Abstract
The pi-class glutathione S-transferase (GSTP1) actively protect cells from carcinogens and electrophilic compounds. Loss of GSTP1 expression via promoter hypermethylation is the most common epigenetic alteration observed in human prostate cancer. Silencing of GSTP1 can increase generation of reactive oxygen species (ROS) and DNA damage in cells. In this study we investigated whether loss of GSTP1 contributes to increased DNA damage that may predispose men to a higher risk of prostate cancer. We found significantly elevated (103%; P<0.0001) levels of 8-oxo-2′-deoxogunosine (8-OHdG), an oxidative DNA damage marker, in adenocarcinomas, compared to benign counterparts, which positively correlated (r=0.2) with loss of GSTP1 activity (34%; P<0.0001). Silencing of GSTP1 using siRNA approach in normal human prostate epithelial RWPE1 cells caused increased intracellular production of ROS and higher susceptibility of cells to H2O2-mediated oxidative stress. Additionally, human prostate carcinoma LNCaP cells, which contain a silenced GSTP1 gene, were genetically modified to constitutively express high levels of GSTP1. Induction of GSTP1 activity lowered endogenous ROS levels in LNCaP-pLPCX-GSTP1 cells, and when exposed to H2O2, these cells exhibited significantly reduced production of ROS and 8-OHdG levels, compared to vector control LNCaP-pLPCX cells. Furthermore, exposure of LNCaP cells to green tea polyphenols caused re-expression of GSTP1, which protected the cells from H2O2-mediated DNA damage through decreased ROS production compared to non-exposed cells. These results suggest that loss of GSTP1 expression in human prostate cells, a process that increases their susceptibility to oxidative stress-induced DNA damage, may be an important target for primary prevention of prostate cancer.
Keywords: prostate cancer, oxidative stress, DNA damage, reactive oxygen species, glutathione S-transferase P1
INTRODUCTION
Glutathione S-transferases (GST) are a group of isoenzymes that catalyze intracellular detoxification reactions by conjugating glutathione with electrophilic compounds including carcinogens, natural toxins and exogenous drugs (1, 2). The resulting complexes generated by this reaction are usually less toxic than the parent xenobiotic; these complexes are eventually metabolized and exported via a glutathione-dependent transport system (3). Among the isoenzymes, the role of pi class GST (GSTP1) is of particular interest in cancer biology (4, 5). In humans, overexpression of pi-class GST has been associated with tumor progression and drug resistance (5). GSTP1 over-expression has been reported in many human tumors, and has been shown to be correlated with advanced stage, disease aggressiveness, drug resistance and poor survival (6–8). In contrast, early loss of GSTP expression results in increased cancer susceptibility. For example, GSTP−/− mice display a strong tendency to develop skin papillomas and lung cancer following carcinogen exposure (9, 10), and loss of GSTP markedly enhances colon tumorigenesis in Apc (Min) mice. These findings suggest that GSTP1 possesses tumor suppressor functions as well (11).
In prostatic epithelium, it has been shown that the vast majority of high-grade PIN lesions and adenocarcinomas exhibit early loss of GSTP1 expression, associated with hypermethylation of the CpG islands encompassing the GSTP1 promoter (12, 13). It has been proposed that GSTP1 is a caretaker gene, protecting the cells against genomic damage mediated by oxidants and electrophiles from inflammation or dietary exposures (14). Reports suggest that loss of GSTP1 shifts the pro-oxidant-antioxidant balance towards an oxidative state, resulting in increased inflammation and oxidative stress to prostate epithelial cells (15). Studies have suggested age-related structural changes in the DNA of prostate tissue which is likely a result of oxidative damage induced by hydroxyl radicals (16). Age-related oxidative DNA damage and increased accumulation of 8-oxo-2′-deoxyguanosine (8-OHdG) have been shown to be more pronounced in prostate neoplasms than in benign prostate tissue (17). We have recently demonstrated that chronic intraprostatic inflammation causes pre-malignant and malignant changes in prostatic epithelium which may be due at least in part to accumulation of oxidative DNA products as a result of loss of GSTP1 (18). To explore the hypothesis that malignancies may result from exposure to oxidative stress, we examined 8-OHdG levels in the DNA of paired cancerous and benign tissues and examined the relationship between 8-OHdG levels and GSTP1 activity and expression. In addition, we examined whether loss of GSTP1 in prostate epithelial cells increases their susceptibility to oxidative stress; and also examined whether re-establishment of GSTP1 activity results in tumor suppression and caretaker activities during oxidative stress-mediated DNA damage.
MATERIALS AND METHODS
Chemicals and reagents
All chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless otherwise specified. Tissue culture supplies were procured from Falcon (Becton Dickinson Labware, Franklin Lakes, NJ). All tissue culture reagents were obtained from Gibco–Invitrogen Cell Culture (Grand Island, NY) and the fetal bovine serum was purchased from Tissue Culture Biologicals (Tulare, CA). 2′, 7′-dichlorofluorescein diacetate (DCF-DA) was purchased from Invitrogen. Epigalocatechin-3-gallate (EGCG) and Polyphenon E® hereafter referred as green tea polyphenols (GTP) were procured from Mitsui Norin, Japan. Concentrations of 10μg/mL Polyphenon E correspond to 14μM EGCG. The constituents present in Polyphenon E® are mentioned in our previous publication (19).
Human prostate tissue specimens
Discarded benign and malignant prostate tissue from patients without any previous form of adjuvant therapy and who underwent surgery was obtained from the Tissue Procurement Facility of University Hospitals Case Medical Center and the Midwestern Division of the Cooperative Human Tissue Network. The Gleason grade and score of adenocarcinoma specimens were assigned by a surgical pathologist experienced in genitourinary pathology. Immediately after procurement, samples were snap frozen in liquid nitrogen and stored at −80°C till further use. These studies were approved by the Institutional Review Board at Case Western Reserve University.
Cell culture
Human prostate cancer LNCaP cells and virally transformed normal human prostate epithelial cells RWPE1 were obtained from American Type Culture Collection (Manassas, VA). LNCaP cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum and RWPE1 cells were cultured in keratinocyte growth medium supplemented with 5ng/mL human recombinant epidermal growth factor and 0.05mg/mL bovine pituitary extract (Gibco/Invitrogen, Carlsbad, CA) and maintained in 5% CO2 atmosphere at 37°C.
GSTP1 activity assay
GSTP1 activity was determined in tissue and cell lysate by a standard ELISA assay according to vendor’s protocol (Biotrin International, Dublin, Ireland). The substrate reaction was read spectrophotometrically at 450nm using the 96-well automated VersaMax Tunable Microplate Reader (Molecular Devices, Sunnyvale, CA).
Genomic DNA isolation from prostate tissues
Genomic DNA was isolated from approximately 50mg of tumor tissue and adjacent non-tumorous tissue using Fast DNA® Spin Kit (Qbiogene) according to manufacturer’s instructions.
8-OHdG measurement
Measurement of 8-OHdG in tissue specimens and cultured cells was performed with OxiSelect™ Oxidative DNA damage ELISA kit, Cell Biolabs Inc. (San Diego, CA) as per vendor’s protocol. Briefly, DNA was converted to single stranded DNA and 8-OHdG was quantified by quantitative ELISA assay. The quantity of 8-OHdG in the specimens were determined by comparing its absorbance with known 8-OHdG standard curve.
ROS measurement assay
Generation of reactive oxygen species (ROS) in cultured cells was monitored by the conversion of 2′, 7′-dichlorofluorescin diacetate (DCF-DA; Molecular Probes, Eugene, OR) to 2′, 7′-dichlorofluorescein, a dye that fluoresces when ROS are generated by measuring the fluorescence intensity using FluoStar Omega Spectrophotometer (BMG Labtech) at 480 nm for excitation and 560 nm for detection of fluorescence emission. The values, expressed in percentage arbitrary fluorescence units, were compared across treatment groups.
We also used confocal microscopy for the measurement of ROS using DCF-DA as previously described (20). Briefly, LNCaP cells were cultured on glass-bottomed 35-mm petri dishes coated with D-lysine subjected to various treatments. The cells pretreated with GTP were exposed to DCF-DA for 20 min followed by addition of H2O2 and then immediately scanned in DCF-DA-free PBS using 568-nm excitation light from an argon/krypton laser, a 560-nm dichroic mirror, and a 590-nm long pass filter. Images of green MitoTracker fluorescence were collected using a 488-nm excitation light from the argon/krypton laser, a 560-nm dichroic mirror, and a 500–550 nm band pass barrier filter. Quantitation was based on analysis of fluorescence per cell or per area from at least three separate experiments and was expressed relative to control preparations.
Protein extraction and Western blotting
Protein extraction from tissue and cultured cells were performed as previously reported (21). The protein concentration was determined by the DC Bio-Rad assay using the manufacturer’s protocol (Bio-Rad Laboratories Hercules, CA). For Western blot analysis, 30μg of cell lysate were resolved in 4–20% Tris–glycine polyacrylamide gel and then transferred onto the nitrocellulose membrane. The blot was blocked in blocking buffer (5% non-fat dry milk 1% Tween 20; in 20 mM TBS, pH 7.6) for 1 hour at room temperature, and probed using appropriate primary antibodies in blocking buffer overnight at 4°C. The membrane was then incubated with appropriate secondary antibody conjugated with horseradish peroxidase (HRP) (Amersham Life Sciences, Inc., Arlington Heights, IL) followed by detection using chemiluminescence ECL kit (Amersham Life Sciences, Inc.). To ensure equal protein loading, the membrane was reprobed with anti-β-actin antibody (Santa Cruz Biotechnologies).
Construction of GSTP1 expression plasmid
GSTP1 (NM_00852) human GSTP1-cDNA clone pCMV6-XL5 was purchased from Origene (Rockville, MD). Full-length GSTP1 cDNA fragment digested from the pCMV6-XL5 by Not I restriction endonuclease enzyme and cloned into the pLPCX mammalian expression plasmid using Clontech T4 DNA ligase (NEB). GSTP1 sequences were confirmed by DNA sequencing. Transfection was performed with FuGene 6 transfection reagents (Roche Applied Science, Mannheim, Germany) according to the manufacturer’s instructions. Control transfection was conducted with the pLPCX empty vector. Stable transfected single cell colonies were selected by incubation of the cells in 200μg/mL of puromycin. Positive stable transfectants were confirmed by Western blot analysis. The resulting clonal cell lines were designated as LNCaP-pLPCX-GSTP1 and LNCaP-pLPCX.
Transient transfection for GSTP1 knockdown
RWPE1 cells were transfected with GSTP1 siRNA or control siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) according to the siRNA transfection protocol provided by the manufacturer. Briefly, the day before transfection, RWPE1 cells were plated into 100mm plates at the density of 105 cells/mL in Keratinocyte medium (Invitrogen). The cells with 60–80% confluent were transfected with 50nmol/L of GSTP1 siRNA or control siRNA in serum-free Opti-MEM® reduced serum media using FuGene 6 (Roche). After 16 hours of the transfection, the medium was replaced with keratinocyte medium and continued to culture the cells for additional 8 hour, and then GSTP1 expression level was determined by Western blotting and RT-PCR.
RNA Extraction and reverse transcriptase-PCR
Total RNA was extracted from GSTP1 siRNA or control siRNA transient transfected RWPE1 cells after 16 hour of the transfection using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Cells were homogenized in TRIzol, and RNA was precipitated in 100% isopropyl alcohol and resuspended in nuclease free water (Ambion/Applied Biosystems, Austin, TX). RNA was stored in −80°C until needed. First strand synthesis was performed using the high-capacity cDNA Reverse transcription Kit (ABI, Veriti thermal cycler). GSTP1 was amplified using the forward primer 5′GCCTCCTGCCTATACGGGCA3′ and reverse primer 5′CGAAGGAGATCTGGTCTCCCACAA3′ and GAPDH forward 5′GAAGGTGAAGGTCGGAGTC3′ and reverse primer 5′GAAGATGGTGATGGGATTTC3′ used as endogenous controls. PCR products were electrophorsed in 2% agarose gels along with 1kb plus DNA (Invitrogen) and were analyzed.
Cell viability assay
Cell viability was measured using the conventional thiazolyl blue tetrazolium bromide (MTT) reduction assay. Cell respiration, an indicator of cell viability, was determined by the mitochondrial-dependent reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) to formazan. After removal of culture media, cells were incubated at 37° with MTT (0.5mg/mL) for 1 hour. The medium was aspirated and cells were solubilized in dimethyl sulfoxide (100μL) for at least 15 min in the dark. The extent of reduction of MTT was quantified by optical density measurement at 540nm. The effect of H2O2 on cell viability was assessed as percentage cell viability compared to vehicle-treated control cells, which were arbitrarily assigned 100% viability.
Statistical analysis
The GSTP1 enzyme and 8-OHdG estimations, ROS production and cell viability assay were performed in duplicate. All experiments were repeated at least three times. Statistical comparisons among 3 or more groups were made by ANOVA followed by a Dunnett’s multiple comparison tests. Data was summarized as Mean ± SD (standard deviation). The difference of GSTP1 enzyme activity and 8-OH-DG between benign and prostate cancer specimen (paired sample) was examined using paired T-test. The association between GSTP1 enzyme activity and 8-OH-DG (either % decrease/increase or absolute decrease/increase) was estimated using Pearson correlation coefficient. All tests are two-tailed and p-value less than 0.05 are considered to be statistically significant.
RESULTS
GSTP1 activity is decreased in prostate cancer
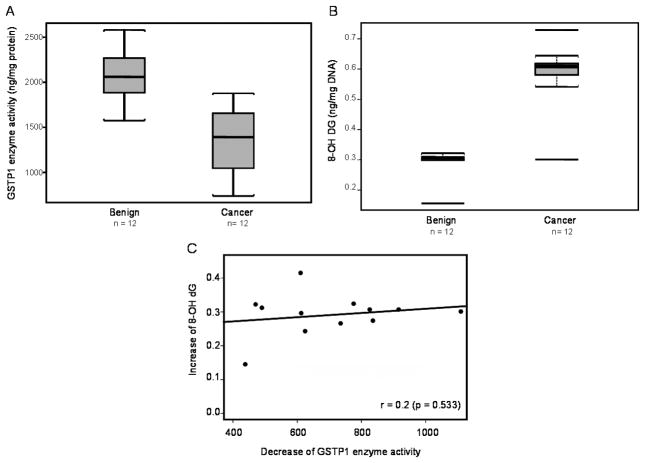
We determined GSTP1 enzyme activity in paired benign and cancer specimens of the prostate obtained from the same individuals. GSTP1 activity was measured using an enzyme-linked immunoassay kit obtained from Biotrin International (Dublin Ireland). A wide inter-individual variation in GSTP1 activity was observed among the individuals examined. As shown in figure 1 A, GSTP1 activity in benign tissue range from 1728.6 to 2580.4 ng/mg protein and in cancer specimens from 738.2 to 1754.4 ng/mg protein. The average GSTP1 activity in benign tissue was 2063.81 ± 289.54 ng/mg protein (Mean ± SD) and in cancer 1359.97 ± 365.16 ng/mg protein (Mean ± SD), which represents a 34% decrease in cancer (P<0.0001).
Figure 1.
Effect of GSTP1 loss on oxidative DNA damage in human prostate cancer. (A) Box-plot for GSTP1 enzyme activity, (B) Box-plot for 8-oxo-2′-deoxogunosine (8-OHdG), and (C) Correlation of 8-OHdG levels and GSTP1 enzyme activity in benign and prostate cancer. The paired benign and cancer tissue specimens were obtained from patients with prostate cancer. Black bar= median; box= 25th to 75th percentiles, bars= entire range. The correlation coefficient was determine by linear regression analysis of 8-OHdG as a function of GSTP1 activity. The details are described in ‘materials and methods’ section.
Oxidative DNA damage is higher in prostate cancer
Next we determined the 8-OHdG levels in the paired prostate tissues that were evaluated for GSTP1 activity. The 8-OHdG levels in DNA in benign prostate tissue ranged from 0.156 to 0.322 ng/μg DNA and in cancer tissue from 0.307 to 0.609 ng/μg DNA, respectively. The average 8-OHdG level in benign tissue was 0.29 ± 0.004 ng/μg DNA (Mean ± SD) and in cancer 0.59 ± 0.101 (Mean ± SD), which represents a 103% increase in cancer (P<0.0001).
Relationship between 8-OHdG levels and GSTP1 activity in prostate tissue
Next we determined whether an association exists between 8-OhdG levels and GSTP1 activity. The association between percent decrease in GSTP1 and percent increase of 8-OHdG with Pearson correlation coefficient r= −0.18 (P=0.534). Using the absolute changes (decrease of GSTP1, benign versus cancer and increase of 8-OHdG, benign versus cancer) instead of percent changes the association was r= 0.2 (p=0.533). In both analyses the association between the two parameters was not significant (Figure 1C).
GSTP1 protein expression is decreased in prostate cancer tissue
To further confirm the differential expression of GSTP1 in benign and cancer tissue, the immunoblot analysis was performed on tissue specimens. As shown in figure 2A, the protein expression of GSTP1 was decreased in 10 of 12 cancer tissues of each individual compared with the paired benign tissue. In additional studies we also determined the extent of GSTP1 promoter methylation in benign and cancer specimens. Indeed, a significant increase in GSTP1 promoter methylation was noted in 8 of 12 cancer specimens compared to benign tissue (Supplemental Figure 1).
Figure 2.
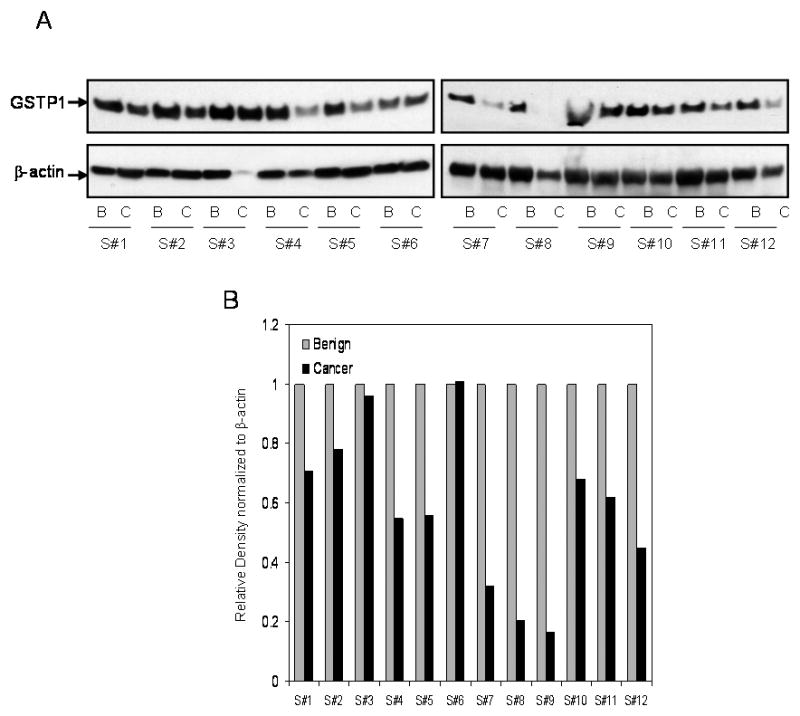
GSTP1 protein expression in paired benign and cancer tissue obtained from patients with prostate cancer. (A) The protein expression was measured by Western blot analysis in the tissue obtained from the benign and cancer specimens of the same patient. Total cell lysates were prepared from the benign and prostate cancer tissues. Protein (30μg) was subjected to SDS-PAGE, followed by Western blot analysis and the detection of protein. The protein expression in the benign and prostate cancer tissue from the same patients is represented from #1 to#12, respectively. For normalization of protein loading, the membrane was stripped and reprobed with β-actin primary antibody and appropriate secondary HRP conjugate. (B) Densitometric analysis of GSTP1 expression normalized to β-actin. The details are described in ‘materials and methods’ section.
Next we determined the ratio of 5-methyl-deoxycytidine (5mdC) to total deoxycytidine (dC) in selected tumor specimens and benign tissue counterparts. The levels of 5mdC and dC were detected in pmol. The ratio of 5mdC/dC in benign tissue ranges from 0.24 to 0.89 and in cancer specimens from 0.60 to 0.93; which may be due to increase methylation of DNA bases in cancer tissue (Supplemental Figure 2).
Oxidative DNA damage and ROS generation in human prostate epithelial cells
Next we sought to determine the consequence of oxidative stress on cell viability, generation of intracellular reactive oxygen species and oxidative DNA damage in response to oxidative stress. For these studies we used virally transformed human prostate epithelial RWPE1 cells and H2O2 as a stable agent which causes oxidative stress and DNA damage resulting in altered cell viability. As shown in the Supplemental figure 3A, treatment of RWPE1 cells with 100μM H2O2 caused a significant decrease in cell viability which decreased to 56.6% from 0.5 hour to 9 hours. Treatment with H2O2 caused an increase i n ROS generation which peaked at 3 hours (51.1%) followed by gradual decrease up to 9 hours. Simultaneously, increased accumulation of 8-OHdG was noted which progressively increased in a time-dependent fashion from 88.2% at 0.5 hour to 158.3% at 9 hours, which correlated with changes in cellular morphology (Supplemental Figure 3B–D). For further studies we used a 3 hour time point for measuring ROS generation and 9 hour for DNA damage measurement.
Loss of GSTP1 increases ROS generation and oxidative DNA damage in human prostate epithelial cells
To determine whether loss of GSTP1 in RWPE1 cells causes changes in ROS generation and 8-OHdG levels, we used a knockdown approach for silencing GSTP1 expression. We used commercially available siRNA for GSTP1 knockdown, where a maximum inhibition of 67.8% in GSTP1 at the message level and 87.8% in GSTP1 inhibition at the protein level was achieved with GSTP1 siRNA after 16 hour post-transfection (Figure 3A).
Figure 3.

Effect of GSTP1 knockdown on oxidative stress and DNA damage in normal human prostate epithelial RWPE1 cells. (A) GSTP1 mRNA and protein expression in RWPE1 cells after knockdown using siRNA. (B) ROS production in RWPE1 cells and after GSTP1 knockdown to H2O2-mediated oxidative stress, and (C) 8-OHdG levels in RWPE1 cells and after GSTP1 knockdown to H2O2-mediated oxidative stress. A significant protection was exhibited in cells treated with N-Acetyl Cysteine (NAC), a well known quencher of ROS. The bars represent mean ± SD of at least 3 independent experiment each performed in triplicate, *p< 0.05 and **p< 0.001 represent significant differences as compared with the H2O2 treated group. The details are described in ‘materials and methods’ section.
Sixteen hours after GSTP1 knockdown, the cells were exposed to 100μM H2O2 and treatment with N-Acetyl Cysteine (NAC), a well known quencher of ROS. Treatment of cells with H2O2 caused a significant increase in ROS generation and 8-OHdG levels in the cells after GSTP1 knockdown. As shown in figure 3B, H2O2 exposure caused a 51% increase in ROS production in RWPE1 cells, which further increased to 108% after GSTP1 knockdown. Treatment with NAC at 10 and 20μM concentrations resulted in a 48–39% decrease in ROS production in RWPE1 cells whereas 27–42% reduction in ROS production was achieved after GSTP1 knockdown in these cells. Similarly, H2O2 exposure to RWPE1 cells caused 135% increase in 8-OHdG which markedly increased to 345% after GSTP1 knockdown. Treatment with NAC at 10 and 20μM concentrations resulted in 16–19% decrease in 8-OHdG in RWPE1 cells, whereas 55–58% reduction in 8-OHdG levels were observed after GSTP1 knockdown in these cells (Figure 3C). This significant increase in ROS production and 8-OHdG levels noted after GSTP1 knockdown and defense after NAC treatment strongly supports the notion that GSTP1 plays a protective role during oxidative stress.
GSTP1 expression in human prostate cancer LNCaP cells
Human prostate cancer LNCaP cells are devoid of GSTP1 expression and cell lysates obtained from LNCaP exhibit a very low basal level of GSTP1 activity. Treatment of LNCaP cells with 10μg/ml GTP, 20μM EGCG and 10nM Aza-dC for 72 hours caused re-expression of GSTP1 enzyme activity in these cells. Exposure of cells resulted in marked increase of 44% with GTP; 27% with EGCG and 33% with Aza-dC in GSTP1 activity (Figure 4A). In the next approach LNCaP cells were stably transfected with GSTP1 expression plasmid. Infection of LNCaP cells with pLPCX vector exhibit a very low basal level of GSTP1 expression, whereas LNCaP-pLPCX-GSTP1 showed significantly higher (266%) overall GSTP1 activity (Figure 4A). Assessment of GSTP1 content in LNCaP cells after treatment with 10μg/ml GTP, 20μM EGCG and 10nM Aza-dC by Western blot analysis indicates that LNCaP and LNCaP-pLPCX cells have essentially no expression of GSTP1 protein, whereas LNCaP-pLPXC-GSTP1 cells express significantly higher levels of protein expression. Similarly, an increase in GSTP1 protein expression was observed after treatment of cells with GTP, EGCG and Aza-dC that coincides with the activity assay (Figure 4B).
Figure 4.
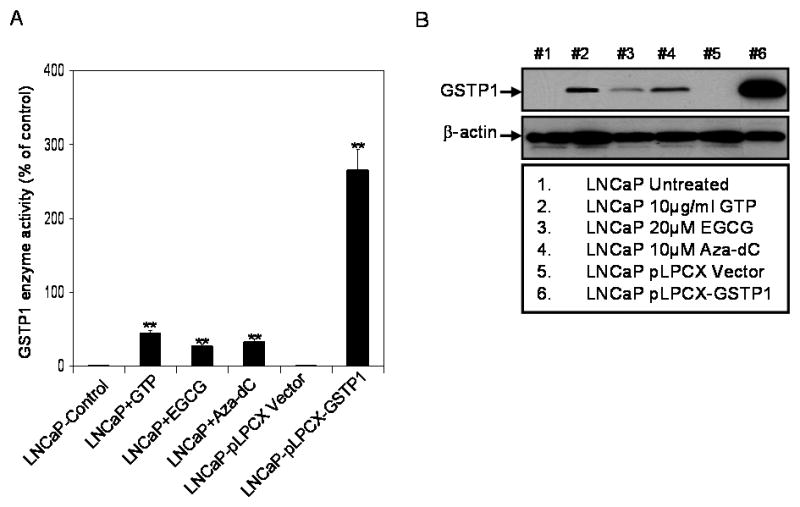
GSTP1 activity, and GSTP1-specific protein expression in LNCaP and LNCaP cells stably transfected with a control vector (LNCaP-pLPCX) or a constitutively expressing GSTP1 vector (LNCaP-pLPCX-GSTP1) or after 72 hours treatment with 10nm 5-aza-2-deoxycytidine (Aza-dC), 10μg/ml green tea polyphenols (GTP) and 20μM with epigallocatechin-3-gallate (EGCG). (A) GSTP1 activity was measured using human Biotrin GSTP1 ELISA assay. The bars represent mean ± SD of at least 3 independent experiment each performed in triplicate, **p< 0.001 represent significant differences as compared with the corresponding controls (B) Western blot analysis of total cell lysate (30μg protein) from indicated cell lines using a rabbit antihuman GSTP1 polyclonal antibody. The details are described in ‘materials and methods’ section.
H2O2-induced oxidative stress in parental LNCaP cells and LNCaP cells with elevated GSTP1 expression
The MTT assay performed demonstrate that exposure of LNCaP cells with H2O2 (100μM) up to 9 hour was cytotoxic to cells and resulted in decrease in cell viability in a time-dependent manner (Data not shown). Clones of LNCaP-pLPCX-GSTP1 cells were more resistant to H2O2-mediated oxidative stress compared with parental cells. A significant decrease in ROS generation and 8-OHdG levels were noted after H2O2 exposure. Compared to cells treated with H2O2, a decrease of 28.5% in ROS production with GTP, 39.5% with EGCG, 44.4% with Aza-dC and 64.2% in LNCaP-pLPCX-GSTP1 clone was observed (Figure 5A). Similarly, a decrease of 63.2% with GTP, 52.8% with EGCG, 62.5% with Aza-dC and 68.3% in LNCaP-pLPCX-GSTP1 clone was observed in 8-OHdG levels, compared to cells treated with H2O2 (P<0.001 paired Student’s t test).
Figure 5.
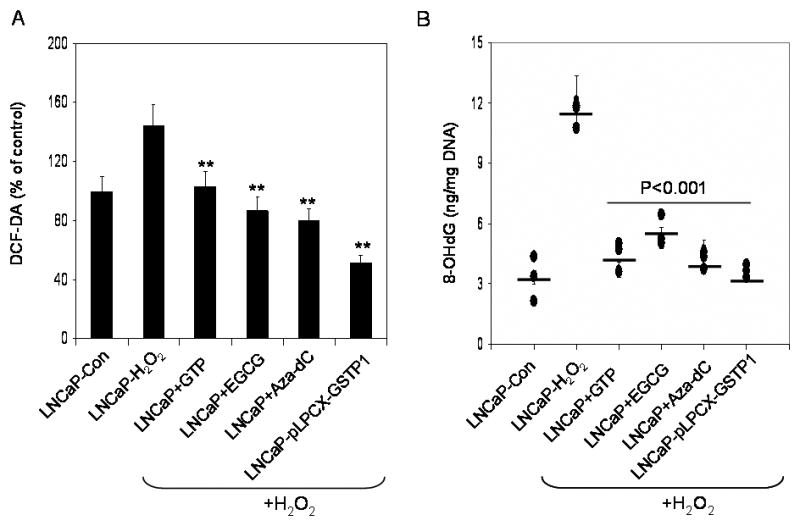
Effect of H2O2-mediated ROS production and 8-OHdG levels in LNCaP and LNCaP cells stably transfected with a control vector (LNCaP-pLPCX) or a constitutively expressing GSTP1 vector (LNCaP-pLPCX-GSTP1) or after 72 hours treatment with 10nm 5-aza-2-deoxycytidine (Aza-dC), 10μg/ml green tea polyphenols (GTP) and 20μM with epigallocatechin-3-gallate (EGCG). (A) Measurement of ROS production by using 2′, 7′-dichlorofluorescein diacetate, a dye that fluoresces when ROS are generated, and (B) oxidative DNA damage by measurement of 8-OHdG. The bars and solid line represent mean ± SD of at least 3 independent experiment each performed in triplicate, **p< 0.001 represent significant differences as compared with the H2O2 treated group. The details are described in ‘materials and methods’ section.
Re-expression of GSTP1 by GTP and EGCG has functional activity
Previous studies have demonstrated that loss of GSTP1 expression in LNCaP cells is due to epigenetic alterations related to promoter DNA hypermethylation and chromatin remodeling (22, 23). Since GTP and EGCG cause re-expression of GSTP1 in LNCaP cells (19), we sought to determine whether the re-expressed GSTP1 has functional significance. For these studies, LNCaP cells were exposed to 10μg/ml GTP and 20μM EGCG for 72 hours and later the cells were washed with PBS and 1mM H2O2 was added and were followed in time-dependent fashion. Using DCF-DA, a dye that fluoresces in the presence of H2O2 or hydroxyl radicals, we confirmed that pre-exposure of cells with GTP markedly reduced ROS generation in these cells. Scans of fluorescence-representing intensities are shown with LNCaP cells as control and cells pre-exposed to GTP only and cells treated with H2O2 and GTP+ H2O2. ROS production was detected in LNCaP cells treated with H2O2 and followed saturation kinetics consistent with a channel-mediated ROS uptake (Figure 6A). Pre-treatment of cells with GTP their exposure to H2O2 caused a significant decrease in the intensity in these cells (Figure 6B). These findings are similar to those of other investigators, supporting the concept that GSTP1 provides protective effects against ROS, and furthermore suggesting that re-expression of GSTP1 may in part be responsible for suppression of the adverse effects of oxidative stress (24, 25).
Figure 6.
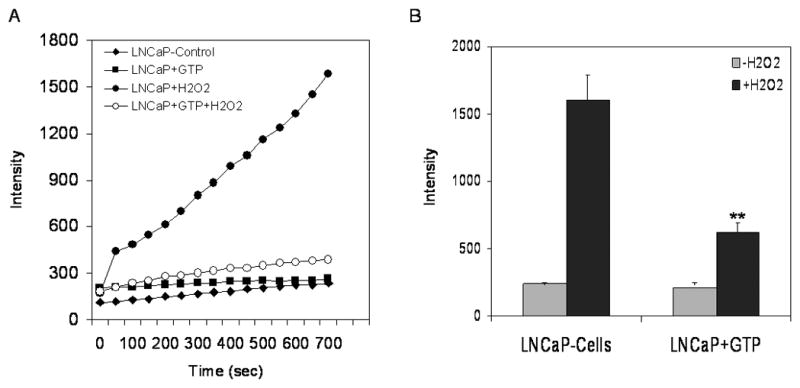
Effect of green tea polyphenols (GTP) in inhibiting reactive oxygen species (ROS) generation in LNCaP cells. The cells were incubated with or without 10μg/ml GTP for 72 hours. ROS was generated by exposure of cells to 1mM H2O2 and tested with ROS sensitive and highly fluorescent 2′, 7′-dichlorofluorescein (DCF) dye. (A) Fluorescence quantification was recorded overtime as described under Materials and Methods. (B) Fluorescence intensity at the termination of the experiment. The bars represent mean ± SD of at least 3 independent experiment each performed in triplicate, **p< 0.001 represent significant differences as compared with the H2O2 treated group.
DISCUSSION
Oxidative stress and accumulated genomic DNA damage may contribute to prostate carcinogenesis (15–18). In this study we have shown that prostate cancer tissues contain significantly higher levels of 8-OHdG than their matched benign tissue, and in addition, strikingly reduced GSTP1 expression is observed in prostate cancer specimens compared to their benign counterparts. While increased DNA damage and altered GSTP1 expression are common observations in prostate cancer, the relationship between these two markers in prostate cancer has not previously been demonstrated. Based on our studies it is reasonable to suggest that loss of GSTP1 activity has an association with 8-OHdG levels that might contribute to the generation of reactive intermediates and oxidized DNA lesions in tumor specimens.
Oxidative stress induced by reactive oxygen species has been shown to be involved in several patho-physiological processes, including carcinogenesis (26, 27). Growing evidence suggests that chronic inflammation with low levels of ROS production plays an important role in causing DNA damage and development of cancer (28). Our previous findings in a prospective five year follow-up study in needle biopsy specimens demonstrate a strong association between chronic prostatic inflammation, pre-malignant, and malignant changes in the prostatic epithelium (18). Studies have shown that 42% of men aged 55 to 80 years exhibit prostatic DNA damage, reflected by levels of 8-hydroxy 2′deoxygunosine (8-OHdG), which results from oxidative modification of guanine (29). Oxidative damage to the DNA base 2′-deoxyguanosine produces 8-hydroxy 2′deoxygunosine (8-OHdG), leading to a point mutation by an A to T substitution when incorporated into DNA (30). It has been demonstrated that hydroxyl radical (OH•), singlet oxygen (O2−) or peroxinitrite anion (ONOO−) is responsible for the formation of 8-OHdG (31, 32). Levels of 8-OHdG in tissue may increase either because there is a strong DNA damaging stimulus or because one of the specific DNA repair mechanism is deficient. Our results suggest that DNA damage is largely due to oxidative stress as indicated by 8-OHdG and subsequent loss of GSTP1 expression as evident in the prostate of the majority of tumor counterpart compared to non-tumorous tissue.
Studies have shown that GST serves a caretaker function, protecting cells against as much as 90% of damage induced by electrophiles and other free radicals (4, 5). Loss of GSTP1 has been shown to increase the inflammatory response in tissues significantly infiltrated by leukocytes (33). It has been demonstrated that disruption of GSPT1/2 allele in rodent model increases the potential of skin and lung tumorigenesis in response to carcinogens (9, 10). Furthermore, GSTP can alter colon tumorigenicity in a mouse model that does not involve carcinogens, suggesting that variations in GSTP expression may influence tumor progression (11). Considering that GSTP1 is an anti-oxidative enzyme and tumor suppressor, its loss caused by epigenetic and genetic changes might lead to tissue damage and promote carcinogenesis. This hypothesis is strongly supported by our findings, which demonstrate that GSTP1 promoter methylation is higher in cancer tissue than in benign tissue from same individual. With the limited sample size and grossly examined tissues for benign and cancer counterparts, our present data suggest a correlation between the loss of GSTP1 activity and DNA damage associated with prostate cancer. It would be worthwhile to design studies with larger sample size and precise separation of cancer and benign tissue are needed to substantiate these findings.
Reactive oxygen species such as H2O2, OH• and O2−, NO levels are relatively higher in prostate epithelial cells than in most tissues (31, 32). Direct evidence linking ROS with an increase in tumor development in the prostate has been established (16–18, 30). This might be due to the loss of genes such as GSTP1, which are caretakers of the electrophile and carcinogenic metabolism in the tissue. The current study sought to assess the role of GSTP1 in protection against H2O2-mediated cytotoxicity and mitigation of oxidative stress in benign and malignant prostatic epithelial cells. Our results demonstrate that silencing of GSTP1 in normal human prostate epithelial RWPE1 cells leads to increased vulnerability to ROS and oxidative stress. Our findings suggest that it may be possible to develop strategies for the prevention of the initial and cumulative DNA damaging events that may contribute to prostate carcinogenesis.
In many prostate cancers, GSTP1 expression is completely abolished by hypermethylation of the promoter (22, 23). For a number of years our laboratory has been involved in studying the role of GSTP1 in prostate cancer and unraveling the mechanism(s) of transcriptional regulation by natural dietary agents (19, 34, 35). Many of our studies have been conducted on human prostate cancer LNCaP cells, a well characterized prostate adenocarcinoma cell line which serves as a model for understanding many aspects of prostatic epithelial cell biochemistry and molecular biology. In LNCaP cells, the GSTP1 gene has been silenced because of CpG island hypermethylation of the promoter region (19, 34). This silencing occurs in >90% of prostatic carcinomas and is increased in HGPIN, and may be an early genetic lesion that predisposes selected cells to carcinogenic insult (15, 16, 23). The relevance of this model system is previously supported by reports demonstrating protection against PhIP-induced cytotoxicity and DNA adducts formation in GSTP1 over-expressing cells (36). In our studies we demonstrate that restoration of the expression of GSTP1 in LNCaP cells protects these cells from toxicity in the form of oxidative stress-induced DNA damage associated with exposure of electrophiles. Further studies are needed on determining the role of other antioxidant enzymes responsible for xenobiotic metabolism and DNA repair enhancement to inhibit the deleterious effects of ROS.
Silencing of GSTP1 in prostate cells may lead to increase vulnerability to electrophilic compounds (4, 5). We demonstrated that restoration of GSTP1 expression in prostate derived LNCaP cells by green tea polyphenols and its major constituent epigallocatechin-3-gallate (EGCG) can mitigate cytotoxicity caused by H2O2 in a similar fashion as demonstrated by genetic overexpression (19, 34). Hence a strong rationale exists for the use of interventional strategies, including green tea intake, to block or decrease DNA damage in prostatic epithelium, and thereby to prevent or delay carcinogenesis. Epidemiological studies have demonstrated a link between green tea intake and reduced prostate cancer risk (37, 38). Our previous studies have shown that regular consumption of green tea polyphenols in amounts comparable with those readily achievable in humans inhibits the development of prostate cancer in an autochthonous mouse model of prostate cancer (39). A recent study suggested that consumption of green tea polyphenols leads to increased GSTP1 expression (40). Induction of GSTP in the prostate by chemopreventive agents may therefore be a viable prevention strategy either alone or in concert with other strategies (41). Accumulated evidence may also explain the inverse correlation between prostate cancer incidence and consumption of plant-based diets rich in polyphenols, which may be GSTP inducers (42, 43). These conclusions support the contention that induction of GSTP expression is an important component of the anti-carcinogenic activity of some dietary agents, and support their inclusion in strategies aimed at chemoprevention of prostate cancer.
Supplementary Material
Methyl-specific PCR analysis for GSTP1 in paired benign and cancer tissue obtained from patients with prostate cancer. AC, adenocarcinoma and BP, matched benign tissue from same individuals. Lanes M and U correspond to methylated and unmethylated DNA, respectively. In each case DNA from LNCaP cells were used as positive control for methylation. Ladd, represents molecular weight marker; and water control for contamination of the PCR reaction. The details are described in ‘materials and methods’ section.
Measurement of 5-methyl-deoxycytidine (5mdC) and total deoxycytidine (dC) in select benign and cancer specimens from same individuals. The procedure requires a thorough purification of the DNA and hydrolysis of bases as nucleoside used for detection of methylation by HPLC using a standard. The levels of 5mdC and dC were detected in pmol. The details are described in ‘materials and methods’ section.
Time-dependent effect of H2O2-mediated oxidative stress in normal human prostate epithelial RWPE1 cells. (A) Measurement of cell viability by MTT assay, (B) Measurement of ROS production by using 2′, 7′-dichlorofluorescein diacetate, a dye that fluoresces when ROS are generated, (C) oxidative DNA damage by measurement of 8-OHdG, and (D) photograph of cell by light microscopy. The details are described in ‘materials and methods’ section.
Acknowledgments
The research work is supported by United States Public Health Service Grants RO1 CA115491, RO1 CA108512, RO1 AT002709 and R21 CA109424 to SG. We are thankful to Dr. Yukihiko Hara at Mitsui Norin Ltd., Japan for kindly providing the Polyphenon E® and EGCG for the study. The authors also acknowledge technical assistance provided by Dr. Minh Lam at the confocal microscopy core facility for conducting experiments using confocal laser microscopy and Dr. Haripaul Sharma for the measurement of 5-methyl cytosine by HPLC in the Urology Department at Case Western Reserve University.
References
- 1.Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- 2.Sau A, Pellizzari Tregno F, Valentino F, Federici G, Caccuri AM. Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys. 2010;500:116–122. doi: 10.1016/j.abb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava SK, Watkins SC, Schuetz E, Singh SV. Role of glutathione conjugate efflux in cellular protection against benzo[a]pyrene-7,8-diol-9,10-epoxide-induced DNA damage. Mol Carcinog. 2002;33:156–162. [PubMed] [Google Scholar]
- 4.Vasieva O. The many faces of glutathione transferase pi. Curr Mol Med. 2011;11:129–139. doi: 10.2174/156652411794859278. [DOI] [PubMed] [Google Scholar]
- 5.Henderson CJ, McLaren AW, Moffat GJ, Bacon EJ, Wolf CR. Pi-class glutathione S-transferase: regulation and function. Chem Biol Interact. 1998;111–112:69–82. doi: 10.1016/s0009-2797(97)00176-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Deng X, Ren X, Zhang B, Chen X, Yang J, Ding H, Sui J, Song X. Expression of mutant p53 and of the multidrug resistant proteins P-glycoprotein and glutathione S-transferase-pi correlated in colorectal adenocarcinoma. Scand J Gastroenterol. 2010;45:925–934. doi: 10.3109/00365521003734117. [DOI] [PubMed] [Google Scholar]
- 7.Pasello M, Michelacci F, Scionti I, Hattinger CM, Zuntini M, Caccuri AM, Scotlandi K, Picci P, Serra M. Overcoming glutathione S-transferase P1-related cisplatin resistance in osteosarcoma. Cancer Res. 2008;68:6661–6668. doi: 10.1158/0008-5472.CAN-07-5840. [DOI] [PubMed] [Google Scholar]
- 8.Arai T, Miyoshi Y, Kim SJ, Akazawa K, Maruyama N, Taguchi T, Tamaki Y, Noguchi S. Association of GSTP1 expression with resistance to docetaxel and paclitaxel in human breast cancers. Eur J Surg Oncol. 2008;34:734–738. doi: 10.1016/j.ejso.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci U S A. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritchie KJ, Henderson CJ, Wang XJ, Vassieva O, Carrie D, Farmer PB, Gaskell M, Park K, Wolf CR. Glutathione transferase pi plays a critical role in the development of lung carcinogenesis following exposure to tobacco-related carcinogens and urethane. Cancer Res. 2007;67:9248–9257. doi: 10.1158/0008-5472.CAN-07-1764. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie KJ, Walsh S, Sansom OJ, Henderson CJ, Wolf CR. Markedly enhanced colon tumorigenesis in Apc(Min) mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci U S A. 2009;106:20859–20864. doi: 10.1073/pnas.0911351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama M, Bennett CJ, Hicks JL, Epstein JI, Platz EA, Nelson WG, De Marzo AM. Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection. Am J Pathol. 2003;163:923–933. doi: 10.1016/s0002-9440(10)63452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostwick DG, Meiers I, Shanks JH. Glutathione S-transferase: differential expression of alpha, mu, and pi isoenzymes in benign prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma. Hum Pathol. 2007;38:1394–1401. doi: 10.1016/j.humpath.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Nelson WG, De Marzo AM, Deweese TL, Lin X, Brooks JD, Putzi MJ, Nelson CP, Groopman JD, Kensler TW. Preneoplastic prostate lesions: an opportunity for prostate cancer prevention. Ann N Y Acad Sci. 2001;952:135–144. doi: 10.1111/j.1749-6632.2001.tb02734.x. [DOI] [PubMed] [Google Scholar]
- 15.Nelson WG, DeWeese TL, DeMarzo AM. The diet, prostate inflammation, and the development of prostate cancer. Cancer Metastasis Rev. 2002;21:3–16. doi: 10.1023/a:1020110718701. [DOI] [PubMed] [Google Scholar]
- 16.Miyake H, Hara I, Kamidono S, Eto H. Oxidative DNA damage in patients with prostate cancer and its response to treatment. J Urol. 2004;171:1533–1536. doi: 10.1097/01.ju.0000116617.32728.ca. [DOI] [PubMed] [Google Scholar]
- 17.Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61:6025–6028. [PubMed] [Google Scholar]
- 18.MacLennan GT, Eisenberg R, Fleshman RL, Taylor JM, Fu P, Resnick MI, Gupta S. The influence of chronic inflammation in prostatic carcinogenesis: a 5-year followup study. J Urol. 2006;176:1012–1016. doi: 10.1016/j.juro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer. 2010;126:2520–2533. doi: 10.1002/ijc.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001;276:47379–47386. doi: 10.1074/jbc.M107678200. [DOI] [PubMed] [Google Scholar]
- 21.Shukla S, Shukla M, Maclennan GT, Fu P, Gupta S. Deregulation of FOXO3A during prostate cancer progression. Int J Oncol. 2009;34:1613–1620. doi: 10.3892/ijo_00000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singal R, van Wert J, Bashambu M. Cytosine methylation represses glutathione S-transferase P1 (GSTP1) gene expression in human prostate cancer cells. Cancer Res. 2001;61:4820–4826. [PubMed] [Google Scholar]
- 23.Lin X, Tascilar M, Lee WH, Vles WJ, Lee BH, Veeraswamy R, Asgari K, Freije D, van Rees B, Gage WR, Bova GS, Isaacs WB, Brooks JD, DeWeese TL, De Marzo AM, Nelson WG. GSTP1 CpG island hypermethylation is responsible for the absence of GSTP1 expression in human prostate cancer cells. Am J Pathol. 2001;159:1815–1826. doi: 10.1016/S0002-9440(10)63028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YH, Han DW, Shu H, Ryu GH, Hyon SH, Cho BK, Park JC. Protective effects of green tea polyphenol against reactive oxygen species induced oxidative stress in cultured rat calvarial osteoblast. Cell Biol and Toxicology. 2003;19:325–337. doi: 10.1023/b:cbto.0000004986.51081.c5. [DOI] [PubMed] [Google Scholar]
- 25.Elbling L, Herbacek I, Weiss RM, Jantschitsch C, Micksche M, Gerner C, Pangratz H, Grusch M, Knasmüller S, Berger W. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free Rad Biol Med. 2010;49:1444–1452. doi: 10.1016/j.freeradbiomed.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 28.Koul HK, Kumar B, Koul S, Deb AA, Hwa JS, Maroni P, van Bokhoven A, Lucia MS, Kim FJ, Meacham RB. The role of inflammation and infection in prostate cancer: Importance in prevention, diagnosis and treatment. Drugs Today (Barc) 2010;46:929–943. doi: 10.1358/dot.2010.46.12.1537942. [DOI] [PubMed] [Google Scholar]
- 29.Lockett KL, Hall MC, Clark PE, Chuang SC, Robinson B, Lin HY, Su LJ, Hu JJ. DNA damage levels in prostate cancer cases and controls. Carcinogenesis. 2006;27:1187–1193. doi: 10.1093/carcin/bgi288. [DOI] [PubMed] [Google Scholar]
- 30.Asami S, Manabe H, Miyake J, Tsurudome Y, Hirano T, Yamaguchi R, Itoh H, Kasai H. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis. 1997;18:1763–1766. doi: 10.1093/carcin/18.9.1763. [DOI] [PubMed] [Google Scholar]
- 31.Tam NN, Leav I, Ho SM. Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat. Am J Pathol. 2007;171:1334–1341. doi: 10.2353/ajpath.2007.070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arsova-Sarafinovska Z, Eken A, Matevska N, Erdem O, Sayal A, Savaser A, Banev S, Petrovski D, Dzikova S, Georgiev V, Sikole A, Ozgök Y, Suturkova L, Dimovski AJ, Aydin A. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin Biochem. 2009;42:1228–1235. doi: 10.1016/j.clinbiochem.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Bergh A, Damber JE. Increased p53 immunoreactivity in proliferative inflammatory atrophy of prostate is related to focal acute inflammation. APMIS. 2009;117:185–195. doi: 10.1111/j.1600-0463.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- 34.Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012;33:377–384. doi: 10.1093/carcin/bgr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S. Prostate cancer chemoprevention: current status and future prospects. Toxicol Appl Pharmacol. 2007;224:369–376. doi: 10.1016/j.taap.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, Nelson WG, Kensler TW. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res. 2001;61:103–109. [PubMed] [Google Scholar]
- 37.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S JPHC Study Group. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 38.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow HH, Hakim IA, Vining DR, Crowell JA, Tome ME, Ranger-Moore J, Cordova CA, Mikhael DM, Briehl MM, Alberts DS. Modulation of human glutathione s-transferases by polyphenon E intervention. Cancer Epidemiol Biomarkers Prev. 2007;16:1662–1666. doi: 10.1158/1055-9965.EPI-06-0830. [DOI] [PubMed] [Google Scholar]
- 41.Lii CK, Liu KL, Cheng YP, Lin AH, Chen HW, Tsai CW. Sulforaphane and alpha-lipoic acid upregulate the expression of the pi class of glutathione S-transferase through c-jun and Nrf2 activation. J Nutr. 2010;140:885–892. doi: 10.3945/jn.110.121418. [DOI] [PubMed] [Google Scholar]
- 42.Parsons JK, Newman VA, Mohler JL, Pierce JP, Flatt S, Marshall J. Dietary modification in patients with prostate cancer on active surveillance: a randomized, multicentre feasibility study. BJU Int. 2008;101:1227–1231. doi: 10.1111/j.1464-410X.2007.07365.x. [DOI] [PubMed] [Google Scholar]
- 43.Richman EL, Carroll PR, Chan JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer. 2012;131:201–210. doi: 10.1002/ijc.26348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methyl-specific PCR analysis for GSTP1 in paired benign and cancer tissue obtained from patients with prostate cancer. AC, adenocarcinoma and BP, matched benign tissue from same individuals. Lanes M and U correspond to methylated and unmethylated DNA, respectively. In each case DNA from LNCaP cells were used as positive control for methylation. Ladd, represents molecular weight marker; and water control for contamination of the PCR reaction. The details are described in ‘materials and methods’ section.
Measurement of 5-methyl-deoxycytidine (5mdC) and total deoxycytidine (dC) in select benign and cancer specimens from same individuals. The procedure requires a thorough purification of the DNA and hydrolysis of bases as nucleoside used for detection of methylation by HPLC using a standard. The levels of 5mdC and dC were detected in pmol. The details are described in ‘materials and methods’ section.
Time-dependent effect of H2O2-mediated oxidative stress in normal human prostate epithelial RWPE1 cells. (A) Measurement of cell viability by MTT assay, (B) Measurement of ROS production by using 2′, 7′-dichlorofluorescein diacetate, a dye that fluoresces when ROS are generated, (C) oxidative DNA damage by measurement of 8-OHdG, and (D) photograph of cell by light microscopy. The details are described in ‘materials and methods’ section.