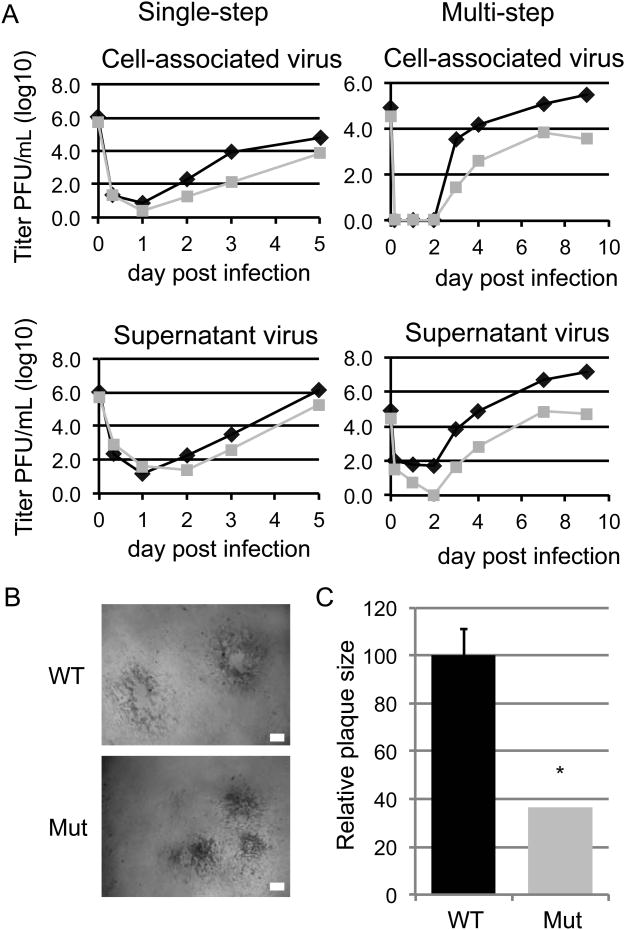
Analysis of the growth kinetics of HCMV miRNA triple mutant revealed a 2-log growth defect in NHDFs and small plaque phenotype without noticeable defects in immediate early, early, and late gene expression, viral genome replication, or incorporation of major envelope or late tegument proteins into the virion (Fig.4 AC)(Fig.S4 B-E). This reduction in viral production by the HCMV mutant may be due to inefficient and/or aberrant assembly of virus secondary to the disruption of the VAC. Therefore the plaque-forming unit (PFU) to genome copy number (GCN) ratio was analyzed for WT and miRNA triple mutant virus obtained from infected cell supernatants. Comparison of WT and miRNA mutant virus secreted into the supernatant revealed a 400-fold and 100-fold increase in the production of noninfectious particles for the mutant virus at 72 and 96 hours post infection (Table 1). A 5-fold increase in PFU:GCN ratio was detected in cells infected with the HCMV miRNA triple mutant virus that were transfected with siRNAs targeting RAB5C, RAB11A, SNAP23, and CDC42 (Table 2). These results indicate that HCMV miRNA targeting of endocytic proteins to restructure the Golgi to form the VAC is essential for efficient production of infectious virus.
Fig. 4. Mutation of the HCMV miRNAs results in reduced virus yield and small plaque phenotype.
(A) NHDFs were infected with the AD169 wild type virus (WT-black diamonds) or the AD169 miR-US5-1, -US5-2, -UL112-1 mutant virus (Mut-grey squares) (MOI=3, single step; MOI=0.05, multi-step) and cell-associated and supernatant virus were harvested at the dpi indicated. Titers were determined by plaque assay. (B) Plaque phenotype of the WT and Mut virus 72 hpi on NHDFs. White bar= 0.1mm (C) Relative plaque size of the WT-black bar compared with the Mut-grey bar. Mean area was determined 72 hpi on NHDFs and normalized to WT. *P < 0.05 by two-tailed Student's t test. Data are represented as mean +/- SD. Figure 4, related to Figures S2, S3, and S4.
Table 1. Mutation of the HCMV miRNAs results in altered virion fitness.
NHDFs were infected with either the AD169 wild type (WT) or the AD169 miR-US5-1, -US5-2, -UL112-1 triple mutant virus (Mut) (MOI=3, 2hrs) followed by acid wash to remove extracellular virus. 72 and 96 hpi supernatant virus was harvested and the PFU/genome copy was determined by virus titration on NHDFs and real-time PCR.
| Supernatant virus | Genome copy/mL | PFU/mL | PFU:genome copy | |||
|---|---|---|---|---|---|---|
| 72hpi | 96hpi | 72hpi | 96hpi | 72hpi | 96hpi | |
| WT | 630538 | 1683477 | 65000 | 110000 | 1:10 | 1:15 |
| Mut | 221094 | 653125 | 5 | 60 | 1:44,219 | 1:10,885 |
Table 2. Transfection with siRNAs against endocytic compartment genes restores the PFU:GCN ratio.
NHDFs were transfected with siRNAs targeting RAB5C, RAB11A, SNAP23, and CDC42 or negative control and were then infected with either the AD169 wild type (WT) or the AD169 miR-US5-1, -US5-2, -UL112-1 triple mutant virus (Mut) (MOI=3, 2hrs). 72 and 96 hpi supernatant virus was harvested and the PFU/genome copy was determined by virus titration on NHDFs and real-time PCR.
| Transfection group | |||||||
|---|---|---|---|---|---|---|---|
| Genome copy/mL | PFU/mL | PFU:genome copy | |||||
| Neg | RAB5C, RAB11A, SNAP23, CDC42 | Neg | RAB5C, RAB11A, SNAP23, CDC42 | Neg | RAB5C, RAB11A, SNAP23, CDC42 | ||
| 72 hpi | WT | 1707942 | 1099182 | 165000 | 350000 | 1:10 | 1:3 |
| Mut | 161927 | 222080 | 250 | 1800 | 1:648 | 1:123 | |
| 96 hpi | WT | 755499 | 518324 | 650000 | 550000 | 1:1 | 1:1 |
| Mut | 816361 | 1337559 | 1550 | 11100 | 1:527 | 1:121 | |
HCMV miRNA targeting of the secretory/endocytic pathway results in accumulation of transferrin in the endocytic recycling compartment
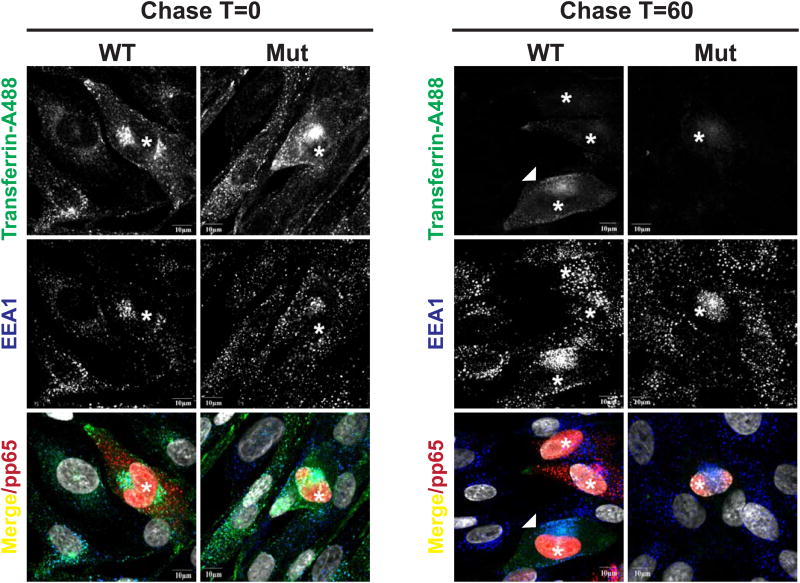
Previous studies of the origins of the VAC indicated that virion glycoproteins accumulated in a membranous compartment that could be labeled with markers of the endocytic recycling compartment (ERC) (Krzyzaniak et al., 2009). In this earlier study, we demonstrated that accumulation of the major envelope glycoprotein of HCMV, gM, was dependent on interaction of this protein with Rab11 effector protein, Fip4, and that these proteins along with endocytosed transferrin accumulate in the ERC (Krzyzaniak et al., 2009). The targeting of several components of the endocytic pathway by HCMV miRNA raised the possibility that these miRNA could impact either endocytosis or endocytic recycling in virus infected cells. We explored this possibility by utilizing fluorochrome conjugated transferrin in an assay of endocytosis and recycling of endocytosed transferrin in cells infected with the WT virus expressing the miRNAs and the viral miRNA mutant. After depletion of endogenous transferrin, fluorochrome labeled transferrin was allowed to internalize into infected cells at 37° followed by a chase with unlabeled transferrin to analyze the recycling of this protein. As can be seen in Figure 5, cells infected with WT and mutant virus bound and internalized transferrin similarly. Interestingly, after a 60 min chase period similar amounts of transferrin were present in a juxtanuclear site of cells infected with the WT virus whereas minimal to undetectable amounts of transferrin were present in cells infected with the mutant virus and in uninfected adjacent cells (Fig.5). These findings support the hypothesis that the miRNA targeting the components of the secretory/endocytic pathway alter the kinetics of transferrin recycling favoring accumulation of the protein in the ERC. This result is consistent with previous observations that demonstrate the localization of transferrin in the VAC of WT HCMV infected cells (Krzyzaniak et al., 2009).
Fig. 5. HCMV miRNAs inhibit transferrin recycling.
NHDFs were infected with the AD169 wild type (WT) or the AD169 miR-US5-1, -US5-2, -UL112-1 mutant virus (Mut) (MOI=0.8). At 72hpi NHDFs were labeled with transferrin conjugated with AlexaFluor488 for 30min. Following a 0 and 60 min chase in unlabeled media containing 10%FBS, the cells were imaged for transferrin (green), the early endosome marker EEA1 (blue), HCMV pp65 (red), and DAPI. While bar=10μM (*) indicates infected cells. White arrow indicates WT infected cell with mature VAC.
Discussion
In summary our studies indicate that multiple members of the endocytic pathway including VAMP3, RAB5C, RAB11A, SNAP23, and CDC42 are targeted by the HCMV miRs UL112-1, US5-1, and US5-2. Mutation of these miRNAs resulted in significant consequences for virus infectivity including increased release of pro-inflammatory cytokines, and major impacts on viral phenotypes including malformation of the VAC, reduction of supernatant virus, and increased production of defective particles. We show that the targeting of the endocytic pathway genes by multiple HCMV encoded miRNAs serves at least two purposes for the virus. The first is to interfere with the trafficking and release of pro-inflammatory cytokines providing the virus with a unique immune evasion strategy, while the second is to restructure components of the secretory pathway, including the Golgi and endocytic compartment to form the VAC leading to increased production of infectious particles.
Both IL-6 and TNFα are pro-inflammatory cytokines that play important roles in both innate and acquired immunity. IL-6 is the predominant inducer of the acute-phase response, while TNFα plays a key role in controlling viral infections, exemplified by experiments using TNFα inhibitors that result in both increased viral replication and pathogenicity in vivo (Yerkovich et al., 1997). The importance of these cytokines in controlling viral infections is underscored by the fact that multiple viruses, including HCMV, have evolved strategies to interfere with IL-6 and TNFα production and release or that mitigate their effects. These strategies include down-regulating cytokine receptors, interfering with recruitment of adaptor proteins and subsequent downstream signaling events, and neutralizing newly released cytokines (Rahman and McFadden, 2006). Additionally, viral proteins have been reported to interfere with release of cytokines from infected cells. For example, the HIV-encoded protein nef, interferes with delivery of TNFα to the plasma membrane in HIV-infected macrophages by preventing recruitment of AP1 (Mazzolini et al., 2010). By reducing or preventing cytokine release from infected cells HCMV creates an environment more favorable for virus replication and spread. Unlike previously identified mechanisms which rely on the expression of exogenous proteins that likely elicit an immune response, HCMV accomplishes this using multiple viral miRNAs which are not immunogenic.
Over the past two decades a significant amount of effort has been devoted to characterizing the formation and composition of the HCMV VAC. The final stages of HCMV particle formation occurs in the VAC where final tegumentation and acquisition of envelope proteins occurs followed by egress from the cell using the secretory machinery. The mature VAC is most readily detected late during the virus replication cycle when HCMV miRNA expression peaks. VAC formation also occurs at an interval when altered distribution of secretory pathway proteins is observed in infected cells. Analysis of the VAC for components of the secretory pathway indicated the presence of markers for the ER to Golgi intermediate compartment, trans-Golgi network (TGN) as well as early and recycling endosomes (Das and Pellett, 2007, Das and Pellett, 2011, Sanchez et al., 2000a, Sanchez et al., 2000b). While HCMV proteins are considered to regulate the formation of the VAC, the observations in this study indicate that viral miRNAs that down-regulate key components of the secretory pathway also contribute to the remodeling of the secretory pathway during infection. The reorganization of the secretory pathway that facilitates VAC formation may be related to the membrane remodeling that occurs during induction of either cell death or cytokinesis (Grant and Donaldson, 2009, Landry et al., 2009). In each case, a significant amount of the plasma membrane is ingested into the cell through the endosomal compartment by temporarily blocking endosomal recycling while allowing endocytosis to proceed (Grant and Donaldson, 2009, Landry et al., 2009, Boucrot and Kirchhausen, 2007). This event leads to loss of plasma membrane area and accumulation of membrane in the endosomal system. Endosomal recycling depends upon many proteins including RABs 5, 7, and 11, CDC42, and actin (Grant and Donaldson, 2009). Interfering with these proteins can inhibit recycling as well as alter position of the endosomal recycling compartment within the cell (Grant and Donaldson, 2009, Tomas et al., 2010, Hehnly et al., 2010). Our findings that demonstrate an inhibition of transferrin recycling in WT HCMV but not in mutant HCMV infected cells is consistent with these previous studies and suggest that formation of the VAC may require inhibition of anterograde trafficking in more distal compartments of the secretory pathways including the endocytic recycling pathway leading to the accumulation of viral proteins in proximity of the ERC. Finally, it is noteworthy that both decreased secretion of TNF-α and IL-6 and inhibition of transferrin recycling represent functional phenotypes of cells infected with viruses expressing these viral miRNAs, an observation that further supports the role of these miRNAs in remodeling the secretory pathway to optimize virus assembly and replication.
The ultimate effect of HCMV miRNA-mediated reduction of the RAB proteins, SNAP23, and CDC42 is the condensation and reorganization of the Golgi, TGN and endosomes into the VAC adjacent to the nucleus. Transfection of a pool of either the HCMV miRNAs or siRNAs targeting the endocytic proteins was sufficient to disrupt the morphology of the Golgi and to generate structures that resemble the VAC. These results in combination with the observation that infection of cells with the HCMV miRNA mutant results in lack of VAC formation and normal formation of Golgi stacks in the cell indicate that the viral miRNAs are responsible for VAC formation. Lastly, the observation that mutation of the miRNAs in the virus resulted in up to a 3-log increase in noninfectious particles is consistent with the role of the VAC in the concentration of viral proteins into a single structure within the secretory machinery that can in turn, lead to more efficient infectious particle formation (Sanchez et al., 2000a, Sanchez et al., 2000b). An important question raised by these results is how particles that mature in the VAC egress from the cell with the loss of the secretory pathway machinery. Do recycling endosomal vesicles with infectious virion cargo traffic between the VAC and the plasma membrane for particle release and if so how does this occur with the loss of docking molecules? The ability to genetically regulate formation of the VAC will provide us with a tool to dissect these and other mechanisms involved in viral assembly and egress from the cell.
Rescuing the HCMV triple mutant with the viral miRNAs is complicated by the fact that each individual miRNA has the potential to target greater than 100 genes. Therefore using the viral miRNAs mutated in the virus to rescue the HCMV mutant would not conclusively demonstrate that the down-regulation of the secretory genes by the miRNAs is solely responsible for the phenotype. In addition since miR-UL112-1 also targets the viral transcriptional activator IE72 and UL112/113 that are involved in viral DNA replication as well as UL120/121 that has an unknown function (Grey et al., 2007), using this miRNA to rescue the mutant viral phenotype would be complicated by the miRNA off-target effects. An additional complicating factor is that miRs US5-1 and US5-2 target US7 that is an HCMV gene with unknown function. Therefore to rule out off-target effects of the HCMV miRNAs, we utilized siRNAs targeting RAB5C, RAB11A, SNAP23, and CDC42 to rescue the HCMV miRNA triple mutant. Using this approach we were able to reconstitute WT HCMV VAC formation as well as reduce production of noninfectious particles. Similarly, siRNAs directed against the secretory genes also rescued the ability of the HCMV triple miRNA mutant to reduce the release of pro-inflammatory cytokines. Importantly, we observed that transfection of the viral miRNAs or siRNAs to the secretory gene targets is sufficient to form the VAC or decrease secretion of pro-inflammatory cytokines in the absence of virus infection indicating that the phenotype is solely dependent on the viral miRNAs targeting the secretory pathway genes.
These results indicate that multiple viral miRNAs coordinately target multiple genes in a single cellular pathway that are an essential part of the viral replication process and provide a mechanistic explanation for formation of the VAC. These results also indicate that viral miRNAs are a critical part of the virus lytic life-cycle and that the full extent of viral mutant phenotypes can only be observed following mutation of multiple miRNA in the virus that coordinately regulate pathways. The future identification of cellular pathways targeted by HCMV miRNAs will allow the targeted mutation of these regulatory RNAs to explore the importance of these pathways in viral replication and latency.
Experimental Procedures
Cells and viruses
HEK293T cells (293T), normal human dermal fibroblasts (NHDFs), and HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1.0 unit/mL penicillin, 1.0 ug/mL streptomycin, and 292 ng/mL L-glutamine (PSG) (Life Technologies, Rockville, MD). HEK293T cells that stably express a c-myc-tagged Argonaute 2 protein (A2) were grown in the medium above supplemented with 300 ug/mL gentamicin (Invitrogen, Carlsbad, CA)(Karginov et al., 2007). A human monocyte-derived cell line (THP-1) was grown in RPMI-1640 supplemented with 10% heat-inactivated FBS and PSG. Monocytic differentiation was induced by adding 10 ng/mL 12-O-tetradecanoylphorbol-13-acetate (TPA) to the medium. Stocks of HCMV were grown and titered in NHDFs using standard techniques. For viral growth curves, NHDFs were infected in duplicate at an MOI of 3 for single-step or MOI 0.05 for multi-step for two hours. Cells were then washed extensively and both cell-associated and supernatant virus was harvested at multiple time points post infection. Titers were determined by plaque assay on NHDFs. In some cases, average plaque size was determined 72 hpi on NHDFs and normalized to WT. PFU/genome copy number was determined by isolating supernatant virus at 72 and 96 hpi, followed by virus titration on NHDFs and real-time PCR to detect genome copy number. In some cases, NHDFs were transfected with pools of miRNAs, siRNAs, or negative controls 48 hours prior to infection using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions with the modification that cells were plated and maintained in DMEM supplemented with 1%FBS for the length of the experiment. For experiments evaluating knockdown of VAMP3 in 293T cells, a vector containing the VAMP3 full-length cDNA clone was transfected into cells using Lipofectamine 2000 along with the small RNAs (OriGene Technologies).
RIP-CHIP
RIP-CHIP was performed as previously described (Grey et al., 2010). Briefly, A2 cells were transfected with a pSIREN expression plasmid encoding the HCMV miR-UL112-1 pre-miR hairpin or a negative control using Fugene according to the manufacturers instructions (Roche). 72 hpt cell lysates were harvested and mRNA associated with the RISC complex was isolated with anti-cmyc agarose beads (Sigma). RNA was isolated from both the total and immunoprecipitated cellular lysates using Trizol (Invitrogen) and analyzed for quality using an Agilent Bioanalyzer. mRNA transcript levels were determined using the Illumina HumanRef-8 platform and analyzed using Gene Sifter software. To identify cellular mRNA transcripts specifically enriched within RISC complex containing miR-UL112-1, the enrichment profile in miR-UL112-1 transfected cells was compared to cells transfected with the negative control vector such that the enrichment value of any given mRNA transcript = (IP112/Total112)/(IPNeg/TotNeg) (Grey et al., 2010). mRNA transcripts were then ranked according to level of enrichment with the highest enriched transcripts considered potential targets of miR-UL112-1. Two independent experiments were performed to increase confidence in the identified targets. Predicted binding sites between miRNAs and potential targets were determined by looking for seed sequence matches or by using the online software RNAhybrid (Grey et al., 2010, Tirabassi et al., 2011). For analysis of HCMV miRNA targets during infection, NHDFs were infected with HCMV strain TR (MOI=3). 72 hpi cells were lysed, samples were taken for total RNA, and miRNA in complex with endogenous Ago2 was immunoprecipitated using an anti-Ago2 antibody followed by streptavidin bead pull-down. RNA was isolated using Trizol and analyzed for quality using an Agilent Bioanalyzer and transcript levels determined on the Illumina HumanRef-8 platform. Microarray data was analyzed using Gene Sifter software. Enrichment of specific transcripts, through association with miRNP complexes was determined by dividing the immunoprecipitated levels of transcripts by the total levels. Transcripts were then ranked according to the level of enrichment with the highest enriched transcripts considered potential targets of HCMV miRNAs.
Reagents
Double stranded miRNA mimics for the HCMV-encoded miRNAs were designed using the published miRNA sequences (www.mirbase.org) as described previously (Grey et al., 2010, Tirabassi et al., 2011). VAMP3, RAB5C, RAB11A, SNAP23, CDC42, and negative control siRNAs were purchased from Ambion (www.ambion.com).
Cloning and site directed mutagenesis
The 3′UTRs of VAMP3, RAB5C, Rab11A, SNAP23, and CDC42 were PCR amplified from 293T cDNA and cloned downstream of the renilla luciferase gene in a psiCHECK-2 dual reporter construct (Promega). A PCR based site-directed mutagenesis protocol was used to introduce mutations into potential miRNA binding sites.
Luciferase assay
293T cells were cotransfected with the 3′UTR dual-luciferase reporter constructs and miRNA mimics using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were harvested 18 hours posttransfection and renilla and luciferase levels were measured using Promega's dual reporter assay.
Northern blot analysis for miRNAs
NHDFs were infected at an MOI of 3 and total RNA was harvested and subjected to Northern blot analysis using probes specific for predicted viral miRNA sequences.
RT-PCR analysis
Total RNA was harvested using Trizol and reverse transcribed using either random hexamers or specific RT primers for miRNA RT-PCR. Gene specific primer-probe sets (Taqman, ABI) were then used for real-time amplification.
Western blot analysis
Extracts were run on an 8-12% SDS-PAGE, transferred to Immobilon-P Transfer Membranes (Milipore Corp., Bedford, MA), and visualized with antibodies specific for VAMP3 (SySy), RAB5C (Sigma), RAB11A (Cell Signaling), SNAP23 (SySy), CDC42 (Sigma), flag (Sigma) and GAPDH (Abcam).
ELISA
THP-1 cells were seeded at a density of 2.5×105 cells/well in 24-well plates and treated with TPA to induce monocytic differentiation. 24 hours later cells were transfected with HCMV miRNAs, siRNAs directed against VAMP3, RAB5C, RAB11A, and SNAP23, or negative control siRNA using Lipofectamine 2000. 72 hpt cells were treated with LPS to induce TNFα and IL-6 secretion. Supernatants were collected 8 hrs post-treatment, centrifuged to remove cell debris, and analyzed for TNFα and IL-6 by ELISA according the manufacturer's instructions (BD OptEIA). In experiments comparing IL-6 release from WT and AD169 miR-US5-1, -US5-2, -UL112-1 triple mutant virus infected cells, NHDFs were infected at an MOI of 3 as described above and IL-6 release was determined by ELISA.
Immunofluorescence
Localization of viral and cellular proteins was determined by indirect immunofluorescence. NHDFs grown on 13-mm glass cover slips were infected with the AD169 WT or AD169 miR-US5-1, -US5-2, -UL112-1 triple mutant virus at an MOI of 0.1. At 6 dpi, coverslips were washed with DPBS and fixed in DPBS containing 4% paraformaldehyde. Cells were permeabilized with 0.1% Triton X-100, and blocked with 10-50% normal goat serum (Invitrogen). The coverslips were then incubated with primary antibody including anti-calreticulin, anti-golgin245, anti-gm130, anti-TGN46, anti-HCMV IE-1 (mab p63-27), anti-gM (mab IMP) and anti-gM/gN (mab 14-16A). Cells were then washed and incubated with the appropriate fluorophore-conjugated secondary antibody (Southern Biotech). In some experiments, HeLa cells were electroporated with siRNAs using the basic nucleofector kit (Amaxa Bioscience) prior to the immunostaining. In experiments evaluating transferrin internalization and recycling, NHDFs were infected with the WT or Mut virus (MOI=0.8, 72hrs), starved 2hrs in serum-free media and then labeled with transferrin conjugated with AlexaFluor488 for 30min at 37°C. The coverslips were then washed three times with cold PBS, placed in complete media for 0, 10, 20, 30, 45, and 60 minute chase periods at 37°C and imaged for transferrin, EEA1, DAPI, and HCMV pp65 (mab 28-19). Fluorescence was visualized using an Olympus Fluoview 1000 confocal microscope utilizing identical laser and gain settings for comparative studies.
Statistical analysis
The Student's t test (Microsoft Excel software) was used to determine P values. Results were considered significant at a probability (P) < 0.05.
Supplementary Material
Highlight.
HCMV miRNAs US5-1, US5-2, and UL112-1 target multiple endocytic pathway components
HCMV miRNA targeting of the endocytic pathway limits TNF-α and IL-6 release
Endocytic pathway targeting facilitates virion assembly compartment formation
HCMV miRNAs inhibit endocytic recycling of transferrin
Acknowledgments
We wish to thank Drs. Richard Goodman, Louis Picker, Klaus Frueh and Patrizia Caposio for their helpful comments on this paper and Andrew Townsend for help with graphics. We thank Renee Espinoza-Najera and Helen Hewitt for technical assistance. This research was supported by grants AI21640 (JAN) and AI50189 (WJB) and AI035602 (WJB) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abend JR, Uldrick T, Ziegelbauer JM. Regulation of tumor necrosis factor-like weak inducer of apoptosis receptor protein (TWEAKR) expression by Kaposi's sarcoma-associated herpesvirus microRNA prevents TWEAK-induced apoptosis and inflammatory cytokine expression. J Virol. 2010;84:12139–51. doi: 10.1128/JVI.00884-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine JC. The human cytomegalovirus assembly compartment: a masterpiece of viral manipulation of cellular processes that facilitates assembly and egress. PLoS Pathog. 2012;8:e1002878. doi: 10.1371/journal.ppat.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie J, Sahlender DA, Sinclair JH. Human cytomegalovirus infection inhibits tumor necrosis factor alpha (TNF-alpha) signaling by targeting the 55-kilodalton TNF-alpha receptor. J Virol. 2003;77:7007–16. doi: 10.1128/JVI.77.12.7007-7016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein AM, Whiteheart SW. Identification of a cellubrevin/vesicle associated membrane protein 3 homologue in human platelets. Blood. 1999;93:571–9. [PubMed] [Google Scholar]
- Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci U S A. 2007;104:7939–44. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich NJ, Maguire TG, Paton AW, Paton JC, Alwine JC. The endoplasmic reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment. J Virol. 2009;83:11421–8. doi: 10.1128/JVI.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda V, Esteban M, Fraile-Ramos A. Human cytomegalovirus final envelopment on membranes containing both trans-Golgi network and endosomal markers. Cell Microbiol. 2010;12:386–404. doi: 10.1111/j.1462-5822.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–33. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Pellett PE. Members of the HCMV US12 family of predicted heptaspanning membrane proteins have unique intracellular distributions, including association with the cytoplasmic virion assembly complex. Virology. 2007;361:263–73. doi: 10.1016/j.virol.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Das S, Pellett PE. Spatial relationships between markers for secretory and endosomal machinery in human cytomegalovirus-infected cells versus those in uninfected cells. J Virol. 2011;85:5864–79. doi: 10.1128/JVI.00155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Vasanji A, Pellett PE. Three-dimensional structure of the human cytomegalovirus cytoplasmic virion assembly complex includes a reoriented secretory apparatus. J Virol. 2007;81:11861–9. doi: 10.1128/JVI.01077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealy C, Denson M, Humphreys C, Mcsharry B, Wilkinson G, Caswell R. Posttranscriptional suppression of interleukin-6 production by human cytomegalovirus. J Virol. 2005;79:472–85. doi: 10.1128/JVI.79.1.472-485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Corcoran DL, Mukherjee N, Skalsky RL, Hafner M, Nusbaum JD, Shamulailatpam P, Love CL, Dave SS, Tuschl T, Ohler U, Cullen BR. Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe. 2011;10:515–26. doi: 10.1016/j.chom.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey F, Antoniewicz A, Allen E, Saugstad J, Mcshea A, Carrington JC, Nelson J. Identification and characterization of human cytomegalovirus-encoded microRNAs. J Virol. 2005;79:12095–9. doi: 10.1128/JVI.79.18.12095-12099.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey F, Meyers H, White EA, Spector DH, Nelson J. A human cytomegalovirus-encoded microRNA regulates expression of multiple viral genes involved in replication. PLoS Pathog. 2007;3:e163. doi: 10.1371/journal.ppat.0030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey F, Tirabassi R, Meyers H, Wu G, Mcweeney S, Hook L, Nelson JA. A viral microRNA down-regulates multiple cell cycle genes through mRNA 5′UTRs. PLoS Pathog. 2010;6:e1000967. doi: 10.1371/journal.ppat.1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehnly H, Xu W, Chen JL, Stamnes M. Cdc42 regulates microtubule-dependent Golgi positioning. Traffic. 2010;11:1067–78. doi: 10.1111/j.1600-0854.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MA, Borton JA, Keech AM, Wong J, Britt WJ, Magun BE, Nelson JA. Human cytomegalovirus attenuates interleukin-1beta and tumor necrosis factor alpha proinflammatory signaling by inhibition of NF-kappaB activation. J Virol. 2006;80:5588–98. doi: 10.1128/JVI.00060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karginov FV, Conaco C, Xuan Z, Schmidt BH, Parker JS, Mandel G, Hannon GJ. A biochemical approach to identifying microRNA targets. Proc Natl Acad Sci U S A. 2007;104:19291–6. doi: 10.1073/pnas.0709971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Lee S, Kim S, Kim D, Ahn JH, Ahn K. Human cytomegalovirus clinical strain-specific microRNA miR-UL148D targets the human chemokine RANTES during infection. PLoS Pathog. 2012;8:e1002577. doi: 10.1371/journal.ppat.1002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalik TF, Wing B, Haskill JS, Azizkhan JC, Baldwin AS, JR, Huang ES. Multiple mechanisms are implicated in the regulation of NF-kappa B activity during human cytomegalovirus infection. Proc Natl Acad Sci U S A. 1993;90:1107–11. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzaniak MA, Mach M, Britt WJ. HCMV-encoded glycoprotein M (UL100) interacts with Rab11 effector protein FIP4. Traffic. 2009;10:1439–57. doi: 10.1111/j.1600-0854.2009.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry MC, Sicotte A, Champagne C, Lavoie JN. Regulation of cell death by recycling endosomes and golgi membrane dynamics via a pathway involving Src-family kinases, Cdc42 and Rab11a. Mol Biol Cell. 2009;20:4091–106. doi: 10.1091/mbc.E09-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Bai Z, Ye F, Xie J, Kim CG, Huang Y, Gao SJ. Regulation of NFkappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol. 2010;12:193–9. doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Weinstein EG, Abdelhakim A, Yekta S, Rhoades MW, Burge CB, Bartel DP. The microRNAs of Caenorhabditis elegans. Genes Dev. 2003;17:991–1008. doi: 10.1101/gad.1074403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini J, Herit F, Bouchet J, Benmerah A, Benichou S, Niedergang F. Inhibition of phagocytosis in HIV-1-infected macrophages relies on Nef-dependent alteration of focal delivery of recycling compartments. Blood. 2010;115:4226–36. doi: 10.1182/blood-2009-12-259473. [DOI] [PubMed] [Google Scholar]
- Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–5. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;304:734–6. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Mcfadden G. Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2006;2:e4. doi: 10.1371/journal.ppat.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols MA, Skalsky RL, Maldonado AM, Riva A, Lopez MC, Baker HV, Renne R. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007;3:e65. doi: 10.1371/journal.ppat.0030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Greis KD, Sztul E, Britt WJ. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J Virol. 2000a;74:975–86. doi: 10.1128/jvi.74.2.975-986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Sztul E, Britt WJ. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-golgi-intermediate compartment. J Virol. 2000b;74:3842–51. doi: 10.1128/jvi.74.8.3842-3851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, Hafner M, Nusbaum JD, Feederle R, Delecluse HJ, Luftig MA, Tuschl T, Ohler U, Cullen BR. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 2012;8:e1002484. doi: 10.1371/journal.ppat.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark TJ, Arnold JD, Spector DH, Yeo GW. High-resolution profiling and analysis of viral and host small RNAs during human cytomegalovirus infection. J Virol. 2012;86:226–35. doi: 10.1128/JVI.05903-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Ginossar N, Elefant N, Zimmermann A, Wolf DG, Saleh N, Biton M, Horwitz E, Prokocimer Z, Prichard M, Hahn G, Goldman-Wohl D, Greenfield C, Yagel S, Hengel H, Altuvia Y, Margalit H, Mandelboim O. Host immune system gene targeting by a viral miRNA. Science. 2007;317:376–81. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–29. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- Tirabassi R, Hook L, Landais I, Grey F, Meyers H, Hewitt H, Nelson J. Human cytomegalovirus US7 is regulated synergistically by two virally encoded microRNAs and by two distinct mechanisms. J Virol. 2011;85:11938–44. doi: 10.1128/JVI.05443-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas MI, Kucic N, Mahmutefendic H, Blagojevic G, Lucin P. Murine cytomegalovirus perturbs endosomal trafficking of major histocompatibility complex class I molecules in the early phase of infection. J Virol. 2010;84:11101–12. doi: 10.1128/JVI.00988-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–3. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkovich ST, Olver SD, Lenzo JC, Peacock CD, Price P. The roles of tumour necrosis factor-alpha, interleukin-1 and interleukin-12 in murine cytomegalovirus infection. Immunology. 1997;91:45–52. doi: 10.1046/j.1365-2567.1997.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko AD, Hwang ES, Rasmussen L, Keay S, Pereira L, Huang ES. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-kappaB during infection. J Virol. 1997a;71:5051–9. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko AD, Mayo MW, Poma EE, Baldwin AS, JR, Huang ES. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J Virol. 1997b;71:4638–48. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, Mcbride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer JM, Sullivan CS, Ganem D. Tandem array-based expression screens identify host mRNA targets of virus-encoded microRNAs. Nat Genet. 2009;41:130–4. doi: 10.1038/ng.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.