Abstract
The viral immediate-early transactivator Rta/Orf50 is necessary and sufficient to initiate Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) reactivation from latently infected cells. Since Rta/Orf50 is conserved among all known gamma-2-herpesviruses, we investigated whether the murine gamma-68-herpesvirus (MHV-68) and rhesus monkey rhadinovirus (RRV) homologs can functionally substitute for KSHV Rta/Orf50. (i) Our comparison of 12 KSHV promoters showed that most responded to all three Rta/Orf50proteins, but three promoters (vGPCR, K8, and gB) responded only to the KSHV Rta/Orf50 transactivator. Overall, the activation of KSHV promoters was higher with KSHV Rta than with the RRV and MHV-68 Rta. (ii) Only the primate Rta/Orf50 homologs were able to interfere with human p53-depedent transcriptional activation. (iii) Transcriptional profiling showed that the KSHV Rta/Orf50 was more efficient than it's homologs in inducing KSHV lytic transcription from the latent state. These results suggest that the core functionality of Rta/Orf50 is conserved and independent of its host, but the human protein has evolved additional, human-specific capabilities.
Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) is convincingly associated with Kaposi's sarcoma (KS) and the AIDS-associated lymphoproliferative disorders, primary effusion lymphoma and multicentric Castleman's disease (reviewed in reference 1). Animal models represent important systems for the study of the biology and pathogenesis of KSHV, in light of the fact that the human virus replicates poorly in culture. Currently, there are two animal model systems that are widely used to investigate KSHV biology based on their sequence relatedness to KSHV. These include murine gamma-68-herpesvirus (MHV-68) and rhesus monkey rhadinovirus (RRV), both of which replicate to high titers in tissue culture and are capable of establishing persistent infection in their respective hosts (2, 14, 37, 55, 57). In order to increase the applicability of these systems, it is important to compare key genes (and potential therapeutic targets) encoded by these three viruses.
Lytic replication of herpesviruses follows an ordered cascade of gene expression. Immediate-early (alpha) proteins are transcribed first, followed by early (beta) and finally late (gamma) proteins. The three gamma-2-herpesviruses, KSHV, RRV, and MHV-68, all encode a viral immediate-early transactivator protein named Rta/Orf50 in a similar genomic location. Moreover, all three rhadinovirus Rta transcripts share a similar architecture. They comprise two exons separated by an intron, which is essentially composed of the Orf49 gene (10, 34, 50, 57). In each case, splicing of the two exons and excision of the intervening intron results in a single, major Rta transcript, but there are also many bicistronic alternatively spliced Rta-containing transcripts. The KSHV Rta/Orf50 protein is indispensable for reactivation from latency. It also activates a variety of cellular and viral promoters. Introduction of KSHV Rta/Orf50 alone into latently infected cells is sufficient for initiating the entire viral lytic cascade (19, 34, 50), while a dominant-negative Rta/Orf50 mutant protein is able to abolish KSHV replication (35). The Rta/Orf50 protein in the related murine rhadinovirus MHV-68 fulfills the same function (57). KSHV Rta/Orf50 can transactivate a number of KSHV early promoters by several different mechanisms. (i) Purified Rta/Orf50 binds directly to its consensus sequence in the KSHV KbZIP/K8, Mta/Orf57, Nut-1/Pan, and viral interleukin-6 (vIL-6) promoters (5, 8, 13, 33, 49). (ii) It can interact with RBP-jk, and this interaction positively regulates the Nut-1, Orf57, ssB, and thymidine kinase (TK) promoters (31). (iii) It autoregulates its own promoter via the Oct-1 transcription factor. (iv) It may also aid in Sp-1-mediated and general transcription (9, 21, 43, 48, 53, 59). It also induces the K5 promoter (24). Studies with RRV Rta/Orf50 by our laboratory and others have demonstrated that this protein can dramatically transactivate the RRV R8, and RRV Orf57 promoters (10, 32). The KSHV Rta/Orf50 was also able to transactivate these RRV early promoters, suggesting that the functionality between the RRV and KSHV Rta proteins is conserved (10). KSHV encodes other immediate-early proteins, namely, Orf45 and Orf K8/KbZIP, which also contribute to lytic replication. However, unlike Rta/Orf50, none of these proteins alone can induce KSHV lytic reactivation from latently infected primary effusion lymphoma cells (50, 51). Recent evidence suggests that these proteins are primarily involved with the regulation of cellular pathways that pave the way for complete viral lytic replication in more restrictive cell types (26, 56, 61).
The Rta/Orf50 proteins of KSHV, RRV, and MHV-68 are homologous and show a high degree of sequence similarity at the amino acid level. In order to determine whether this sequence similarity translated into a conservation of function, we analyzed the ability of the KSHV, RRV, and MHV-68 Rta/Orf50 proteins to activate a set of KSHV immediate-early, early, and late gene promoters in epithelial, endothelial, and B-cell lines. Here we report that all three Rta/Orf50 proteins were able to activate several KSHV promoters. However, the RRV and MHV-68 Rta/Orf50 proteins were significantly depressed in their ability to activate KSHV promoters compared to the KSHV Rta/Orf50 protein. In particular the KSHV K8, vGPCR, kaposin, and gB promoters only responded to KSHV Rta/Orf50, but not to any of its homologs. In addition, only KSHV and RRV Rta/Orf50, but not the murine homolog, were able to inhibit p53's transactivation function, a property of Rta/Orf50 that has been described by Gwack et al. (22). Finally, we compared the ability of the three proteins to reactivate the KSHV lytic cycle when transfected into BCBL-1 cells, which are latently infected with KSHV. We found that although the KSHV, RRV, and MHV-68 Rta/Orf50 proteins could induce the complete KSHV reactivation program as previously defined (16, 27, 40, 44), RRV and MHV-68 Rta/Orf50 were less effective than KSHV Rta/Orf50.
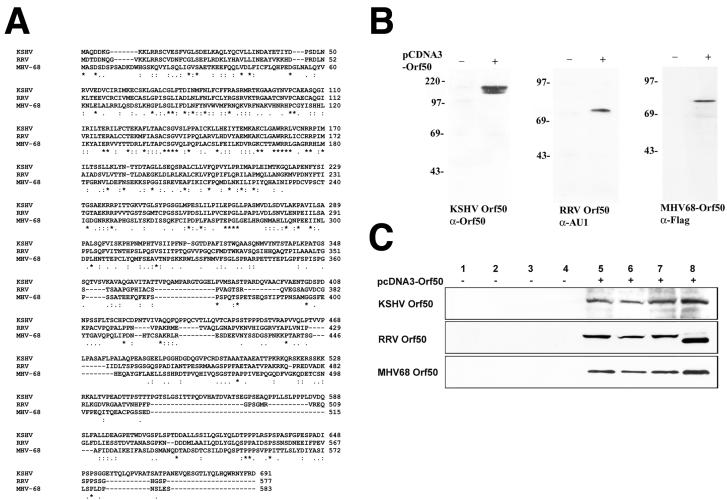
Alignment of the rhadinovirus Rta/Orf50 proteins.
The three Rta/Orf50 proteins of KSHV, RRV, and MHV-68 are well conserved, raising the possibility that the transactivation functions of the Rta/Orf50 proteins are likewise conserved among this group of proteins. Figure 1A depicts the alignment of the amino acid sequences encoded by the Rta/Orf50 proteins of KSHV, RRV, and MHV-68, which was performed using the ClustalW program. The amino acid similarity between KSHV and RRV Rta/Orf50 is 55%, the amino acid similarity between KSHV and MHV-68 Rta/Orf50 is 43%, and the amino acid similarity between RRV Rta/Orf50 and MHV-68 Rta/Orf50 is 47%. Amino acids 1 to 380 are conserved in all three proteins, followed by a more deletion-prone region and two more conserved blocks at amino acids 500 to 570 and 615 to 660. Expression of truncated versions of the KSHV Rta/Orf50 protein revealed that DNA binding was mediated by the amino-terminal 272 amino acids (33), which is consistent with our alignment in Fig. 1A, where this region is conserved among all three Rta/Orf50 rhadinovirus proteins. Figure 1B depicts a Western blot of the three Rta/Orf50 proteins expressed in 293 cells. All three proteins exhibit a higher apparent molecular weight than that predicted by their amino acid sequence. This is consistent with prior reports that showed that the KSHV and MHV-68 proteins are posttranslationally modified by phosphorylation (8, 31, 34, 47, 49, 57).
FIG. 1.
Three Rta/Orf50 proteins of the gamma-2-herpesvirus family. (A) ClustalW alignment of the three Rta/Orf50 proteins of KSHV, RRV, and MHV-68. Conserved amino acids are indicated by stars, and similarly charged residues are indicated by a semicolon. (B) Western blot of Rta/Orf50 protein expression after transient transfection into 293 cells. KSHV Rta/Orf50 was detected with an antibody against Rta/Orf50, and RRV and MHV-68 Rta/Orf50 proteins were epitope tagged and detected with antibodies against either an AU1 or Flag tag, respectively. (C) Western blot of the three Rta/Orf50 proteins in the four different cell lines. Lanes: 1 and 5, BJAB B cells; 2 and 6, BCBL-1 B cells; 3 and 7, SLK endothelial cells; 4 and 8, 293 epithelial cells. One hundred-fifty micrograms of total cell extract from the BJAB, BCBL, and SLK cells and 80 μg of cell extract from the 293 cells were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blots were performed with either an AU1 antibody to detect RRV Orf50, Flag antibody to detect MHV-68 Orf50, or Rta antibody (K. Ueda) to detect KSHV Orf50.
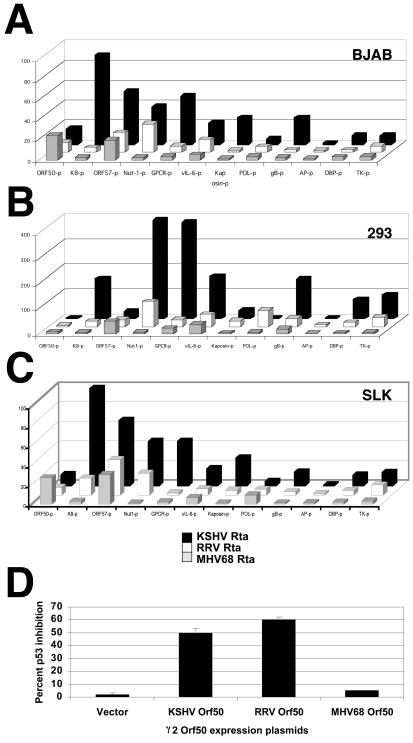
Comparison of KSHV, RRV, and MHV-68 Rta/Orf50 activities on a panel of KSHV promoters.
In order to evaluate the impact of the different Rta/Orf50 proteins with regard to the KSHV viral life cycle, we compared the ability of the KSHV, RRV, and MHV-68 transactivators to activate KSHV promoters in three relevant cell lines: KSHV-negative SLK endothelial cells, KSHV-negative BJAB B lymphocytes, and 293 epithelial cells. KSHV has been shown to be tropic for both B cells and endothelial cells and has also previously been shown to infect and replicate in 293 epithelial cells (17, 41, 42, 60). The KSHV promoters we used have been previously described (28, 31, 33-35). These include the immediate-early/early promoters KSHV Rta/Orf50, K8, and Mta/Orf57. The set of delayed early promoters included promoter regions for KSHV Nut-1 (also called Pan), viral G-protein-coupled receptor (vGPCR), DNA binding protein (DBP), vIL-6, TK, kaposin (or K12), and DNA polymerase (POL). The set of late gene promoters included those for glycoprotein B (gB) and assembly protein (AP). To control for experimental variation, transfections were performed in duplicate and repeated five times in each cell line. To control for transfection efficiency, the results were normalized using a β-galactosidase expression vector, which was cotransfected with the Rta expression plasmids and all the luciferase reporter plasmids in each experiment. The same cell extracts were used to measure the β-galactosidase activity and luciferase activity of each sample. To control for similar expression levels of all three Rta/Orf50 proteins, the three gamma-2-herpesvirus Rta/Orf50 cDNAs were all expressed from a pcDNA3 expression vector (Invitrogen). The three Rta/Orf50 proteins showed similar levels of expression in each cell line when expressed from this vector (Fig. 1C). To determine the effect of Rta/Orf50 on the different promoters, transactivation by Rta/Orf50 was calculated as fold activation compared to pcDNA3 vector alone. This normalization procedure allowed us to compare the relative effect of the three Rta/Orf50 proteins on all promoters in all cell lines.
BJAB is an Epstein-Barr virus- and KSHV-negative B-cell line. BJAB cells were electroporated with the promoter constructs described above and one of the three Rta/Orf50 expression plasmids. Table 1 depicts the transcriptional activation seen with these constructs in BJAB B lymphocytes. (i) Promoter activity, which was measured in luciferase units for each of the plasmids and normalized to β-galactosidase activity, is represented as fold activation over the pcDNA3 vector control (see also Fig. 2A). (ii) To compare the efficiency of the three Rta/Orf50 proteins, the activation of each KSHV promoter by the KSHV Rta/Orf50 transactivator was set at 100%, and the ability of RRV and MHV-68 transactivators to activate the same promoter was calculated as a percentage of the activity seen with the KSHV Rta/Orf50 protein. Although the RRV and MHV-68 proteins could transactivate the KSHV promoters of all three groups of immediate-early, early, and late genes, overall transactivation efficiencies were lower than that seen with the autologous KSHV Rta/Orf50 transactivator. In BJAB cells, KSHV Rta/Orf50 was able to transactivate the immediate-early promoter K8 significantly, whereas the other two Rta/Orf50 homologs failed to do so to any significant degree above background. This was also true for the delayed early promoters, vGPCR and kaposin, and the gB promoter. This phenotype is consistent with a report by Chang et al. (4) and KSHV array analysis (27, 40) that found gB mRNA and the gB promoter fragment used here exhibit viral early kinetics. The late AP promoter was not significantly activated by any of the Rta/Orf50 proteins. Both primate Rta/Orf50 proteins could activate the KSHV Nut-1 promoter, whereas the MHV-68 Rta/Orf50 protein was not able to significantly transactivate this promoter.
TABLE 1.
Transactivation of KSHV promoters by rhadinovirus Rta/Orf50 proteinsa
| Cells and promoters | Activation (fold)
|
% of KSHV activity
|
Class | ||||
|---|---|---|---|---|---|---|---|
| KSHV | RRV | MHV-68 | KSHV | RRV | MHV-68 | ||
| BJAB cells | |||||||
| Immediate early/early | |||||||
| Orf50-p | 17 ± 5 | 10 ± 4 | 24 ± 1 | 100 | 60 | 147 | All |
| K8-p | 90 ± 8 | 5 ± 0 | 2 ± 0 | 100 | 5 | 2 | Human |
| Orf57-p | 54 ± 0 | 20 ± 1 | 19 ± 1 | 100 | 37 | 36 | All |
| Delayed early | |||||||
| Nut1-p | 39 ± 3 | 29 ± 0 | 2 ± 0 | 100 | 74 | 6 | Primate |
| GPCR-p | 49 ± 8 | 7 ± 0 | 3 ± 1 | 100 | 14 | 7 | Human |
| DBP-p | 9 ± 1 | 3 ± 1 | 3 ± 0 | 100 | 27 | 33 | All |
| vIL-6-p | 22 ± 8 | 13 ± 3 | 5 ± 1 | 100 | 57 | 22 | All |
| TK-p | 10 ± 2 | 6 ± 0 | 4 ± 1 | 100 | 65 | 39 | All |
| Kaposin-p | 27 ± 4 | 2 ± 2 | 1 ± 0 | 100 | 8 | 5 | Human |
| POL-p | 6 ± 0 | 6 ± 0 | 4 ± 1 | 100 | 90 | 57 | All |
| Late | |||||||
| gB-p | 28 ± 2 | 2 ± 0 | 3 ± 0 | 100 | 8 | 9 | Human |
| AP-p | 2 ± 0 | 2 ± 0 | 1 ± 0 | NA | NA | NA | None |
| HEK293 | |||||||
| Immediate early/early | |||||||
| Orf50-p | 5 ± 1 | 5 ± 0 | 8 ± 0 | 100 | 100 | 150 | All |
| K8-p | 161 ± 10 | 19 ± 1 | 5 ± 1 | 100 | 12 | 3 | Human |
| Orf57-p | 31 ± 0 | 26 ± 1 | 50 ± 4 | 100 | 84 | 161 | All |
| Delayed early | |||||||
| Nut1-p | 393 ± 8 | 99 ± 8 | 5 ± 0 | 100 | 25 | 1 | Primate |
| GPCR-p | 386 ± 10 | 27 ± 0 | 21 ± 0 | 100 | 7 | 6 | Human |
| DBP-p | 78 ± 26 | 15 ± 1 | 5 ± 0 | 100 | 20 | 6 | Primate |
| vIL-6-p | 171 ± 10 | 48 ± 2 | 39 ± 1 | 100 | 28 | 23 | Human |
| TK-p | 96 ± 1 | 37 ± 1 | 6 ± 1 | 100 | 38 | 6 | Primate |
| Kaposin-p | 34 ± 1 | 22 ± 1 | 3 ± 1 | 100 | 64 | 10 | Primate |
| POL-p | 5 ± 0 | 63 ± 1 | 7 ± 1 | 100 | 1163 | 124 | All |
| Late | |||||||
| gB-p | 159 ± 12 | 32 ± 1 | 20 ± 1 | 100 | 20 | 13 | Human |
| AP-p | 4 ± 0 | 7 ± 0 | 1 ± 1 | 100 | 205 | 21 | Primate |
| SLK cells | |||||||
| Immediate early/early | |||||||
| Orf50-p | 12 ± 2 | 8 ± 3 | 27 ± 10 | 100 | 68 | 230 | All |
| K8-p | 100 ± 15 | 17 ± 1 | 3 ± 1 | 100 | 17 | 3 | Human |
| Orf57-p | 67 ± 3 | 36 ± 3 | 30 ± 1 | 100 | 54 | 45 | All |
| Delayed early | |||||||
| Nut1-p | 46 ± 10 | 22 ± 7 | 1 ± 0 | 100 | 49 | 2 | Primate |
| GPCR-p | 46 ± 8 | 2 ± 0 | 2 ± 0 | 100 | 5 | 5 | Human |
| DBP-p | 11 ± 3 | 5 ± 2 | 2 ± 1 | 100 | 42 | 19 | All |
| vIL-6-p | 18 ± 1 | 6 ± 1 | 7 ± 1 | 100 | 35 | 38 | All |
| TK-p | 14 ± 2 | 10 ± 0 | 3 ± 1 | 100 | 72 | 23 | All |
| Kaposin-p | 29 ± 1 | 5 ± 3 | 1 ± 1 | 100 | 17 | 3 | Human |
| POL-p | 5 ± 0 | 6 ± 0 | 9 ± 2 | 100 | 120 | 192 | All |
| Late | |||||||
| gB-p | 15 ± 1 | 3 ± 0 | 1 ± 0 | 100 | 23 | 9 | Human |
| AP-p | 1 ± 0 | 1 ± 0 | 2 ± 0 | NA | NA | NA | None |
Shown is a summary of the results obtained by cotransfection experiments between KSHV, RRV, and MHV-68 Rta/Orf50 proteins and a panel of KSHV promoters fused to a luciferase reporter gene. All promoter activity was normalized to β-galactosidase activity and is shown as fold induction over empty expression vector and as percent activity relative to KSHV Rta/Orf50.
FIG. 2.
Transactivation functions of KSHV, RRV, and MHV-68 Rta/Orf50. Panels A, B, and C depict the results of the transfection data from Table 1 for BJAB, 293, and SLK cells, respectively. (D) Inhibition of human p53 transactivation of a p53-responsive promoter/luciferase construct in p53-negative (10)1 cells. Promoter activity was measured as relative light units normalized to a cotransfected β-galactosidase promoter. The ability of all three Rta/Orf50 proteins to inhibit p53 activity is quantified as a percentage of inhibition.
An identical set of experiments were performed in 293 epithelial cells. 293 epithelial cells have been previously shown to support KSHV infection in vitro (17, 41, 42, 60). We transfected the aforementioned promoter constructs into these cells along with the KSHV, RRV, or MHV-68 Rta/Orf50 expression plasmids as described above. Promoter activity was measured by luciferase units for each of the plasmids and depicted as fold activation over the pcDNA3 vector control (Table 1 and Fig. 2B). As can be seen in Table 1, although the RRV and MHV-68 proteins could transactivate all three classes of promoters, their efficiencies were lower compared to the autologous KSHV protein, as was the case in BJAB cells. In 293 cells, the KSHV Rta/Orf50 protein was able to transactivate the immediate-early/early K8 promoter significantly, whereas the other Rta/Orf50 transactivators could not. This was also true for the delayed early promoter, vGPCR, and the late gB promoter. However, in 293 epithelial cells, the RRV Rta/Orf50 transactivator was able to moderately activate the Nut-1, DBP, kaposin, TK, and AP promoters, whereas MHV68 Rta/Orf50 did not significantly activate these promoters.
SLK is a KS tumor-derived endothelial cell line that is KSHV negative (25, 36, 41). It was of particular interest to measure promoter activity in these cells since KSHV can infect endothelial cells in vitro and KS lesions are comprised of a large number of infected endothelial cells. Furthermore, KSHV Rta/Orf50 can induce KSHV lytic transcription experimentally in in vitro KSHV- infected SLK cell populations (R. Renne and D. Dittmer, unpublished observation). We transfected the aforementioned promoter constructs into these cells along with the KSHV, RRV, or MHV-68 Rta/Orf50 expression plasmids as described above. Table 1 and Fig. 2C depicts the transcriptional activation seen with these constructs in SLK endothelial cells. Once again, the promoter activity for each construct is represented as fold activation over the pcDNA3 vector control. As can be seen in Table 1, although the RRV and MHV-68 proteins could transactivate all three classes of promoters, their transactivation efficiencies were lower than that seen with the autologous KSHV protein, a pattern similar to the results we obtained in BJAB and 293 cells. Furthermore, the KSHV Rta/Orf50 protein was able to transactivate the immediate-early/early promoter, K8, significantly, whereas the two other Rta/Orf50 transactivators could not. This was also true for the delayed early promoter, vGPCR, as well as the kaposin promoter and the gB promoter. In SLK cells, the RRV Rta/Orf50 transactivator was able to moderately activate the Nut-1 promoter whereas MHV-68 Rta/Orf50 did not significantly activate this promoter.
In summary, we tested a panel of 12 KSHV promoters in three different cell lines that are relevant for KSHV tropism/infection, which include B cells, endothelial cells, and epithelial cells. In all three cell lines, the KSHV viral transactivator activated the autologous KSHV promoter significantly, as has been previously described (3, 6, 7, 10, 19, 30, 33, 34, 39, 43, 46, 49, 51, 57, 59, 61). These studies provide for the first simultaneous comparison of primate Rta/Orf50 proteins. In contrast to KSHV, the RRV and MHV-68 Rta/Orf50 transactivators were notably reduced in their ability to transactivate this panel of KSHV promoters. In this set of KSHV promoters, the overall response patterns to KSHV Rta/Orf50 were similar in BJAB B-cells and SLK endothelial cells (Fig. 2). Surprisingly, in 293 cells the Mta/Orf57 promoter was less responsive to KSHV Rta/Orf50 relative to the Nut-1 promoter, while the opposite was true in SLK and BJAB cells. This suggests that the compositions of host factors that mediate Rta/Orf50's effects differ between 293 and the other two cell lines and underscores the need to conduct transactivation studies in multiple cell lines. Neither the RRV nor MHV-68 Rta/Orf50 proteins were able to activate the KSHV K8, vGPCR GPCR, or the gB promoter. This suggests that some interactions between human cellular transcription factors and KSHV Rta/Orf50 are not evolutionary conserved but are required for at least this subset of KSHV promoters. We would predict that a subset of basic transcription factors that interacts with KSHV Rta/Orf50 (20) includes proteins that would not interact with either the RRV or MHV-68 Rta/Orf50 proteins. The regulation of vGPCR, K8, and gB promoters by KSHV Rta/Orf50 appears to be unique to the human virus and may explain some aspects of its biology.
Rta/Orf50's interaction with p53 is restricted to the primate viruses.
KSHV Rta/Orf50 has been demonstrated to inhibit p53's transactivation function (23), which is independent of Rta/Orf50's specific DNA-binding function. We compared the ability of MHV-68 and RRV Rta/Orf50 transactivators to inhibit p53's transactivation function by using a well-established p53-responsive element (58). As seen in Fig. 2D, only RRV and KSHV Rta/Orf50 were able to interfere with human p53's transactivation function, but MHV-68 Rta/Orf50 could not. This suggests that the interaction between the primate Rta/Orf50 proteins and human p53 has evolved later during speciation. Similar to the interaction between the human papillomavirus E6 proteins and p53 (45, 54), which can be interrupted by slight amino acid variation between “high-risk” and “low-risk” strains, the interaction between Rta/Orf50 and p53 also shows high specificity.
Comparison of the genome-wide effects of KSHV, RRV, and MHV-68 Rta/Orf50 proteins on KSHV reactivation.
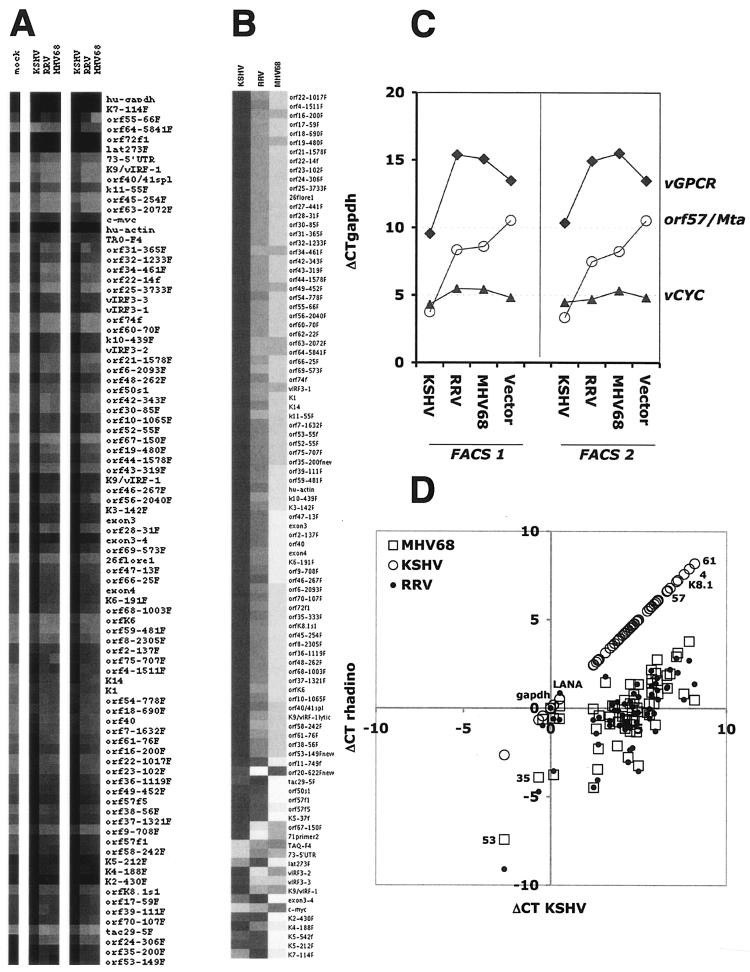
In order to evaluate the impact of the different Rta/Orf50 proteins on the KSHV life cycle, we analyzed the effect of expression of the individual Rta/Orf50 proteins on KSHV reactivation from latently infected BCBL-1 cells. KSHV Rta/Orf50 has previously been shown to efficiently induce KSHV lytic replication in these cells (34, 50, 51), and KSHV Rta/Orf50 alone is necessary and sufficient to induce the latent to lytic switch. We electroporated 107 BCBL-1 cells with 20 μg of empty vector pcDNA3 or the three different pcDNA3-Orf50 expression constructs along with 5 μg of pEGFP plasmid (Clontech, Inc.). Forty-eight hours postelectroporation, the cells were sorted based upon GFP fluorescence. No cells were excluded based on scatter, since KSHV reactivation in BCBL-1 cells induces a blast phenotype, which changes the light scatter profile of the cells. In independent experiments, between 5 × 103 and 10 × 103 GFP-positive cells were sorted and mRNA was isolated, reverse transcribed, and analyzed using our previously developed real-time quantitative reverse transcription-PCR (RT-PCR) array for KSHV (12, 16). We previously showed that glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels do not change upon KSHV reactivation in BCBL-1 cells (16). We also tested several other cellular mRNAs in this system, including those for actin, 18S rRNA, and hypoxanthine phosphoribosyltransferase. These did not change in response to KSHV reactivation (data not shown). Since these mRNAs correlated with each other, as expected based on prior studies (12, 16), we chose to normalize all data based upon the level of GAPDH mRNA and subjected the normalized data to statistical analysis (52). Figure 3A shows a Treeview representation after hierarchical clustering of the data from cells sorted with a fluorescence-activated cell sorter 48 h after treatment (15). Overall, the repeat experiments showed an identical pattern: relative to mock-treated cells, KSHV Rta/Orf50 significantly induced the transcription of all KSHV lytic mRNAs (indicated by the dark shade of gray) but not cellular mRNAs or latency type I mRNAs. Electroporation of an RRV or MHV-68 Rta/Orf50 expression plasmid also induced KSHV transcription, but to a lesser degree than KSHV Rta/Orf50. This could presumably have occurred due to the nonhuman Rta/Orf50 proteins activating the KSHV Rta/Orf50 promoter and thus initiating a complete, albeit delayed, lytic replication cascade. Figure 3B shows the data from the total culture at 72 h postelectroporation. Here data were also normalized relative to mock-transfected cells as described in references 12 and 16. Relative to mock-transduced cultures, KSHV Rta/Orf50-transfected BCBL-1 cultures induced virtually all mRNAs, RRV Rta/Orf50-transfected cultures likewise induced all mRNAs, but to a lesser degree. Consistently, for every time point tested, MHV-68- Rta/Orf50-transfected BCBL cells exhibited the least amount of induction at this time point. At the 24-h time point, no significant differences in KSHV transcription were observed in cells transfected with three Rta/Orf50 homologs and cells transfected with a GFP expression plasmid (data not shown).
FIG.3.
Ability of the three Rta/Orf50 proteins to reactivate KSHV from latently infected BCBL-1 cells. (A) “Heatmap” representation of KSHV mRNA after transfection with KSHV, RRV, or MHV-68 Rta/Orf50 as determined by real-time quantitative RT-PCR (16). The three leftmost panels represent mock-treated cells and two replicate experiments of sorted, Rta/Orf50-transfected cells after 48 h. (B) KSHV mRNA levels in the total culture 72 h after transfection with KSHV, RRV, or MHV-68 Rta/Orf50. Darker shades of gray correspond to higher mRNA levels on a log 2 scale. (C) Plots of three individual relative mRNA (Δ CT) levels selected from panel A for KSHV v-cyclin (CYC), Mta/Orf57, and vGPCR on the vertical axis for each of the experiments on the horizontal axis. (D) mRNA levels (Δ CT) after electroporation with KSHV, RRV, or MHV68 Rta/Orf50 on the horizontal axis relative to the mRNA levels after electroporation with KSHV Rta/Orf50.
As in our transfection experiments, we could discern genes that were responsive to the Rta/Orf50 viral transactivator of any species. We also identified genes that were only activated by KSHV Orf50/Rta. This is exemplified in Fig. 3C, which shows the results of two independent experiments. Shown here are the normalized (ΔCTgapdh) values for a latent mRNA, v-cyclin (vCYC), and two early gene mRNA transcripts, Orf57/Mta and vGPCR. In this representation, lower ΔCT (change in cycle threshold [CT]) values correspond to higher mRNA levels on a logarithmic scale. As expected, v-cyclin mRNA was unaffected by KSHV, RRV, or MHV-68 Rta/Orf50 as compared to the vector-only-transfected BCBL-1 cells. We had previously shown that the LANA promoter, which also drives v-cyclin expression, is unaffected by KSHV Rta/Orf50 (28). The mRNA for Mta/Orf57 is significantly induced by RRV or MHV-68 Rta/Orf50 and even more so by KSHV Rta/Orf50 as compared to the vector-transfected cells. In contrast, only the KSHV Rta/Orf50 protein, but not the RRV or MHV-68 Rta/Orf50 protein, induces the mRNA for vGPCR, as evidenced by a 5-U decrease in ΔCT (corresponding to a 1.85- or ∼19-fold difference). The pattern of transactivation of KSHV mRNA transcripts in the context of the viral genome parallels the pattern seen with the individual KSHV promoters, as shown in Table 1.
Figure 3D shows a plot of the levels of induction (ΔCT) by RRV or MHV-68 Rta/Orf50 for each of the KSHV genes (y axis) relative to the induction that was achieved by KSHV Rta/Orf50 alone (x axis). The values are represented on a logarithmic scale, to allow for a more robust statistical analysis (18). As can be gleaned from this two-dimensional representation, neither the MHV-68 nor RRV Rta/Orf50 proteins induced KSHV lytic mRNAs to the levels seen after transfection of KSHV Rta/Orf50 expression plasmid. However, both RRV Rta/Orf50 and MHV-68 Rta/Orf50 induced the complete KSHV reactivation program. Although the RRV Rta/Orf50 protein could activate KSHV promoters better than the MHV-68 protein in both reporter assays (Table 1) and the viral microarray (Fig. 3A and B), the primate Rta/Orf50 protein was not dramatically better at inducing KSHV reactivation as a whole than the MHV-68 protein, as evidenced by their overlapping expression profiles (Fig. 3D). This suggests a mechanism whereby MHV-68 or RRV Rta/Orf50 cannot fully substitute for KSHV Rta/Orf50 but may instead initiate lytic replication by inducing the KSHV immediate-early promoters, such as the KSHV Rta/Orf50 promoter. In this scenario, the rate-limiting step for complete, ordered lytic replication may also be dependent on the time postelectroporation of the BCBL-1 cells.
To validate our statistical analysis, we performed Gaussian clustering using a second different, genetic optimization algorithm with ArrayMiner 2.6 under Macintosh OsX (Optimal Design, Inc., Brussels, Belgium). We analyzed the complete data sets for these experiments followed by flow cytometry sorting for each rhadinovirus Rta/Orf50 as well as vector control (595 data points after exclusion of some PCR failures). The algorithm terminated with five essentially similar clusters (data not shown), as well as genes that did not change expression upon electroporation. The latter group included the cellular housekeeping mRNAs c-myc and hu-actin, as well as the KSHV latent mRNAs for LANA and v-cyclin (not shown). This is consistent with our prior observations, which found that neither LANA mRNA nor the LANA promoter is activated upon KSHV reactivation in BCBL-1 cells (11, 16, 28). All other KSHV mRNAs were induced by KSHV Rta/Orf50 relative to the vector. Four mRNAs that were most highly induced by KSHV Rta/Orf50 relative to vector were the KSHV K2, K4, Rta/Orf50, and K8 transcripts. RRV and MHV Rta/Orf50 also induced KSHV lytic mRNAs but much less efficiently.
Summary.
All herpesvirus lytic replication occurs in an ordered sequence of gene expression. Immediate-early mRNAs are transcribed in the absence of de novo protein synthesis, early mRNAs are activated by the immediate-early transactivating factors, and late mRNA transcription is blocked by inhibiting viral DNA replication. Once the immediate-early protein(s) is made, this transcriptional cascade culminates in capsid assembly, virion egress, and death of the host cell.
One question regarding the evolution of the gammaherpesvirus subfamily pertains to the degree of functional conservation that exists among the human, primate, and vole rhadinoviruses. We find that the immediate-early Rta/Orf50 proteins of KSHV, RRV, and MHV-68 are highly conserved in amino acid sequence (Fig. 1), as well as in their ability to activate relevant KSHV viral promoters (Fig. 2). KSHV Orf50 alone is necessary and sufficient to induce KSHV lytic reactivation in BCBL-1 cells (33-35, 50, 51). We demonstrate here that the RRV and MHV-68 Rta/Orf50 protein can function similarly. In addition, we found important qualitative as well as quantitative differences among the three homologous proteins: Some KSHV promoters (K8, vGPCR, gB, and kaposin) only responded to KSHV Rta/Orf50, but not to RRV or MHV-68 Rta/Orf50, while other promoters responded to all three (Rta/Orf50, Mta/Orf57, DBP, vIL-6, TK, Pol, and AP). This is not unexpected since Rta/Orf50 has multiple modes of action. It transactivates the Nut-1, Orf57, and K8 promoters by binding directly to DNA (33, 47, 49), while other promoters may be activated indirectly through protein-protein interactions. Interestingly, we had previously observed that KSHV Rta activated the RRV R8, Orf57, and gB promoters but only weakly activated the RRV v-IRF and Orf50 promoters (10).
In the context of latently infected BCBL-1 cells, addition of KSHV Rta/Orf50 was sufficient to induce the complete lytic transcription program, which agrees with a recent study by Nakamura et al. (38). Extending their work, we found that KSHV Rta/Orf50 was much better at inducing complete KSHV reactivation than either the RRV or MHV-68 Rta/Orf50 homologs (Fig. 3). This suggests that while either Rta/Orf50 homolog is sufficient to induce KSHV reactivation, other KSHV immediate-early or early proteins, such as KbZIP/K8 (56, 61), Mta/Orf57 (29), or Orf45 (61), cooperate with Rta/Orf50 to complete the lytic cascade. We surmise that this cooperation is species specific. The delayed replication kinetics in cells that are transfected by RRV or MHV-68 Rta/Orf50 suggests that enough KSHV Rta/Orf50 protein needs to accumulate in the BCBL-1 cells to circumvent this species barrier and activate complete lytic protein expression. Ultimately, mature KSHV virions are produced in this system, regardless of which rhadinovirus Rta/Orf50 protein initiates the process.
Acknowledgments
We thank K. Ueda for the anti-KSHV Rta/Orf50 antibody and Don Ganem and Gerry Zambetti for cell lines and plasmids. We thank Ren Sun for the pcDNA3-MHV-68 Rta plasmid.
This work was supported by NIH grants RR1555777 and CA109232 to D.P.D. and the American Heart Association (0355852U) and NCI grant CA096500 to B.D.
REFERENCES
- 1.Ablashi, D. V., L. G. Chatlynne, J. E. Whitman, Jr., and E. Cesarman. 2002. Spectrum of Kaposi's sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin. Microbiol. Rev. 15:439-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowser, B. S., S. M. DeWire, and B. Damania. 2002. Transcriptional regulation of the K1 gene product of Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:12574-12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, J., and D. Ganem. 2000. On the control of late gene expression in Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8). J. Gen. Virol. 81:2039-2047. [DOI] [PubMed] [Google Scholar]
- 5.Chang, P.-J., D. Shedd, L. Gradoville, M.-S. Cho, L.-W. Chen, J. Chang, and G. Miller. 2002. Open reading frame 50 protein of Kaposi's sarcoma-associated herpesvirus directly activates the viral PAN and K12 genes by binding to related response elements. J. Virol. 76:3168-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiou, C.-J., L. J. Poole, P. S. Kim, D. M. Ciufo, J. S. Cannon, C. M. ap Rhys, D. J. Alcendor, J.-C. Zong, R. F. Ambinder, and G. S. Hayward. 2002. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3421-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curreli, F., F. Cerimele, S. Muralidhar, L. J. Rosenthal, E. Cesarman, A. E. Friedman-Kien, and O. Flore. 2002. Transcriptional downregulation of ORF50/Rta by methotrexate inhibits the switch of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 from latency to lytic replication. J. Virol. 76:5208-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, H., M. J. Song, J. T. Chu, and R. Sun. 2002. Transcriptional regulation of the interleukin-6 gene of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 76:8252-8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng, H., A. Young, and R. Sun. 2000. Auto-activation of the rta gene of human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 81:3043-3048. [DOI] [PubMed] [Google Scholar]
- 10.DeWire, S. M., M. A. McVoy, and B. Damania. 2002. Kinetics of expression of rhesus monkey rhadinovirus (RRV) and identification and characterization of a polycistronic transcript encoding the RRV Orf50/Rta, RRV R8, and R8.1 genes. J. Virol. 76:9819-9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittmer, D., M. Lagunoff, R. Renne, K. Staskus, A. Haase, and D. Ganem. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmer, D. P. 2003. Transcription profile of Kaposi's sarcoma-associated herpesvirus in primary Kaposi's sarcoma lesions as determined by real-time PCR arrays. Cancer Res. 63:2010-2015. [PubMed] [Google Scholar]
- 13.Duan, W., S. Wang, S. Liu, and C. Wood. 2001. Characterization of Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 ORF57 promoter. Arch. Virol. 146:403-413. [DOI] [PubMed] [Google Scholar]
- 14.Dutia, B. M., C. J. Clarke, D. J. Allen, and A. A. Nash. 1997. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J. Virol. 71:4278-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakhari, F. D., and D. P. Dittmer. 2002. Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 76:6213-6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foreman, K. E., J. Friborg, Jr., W. P. Kong, C. Woffendin, P. J. Polverini, B. J. Nickoloff, and G. J. Nabel. 1997. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336:163-171. [DOI] [PubMed] [Google Scholar]
- 18.Glantz, S., and B. Slinker. 2000. Primer of applied regression and analysis of variance. McGraw-Hill, New York, N.Y.
- 19.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwack, Y., H. J. Baek, H. Nakamura, S. H. Lee, M. Meisterernst, R. G. Roeder, and J. U. Jung. 2003. Principal role of TRAP/mediator and SWI/SNF complexes in Kaposi's sarcoma-associated herpesvirus RTA-mediated lytic reactivation. Mol. Cell. Biol. 23:2055-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwack, Y., H. Byun, S. Hwang, C. Lim, and J. Choe. 2001. CREB-binding protein and histone deacetylase regulate the transcriptional activity of Kaposi's sarcoma-associated herpesvirus open reading frame 50. J. Virol. 75:1909-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwack, Y., S. Hwang, H. Byun, C. Lim, J. W. Kim, E.-J. Choi, and J. Choe. 2001. Kaposi's sarcoma-associated herpesvirus open reading frame 50 represses p53-induced transcriptional activity and apoptosis. J. Virol. 75:6245-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwack, Y., S. Hwang, C. Lim, Y. S. Won, C. H. Lee, and J. Choe. 2002. Kaposi's sarcoma-associated herpesvirus open reading frame 50 stimulates the transcriptional activity of STAT3. J. Biol. Chem. 277:6438-6442. [DOI] [PubMed] [Google Scholar]
- 24.Haque, M., J. Chen, K. Ueda, Y. Mori, K. Nakano, Y. Hirata, S. Kanamori, Y. Uchiyama, R. Inagi, T. Okuno, and K. Yamanishi. 2000. Identification and analysis of the K5 gene of Kaposi's sarcoma-associated herpesvirus. J. Virol. 74:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herndier, B. G., A. Werner, P. Arnstein, N. W. Abbey, F. Demartis, R. L. Cohen, M. A. Shuman, and J. A. Levy. 1994. Characterization of a human Kaposi's sarcoma cell line that induces angiogenic tumors in animals. AIDS 8:575-581. [DOI] [PubMed] [Google Scholar]
- 26.Izumiya, Y., S.-F. Lin, T. Ellison, L.-Y. Chen, C. Izumiya, P. Luciw, and H.-J. Kung. 2003. Kaposi's sarcoma-associated herpesvirus K-bZIP is a coregulator of K-Rta: physical association and promoter-dependent transcriptional repression. J. Virol. 77:1441-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenner, R. G., M. M. Albà, C. Boshoff, and P. Kellam. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong, J., J. Papin, and D. Dittmer. 2001. Differential regulation of the overlapping Kaposi's sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoter. J. Virol. 75:1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirshner, J. R., D. M. Lukac, J. Chang, and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 57 encodes a posttranscriptional regulator with multiple distinct activities. J. Virol. 74:3586-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagunoff, M., D. M. Lukac, and D. Ganem. 2001. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J. Virol. 75:5891-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang, Y., J. Chang, S. J. Lynch, D. M. Lukac, and D. Ganem. 2002. The lytic switch protein of KSHV activates gene expression via functional interaction with RBP-Jkappa (CSL), the target of the Notch signaling pathway. Genes Dev. 16:1977-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, S. F., D. R. Robinson, J. Oh, J. U. Jung, P. A. Luciw, and H. J. Kung. 2002. Identification of the bZIP and Rta homologues in the genome of rhesus monkey rhadinovirus. Virology 298:181-188. [DOI] [PubMed] [Google Scholar]
- 33.Lukac, D. M., L. Garibyan, J. R. Kirshner, D. Palmeri, and D. Ganem. 2001. DNA binding by Kaposi's sarcoma-associated herpesvirus lytic switch protein is necessary for transcriptional activation of two viral delayed early promoters. J. Virol. 75:6786-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 36.Lunardi-Iskandar, Y., J. L. Bryant, R. A. Zeman, V. H. Lam, F. Samaniego, J. M. Besnier, P. Hermans, A. R. Thierry, P. Gill, and R. C. Gallo. 1995. Tumorigenesis and metastasis of neoplastic Kaposi's sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature 375:64-68. [DOI] [PubMed] [Google Scholar]
- 37.Mansfield, K. G., S. V. Westmoreland, C. D. DeBakker, S. Czajak, A. A. Lackner, and R. C. Desrosiers. 1999. Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses. J. Virol. 73:10320-10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura, H., M. Lu, Y. Gwack, J. Souvlis, S. L. Zeichner, and J. U. Jung. 2003. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J. Virol. 77:4205-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okuno, T., Y. B. Jiang, K. Ueda, K. Nishimura, T. Tamura, and K. Yamanishi. 2002. Activation of human herpesvirus 8 open reading frame K5 independent of ORF50 expression. Virus Res. 90:77-89. [DOI] [PubMed] [Google Scholar]
- 40.Paulose-Murphy, M., N.-K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 43.Sakakibara, S., K. Ueda, J. Chen, T. Okuno, and K. Yamanishi. 2001. Octamer-binding sequence is a key element for the autoregulation of Kaposi's sarcoma-associated herpesvirus ORF50/Lyta gene expression. J. Virol. 75:6894-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarid, R., O. Flore, R. A. Bohenzky, Y. Chang, and P. S. Moore. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 46.Seaman, W. T., D. Ye, R. X. Wang, E. E. Hale, M. Weisse, and E. B. Quinlivan. 1999. Gene expression from the ORF50/K8 region of Kaposi's sarcoma-associated herpesvirus. Virology 263:436-449. [DOI] [PubMed] [Google Scholar]
- 47.Song, M. J., H. J. Brown, T.-T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by Rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song, M. J., H. Deng, and R. Sun. 2003. Comparative study of regulation of RTA-responsive genes in Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 77:9451-9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song, M. J., X. Li, H. J. Brown, and R. Sun. 2002. Characterization of interactions between RTA and the promoter of polyadenylated nuclear RNA in Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 76:5000-5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun, R., S.-F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandesompele, J., K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe, and F. Speleman. 18 June 2002. posting date. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. [Online.] http://genomebiology.com/2002/3/7/RESEARCH/0034. [DOI] [PMC free article] [PubMed]
- 53.Wang, S., S. Liu, M.-H. Wu, Y. Geng, and C. Wood. 2001. Identification of a cellular protein that interacts and synergizes with the RTA (ORF50) protein of Kaposi's sarcoma-associated herpesvirus in transcriptional activation. J. Virol. 75:11961-11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 55.Wong, S. W., E. P. Bergquam, R. M. Swanson, F. W. Lee, S. M. Shiigi, N. A. Avery, J. W. Fanton, and M. K. Axthelm. 1999. Induction of B cell hyperplasia in simian immunodeficiency virus- infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J. Exp Med. 190:827-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu, F. Y., Q. Q. Tang, H. Chen, C. ApRhys, C. Farrell, J. Chen, M. Fujimuro, M. D. Lane, and G. S. Hayward. 2002. Lytic replication-associated protein (RAP) encoded by Kaposi sarcoma-associated herpesvirus causes p21CIP-1-mediated G1 cell cycle arrest through CCAAT/enhancer-binding protein-alpha. Proc. Natl. Acad. Sci. USA 99:10683-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu, T.-T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zambetti, G. P., J. Bargonetti, K. Walker, C. Prives, and A. J. Levine. 1992. Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev. 6:1143-1152. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, L., J. Chiu, and J. C. Lin. 1998. Activation of human herpesvirus 8 (HHV-8) thymidine kinase (TK) TATAA-less promoter by HHV-8 ORF50 gene product is SP1 dependent. DNA Cell Biol. 17:735-742. [DOI] [PubMed] [Google Scholar]
- 60.Zhou, F.-C., Y.-J. Zhang, J.-H. Deng, X.-P. Wang, H.-Y. Pan, E. Hettler, and S.-J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu, F. X., T. Cusano, and Y. Yuan. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5556-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]