Abstract
The appropriate use of conventional or potential treatments for hepatocellular carcinoma requires that benefit can be shown. Therefore, the accurate assessment of response is both critical and essential. Demonstration of benefit observed will be determined by the criteria used. However, the use of conventional criteria based on anatomical imaging to assess response and progression is inadequate. Limitations occur due to the unique nature, presentation and course of hepatocellular cancer, any underlying concomitant disease, the multiplicity of treatment options, and the challenges in assessing viable tumor. Locoregional therapies or cytostatic therapies can have beneficial effects and induce tumor necrosis without appreciable changes in tumor size. In recognition of the inherent limitations in conventional imaging criteria, various modifications have been proposed. In this review, the goals of assessing tumor response in clinical practice and in clinical trials are outlined. The varying patterns of response to different therapeutic modalities such as locoregional therapy and molecularly targeted therapy are reviewed, and an approach to the assessment of response based on clinical, biochemical, morphological and functional criteria has been outlined. The implications of current and proposed approaches of assessing response for clinical practice or design of clinical trials are reviewed.
Keywords: Clinical trials, Imaging, liver cancer, magnetic resonance imaging
Introduction
The determination of tumor response during treatment is a critical and essential in the management of patients with liver cancers. A variety of therapeutic modalities are available for the treatment of these cancers, and include surgical resection or liver transplantation, local or regional therapy or systemic therapies (Table 1). In clinical practice, a rapid, reproducible and accurate assessment of tumor response is needed to determine the response to therapeutic interventions that do not involve surgical resection, to plan future treatments and to assess prognosis while minimizing morbidity of ineffective therapies. In clinical trials and experimental protocols, accurate and meaningful response measurements form the basis for determining the efficacy of response, for comparison of different treatment strategies, and for regulatory approval prior to registration of a new therapeutic agent.
Table 1. Overview of current therapeutic modalities for HCC.
Surgery offers the potential for cure. Local therapies my result in cure for very small lesions that are completely ablated, whereas other modalities result in reduction of tumor burden or delay in tumor progression.
| Surgery | Transplantation Resection |
| Local therapy | Radiofrequency ablation Microwave ablation Cryotherapy Ethanol or acetic acid ablation |
| Regional therapy | Trans-arterial chemotherapy Trans-arterial radiation therapy External beam radiation Stereotactic body radiation therapy |
| Systemic therapy | Doxorubicin Sorafenib |
Tumor response criteria such as the World Health Organization (WHO) (1) criteria and Response Evaluation Criteria in Solid Tumors (RECIST) (2) (3) are familiar to most practitioners and investigators. These criteria are based on linear measurements of tumor size on radiological images, and allow for the quantitation of tumor shrinkage as a marker of response to therapy. They have gained acceptance by clinicians, clinical trials specialists and regulatory bodies for determining tumor response to therapy. However, these size-based imaging criteria are unreliable in assessing the benefit of conventional and future treatments for hepatocellular carcinoma (HCC), and have significant limitations for assessment of therapeutic response or progression in these cancers. These criteria do not always accurately reflect tumor burden, which reflects the amount of viable tumor present. Liver directed therapies such as ablation or embolization may induce tumor necrosis without appreciable changes in tumor size. Similarly, molecular targeted therapies such as sorafenib have been shown to have survival benefits without significant alterations in tumor size. Moreover, HCC often arises in the presence of cirrhosis with regenerative nodules. This setting of a field defect promoting tumorigenesis results in a propensity towards multifocality and unique patterns of progression within the liver. Thus, an important determinant of response or progression requires an accurate evaluation for malignancy in all new hepatic mass lesions in a cirrhotic liver. In recognition of the inherent limitations in conventional imaging criteria, modifications have been proposed and endorsed in guidelines by professional guidelines such as the American Association for Study of Liver Disease (AASLD) and the European Association for Study of Liver diseases (EASL).
The use of a standard approach to assess response is essential for the evaluation and interpretation of treatment response, comparison of different clinical trials and for approval of new therapeutic agents by regulatory authorities. In this brief review, we will outline the unique challenges of assessing response for hepatocellular cancer, the wide range of therapeutic options, and current approaches to determine treatment responses, along with their application in clinical practice or trials.
Assessment of response
The response to therapy can help predict tumor biology and guide subsequent therapy or management such as listing for orthotopic liver transplantation (OLT). The criteria of number and size of HCC mass lesions form the basis of decisions for listing patients for OLT. Patients with tumors that expand beyond accepted criteria will be removed from transplant lists. Newer expanded criteria require a treatment response in order to list for transplant and therefore uniformity in assessing response amongst transplant centers is critical.
Assessment of response in clinical trials of therapeutic interventions for HCC have been challenging. The criteria used should be able to identify meaningful benefit of an intervention in reducing mortality and morbidity. Evaluation and response criteria used have varied between studies, study groups, and cancer centers with respect to the methods and approach used to evaluate tumor response, identification of an index lesion, assessment of progression, and in the distinction between therapeutically targeted and non-targeted lesions during treatment of multifocal lesions.
In order to assess response to therapy for HCC, an approach based on clinical characteristics, accurate assessment of tumor burden and objective evidence of disease progression is necessary. In practice, imaging criteria are relied on to identify therapeutic response or disease progression. The specific criteria that are currently used are based on anatomical or morphological features, namely size and arterial vascularization (Table 2). Emerging criteria are based on functional imaging encompassing biochemical, biological and molecular features. While they may offer some attractive benefits, functional imaging based criteria are in early stages of development and are not routinely used at this time.
Table 2.
Comparison of anatomical and morphological imaging based criteria
| WHO(1) | Recist 1.0(2) |
Recist 1.1(3) |
EASL(20) | AASLD/ JNCI mRECIST( 18, 19) |
|
|---|---|---|---|---|---|
| Size of lesion | + | + | + | + | + |
| Vascularity – contrast enhancement | − | − | − | + | + |
| Dimensions | 2 | 1 | 1 | 2 | 1 |
| Number of lesions | 5 target 5 non-target |
2 target 5 non-target |
Clinical markers of response
The monitoring, assessment of treatment response and management of patients undergoing treatment for HCC involves clinical judgment based on a knowledge of patterns of response and disease progression and experience in managing co-existing chronic liver disease. Thus, the requisite training and experience may overlap that provided in traditional graduate medical education programs in oncology, hepatology, radiology or surgery.
Presentation
The value of a clinical history and examination is unclear, given that many patients can present with advanced disease despite tumor growth, and that systemic symptoms can reflect the underlying hepatic disease rather than tumor. For these reasons, assessment depends on accurate evaluation of tumor growth by tumor markers or imaging.
Tumor progression
Disease progression can occur due to continued growth of tumor, spread within or outside the liver, or the development of new lesions within the liver. Imaging can provide an objective assessment of change in tumor size, while contrast enhancement can provide an assessment of extent of tumor necrosis. Correlative pathological analysis show that change in size of a lesion as well as contrast enhancement can reliably predict tumor necrosis (4).
Tumor recurrence or intrahepatic metastases
Distinguishing between new HCC and cirrhotic or dysplastic nodules is challenging in patients with cirrhosis. The identification of new tumors is best done by contrast enhanced imaging. Lesions should be examined for contrast enhancement characteristics that have high sensitivity and specificity for HCC in this setting. Conventionally, new lesions > 1 cm with contrast enhancement in the arterial phase and washout in the portal venous phase, or lesions showing enlargement of at least 1 cm over serial imaging are considered to be HCC.
Liver Disease progression
The underlying liver disease can progress in several ways that should be distinguished from tumor progression. These include ascites, pleural effusion, portal vein thrombosis, hepatic vein thrombosis, and regional lymphadenopathy.
Ascites
The presence of ascites can result in further decompensation of liver disease due to treatment effect, or natural progression of disease. Cytological analysis is recommended, and incorporated in the RECIST criteria.
Coagulopathy
Worsening of coagulopathy such as a prolongation of prothrombin time and INR may represent liver disease progression and as such is included in the Model of End Stage Liver Disease prognostic score.
Pleural effusion
Similar to ascites, the presence of a pleural effusion, particularly right sided effusions, are likely to be manifestations of underlying disease rather than tumor spread. Cytological analysis is required prior to considering these to be related to tumor progression.
Venous thrombosis
The development of a bland thrombus in the portal vein is common in cirrhosis. There is always a concern that a thrombus in patients with hepatocellular carcinoma may represent a tumor thrombus; however if the thrombus does not enhance on imaging and in particular if it is not immediately adjacent to the tumor, it does not necessarily reflect tumor progression. Hepatic vein thrombosis is usually a result of tumor spread, and portends a poor prognosis.
Lymphadenopathy
Enlarged lymph nodes, in particular hilar lymph nodes are common in patients with chronic liver disease. By RECIST criteria lymph nodes greater than 2 cm are considered significant.
Hepatic failure
Progressive increases in bilirubin is most often a result of underlying liver disease due to functional inadequacy of hepatic parenchymal function.
Survival
The traditional oncological endpoint of overall survival cannot be relied on as a sole measure in evaluating therapeutic interventions in HCC. When HCC arises in the setting of cirrhosis or advanced fibrosis, overall survival can be impacted by factors such as progression of underlying disease and its complications, such as liver failure, and portal hypertension, as well as the impact of the therapeutic efforts on the underlying disease. Thus, overall survival may be adversely affected by these confounding factors even with the use of the most effective anti-cancer therapies. A more relevant endpoint in evaluating interventions in HCC and in reporting results in clinical trials may be time to progression (TTP) of tumor in response to treatment. Overall survival still has relevance in the analysis of therapeutic response to treatments in HCC but it should be used in combination with TTP and must be used with caution when establishing success of clinical interventions.
Biochemical markers of response
An ideal serum biomarker of response should (a) consistently correlate with tumor burden, (b) show meaningful correlation with responses to therapy, and (c) correlate with prognosis. There are no currently available biomarkers that meet these criteria and are predictive of tumor burden or prognosis in HCC. The alpha fetoprotein (AFP) tumor marker is elevated in the serum of some, but not all patients with HCC, and is often used in clinical practice to evaluate for a therapeutic response. For patients with an elevated baseline serum AFP, a reduction in serum AFP concentration may reflect changes in tumor burden with therapy and be used as an indicator of tumor response. The definitions of response based on changes in AFP in clinical reports have varied from 20% to 50% decreases in AFP (5–7). Responses in AFP have correlated with overall survival following surgical resection, systemic chemotherapy, and locoregional therapy (5–10). Changes in AFP following surgery and rate of change in AFP before OLT can also predict recurrence (11, 12).
To date, the superiority or equivalence of AFP compared to conventional radiological measures of response has not been shown (13). There are several limitations regarding the use of AFP. These include the heterogenous nature of elevations of AFP that can vary over several orders of magnitude. Moreover, up to a half of HCC may not produce AFP. In addition, AFP levels may not normalize completely even with complete eradication of tumor in the setting of chronic hepatic inflammation and regeneration such as with chronic Hepatitis C virus infection. Baseline elevations of AFP may be unrelated to tumor burden in these conditions. The value of AFP with molecular targeted or cytostatic therapies that do not reduce tumor burden despite a survival advantage remains to be shown. There have been several retrospective studies which have looked at the utility of AFP for analysis of therapeutic response to treatment. These studies have several limitations. There is no clearly defined cut-off value for AFP, they were not prospectively designed to evaluate treatment responses, and there was variability in the radiographic criteria used in assessment of treatment response. Although some authors have called for replacing radiological follow-up studies with AFP determinations, there is insufficient data to validate this approach at this time and AFP measurements are best used in conjunction with rather than in place of radiological assessments.
There are several additional biochemical markers which have been evaluated in HCC. Lens culinaris agglutinin-reactive AFP (AFP-L3) is the glycosylated subfraction of AFP and is more specific to malignant hepatocytes than AFP. It may have a role in distinguishing between benign and malignant elevations of AFP such as in the setting of hepatitis C; however, it has a low utility in cases where the AFP is not markedly elevated (14). The clinical utility of the AFP-L3 level is limited because of variability in comparison to tumor size. Another marker, des-gamma carboxyprothrombin (DCP) has been investigated as a serological marker for HCC detection. Initially developed as a radioimmunoassay, the DCP assay has now been modified as a monoclonal antibody enzyme immunoassay (EIA) to quantify plasma DCP levels. Mita and colleagues showed that determination of DCP levels using the more sensitive EIA method at a cutoff value of 40 mAU/ml had a moderate sensitivity (61.5%) and a high specificity (94.7%) for diagnosing HCC in high-risk populations (15). Because elevated DCP levels may not be associated with increased AFP or AFP-L3/AFP levels in HCC patients, studies have demonstrated that a combination of these markers has a greater sensitivity in diagnosing HCC than the DCP alone. Glypican-3 (GPC-3) is a heparin sulfate proteoglycan that interacts with several growth factors by binding to the cell membrane via glycosylphosphatidylinositol anchors. Because GPC-3 has only been detected in HCC cells and not in benign liver tissues, it has been investigated as a potential biomarker for the diagnosis of early-stage HCC (16). Serum GPC-3 levels at a cutoff value of 300 ng/L had a sensitivity and specificity for HCC diagnosis of 47.0% and 93.5%, respectively making it a potential biomarker for HCC. There may be a value in integrating these markers in the diagnostic algorithm for HCC in the future, however currently they have no clear role as markers of therapeutic response.
Anatomical or morphological imaging-based criteria to assess response
Anatomical and morphological based imaging is the main approach to assessment of response. Guidelines for assessment of response based on radiological characteristics have evolved from evaluation of tumor shrinkage by measuring size (WHO, RECIST, RECIST 1.1), to evaluation of viable tumor burden by measuring vascularization (EASL, mRECIST) (17).
Tumor size
Tumor size can be assessed on either computed tomography (CT) or magnetic resonance imaging (MRI). The RECIST and WHO criteria describe size based measurements in either one or two dimensions respectively. Changes in size can generally be quantitated accurately. However, tumor necrosis, a desirable effect of therapies with an impact on survival, can occur in the absence of change in size. Furthermore, interventions such as local ablation will alter imaging characteristics and size determinations of target lesion. Following radiofrequency ablation (RFA), transarterial chemoembolization (TACE) or transarterial radioembolization (TARE), the size of lesions on imaging studies may actually increase due to the intervention. An example of such a change is shown in Figure 1. Thus tumor responses based on size alone (RECIST or WHO) cannot be used to assess response with these modalities. These limitations have prompted efforts to incorporate measures to detect and monitor response based on the presence of viable, non necrotic tumor burden.
Figure 1. MR imaging of HCC.
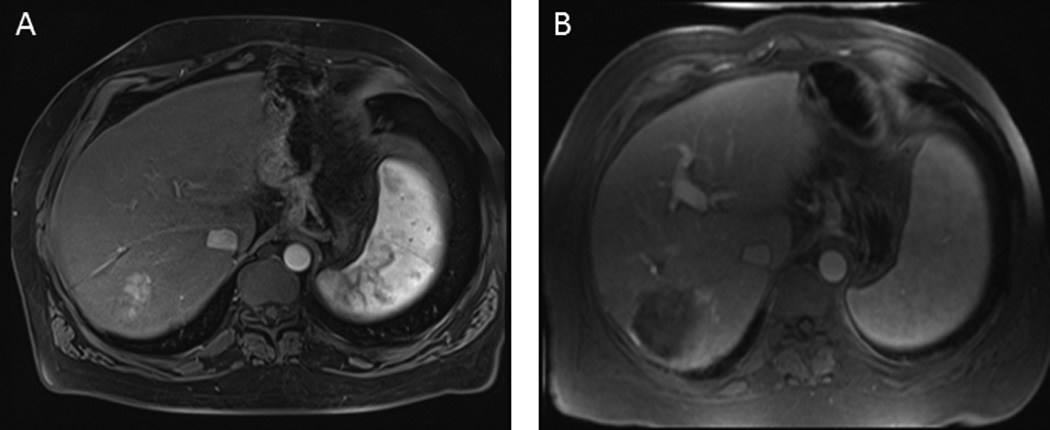
A. Pre-treatment. A solitary enhancing HCC is present in the right lobe. B. Post-transarterial chemoembolization. The HCC has been successfully treated, and there is an absence of viable tumor despite an increase in size of lesion.
Viable tumor burden
The evaluation of tumoral arterial enhancement by contrast to identify necrosis has been proposed to more accurately identify response to therapeutic interventions, particularly with liver directed locoregional therapies or molecular therapies that may improve survival by causing tumor necrosis or stopping tumor growth in the absence of major changes in tumor size. A loss of uptake of contrast agent during the arterial phase of dynamic contrast enhanced CT scanning correlates with necrosis, and thus the presence of contrast enhancement can be used to determine the presence of viable tumor. Criteria based on quantitation of contrast enhancement to assess viable tumor area are outlined in the EASL and AASLD/JNCI criteria which have recently been embodied as the modified RECIST (mRECIST) criteria (18–20). According to these criteria, the response to therapy can be assessed by a reduction in contrast enhanced tumor, and subsequent tumor progression detected by an increase in size or an increase in contrast enhancement. However, accurate quantitation of contrast enhancing regions is difficult in tumors with heterogeneous regions of necrosis. For liver-directed therapies, the optimal assessment of response requires resolution of any transient effects of the intervention (21–26). The choice of imaging modality used also depends on the context. Thus, MRI is preferable for chemoembolization using lipiodol as its retention within the tumor will obscure any enhancement due to contrast. However, the use of lipiodol retention as a marker of tumor response has been reported.
Volumetry
Size measurements may not reflect tumor burden, which is more accurately evaluated by volumetric analysis. Such analysis can be automated providing reproducibility and reduced observer bias. Volumetry of contrast enhanced regions similarly can provide an approximation of viable tumor based on vascularization as reflected by regions of contrast enhancement. The use of pre-defined algorithms for volumetric analysis may obviate some of the concerns regarding by accuracy of quantitation of viable tumor burden, or of tumor necrosis, but their predictive value will need to be validated prior to adoption. This could be done by incorporating these assessments in ongoing trials.
Functional imaging based markers of response
An earlier assessment of tumor response may be possible by functional imaging that can detect tumor biochemical or micro-environmental changes that precede tumor shrinkage or growth. Several emerging techniques can functionally image perfusion, oxygenation and metabolism (Table 3). These are being evaluated in exploratory early phase studies. Their ultimate use in clinical practice as predictors of response will require comparisons with existing approaches, standardization of techniques of image analysis and acquisition, validation of reproducibility and utility in large multi-center studies, and correlations with pathological changes and survival. The assessment of functional markers is hampered by the lack of appropriate gold standards for comparison of their efficacy.
Table 3.
Measures of response to therapy
| Criterion | Description | Limitations | |
|---|---|---|---|
| Clinical | Symptomatic relief or progression | Reduction or progression of symptoms, change in QOL measures, transfusion requirements or other parameters | Lack of validated tools to measure QOL, or symptom response Lack of defining symptoms |
| Tumor recurrence | Lack of standardized time to assess response based on size or tumor necrosis | ||
| New lesions and tumor progression | Challenging to document new tumors in cirrhotic livers and distinguish from cirrhotic nodules Progression of underlying liver disease can mask tumor progression |
||
| Survival | Time from treatment initiation to death | Confounded by competing causes of mortality from liver disease or therapy | |
|
Biochemical (Tumor markers) |
AFP | Serum tumor marker | AFP is not elevated in upto 50% patients with HCC. AFP levels may not normalize even with complete tumor response in patients with underlying chronic hepatitis |
|
Structural imaging (Anatomical-morphological) |
RECIST | Tumor size, largest diameter | Size measurements Observer variations Irregular or infiltrative lesions difficult to quantitate Changes may lag behind biochemical or molecular changes |
| RECIST 1.1 | Unidimensional measurement of tumor size | ||
| WHO | Cross-product of tumor size in two directions | ||
| Volumetry | Tumor volume assessment | ||
| EASL | Contrast enhanced lesion (= viable tumor) | Contrast enhanced region | |
| mRECIST | Unidimensional? assessment of viable tumor | ||
| Volumetry | Assessment of viable tumor volume based on contrast enhancement | ||
|
Functional imaging (cellular-molecular-biological) |
18F FDG- PET | Metabolic / proliferative activity within tumors | Not widely available Not standardized, other than for PET imaging Not yet validated as markers of outcomes, or viable tumor |
| DW-MRI | Water motion and tumor cellularity | ||
| DCE-MRI | Contrast biodistribution within tumors | ||
| MRS | Relative amounts of biochemical components within tumors |
EASL: European association for study of liver disease, RECIST: Response Evaluation Criteria in Solid Tumors, AFP: Alpha-feto-protein, WHO: World Health Organization, DW-MRI: Diffusion-weighted MRI, DCE-MRI: Diffusion contrast enhanced MRI, MRS: Magnetic resonance spectroscopy, 18F FDG-PET: 18F fludeoxyglucose positron emission tomography Modalities in italics are currently under investigation.
Magnetic resonance perfusion imaging
Using dynamic MRI imaging, measurements for permeability and other kinetic parameters related to perfusion can be quantitated. These include the transfer constant (Ktrans) and redistribution rate constants. These parameters have been shown to be more sensitive in predicting response to sunitinib than the RECIST or mRECIST criteria (27).
Diffusion weighted imaging (DWI)
DWI by MRI allows the quantification of the diffusivity of water molecules in biological tissue by means of apparent diffusion coefficient measurements (ADC). In the presence of intact cell membranes, the motion of water molecules within tissues is restricted. With a loss of membrane integrity, such as after treatment, the flow of water molecules is not restricted and their distribution is more homogeneous which is detected as an enhanced ADC signal. ADC correlates inversely with cellularity and has been shown to correlate with pathological findings (28). An early increase in tumor ADC may correspond to tumor necrosis and be useful after TACE or following radiation therapy (29, 30). To date, there is limited data on the ability of this technique to discriminate responders from non-responders, or for this technique to predict outcomes.
How should response be assessed in clinical practice?
An overview of the clinical, biochemical, and imaging measures of response are outlined in Table 3. Accurate assessment of HCC tumor burden, response or progression is dictated primarily by imaging, and to a lesser extent by tumor markers and never by clinical features alone. Understanding imaging-based response criteria and their appropriate use is therefore essential for optimal clinical management. Although some practicing clinicians may not be familiar with the current revisions or modifications of the RECIST response criteria, awareness of these and their application for routine use in management of HCC would be expected to increase with use. Despite the complexities involved with the routine use of these criteria, an accurate assessment of disease progression in patients with HCC provides a critical and essential basis for the ongoing management and planning of additional treatments.
The assessment of response will depend on the intent of treatment, namely whether or not treatment is with curative or with palliative intent. For curative treatments such as resection, transplantation, or locally ablative treatments of small solitary lesions without residual tumor, the goals are to detect tumor recurrence or new tumor formation. A suggested surveillance program would include both imaging and tumor marker assessments at 3 month intervals for the first year, 6 month intervals for the second year, and annually thereafter. For treatments with the intention of palliation or control of tumor growth, such as with regional or systemic therapies, the goals of response assessments are to determine disease progression and symptom control, if present. Following chemoembolization, an evaluation of therapeutic response should be performed 1–6 months following the intervention, with a determination of impact on both lesions that were therapeutically targeted and any others that may not have been therapeutically targeted. Following ablation, imaging at 3 months may provide baseline information on response once the inflammatory changes and hemorrhage related to the procedure have resolved. Subsequent imaging and tumor marker assessments every 3 months for the first 2 years will allow documentation of stability or disease progression, and guide further interventions if disease progression is noted. If there is no disease progression for two years, these evaluations could be done annually.
The criteria used to assess response will determine the reported benefit (Figure 2). Following TACE, mRECIST and EASL criteria for response 1 month after initial TACE more consistently predicted the differences in overall survival between responders and non-responders than conventional RECIST 1.1 criteria (31). Following TARE, the EASL assessment provides the greatest anatomical response three months after TARE (32). Neither EASL nor mRECIST could predict complete pathological necrosis in a study of TARE with or without sorafenib (33). While size based criteria agree closely with each other, they show little agreement with viable tumor criteria (EASL). Following chemoembolization of the primary index lesion, the largest tumor targeted during the first treatment session, can be used to determine response. The radiological and biochemical response to pre-OLT locoregional therapy predicts death and tumor recurrence after transplant (34). Moreover, the assessment of a CR after TACE at 1 month may correlate with a favorable biological behavior of the tumor and identify patients beyond standard Milan criteria who may also benefit from OLT (35).
Figure 2. Criteria used for the classification of response to therapy for hepatocellular cancer.
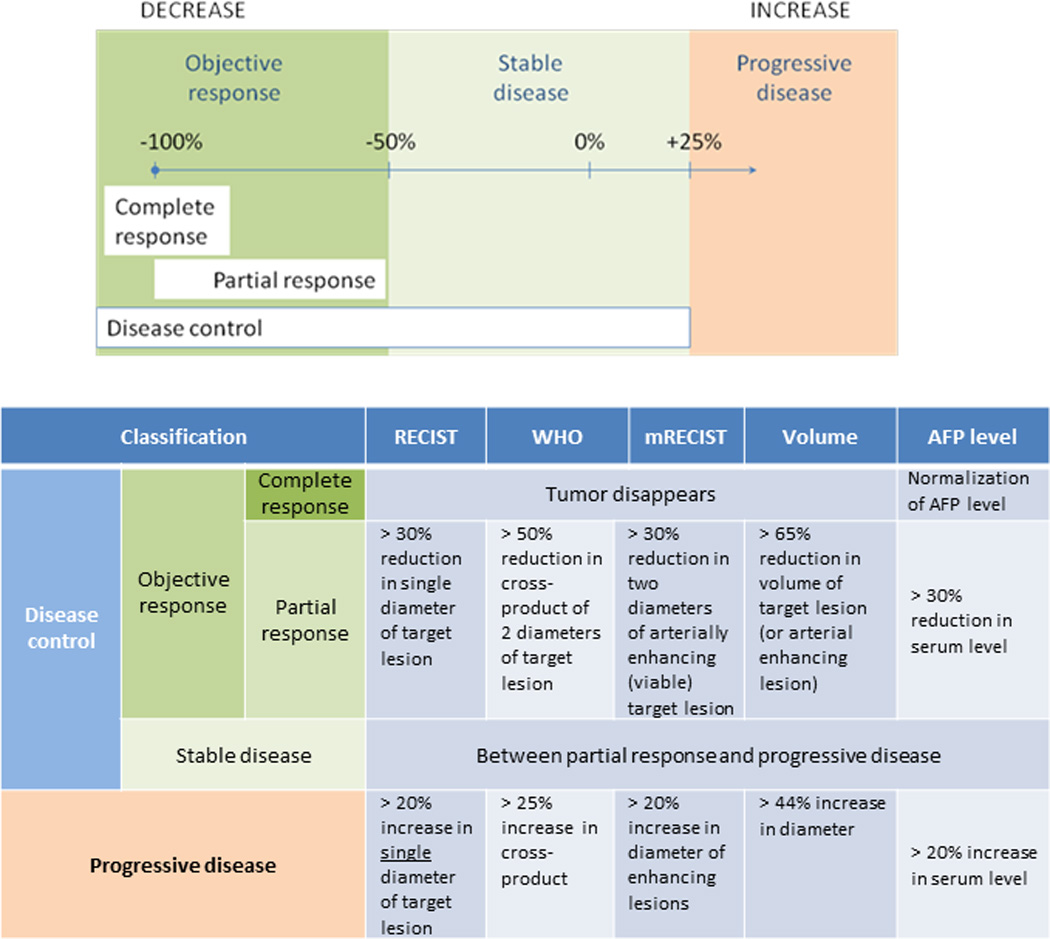
Disease control is defined as stable disease or objective response, whereas progressive disease is defined by change in selected imaging or biochemical parameters. RECIST: Response Evaluation Criteria in Solid Tumors, WHO: World Health Organization mRECIST: Modified response evaluation criteria in solid tumors, AFP: Alpha-feto-protein.
What are the optimum response criteria for use in clinical trials for HCC?
Although overall survival is the least ambiguous measure of response, its use in HCC is impacted in many patients by the presence of coexisting liver disease. Both the extent of disease and the effect of treatment on functioning liver are important determinants of overall survival. Thus, although overall survival should be considered in both intention-to-treat and per-protocol treatment, endpoints that reflect tumor response to therapy are also needed. However, response rates do not necessarily influence other measures of overall clinical benefit or outcome in patients with HCC. Moreover, tumor response is not always sufficient for regulatory approval.
The endpoints chosen should reflect the expected effects of the clinical treatment, objective measures of response, and the specific goals of the study. Treatment efficacy can be shown by objective response rate or time to progression. Endpoints based on these criteria are susceptible to potential bias and observer dependent accuracy and precision because they require subjective clinical assessment or radiological observation. Progression free survival allows a more robust assessment of treatment effects and is not affected by subsequent therapy. While the precise date of progression may not be known, and the relationship or its reflection to clinical benefit will depend on the situation. The assessment of progression free survival will depend on the frequency of testing. Quality of life measures such as relief of tumor-related symptoms and drug toxicity should also be considered in decision-making related to assessment of treatment response. The paucity of well validated tools for these assessments is a limitation.
Several studies of single or combination approaches to HCC are either planned or in progress. The timely adoption of a standardized approach to assessment of response in clinical trials of HCC will ensure comparability of studies with respect to the major end-points of event-free survival, disease-free survival and progression-free survival in these studies. AASLD-JNCI endpoints in clinical trials recommend time to recurrence or time to progression as a primary endpoint for phase 2 studies. Recurrence or progression is based on RECIST criteria. The primary endpoint for phase II studies to proceed to phase III studies has been response assessed by WHO or RECIST criteria.
There are several considerations in implementing a standard for use in clinical trials for HCC because the observed response will be influenced by the specific endpoints that are chosen, and thereby impact on drug approval and efficacy claims. For early phase studies, it would be most appropriate to use mRECIST criteria to determine response after regulatory endorsement for its use has been obtained. The use of independent endpoint and imaging assessment committees as a standard for outcomes assessment in HCC should also be encouraged to enable blinded and uniform assessment, and avoid local bias.
The use of modified or updated criteria such as mRECIST or RECIST 1.1 may not be appropriate if comparing to historical data, previous trials of same agent, or other indications, or for non-inferiority trials. Thus, the choice of the response criteria used may depend on whether or not other completed or ongoing studies of the treatment use RECIST criteria, or if a comparison is planned to a marketed product or treatment where use was earlier established with these criteria. Additional considerations include whether or not lesion measurements have been performed manually or using automated algorithms, and future developments may include the use of volumetric calculations.
The use of response criteria for phase II studies using targeted therapies may not be appropriate based on observations from clinical practice and reported trials of these agents in HCC. Combinations of liver directed, or locoregional, therapies and molecular targeted therapies are underway. While many of these may use the RECIST criteria, often as a requirement by regulatory authorities, it is emphasized that the true response may not be identified by the size of a lesion, or a treated lesion in the case of adjuvant post-therapy. This paves the way for potential confounding effects. A specific consideration for trials of liver directed therapy involves timing of evaluation of responses when different lesions are treated at different times in multistaged procedures. In these studies, the concept of response in the index lesion, the largest initial lesion targeted has recently been proposed with data suggesting that it may have prognostic value for outcomes in multifocal disease treated with locoregional therapies (36).
The use of appropriate surrogate markers could be incorporated into future clinical trials, such as p-ERK and c-KIT in the case of sorafenib (18).
Summary
The increasing diversity and complexity of management options and the rapidly evolving landscape of clinical trials for new agents for HCC emphasize the urgency for the timely adoption of a standardized approach and appropriate criteria by clinicians, clinical trialists, and regulatory agencies. At this time the mRECIST criteria remain the most optimal approach to radiological assessment of tumor response in clinical trials, although accurate quantitation of viable tumor remains a challenge due to heterogeneous responses and false positives related to vascular changes unrelated to tumor necrosis.
Assessment of response in HCC requires the use of HCC specific criteria that encompass biological parameters such as patterns of tumor development and growth, incorporate patterns of response, such as complete disappearance of viable tumor, elimination of preneoplastic background liver, and progression of symptoms. Assessment of the response to therapeutic strategies that involve liver-directed therapies or molecular targeted therapy will need to include quantitation of viable or necrotic tumor tissue. Volumetric analyses may improve the accuracy of these morphological-anatomical measures of response. The emerging function imaging techniques are promising. However, as with all new modalities, a demonstration of a correlation of response criteria with outcomes and validation in clinical studies and practice is essential before adoption for routine use.
Expertise from several specialties such as diagnostic and interventional radiology, hepatology, transplantation surgery, surgical, medical and radiation oncology is required for management of HCC The accurate assessment of response will facilitate communication and multidisciplinary care of patients with HCC. Adoption of uniform criteria such as mRECIST will facilitate the evolution of the increasing complex arena of clinical trials for HCC. Use of appropriate response criteria may require more attention to techniques for imaging, more complex measurements, and require additional training for clinicians and investigators but the advantages of broader adoption and use of such criteria will provide major benefits for clinical practice and for evaluation of new therapeutics.
Acknowledgements
Preparation of this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK069370 and UH2 TR000884. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure: There are no conflicts of interest
Key messages:
The choice of appropriate measures of response is essential in order to assess response to therapy for hepatocellular cancers.
References
- 1.World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva Albany, N.Y.: World Health Organization; 1979. sold by WHO Publications Centre USA. [Google Scholar]
- 2.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000 Feb 2;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Riaz A, Lewandowski RJ, Kulik L, Ryu RK, Mulcahy MF, Baker T, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with chemoembolization. Cardiovasc Intervent Radiol. 2010 Dec;33(6):1143–1152. doi: 10.1007/s00270-009-9766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntire KR, Vogel CL, Primack A, Waldmann TA, Kyalwazi SK. Effect of surgical and chemotherapeutic treatment on alpha-fetoprotein levels in patients with hepatocellular carcinoma. Cancer. 1976 Feb;37(2):677–683. doi: 10.1002/1097-0142(197602)37:2<677::aid-cncr2820370211>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009 Jan 20;27(3):446–452. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 7.Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009 Dec 1;27(34):5734–5742. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 8.Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. Journal of hepatology. 2012 Jul;57(1):101–107. doi: 10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Lee YK, Kim SU, Kim do Y, Ahn SH, Lee KH, Lee do Y, et al. Prognostic value of alpha-fetoprotein and des-gamma-carboxy prothrombin responses in patients with hepatocellular carcinoma treated with transarterial chemoembolization. BMC Cancer. 2013;13:5. doi: 10.1186/1471-2407-13-5. [Research Support, Non-U.S. Gov't]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannini EG, Marenco S, Borgonovo G, Savarino V, Farinati F, Del Poggio P, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012 Oct;56(4):1371–1379. doi: 10.1002/hep.25814. [Comparative Study]. [DOI] [PubMed] [Google Scholar]
- 11.Johnson PJ, Williams R. Serum alpha-fetoprotein estimations and doubling time in hepatocellular carcinoma: influence of therapy and possible value in early detection. J Natl Cancer Inst. 1980 Jun;64(6):1329–1332. doi: 10.1093/jnci/64.6.1329. [DOI] [PubMed] [Google Scholar]
- 12.Han K, Tzimas GN, Barkun JS, Metrakos P, Tchervenkov JL, Hilzenrat N, et al. Preoperative alpha-fetoprotein slope is predictive of hepatocellular carcinoma recurrence after liver transplantation. Can J Gastroenterol. 2007 Jan;21(1):39–45. doi: 10.1155/2007/206383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman M. The resurrection of alphafetoprotein. J Hepatol. 2010 Jun;52(6):939–940. doi: 10.1016/j.jhep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Kanke F, et al. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein <20 ng/mL. Cancer science. 2011 May;102(5):1025–1031. doi: 10.1111/j.1349-7006.2011.01875.x. [DOI] [PubMed] [Google Scholar]
- 15.Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des-gamma-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998 May 1;82(9):1643–1648. doi: 10.1002/(sici)1097-0142(19980501)82:9<1643::aid-cncr8>3.0.co;2-b. [Clinical Trial Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Li P, Zhai Y, Qu CF, Zhang LJ, Tan YF, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World journal of gastroenterology : WJG. 2010 Sep 21;16(35):4410–4415. doi: 10.3748/wjg.v16.i35.4410. [Research Support, Non-U.S. Gov't]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang T, Zhu AX, Sahani DV. Established and novel imaging biomarkers for assessing response to therapy in hepatocellular carcinoma. Journal of hepatology. 2013 Jan;58(1):169–177. doi: 10.1016/j.jhep.2012.08.022. [Research Support, Non-U.S. Gov't Review]. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008 May 21;100(10):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 19.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010 Feb;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001 Sep;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 21.Bryant MK, Dorn DP, Zarzour J, Smith JK, Redden DT, Saddekni S, et al. Computed tomography predictors of hepatocellular carcinoma tumour necrosis after chemoembolization. HPB (Oxford) 2013 Aug 26; doi: 10.1111/hpb.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bargellini I, Bozzi E, Campani D, Carrai P, De Simone P, Pollina L, et al. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. European journal of radiology. 2013 May;82(5):e212–e218. doi: 10.1016/j.ejrad.2012.12.009. [Randomized Controlled Trial]. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Guo Z, Si T, Wang H. EASL and mRECIST responses are independent predictors of survival in hepatocellular carcinoma patients treated with cryoablation. European journal of gastroenterology & hepatology. 2013 May;25(5):620–627. doi: 10.1097/MEG.0b013e32835ced13. [DOI] [PubMed] [Google Scholar]
- 24.Jung ES, Kim JH, Yoon EL, Lee HJ, Lee SJ, Suh SJ, et al. Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Journal of hepatology. 2013 Jun;58(6):1181–1187. doi: 10.1016/j.jhep.2013.01.039. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Watanabe H, Sone M, Onaya H, Sakamoto N, Osuga K, et al. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013 Mar;118(1):16–22. doi: 10.3109/03009734.2012.729104. [Multicenter Study Research Support, Non-U.S. Gov't]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim BK, Kim KA, Park JY, Ahn SH, Chon CY, Han KH, et al. Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolisation. European journal of cancer. 2013 Mar;49(4):826–834. doi: 10.1016/j.ejca.2012.08.022. [Comparative Study Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 27.Sahani DV, Jiang T, Hayano K, Duda DG, Catalano OA, Ancukiewicz M, et al. Magnetic resonance imaging biomarkers in hepatocellular carcinoma: association with response and circulating biomarkers after sunitinib therapy. Journal of hematology & oncology. 2013;6:51. doi: 10.1186/1756-8722-6-51. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamel IR, Bluemke DA, Ramsey D, Abusedera M, Torbenson M, Eng J, et al. Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. AJR American journal of roentgenology. 2003 Sep;181(3):708–710. doi: 10.2214/ajr.181.3.1810708. [Evaluation Studies]. [DOI] [PubMed] [Google Scholar]
- 29.Eccles C, Haider MA, Dawson LA. In reply to letter to the editor by Dr Willems et al. re: Eccles et al. Change in diffusion weighted MRI during liver cancer radiotherapy: Preliminary observations. Acta Oncol. 2010;49(2):256–257. doi: 10.3109/02841860903431121. [DOI] [PubMed] [Google Scholar]
- 30.Eccles CL, Haider EA, Haider MA, Fung S, Lockwood G, Dawson LA. Change in diffusion weighted MRI during liver cancer radiotherapy: preliminary observations. Acta Oncol. 2009;48(7):1034–1043. doi: 10.1080/02841860903099972. [DOI] [PubMed] [Google Scholar]
- 31.Kim CJ, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Radiologic response to transcatheter hepatic arterial chemoembolization and clinical outcomes in patients with hepatocellular carcinoma. Liver international : official journal of the International Association for the Study of the Liver. 2013 Jul 3; doi: 10.1111/liv.12270. [DOI] [PubMed] [Google Scholar]
- 32.Duke E, Deng J, Ibrahim SM, Lewandowski RJ, Ryu RK, Sato KT, et al. Agreement between competing imaging measures of response of hepatocellular carcinoma to yttrium-90 radioembolization. J Vasc Interv Radiol. 2010 Apr;21(4):515–521. doi: 10.1016/j.jvir.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vouche M, Kulik L, Atassi R, Memon K, Hickey R, Ganger D, et al. Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: Imaging analysis from a prospective randomized trial of Y90 +/− sorafenib. Hepatology. 2013 May 22; doi: 10.1002/hep.26487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, et al. Alpha-fetoprotein and modified response evaluation criteria in Solid Tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013 Oct;19(10):1108–1118. doi: 10.1002/lt.23706. [DOI] [PubMed] [Google Scholar]
- 35.Bargellini I, Vignali C, Cioni R, Petruzzi P, Cicorelli A, Campani D, et al. Hepatocellular carcinoma: CT for tumor response after transarterial chemoembolization in patients exceeding Milan criteria--selection parameter for liver transplantation. Radiology. 2010 Apr;255(1):289–300. doi: 10.1148/radiol.09090927. [DOI] [PubMed] [Google Scholar]
- 36.Riaz A, Miller FH, Kulik LM, Nikolaidis P, Yaghmai V, Lewandowski RJ, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010 Mar 17;303(11):1062–1069. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]


