Abstract
Purpose
To determine the dose-limiting toxicities (DLT), maximum tolerated dose (MTD), pharmacokinetics, and pharmacodynamics of sorafenib in children with refractory extracranial solid tumors and evaluate the tolerability of the solid tumor MTD in children with refractory leukemias.
Experimental Design
Sorafenib was administered orally every 12 hours for consecutive 28-day cycles. Pharmacokinetics (day 1 and steady-state) and pharmacodynamics were conducted during cycle 1.
Results
Of 65 patients enrolled, 60 were eligible. In the solid tumor cohort (n = 49), 4 of 6 patients experienced a DLT [hypertension, pain, rash/urticaria, thrombocytopenia, alanine aminotransferase (ALT)/ aspartate aminotransferase (AST)] at the starting dose (150 mg/m2/dose) which resulted in de-escalation to 105 mg/m2/dose. After eligibility criteria modification and dose re-escalation, the MTD was 200 mg/m2/ dose for solid tumors and 150 mg/m2/dose for leukemias. Sorafenib exposure was highly variable between patients but was within the ranges reported in adults. The apparent sorafenib clearance increased with patient age. Diarrhea, rash, fatigue, and increased ALT/AST were the most common sorafenib-related toxicities. Stable disease for 4 or more cycles was observed in 14 solid tumor patients, and 2 patients with acute myeloid leukemia (AML) and FLT3 internal tandem duplication (FLT3ITD) experienced a decrease in bone marrow blasts to less than 5%.
Conclusions
The recommended phase II dose of sorafenib administered every 12 hours continuously for children with solid tumors is 200 mg/m2/dose and 150 mg/m2/dose for children with leukemias. Sorafenib toxicities and distribution in children are similar to adults. The activity of sorafenib in children with AML and FLT3ITD is currently being evaluated, and a phase II study for select solid tumors is ongoing.
Introduction
Sorafenib (Nexavar) is an orally bioavailable small-molecule inhibitor (molecular weight, 465 g/mole) of multiple kinases controlling tumor growth and angiogenesis, including C- and BRAF, VEGFR-1,-2,-3, PDGFR-β, RET, Flt3, c-KIT (1). Sorafenib received U.S. Food and Drug Administration (FDA) approval at a dose of 400 mg twice daily on a continuous schedule for adults with unresectable hepato-cellular carcinoma (HCC; ref. 2) and advanced renal cell carcinoma (RCC; refs. (3, 4). The most common sorafenib toxicities reported are reversible skin rash, hand foot skin reaction, diarrhea, anorexia, alopecia, abdominal pain, fatigue, hypertension, laboratory abnormalities, including hypophosphatemia and asymptomatic lipase elevation, and mild myelosuppression (5, 6).
Evaluation of sorafenib in the pediatric preclinical testing program has shown in vitro activity at low micromolar concentrations. In vivo antitumor activity consisted exclusively of growth inhibition observed in multiple tumor types (7).
Sorafenib has been evaluated in 2 phase I studies for children in combination with other agents (8, 9). In a phase I study of sorafenib, bevacizumab and low-dose cyclophosphamide for solid tumors, rash, and febrile neutropenia were the most common dose-limiting toxicities (DLT), and the recommended phase II doses were 90 mg/m2/dose sorafenib twice daily, bevacizumab 15 mg/kg IV every 3 weeks, and cyclophosphamide 50 mg/m2 once daily (8). In 12 children with refractory leukemias who received sorafenib combined with clofarabine and cytarabine (9), hand foot skin reaction and rash were prominent and dose limiting at the 200 mg/m2/dose level, and a dose of 150 mg/m2/dose was the maximum tolerated dose (MTD). Sorafenib decreased blast percentages in 10 of 12 patients by day 8, and after combination therapy, complete remission was observed in 6 patients [6 with FLT3 internal tandem duplication (FLT3ITD) and 3 with FLT3 wild-type acute myeloid leukemia (AML); ref. 9].
We conducted a phase I trial of single agent sorafenib on a continuous chronic dosing schedule to determine (i) the toxicity profile, DLTs, and MTD of sorafenib administered to children with refractory solid tumors (part A) and (ii) the tolerability of the solid tumor MTD in children with refractory leukemias (part B). Pharmacokinetics and pharmacodynamics of sorafenib were assessed during cycle 1.
Materials and Methods
Patient population
Patients ≥24 months and ≤21 years of age with treatment-refractory, histologically confirmed measurable, or evaluable solid extracranial tumors (part A) or with refractory leukemias with >25% leukemic blasts in the bone marrow (part B) were eligible. Other eligibility criteria included ability to swallow intact tablets; recovery from toxic effects of prior therapy; Karnofsky/Lansky performance score ≥50; interval from prior therapy ≥21 days for myelosuppressive chemotherapy or monoclonal antibody treatment; ≥7 days for hematopoietic growth factors, ≥2 weeks for local palliative radiation; ≥3 months from total body, craniospinal, or ≥50% radiation to the pelvis; ≥6 weeks from other substantial bone marrow radiation; ≥3 months from a stem cell transplant or rescue; and no evidence of active graft versus host disease; adequate renal function [age-adjusted normal serum creatinine, or GFR ≥70 mL/min/1.73 m2); and adequate liver function [total bilirubin ≤1.5 × institutional upper limit of normal (ULN), albumin ≥2 g/dL, and alanine aminotransferase (ALT) ≤110 U/L]. Adequate bone marrow function was required and defined as an absolute neutrophil count (ANC) ≥1,000/μL, transfusion-independent platelet count ≥75,000/μL, and hemoglobin ≥8.0 g/dL for part A; and platelet count ≥20,000/μL and hemoglobin ≥8.0 g/dL for part B.
After dose-limiting serum, ALT/aspartate aminotransferase (AST) elevation was observed at the starting dose level in one patient with hepatic metastases and preexisting grade 1 elevation in AST/ALT, the eligibility criteria were revised to require a normal ALT (≤45 U/L) at enrollment. Eligibility criteria were re-revised in part B due to allow for mild elevation in the ALT (≤225 U/L) as many patients with leukemia have disease-related baseline elevations in serum transaminases. Additional eligibility criteria included normal serum lipase and amylase; diastolic blood pressure within the 95th percentile for age and gender and not receiving anti-hypertensive medication. Exclusion criteria included pregnancy or lactation; uncontrolled infection; concurrent use of other investigational agents, anticancer agents, agents to prevent organ rejection posttransplant, cytochrome P450 enzyme-inducing antiepileptic drugs, rifampin, grapefruit juice, St. Johns Wort, or therapeutic anticoagulants; evidence of bleeding diathesis; leptomeningeal disease; and Gilbert syndrome.
This trial was approved by the Institutional Review Boards of participating sites. All patients or their legal guardians signed a document of informed consent indicating their understanding of the investigational nature and risks of this study. Assent was obtained according to institutional guidelines.
Drug administration and study design
Sorafenib was supplied as 50- and 200-mg tablets by the Cancer Therapy Evaluation Program (NCI, Bethesda, MD) and administered orally, twice daily, continuously for 28-day cycles. The starting dose was 150 mg/m2/d with planned escalations to 200, 250, and 325 mg/m2/dose. Doses were prescribed using a dosing nomogram with a maximum dose of 600 mg/dose, as higher doses were intolerable in adults. Patients maintained a diary to document drug intake.
A traditional 3 +3 phase I dose-escalation scheme was used. Intrapatient dose-escalation was not permitted. At the solid tumor MTD, the trial was expanded to enroll 12 evaluable solid tumor patients (6 < 12 and 6 ≥12 years of age) to study steady-state pharmacokinetics and further define sorafenib toxicities (part A), and children with refractory leukemias to evaluate the tolerability, pharmacokinetics, and pharmacodynamics of sorafenib (part B).
Toxicity assessment and disease evaluations
Monitoring for sorafenib-related toxicity included physical examination with blood pressure measurement (weekly during cycle 1, then every other week); weekly serum chemistries including electrolytes, calcium, phosphate, magnesium, creatinine, ALT, bilirubin, lipase, and amylase; total protein and albumin before each cycle; and complete blood counts (twice weekly during cycle 1, then weekly). Clinical and laboratory adverse events, except hypertension, were graded according to the NCI Common Terminology Criteria for Adverse Events version 3 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Age- and gender-adjusted norms were used to determine whether the blood pressure was elevated (10) and a previously described algorithm for management of sorafenib-related hypertension was used (11).
As inhibition of VEGF has been previously shown to affect bone growth in juvenile animals by thickening of the growth plate and inhibition of trabecular bone formation (12–14), plain radiographs of tibial growth plates were conducted at baseline in patients <18 years of age, and then after every 3 cycles in patients with open growth plates.
Response was evaluated using Response Criteria in Solid Tumors (15) and published criteria for leukemias (16) at baseline and before every odd cycle in part A as well as at the end of cycles 1 and 2, and before every other cycle in part B.
Definition of DLT and MTD
Hematologic DLT was defined as any grade 4 neutropenia (<500/μL) or thrombocytopenia (<25,000/μL). For patients with leukemia, hematologic toxicity was monitored but not used to define the MTD or considered dose-limiting. Non-hematologic DLTs were any sorafenib-related grade 4 toxicity and any grade 3 toxicity with exception of the following: nausea and vomiting of less than 5 days duration, ALT or AST elevations that recovered to grade ≤1 within 7 days of stopping drug, fever, or infection <5 days, and electrolyte abnormalities responsive to oral supplementation. Persistent (≥7 days) grade 2 toxicities intolerable to the patient were also defined as DLTs. Dose-limiting hypertension was initially defined as grade ≥2 hypertension; however, this definition was redefined after dose level 1 accrual to include grade 4 hypertension, a confirmed diastolic blood pressure >25 mm Hg above the ULN, or an elevated diastolic blood pressure not controlled by anti-hypertensive medication within 14 days (11). Patients with a DLT who resolved within 14 days of sorafenib discontinuation were eligible for one dose reduction provided toxicity resolved to meet on study parameters within 14 days. Sorafenib was permanently discontinued for recurrent DLTs.
Patients were considered fully evaluable for toxicity and determination of the MTD provided they either developed a DLT at any time during cycle 1 or did not develop a DLT and received at least 85% of the prescribed sorafenib dose during cycle 1. Patients not meeting these criteria were replaced. The MTD was the dose level immediately below the dose level at which 2 or more patients in a dose level of up to 6 patients experienced a DLT.
Pharmacokinetics and pharmacodynamics
During the dose-escalation phase, pharmacokinetic sampling was conducted in consenting patients after the first dose of sorafenib (day 1). Three milliliters of heparinized blood was collected before and 0.5, 1, 2, 3, 5, 8, 10–12, 24, and 30–36 hours after dose 1 (doses 2 and 3 were held). Trough samples were obtained on days 5, 7, and 27–28 during cycle 1 in all patients. In the expanded solid tumor cohort and the leukemia cohort, sorafenib pharmacokinetics were evaluated at steady state. Blood was collected 12 hours after the last sorafenib dose, which was also used as the level immediately before the next dose, as well as 0.5, 1, 2, 3, 5, and 8 hours after the dose of sorafenib between days 14 and 28 of cycle 1. Sorafenib plasma concentrations were measured using the high-performance liquid chromatography tandem mass spectroscopic (HPLC/MS-MS) method adapted from an assay (17) with a lower limit of quantification of 5 ng/mL (18).
The sorafenib plasma concentration–time data were analyzed using noncompartmental methods. Peak sorafenib concentration (Cmax) and time to peak concentration (Tmax) were determined from each patient’s concentration–time plot. For day 1, the sorafenib exposure [area under curve (AUC)0–24h] was calculated using the linear trapezoidal method. For steady-state pharmacokinetics, the pre-sorafenib trough concentration was also used as the 12-hour after sorafenib concentration based on the assumption that at steady state the trough concentration should be constant. At steady state, the AUC over the dosing interval (AUC0–12h) is equivalent to the AUC0–∞, if no time-dependent changes occur in exposure. Therefore, apparent clearance was calculated by dividing the sorafenib dose by the AUC0–12h. The relationship between day 1 and steady-state drug exposure (normalized AUC0–24h and AUC0–12h, respectively) to gender, DLT, and response (<4 or ≥4 cycles received) were evaluated by the Mann–Whitney U test. The relationship between clearance and age was evaluated by linear regression.
Blood samples for plasma VEGF, soluble VEGFR2, endoglin, and placental growth factor (PlGF) were obtained in EDTA tubes and quantified at baseline and on days 27 and 28 of cycle 1 using a commercially available ELISA assay (Quantikine, R&D Systems; ref. 19). Total circulating endothelial cells (CEC) and circulating endothelial progenitor cells (CEPC) were analyzed from baseline and day 27 or 28 of cycle 1 blood samples using a 4-color fluorescence-activated cell sorter (19). The average paired values obtained before treatment and at steady state were compared (Student t test) and correlations were made to drug exposure for day 1 and steady-state pharmacokinetics and response for each growth factor analyzed (Spearman correlation).
Results
Patient characteristics
Of 65 patients enrolled from August 2006 to February 2010, 5 were ineligible [required pretreatment lipase not conducted (n = 3), preexisting organ dysfunction (n = 2)] and 10 were not fully evaluable for toxicity: disease progression during cycle 1 (n = 4), best patient interest (n = 3), acute death after 4 days of sorafenib in patient with numerous preexisting conditions (n = 1), non–dose-limiting grade 2 allergic reaction (n = 1), never received sorafenib (n = 1). Table 1 shows characteristics of the 60 eligible patients.
Table 1.
Characteristics of all eligible patients (N = 60)
| Patient strata | Solid tumora (n = 49) | Leukemia (n = 11) |
|---|---|---|
| Age in years: Median (range) | 14 (4–21) | 14 (6–19) |
| Sex: Female: Male | 22: 27 | 1: 10 |
| Performance status: Median (range) | 90 (50–100) | 90 (80–100) |
| Karnofsky | 90 (60–100) | 90 (90–100) |
| Lansky | 100 (50–100) | 100 (80–100) |
| Prior chemotherapy regimens: Median (range) | 2 (0–7) | 1 (1–4) |
| Number with prior radiation | 29 | 1 |
| Number with prior BMT | 5 | 4 |
| Diagnosis | ||
| Osteosarcoma | 10 | AML 8 |
| Ewing’s sarcoma | 4 | ALL 3 |
| Alveolar rhabdomyosarcoma | 4 | |
| Synovial sarcoma | 3 | |
| Alveolar soft part sarcoma | 2 | |
| MPNST | 2 | |
| Sarcoma, other | 7 | |
| Hepatoblastoma | 4 | |
| Wilms tumor | 4 | |
| Adrenocortical carcinoma | 3 | |
| Renal cell carcinoma | 2 | |
| Other | 4 |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BMT, bone marrow transplant; MPNST, malignant peripheral nerve sheath tumor.
Dose escalation cohort (n = 34), expanded solid tumor cohort at the maximum tolerated dose (n = 15).
Toxicity and MTD
There were no patient deaths attributed to sorafenib toxicity. Table 2 summarizes cycle 1 DLTs. The MTD was exceeded at the first dose level (150 mg/m2/dose) with DLT in 4 of 6 fully evaluable patients, one of whom developed grade 3 AST/ALT elevation in the presence of preexisting grade 1 transaminase elevation and hepatic metastases, and one of whom developed grade 2 hypertension. No DLTs were observed at the next lower dose (105 mg/m2), which corresponds to a fixed dose of approximately 190 mg, approximately half of the adult recommended dose. The protocol was amended to require normal ALT and bilirubin at enrollment and to provide new guidelines for the grading and management of hypertension (11) as described in the Materials and Methods section. With these changes, sorafenib was re-escalated to an intermediate dose of 130 mg/m2 with no DLT in 3 patients, and then to 150, 200, and 250 mg/m2, at which dose the MTD was exceeded with DLT (rash/hand foot skin reaction and hyponatremia) in 2 of 2 evaluable patients. The 200 mg/m2/dose was defined as the MTD with DLT (grade 3 ALT) in 1 of 7 patients. In the expanded solid tumor cohort, DLTs were similar to those previously observed and occurred in 1 of 6 patients ≥12 years, and in 2 of 6 patients <12 years old, for a combined DLT rate of 21% (4 of 19) in all evaluable solid tumor patients at the MTD. Four patients developed grade 3 DLTs during subsequent treatment cycles: at 200 mg/m2, diarrhea (n = 2), hyponatremia (recurrent DLTs, n = 1); at 150 mg/m2, hand foot skin reaction (n = 1). The solid tumor MTD was not tolerated in children with leukemias with DLT (anorexia and gastrointestinal hemorrhage) in 2 of 2 patients, and the 150 mg/m2/dose was determined as the MTD. No dose-limiting ALT/AST elevation was observed in children with leukemia, 2 of whom had mild ALT elevation (62 and 65 U/L) at study entry. One DLT after completion of cycle 1 (150 mg/m2) was observed (grade 4 hyperglycemia).
Table 2.
Cycle 1 dose-limiting toxicities of sorafenib by stratum and dose level
| Stratum | Dose level mg/m2/dose | Patients (n)
|
Dose-limiting toxicity
|
||
|---|---|---|---|---|---|
| Entered | Evaluable | Pt. no. | CTC toxicity term (grade) | ||
| Solid tumor dose escalation | 150a | 6 | 6 | 1 | Platelets (3)b |
| 4 | Hypertension (2), back pain (3) | ||||
| 6 | Rash/desquamation (3), urticaria (3) | ||||
| 5 | ALT (3), AST (3) | ||||
| 105 | 7 | 6 | — | — | |
| 130c | 4 | 3 | — | — | |
| 150c | 6 | 6 | 23 | Lipase (3) | |
| 200c | 8 | 7 | 22 | ALT (3) | |
| 250c | 3 | 2 | 35 | Rash: Hand-foot skin reaction (3) | |
| 34 | Hyponatremia (3) | ||||
| Solid tumor expansion | 200c | ||||
| ≥12 years | 9 | 6 | 56 | Rash/desquamation (3) | |
| <12 years | 6 | 6 | 41 | Hypertension (3), pain - back and chest (3) | |
| 44 | Rash/desquamation (3) | ||||
| Refractory leukemia | 200c | 2 | 2 | 49 | Anorexia (3) |
| 42 | GI hemorrhage – abdomen (4) | ||||
| 150c | 9 | 6 | - | - | |
Equivalent to an adult fixed dose of 270 mg based on adult body surface area of 1.8 m2.
Dose-limiting due to failure to recover to ≥75,000 within 2 weeks of holding sorafenib.
Dose levels with modified eligibility criteria (see article).
Sorafenib’s most frequent non-DLTs were fatigue, rash, diarrhea, AST/ALT/bilirubin elevation, and predominantly mild myelosuppression (Table 3). No bony abnormalities were reported in 5 patients who underwent serial plain radiographs. Three patients required anti-hypertensive medication in reporting period 2 to 4 and were able to continue sorafenib at unchanged dosing.
Table 3.
Non–dose-limiting toxicities possibly, probably, and definitely related to sorafenib (maximum toxicity grade per patient) observed in more than 10% of evaluable patients
| Solid tumor dose-escalation cohort Toxicity grade CTCv3 | Course 1 (n = 30)a
|
Course ≥ 2 (n = 66)a
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Hematologic | ||||||||
| Hemoglobin | 3 | 1 | 2 | 4 | 1 | 1 | ||
| Leukopenia | 5 | 2 | 2 | 4 | 2 | 2 | ||
| Lymphopenia | 2 | 1 | 2 | 2 | 2 | 1 | ||
| Neutropenia | 5 | 1 | 2 | 3 | 1 | 2 | ||
| Thrombocytopenia | 4 | 5 | 1 | |||||
| Cardiovascular | ||||||||
| Hypertension | 4 | 1 | 3 | 1 | ||||
| Constitutional | ||||||||
| Fatigue | 14 | 3 | 2 | |||||
| Dermatologic | ||||||||
| Rash/Desquamation | 11 | 3 | 4 | |||||
| Hand-foot skin reaction | 1 | 4 | 2 | |||||
| Gastrointestinal | ||||||||
| Diarrhea | 9 | 1 | 3 | 4 | ||||
| Nausea | 4 | 4 | ||||||
| Vomiting | 4 | 1 | ||||||
| Metabolic/laboratory | ||||||||
| Increased ALT | 4 | 1 | 4 | 1 | ||||
| Increased AST | 4 | 1 | 3 | 1 | ||||
| Increased bilirubin | 4 | 1 | 1 | |||||
| Hyperglycemia | 4 | 2 | ||||||
| Hypophosphatemia | 3 | 2 | 3 | 1 | ||||
| Pain | ||||||||
| Headache | 3 | 1 | 1 | 1 | ||||
| Solid tumor expanded PK cohort Toxicity grade CTCv3 |
Course 1 (n = 12)a
|
Course ≥ 2 (n = 31)a
|
||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
|
| ||||||||
| Hematologic | ||||||||
| Lymphopenia | 1 | 1 | 2 | |||||
| Thrombocytopenia | 4 | 1 | 3 | 1 | ||||
| Constitutional | ||||||||
| Fatigue | 4 | 2 | 2 | 2 | ||||
| Fever | 2 | 1 | ||||||
| Dermatologic | ||||||||
| Alopecia | 2 | 1 | ||||||
| Rash/Desquamation | 3 | 1 | 2 | |||||
| Hand-foot skin reaction | 1 | 1 | ||||||
| Gastrointestinal | ||||||||
| Anorexia | 4 | 3 | 1 | |||||
| Diarrhea | 8 | 1 | 3 | 2 | ||||
| Mucositis/stomatitis | 2 | |||||||
| Nausea | 3 | 2 | 2 | 1 | ||||
| Vomiting | 1 | 3 | 1 | 1 | ||||
| Metabolic/laboratory | ||||||||
| Hypoalbuminemia | 1 | 1 | 1 | |||||
| Increased ALT | 5 | 4 | ||||||
| Increased AST | 6 | 6 | ||||||
| Increased bilirubin | 2 | 1 | ||||||
| Hypocalcemia | 3 | 3 | ||||||
| Hyperglycemia | 2 | 1 | ||||||
| Hypophosphatemia | 2 | 4 | 1 | |||||
| Hyponatremia | 3 | 3 | ||||||
| Pain | ||||||||
| Other (forearm) | 1 | |||||||
| Other (jaw) | 1 | |||||||
| Refractory leukemia Toxicity grade CTCv3 |
Course 1 (n = 8)a
|
Course≥ 2 (n = 12)a
|
||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
|
| ||||||||
| Constitutional | ||||||||
| Fatigue | 1 | 2 | ||||||
| Dermatologic | ||||||||
| Rash/Desquamation | 5 | 2 | ||||||
| Hand-foot skin reaction | 2 | |||||||
| Gastrointestinal | ||||||||
| Anorexia | 1 | 1 | 1 | |||||
| Diarrhea | 2 | 1 | 1 | |||||
| Nausea | 2 | 1 | ||||||
| Metabolic/laboratory | ||||||||
| Increased ALT | 1 | 1 | 1 | |||||
| Increased AST | 3 | 1 | 1 | |||||
| Increased bilirubin | 2 | 1 | ||||||
| Hyperglycemia | 2 | 1 | 1 | |||||
| Hypophosphatemia | 1 | 1 | 1 | |||||
| Hypoalbuminemia | 1 | 1 | ||||||
| Hyponatremia | 2 | 1 | ||||||
| Hypocalcemia | 1 | 1 | 1 | |||||
| Hypomagnesemia | 1 | 1 | 2 | |||||
| Hypokalemia | 2 | 1 | ||||||
| Proteinuria | 1 | 1 | ||||||
| Pain | ||||||||
| Headache | 1 | 1 | 1 | |||||
Numbers indicate number of patient courses.
Pharmacokinetics and pharmacodynamics
Twenty patients had pharmacokinetic sampling at day 1 and 17 patients at steady state.
There was substantial interpatient variability for day 1 and steady-state pharmacokinetic parameters (Table 4 and 5). Drug exposure increased only marginally when it was escalated from 150 to 200 mg/m2 for day 1 pharmacokinetics (Table 4). The plasma concentration–time profile captured drug exposure incompletely (Fig. 1A). The terminal half-life appeared to be prolonged (≥24 hours) but could not be estimated. Sorafenib accumulated after multiple doses and steady state appeared to be achieved after 5 to 7 days (Table 4 and Fig. 1B) with the median accumulation ratio (day 27 trough/day 2 trough) ranging from 3.7 to 11.6. No statistically significant relationship between AUC0–24h to DLTs or response was observed.
Table 4.
Sorafenib day 1 plasma pharmacokinetic parameters in children with solid tumors
| Dose Level (mg/m2) | Pt no. | Age (y) | BSA (m2) | Dose (mg) | Actual dose/m2 | Deviation (%) | Cmax (μg/mL) | Tmax (h) | AUC0–24h (μg·h/mL) |
Ctrough (μg/mL)
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D 5 | D 7 | D 27–28 | ||||||||||
| 105 | 7 | 19 | 1.98 | 200 | 101 | −3.8 | 0.1 | 5.0 | 2.5 | — | 2.8 | 1.4 |
| 105 | 10 | 17 | 1.88 | 200 | 106 | 1.3 | 1.1 | 2.0 | 15.1 | — | — | 3.3 |
| 105 | 11 | 14 | 1.50 | 150 | 100 | −4.8 | 1.2 | 8.0 | 20.4 | — | 10.0 | 3.4 |
| 105 | 13 | 21 | 1.86 | 200 | 108 | 2.4 | 0.4 | 5.1 | 9.2 | 5.0 | 2.6 | 6.2 |
| Mean | −1.2 | 0.7 | 5.0 | 11.8 | 5.1 | 3.6 | ||||||
| SD | 0.5 | 2.5 | 7.7 | 4.2 | 2.0 | |||||||
| Median | 0.8 | 5.0 | 12.2 | 2.8 | 3.4 | |||||||
| 130 | 14 | 18 | 1.40 | 200 | 143 | 9.9 | 2.4 | 5.1 | 20.0 | 4.4 | 3.0 | 3.4 |
| 130 | 18 | 14 | 1.22 | 150 | 123 | −5.4 | 0.6 | 7.9 | 9.1 | 3.5 | 4.2 | 3.6 |
| Mean | −2.2 | 1.5 | 6.5 | 14.6 | 4.0 | 3.6 | 3.5 | |||||
| SD | 1.3 | 2.0 | 7.7 | 0.6 | 0.8 | 0.1 | ||||||
| Median | 1.5 | 6.5 | 14.6 | 4.0 | 3.6 | 3.5 | ||||||
| 150 | 1a | 12 | 1.26 | 200 | 159 | 5.8 | 1.0 | 23.5 | 15.5 | 4.9 | 4.2 | 8.1 |
| 150 | 2 | 14 | 1.62 | 250 | 154 | 2.9 | 1.6 | 5.0 | 29.2 | — | 4.9 | 3.4 |
| 150 | 3 | 13 | 1.22 | 200 | 164 | 9.3 | 1.0 | 8.0 | 12.2 | — | — | — |
| 150 | 6a | 12 | 1.05 | 150 | 143 | −4.8 | 1.1 | 5.5 | 18.3 | — | — | — |
| 150 | 19 | 21 | 1.83 | 250 | 137 | −8.9 | 1.8 | 23.3 | 19.2 | 5.8 | 14.6 | |
| 150 | 20 | 16 | 1.91 | 300 | 157 | 4.7 | 0.4 | 24.0 | 6.8 | 1.6 | 2.0 | 2.4 |
| 150 | 21 | 14 | 1.65 | 250 | 152 | 1.0 | 3.8 | 3.0 | 55.0 | — | 11.2 | 11.1 |
| 150 | 23a | 6 | 0.74 | 100 | 135 | −9.9 | 5.1 | 5.1 | 81.5 | 6.6 | 6.6 | — |
| 150 | 24 | 15 | 1.49 | 200 | 134 | −10.5 | 1.1 | 5.0 | 16.9 | 3.2 | 2.8 | 1.9 |
| Mean | −1.0 | 1.9 | 11.4 | 28.3 | 4.1 | 5.4 | 6.9 | |||||
| SD | 1.6 | 9.2 | 24.4 | 2.2 | 3.0 | 5.2 | ||||||
| Median | 1.1 | 5.5 | 18.3 | 4.1 | 4.9 | 5.8 | ||||||
| 200 | 22a | 7 | 1.80 | 350 | 194 | −2.8 | 1.8 | 1.0 | 19.0 | — | — | — |
| 200 | 29 | 16 | 1.48 | 300 | 203 | 1.4 | 3.8 | 3.0 | 46.8 | 3.6 | 0.9 | 1.8 |
| 200 | 30 | 11 | 1.15 | 250 | 217 | 8.7 | 1.4 | 5.0 | — | — | — | — |
| 200 | 32 | 20 | 2 | 400 | 200 | 0 | 1.0 | 3.0 | 16.1 | 8.4 | 6.3 | 5.0 |
| Mean | 0.7 | 2.0 | 3.0 | 27.3 | 6.0 | 3.6 | 3.4 | |||||
| SD | 1.3 | 1.6 | 17.0 | 3.4 | 3.8 | 2.3 | ||||||
| Median | 1.6 | 3.0 | 19.0 | 6.0 | 3.6 | 3.4 | ||||||
| 250 | 35a | 11 | 1.43 | 350 | 245 | −2.1 | 1.8 | 8.0 | 27.6 | 5.5 | 7.8 | — |
Abbreviations: BSA, body surface area; Deviation, percent deviation of actual dose from protocol prescribed dose; Cmax, peak plasma concentration; Tmax, time to reach Cmax; AUC, area under the plasma concentration time curve; Ctrough, sorafenib concentration before administration of the day 5, 7, and 27–28 dose.
Dose-limiting toxicity during cycle 1.
Table 5.
Sorafenib steady-state plasma pharmacokinetics
| Dose Level (mg/m2) | Pt no. | Age (years) | BSA (m2) | Dose (mg) | Actual dose/m2 | Deviation (%) | Cmax (μg/mL) | Tmax (h) | AUC0–12h (μg·h/mL) | CL/F (mL/min/m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Children less than 12 years with solid tumors | ||||||||||
| 200 | 39 | 7 | 0.82 | 150 | 183 | −8.5 | 6.4 | 2 | 65.4 | 46.7 |
| 200 | 40 | 9 | 1.27 | 250 | 197 | −1.6 | 11.3 | 2 | 96 | 34.2 |
| 200 | 41 | 11 | 1.3 | 250 | 192 | −3.9 | 5.9 | 3 | 55.9 | 57.4 |
| 200 | 44a | 7 | 0.83 | 100 | 120 | 20.5 | 10.4 | 5 | 104.6 | 19.2 |
| 200 | 45a | 9 | 1.14 | 250 | 219 | 9.7 | 5.8 | 5 | 54.6 | 67 |
| 200 | 59 | 5 | 0.73 | 150 | 205 | 2.7 | 8.5 | 2.1 | 50.7 | 67.6 |
| 200 | 42a | 6 | 0.9 | 200.0 | 225.0 | 12.4 | 5.0 | 2.0 | 51.4 | 72.8 |
| Mean | 4.5 | 7.2 | 2.7 | 62.3 | 57.6 | |||||
| SD | 2.3 | 1.2 | 17.3 | 14.8 | ||||||
| Median | 6.2 | 2.0 | 55.2 | 62.2 | ||||||
| Children ≥ 12 years with solid tumors | ||||||||||
| 200 | 38 | 15 | 1.65 | 350 | 212 | 6.1 | 5.7 | 5 | 53.9 | 54.7 |
| 200 | 43 | 17 | 2.2 | 400 | 182 | −9.1 | 10.7 | 5 | 87.8 | 34.5 |
| 200 | 50 | 21 | 1.95 | 400 | 205 | −2.6 | 2.5 | 8 | 26.3 | 130 |
| 200 | 51 | 19 | 1.64 | 350 | 213 | 6.7 | 7.7 | 0.6 | 70.7 | 50.3 |
| 200 | 52 | 12 | 1.57 | 300 | 191 | −4.5 | 15.3 | 1 | 55.5 | 57.4 |
| 200 | 53 | 16 | 1.93 | 400 | 207 | 3.6 | 7.9 | 0.5 | 66.8 | 51.7 |
| Mean | 0 | 8.3 | 3.4 | 60.2 | 63.1 | |||||
| SD | 4.4 | 3.1 | 20.6 | 33.7 | ||||||
| Median | 7.8 | 3.0 | 61.2 | 53.2 | ||||||
| Children with refractory leukemias | ||||||||||
| 150 | 55 | 9 | 1.1 | 150.0 | 143.0 | −4.8 | 12.2 | 3.0 | 97.8 | 24.4 |
| 150 | 58 | 19 | 1.7 | 250.0 | 147.0 | −2.0 | 1.9 | 2.0 | 20.3 | 121.0 |
| 150 | 62 | 6 | 0.9 | 150.0 | 163.0 | 8.7 | 6.0 | 1.0 | 50.0 | 54.3 |
| 150 | 63 | 14 | 1.2 | 200.0 | 167.0 | 11.1 | 3.8 | 2.1 | 30.7 | 90.6 |
| Mean | 3.3 | 6.0 | 2.0 | 49.7 | 72.6 | |||||
| SD | 4.5 | 2.0 | 34.4 | 42.2 | ||||||
| Median | 4.9 | 0.8 | 40.3 | 72.5 | ||||||
Abbreviations: BSA, body surface area; Deviation, percent deviation of actual dose from protocol prescribed dose; CL/F, apparent clearance.
Dose-limiting toxicity during cycle 1. Pharmacokinetic sampling performed after dose reduction, and thus patient 44 was not included in any summary calculations.
Figure 1.
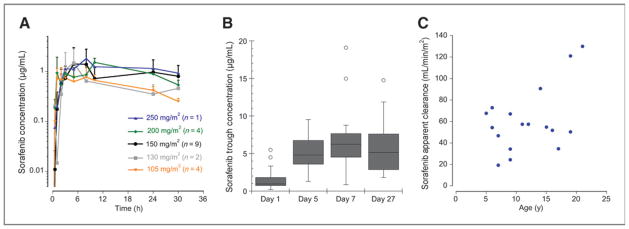
Pharmacokinetics of sorafenib. A, mean plasma concentration × time curves after a single day, 1 dose for each dose level. Error bars, SD of the concentrations. B, cycle 1 plasma sorafenib trough concentrations normalized to 200 mg/m2/dose on days 1 (calculated 12-hour concentration; n = 18), 5 (n = 10), 7 (n = 15), and 27 (n = 14). C, relationship of sorafenib apparent clearance (Cl/F) at steady state to age. The Cl/F increases with patient age (P = 0.048).
For steady-state pharmacokinetics, the accumulation ratio of sorafenib at the MTD [mean (SD) AUC0–12h of 61.2 (18.2) steady state divided by mean (SD) calculated day 1 AUC0–12h of 14.4 (8.9)] was 4.3. Steady-state sorafenib concentrations (AUC0–12h/12) were 4.1 ± 2.1 μg/mL at the 150 mg/m2 and 5.4 ± 1.8 μg/mL at 200 mg/m2 dose levels, respectively. The median apparent sorafenib clearance at the MTD was 56.0 mL/min/m2 (range, 19.2–72.8). The apparent sorafenib clearance increased with patient age (P = 0.048; Fig. 1C). No statistically significant relationship between AUC0–12h and DLTs was observed.
In paired samples for plasma VEGF and endoglin concentrations (n = 26) and paired samples for CEC and CEPC number (n = 21), there was no change comparing baseline with end of cycle 1. A statistically significant decrease in soluble VEGFR2 (P ≤0.001) and increase in PlGF (P ≤ 0.001) was observed at steady state (Supplementary Fig. S2A and S2B). No correlations of the pharmacodynamic parameters to drug exposure or response were observed.
Response evaluation
The median number of sorafenib cycles for solid tumors was 2 (range, 1–22). There were no confirmed objective responses in patients with solid tumors but 1 patient (#24) with synovial sarcoma had more than 40% tumor shrinkage after cycle 1. This patient could not be considered a confirmed partial response, as disease progression was documented after cycle 2. Patients with leukemia received a median of 1 (range, 1–6) cycle. Two patients with AML and FLT3ITD achieved an M1 bone marrow (<5% blasts) after cycles 4 and 5, respectively, and were removed from sorafenib treatment to undergo hematopoietic stem cell transplantation.
Discussion
The MTD of single-agent sorafenib in children with refractory solid tumors is 200 mg/m2/dose twice daily, given orally on a continuous dosing schedule, similar to the recommended 400-mg twice daily fixed dose in adults (5, 6). Children with refractory leukemias did not tolerate the solid tumor MTD, and the recommended dose for this population is 150 mg/m2/dose, which is identical to the recommended dose in children with leukemia in combination with clofarabine and cytarabine (9) and similar to the recommended dose of 300 mg twice daily in adults with AMLs (20). Similar to adults, children with solid tumors and leukemias experienced rash, hand-foot skin reaction, hypertension, ALT/AST elevation, asymptomatic lipase elevation, diarrhea, and mild myelosuppression (5, 6). The DLTs, anorexia and gastrointestinal (GI) hemorrhage, in children with leukemia in our study were different from those with solid tumors (Table 2). In contrast to the toxicities observed in our study, children with leukemia or solid tumors who received sorafenib in combination with other agents, experienced grade 2 and 3 hand foot skin reactions and rashes with much greater incidence (8 of 12 children with leukemia, 5 of 19 children with solid tumors; refs. 8, 9).
In contrast to adults, fatigue in children was predominantly mild, and hypertension was infrequently observed. Up to a third of adults receiving sorafenib require dose reductions with chronic administration, mostly due to skin toxicity and diarrhea (2, 3, 21). While we could not comprehensively assess cumulative toxicity of sorafenib, after cycle 1, only 5 patients developed a DLT, and only 3 patients required initiation of anti-hypertensive medication.
The pharmacokinetics of sorafenib in children were similar to adults. There was substantial interpatient variability both after a single dose and at steady state (22–25). For day 1 pharmacokinetics, only marginal differences between AUC0–24h from 150 to 200 mg/m2/dose were observed. In adults, the AUC0–12h increased only marginally beyond the recommended 400 mg twice daily dose and saturable sorafenib absorption has been described (26). The 400-mg dose is comparable with the pediatric 200 mg/m2/dose twice daily. Sorafenib accumulated and steady state was reached by days 5 to 7, similar to adult studies (6, 23, 24). The median steady-state AUC0–12h at the MTD of 200 mg/ m2 was 55.7 μg h/mL, which is within the range seen in adults (AUC0–12h at 400-mg dose after multiple doses of sorafenib 47.8–76.5 μg h/mL; refs. 6, 23, 24). In 12 children with refractory leukemia, Inaba and colleagues (9) reported sorafenib steady-state (day 7) concentrations of 7.0 ± 1.8 μg/mL at 150 mg/m2 and 6.5 ± 3.6 μg/mL at 200 mg/m2, which is similar to the day 7 concentrations in our study (Table 4) and to the calculated steady-state concentrations of children in our study who had steady-state pharmacokinetics conducted.
In our analysis in 17 patients, the apparent sorafenib clearance at the MTD increased with age (P = 0.048). Additional pharmacokinetic data from young patients receiving sorafenib on other trials will be helpful to more completely analyze this relationship. If confirmed, an analysis of possible etiologies, such as differences in protein binding or drug metabolism, should be explored. Interestingly, the 2 patients (patients 23 and 44) with the highest AUC were the 2 youngest patients (6 and 7 years of age), and both had DLTs. A statistically significant change from baseline to steady-state pharmacodynamics was only observed for soluble VEGFR and PlGF, but there was no correlation to response or sorafenib exposure.
In solid tumor patients, no objective responses were observed; however, stable disease for ≥4 cycles was observed in 14 patients. The objective response of sorafenib has been low in adults with advanced HCCs (2%) and RCCs (10%), and sorafenib was FDA-approved for adults with these diseases based on improvement in progression-free and overall survival (2, 3). The development of sorafenib for pediatric solid tumors has therefore mostly focused on diseases with documented activity in adults. The ongoing Children’s Oncology Group phase II trial of sorafenib includes strata for refractory HCCs and papillary thyroid carcinoma in addition to rhabdomyosarcoma and Wilms tumor. In addition, sorafenib has been evaluated in combination with standard chemotherapy in children with HCCs (27), a phase II study of sorafenib for children with refractory low-grade astrocytomas is ongoing, and sorafenib is undergoing phase I evaluation in combination with irinotecan in children with refractory solid tumors.
Activity of sorafenib in adult AMLs with FLT3ITD has been previously reported in adults (20, 28–30) and in children in combination with additional chemotherapy agents (9, 31). Pharmacokinetic studies showed a much higher rate of conversion of sorafenib to the active N-oxide metabolite than that reported in adult patients and more potent inhibition of FLT3ITD by the N-oxide metabolite providing possible explanations for the promising activity in AMLs (9, 32). In our study, preliminary activity of single-agent sorafenib was observed in 2 children with AMLs and FLT3ITD who cleared their bone marrow blasts and proceeded to hematopoietic stem cell transplantation. On the basis of these findings, our study was expanded to determine the activity and pharmacokinetics of sorafenib and of its active N-oxide metabolite in children with AMLs and FLT3ITD. In addition, a Children’s Oncology Group phase III randomized trial for de novo AMLs incorporating bortezomib and sorafenib for patients with high allelic ratio FLT3ITD was developed and is ongoing (32).
Supplementary Material
Translational Relevance.
Sorafenib is an orally bioavailable inhibitor of multiple kinases controlling tumor growth and angiogenesis. Sorafenib is U.S. Food and Drug Administration (FDA)-approved for adults with unresectable hepatocellular carcinoma and advanced renal cell carcinoma. We conducted a phase I study of single-agent sorafenib in children with refractory solid tumors or leukemias on a chronic dosing schedule. The maximum tolerated dose, toxicity profile, and pharmacokinetics of sorafenib in children are similar to adults. No objective responses were observed in solid tumors. Two children with acute myeloid leukemia (AML) and FLT3 internal tandem duplication (FLT3ITD) cleared their bone marrow blasts and proceeded to hematopoietic stem cell transplantation. On the basis of these findings and the reported activity of sorafenib in select solid tumors and AML and FLT3ITD in children and adults, the activity of single-agent sorafenib is currently being evaluated in a phase II study for children with select solid tumors and in children with AML and FLT3ITD. In addition, sorafenib has been incorporated into a phase III randomized trial for children with de novo AML with high allelic ratio FLT3ITD.
Acknowledgments
The authors thank Elizabeth O’Connor, Biljana Georgievska, and Nicole Stewart from the COG Phase1/Pilot Consortium Operations Center for outstanding data management and administrative support throughout the development and conduct of this trial.
Grant Support
This research was in part supported by the Intramural Research Program of the NIH, National Cancer Institute (NCI), Center for Cancer Research, and by the Phase I/Pilot Consortium Grant U01 CA97452 of the Children’s Oncology Group from the NCI, NIH, Bethesda, MD.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Presented in part at the Annual Meeting of the American Society of Clinical Oncology, 2009, Orlando, FL.
Disclosure of Potential Conflicts of Interest
A. Kim is a consultant/advisory board member of the Nexavar Pediatric Advisory Board. No potential conflicts of interest were disclosed by the other authors.
Disclaimer
The views expressed do not necessarily represent views of the NIH or the U.S. government.
Authors’ Contributions
Conception and design: B.C. Widemann, E. Fox, P.C. Adamson, J.G. Bender, F.M. Balis, S.M. Blaney
Development of methodology: B.C. Widemann, S. Baruchel, P.C. Adamson, F.M. Balis
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): B.C. Widemann, A. Kim, E. Fox, S. Baruchel, J.G. Bender, B. Weigel, F.M. Balis, S.M. Blaney
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): B.C. Widemann, A. Kim, E. Fox, S. Baruchel, A.M. Ingle, J.G. Bender, F.M. Balis, S.M. Blaney
Writing, review, and/or revision of the manuscript: B.C. Widemann, A. Kim, E. Fox, S. Baruchel, P.C. Adamson, A.M. Ingle, J.G. Bender, M. Burke, B. Weigel, D. Stempak, F.M. Balis, S.M. Blaney
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): B.C. Widemann
Study supervision: B.C. Widemann, J.G. Bender, M. Burke, B. Weigel, F.M. Balis, S.M. Blaney
Protocol chair: B.C. Widemann
References
- 1.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–12. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 5.Grandinetti CA, Goldspiel BR. Sorafenib and sunitinib: novel targeted therapies for renal cell cancer. Pharmacotherapy. 2007;27:1125–44. doi: 10.1592/phco.27.8.1125. [DOI] [PubMed] [Google Scholar]
- 6.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–37. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 7.Keir ST, Maris JM, Lock R, Kolb EA, Gorlick R, Carol H, et al. Initial testing (stage 1) of the multi-targeted kinase inhibitor sorafenib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55:1126–33. doi: 10.1002/pbc.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santana V, Baker S, McCarville B, Stewart C, Wu C, Billups C, et al. Phase I study of bevacizumab, sorafenib, and low-dose cyclophophamide (CYC) in children and young adults with refractory solid tumors. J Clin Oncol. 2011;29(suppl):abstr 9500. [Google Scholar]
- 9.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29:3293–300. doi: 10.1200/JCO.2011.34.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 11.Fox E, Aplenc R, Bagatell R, Chuk M, Dombi E, Goodspeed W, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-VEGF-receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28:5174–81. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–8. doi: 10.1016/s1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 13.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 14.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Jain L, Gardner ER, Venitz J, Dahut W, Figg WD. Development of a rapid and sensitive LC-MS/MS assay for the determination of sorafenib in human plasma. J Pharm Biomed Anal. 2008;46:362–7. doi: 10.1016/j.jpba.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim A, McCully C, Cruz R, Cole D, Fox E, Balis F, et al. The plasma and cerebrospinal fluid pharmacokinetics of sorafenib after intravenous administration in non-human primates. Invest New Drugs. 2012;30:524–8. doi: 10.1007/s10637-010-9585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GladeBender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 20.Crump M, Hedley D, Kamel-Reid S, Leber B, Wells R, Brandwein J, et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (National Cancer Institute of Canada) Clinical Trials Group Study. Leuk Lymphoma. 2010;51:252–60. doi: 10.3109/10428190903585286. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 22.Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D, et al. Phase I safety and pharmacokinetics of BAY 43–9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–61. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43–9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–80. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 24.Moore M, Hirte HW, Siu L, Oza A, Hotte SJ, Petrenciuc O, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43–9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16:1688–94. doi: 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 25.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 26.Hornecker M, Blanchet B, Billemont B, Sassi H, Ropert S, Taieb F, et al. Saturable absorption of sorafenib in patients with solid tumors: a population model. Invest New Drugs. 2012;30:1991–2000. doi: 10.1007/s10637-011-9760-z. [DOI] [PubMed] [Google Scholar]
- 27.Schmid I, Haberle B, Albert MH, Corbacioglu S, Frohlich B, Graf N, et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer. 2012;58:539–44. doi: 10.1002/pbc.23295. [DOI] [PubMed] [Google Scholar]
- 28.Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–71. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 29.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–62. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 31.Watt TC, Cooper T. Sorafenib as treatment for relapsed or refractory pediatric acute myelogenous leukemia. Pediatr Blood Cancer. 2012;59:756–7. doi: 10.1002/pbc.23394. [DOI] [PubMed] [Google Scholar]
- 32.Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24:1437–44. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


