Abstract
The importance of Fc-mediated effector function in protective immunity to HIV-1 (hereafter referred to simply as HIV) is becoming increasingly apparent. A large of number of studies in natural infection cohorts, spanning the last 26 years, have associated Fc-mediated effector function, particularly antibody-dependent cellular cytotoxicity, with a favourable clinical course. These studies strongly suggest a role for Fc-mediated effector function in the post-infection control of viraemia. More recently, studies in both humans and non-human primates (NHPs) also implicate Fc-mediated effector function in blocking HIV acquisition. Accordingly, this review will discuss the results supporting a role of Fc-mediated effector function in both blocking acquisition and post-infection control of viraemia. Parallel studies in NHPs and humans will be compared for common themes. Context for this discussion will be provided by summarizing the temporal emergence of key host and virological events over the course of an untreated HIV infection framing where, when and how Fc-mediated effector function might be protective. A hypothesis that Fc-mediated effector function protects primarily in the early stages of both acquisition and post-infection control of viraemia will be developed.
Keywords: Fc-mediated effector function, HIV-1, monocytes, natural killer cells, protective immunity
Course of a typical untreated HIV infection
The course of HIV infection is shown in Fig. 1, which depicts the classical pattern seen in untreated individuals. The advent of potent anti-retroviral therapy dramatically changed this course and deaths from uncontrolled infections are increasingly rare. The course is marked by two major phases leading to AIDS. The first phase is acquisition that occurs during eclipse, which is the time from exposure to HIV to the time of first detectable viraemia (T0). The eclipse phase is approximately 10 days in HIV-infected individuals.1 The precise time it takes HIV to establish an irreversible foothold is unknown but the outer bound is probably the point at which the latent viral reservoir is established in resting memory CD4+ T cells.2,3 This is known to occur as early as 10 days after acute retroviral symptoms appear in humans.4 However, studies using anti-retroviral post-exposure prophylaxis to block infection of non-human primates (NHPs) with simian immunodeficiency virus indicate that the reservoir is established much earlier, between 24 and 72 hr post-exposure,5 which places it significantly before T0.1 Hence, for Fc-mediated effector function to block acquisition it must do so in this ‘window of opportunity’.
Figure 1.
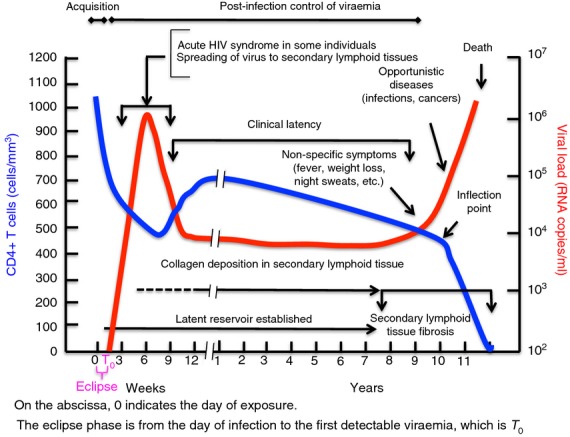
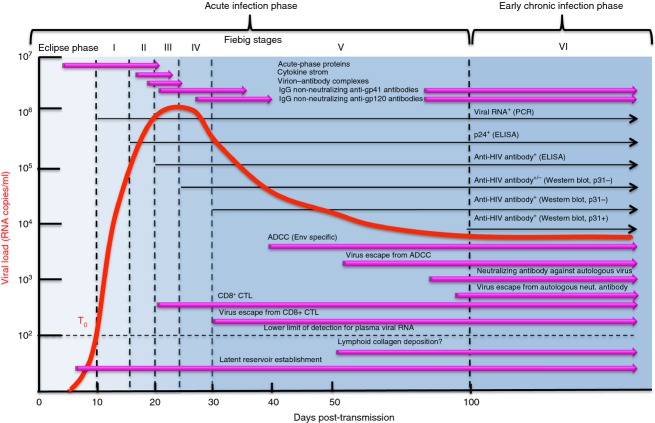
A depiction of the typical course of untreated HIV infection from acquisition to the development of AIDS. General clinical signs and symptoms are shown in addition to key pathological processes leading to failure of lymphocyte homeostasis followed by AIDS and death. This figure is modelled on figures in ref. 21.
The second phase is post-infection control of viraemia, which begins at T0 and continues until control is lost. This phase can last for many years but it is dynamic in both viral replication and host immune response. Before turning to details of where, when and how Fc-mediated effector function might block acquisition or contribute to post-infection control of viraemia, it is useful to consider the dynamics of viral replication, immune responses and pathological changes in an untreated HIV infection. As shown in Fig. 1, peripheral CD4+ T-cell counts are in the normal range during the eclipse phase. HIV establishes a local foothold at this time infecting CD4+ T cells and perhaps other CD4+ cells, such as dendritic cells and monocytes, setting the stage for exponential growth that continues for approximately 6 weeks to peak viraemia. Exponential viral growth is followed by a sharp exponential decline to the viral set-point, which can be stable for many years. Circulating CD4+ T cells are depleted progressively during the exponential phase with a nadir around peak viraemia, followed by a rebound during the exponential decline as the HIV comes under immunological control. Some individuals manifest an acute retroviral syndrome during the burst of early viraemia indicated by mononucleosis-like symptoms, which disappear as the virus is brought under control. As the CD4+ T cells rebound and viraemia exponentially decreases, a phase of clinical latency is entered that can last for many years, although there is continuous steady-state viral replication and accumulating damage to the immune system6–9 even in individuals who control their infections without therapy.10 The clinical latency phase is characterized by a slow decline in circulating CD4+ T cells. As CD4+ T cells decline during this phase, there is an expansion of activated CD8+ T cells, maintaining homeostatic numbers of total CD3+ T cells (reviewed in ref. 11). Eventually, control of the virus is lost leading to increasing viraemia, sharply increased losses of all CD3+ T cells, and AIDS-defining symptoms. Failure of T-cell homeostasis occurs around 18 months before the appearance of AIDS-defining conditions.12 This failure is signalled by an inflection point in the curve quantifying total circulating CD3+ T cells over time as indicated in Fig. 1.12 During this period, there is a catastrophic loss of secondary lymphoid architecture due to fibrosis.6,9,13–15 This is due to progressive collagen accumulation in secondary lymphoid tissues that begins early in infection and continues until lymphocyte homeostasis fails (Fig. 1 and refs 7,9,14,15). Although these pathological changes occur over many years, studies in NHPs show that immunological16–19 and anti-retroviral interventions5 very early in infection have lasting and profound effects on post-infection control of viraemia, even if the intervention is transient.5,16,17 This is also consistent with the relationship between peak viraemia early in HIV infection and viral set-point later in infection.20 Therefore, the discussion will focus on the Fiebig stages that define early infection,1 shown in Fig. 2.
Figure 2.
A map of the specific virological and immunological events to Fiebig stages defined in ref. 1 for acute HIV infections. This figure is modelled on figures in ref. 21.
Fiebig stages
Several recent reviews,21–23 paint an increasingly clear picture of the immunological and pathological events that occur sequentially in the Fiebig stages1 shown in Fig. 2. The discussion will further map the appearance of Fc-mediated effector function in this scheme with emphasis where, when and how it might contribute to blocking acquisition and post-infection control of viraemia. Fiebig stages1 were defined initially by diagnostic measurements such as plasma viraemia and seroconversion as shown in Fig. 2. Intensive characterization of acute infection cohorts enables further mapping of virological and immunological events in Fiebig stages (reviewed in refs 21–23). Figure 2 provides an update of the information originally summarized in the figures of reference21 with information on the emergence of Fc-mediated effector functions during acute infection that probably contribute to post-infection control of viraemia later on.24–27 Additionally, the eclipse phase and early Fiebig stages provide the context for discussion of how Fc-mediated effector function might block acquisition.
As shown in Fig. 2, the first 10 days following infection defines the eclipse phase where there are no systemic virological signs that specifically indicate HIV infection.1 As indicated above, the first 24 to 72 hr after exposure includes the window of opportunity when acquisition can be blocked.5 Its outer limit is establishment of the resting memory CD4+ T-cell reservoir, which no known intervention has depleted (reviewed in ref. 28).
After eclipse, the first specific laboratory sign of HIV infection is plasma viraemia (T0), which occurs approximately 10 days after exposure.1 This defines Fiebig Stage I, which also includes much of the exponential increase in viraemia. Symptoms of acute retroviral syndrome can also appear at this stage but they are not pathognomonic. Detection of the capsid protein, p24, in the circulation defines Fiebig Stage II that also includes the upper part of the exponential virus load curve. Appearance of the first anti-HIV antibodies, determined by ELISA using whole viral lysates, defines Fiebig Stage III around day 20 post-exposure or day 10 post-T0. This stage spans the first part of peak viraemia and symptoms of acute retroviral syndrome can be present. Fiebig Stage IV occurs during the second part of peak viraemia. It is defined by an indeterminate Western blot in which antibodies react with a minority of bands. Fiebig Stages III and IV occur when HIV is starting to be controlled, which continues in to Stages V and VI. Fiebig Stage V is defined by antibodies that react with all bands on a Western blot except for p31. It also includes the exponential decline of plasma viraemia. The temporal association between the appearance of antibodies and exponential decline in plasma viraemia indicates that immunological control is coming to the fore,1 although the protective capacity of these antibodies has been questioned.29 Fiebig Stage VI is defined by antibodies that react will all bands on a Western blot and when plasma viraemia reaches early set-point. The first four stages are approximately 5 days each in duration whereas Stage V lasts for 69 days. Stage VI duration is indeterminate and can last for many years until immunological control fails (Fig. 1). The temporal appearance of functional responses in relation to viral dynamics provides important clues about the mechanisms of immunological control. In this regard, it is also possible to discriminate between recent and chronic infections in Fiebig Stage VI using a sensitive/less-sensitive algorithm that employs a standard HIV ELISA (sensitive) and a ‘detuned HIV ELISA’ (less sensitive) that detects increasing antibody titres that emerge early after infection.30 Hence, the detuned ELISA can discriminate individuals in the early part of Fiebig Stage VI who were recently infected versus those who are chronically infected.
More recent studies show that increased levels of acute-phase proteins, such as serum amyloid precursor A, are elevated as early as the eclipse phase but wane around day 20 post-T0.31 A cytokine storm follows beginning 6 days after T0 in Fiebig Stage II, waning around day 20 post-T0.32 Immune complexes of HIV with either IgM or IgG appear at day 8 post-T0 and become undetectable around day 20 post-T0. Free IgG non-neutralizing antibodies to gp41 appear 13 days after T0, early in Fiebig Stage IV.29 Free IgG non-neutralizing antibodies appear 28 days after T0, midway in Fiebig Stage IV.29 Autologous neutralizing antibodies appear approximately at day 82 post-T0, late in Fiebig Stage V, followed by neutralization insensitive viral variants around 10 days later, apparently selected by neutralization pressure (reviewed in ref. 21). These antibodies are narrowly specific for autologous virus with neutralization breadth increasing slowly over time thereafter.33 Hence, there is a 55-day window between the appearance of the first free IgG antibodies that bind to gp41 or gp120 and the emergence of narrowly specific neutralizing antibodies.21 By contrast, the first CD8+ cytotoxic T-lymphocyte (CTL) responses appear at the beginning of Fiebig Stage III, around day 20, followed by the emergence of CTL escape viruses 10 days later at the beginning of Fiebig Stage V, suggesting that these responses exert immunological pressure on the virus (reviewed in ref. 21). Because there is a 60-day lag between the CD8+ CTL response and neutralizing antibody response, it has been widely accepted that post-infection control of viraemia is largely due to CTLs. This conclusion is also supported by CD8 depletion studies in NHPs.34,35 By contrast, in acutely HIV-infected individuals, there is evidence that antibody-mediated cellular cytotoxicity (ADCC) responses appear around day 36 post-T0, at the beginning of Fiebig Stage V, and that these responses correlate inversely with viral load.24 This is approximately 23 days after the appearance of free non-neutralizing anti-gp41 antibodies and 8 days after the appearance of free non-neutralizing anti-gp120 antibodies. In a more recent study, ADCC responses can induce epitope-specific escape mutations as early as 50 days after T0.26 Taken together, these studies suggest that the first functional antibody responses to Env appear almost concomitantly with binding antibodies, which is approximately 50 days before the emergence of the first detectable neutralizing antibodies against autologous viruses.
HIV acquisition phase
It goes without saying that antibodies must be present at the time of acquisition to block it and this can only be accomplished by active or passive immunization. In recent years, a good picture of the early events during acquisition after vaginal exposure has emerged (reviewed in refs 21,22,36,37). Figure 3 summarizes the virological events that occur during the eclipse phase where the ‘window of opportunity’ is key for blocking acquisition. Passive immunization studies in NHPs using neutralizing antibodies suggest that the window of opportunity is 24 hr at most.38,39 Transmission across the mucosal epithelium is thought to occur within hours of exposure and results in infectious virus reaching susceptible CD4+ target cells. Transmission across the mucosal barrier can be passive through epithelial breaks but an active transport mechanism is also known.40 The nature of the first infected type of CD4+ cell has been debated over the years but recent acute transmission studies strongly suggest that it is a CD4+ CCR5+ memory T cell.41,42 Strikingly, most HIV infections are due to a single founder virus,41,42 which is also true for model AIDS viruses in NHPs.41 It takes approximately 24 hr for an infected CD4+ cell to produce infectious virus,43 so it is likely that the earliest time that HIV can start to spread to other CD4+ CCR5+ T cells is within the first 24–28 hr, a small number of local infected founder cells 2–3 days after exposure44,45 (Fig. 3). Local expansion of the infected founder cells occurs around days 4 to 5 post-exposure,44,45 likely aided by an innate response of the mucosal epithelium that attracts additional target cells to the site (ref. 46 and discussed in ref. 36). Virus or virus-infected cells from the local expansion spread via afferent lymphatics to the draining lymph node, which is rich in additional CD4+ CCR5+ target cells. From there, virus and infected cells spread systemically via the thoracic duct leading to distal and propagating infections in the gut and spleen by haematogenous flow and finally back to lymph nodes. Once the infection spreads from the local focus, it is very likely that the window of opportunity is closed because of the establishment of viral reservoirs and protective niches in distal tissues. The systemic spread of infection ultimately leads to plasma viral loads that cross the 100 copy limit of sensitivity (i.e. T0) around day 10 and exponential expansion of infection during Fiebig Stage 1 (Fig. 2). At this point, the infection is established systemically and comes under immunological control in Fiebig Stage IV. It remains under control until accumulated damage to lymphoid architecture leads to failure of lymphocyte homeostasis and AIDS.
Figure 3.
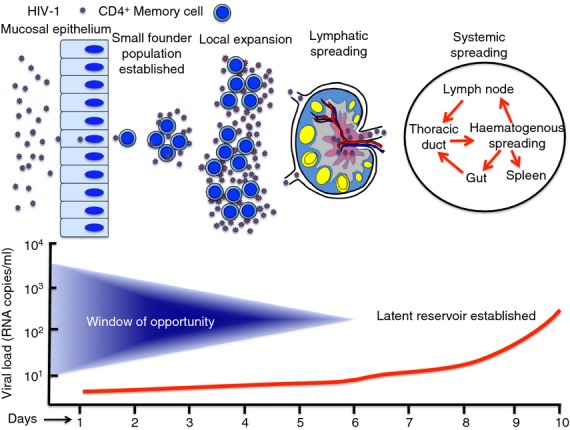
The early virological events during the eclipse phase of HIV infection. The window of opportunity is shown where immunological or pharmacological interventions block acquisition. The window is widest during the first 24 hr after exposure and decreases thereafter until it closes with establishment of the latent CD4+ T-cell viral reservoir.
Now that the key immunological and virological milestones during HIV acquisition and post-infection control have been laid out, the evidence implicating Fc-mediated effector function in protection in each of these phases will be considered. Although acquisition must occur first for there to be post-infection control, the discussion will begin with post-infection control because it provides the earliest and the most comprehensive indication that Fc-mediated effector function contributes to protective immunity to HIV. Details of Fc-receptor expression on various effector populations and binding to distinct IgG subclasses will not be discussed except in the context of specific examples because several excellent reviews deal with these subjects.47–49 Instead, the primary focus will be on the evidence that Fc-mediated effector function contributes to blocking acquisition or post-infection control of viraemia.
Role of Fc-mediated effector function in post-infection control of viraemia
The first point at which Fc-mediated effector function might contribute to post-infection control is around day 8 post-T0 when immune complexes of HIV with IgM and IgG appear in the circulation.29 The coincident appearance of IgM and IgG antibodies in immune complexes so early after infection is surprising. Either immunoglobulin class switching is occurring rapidly or the immune complexes are between virions and naturally occurring ‘innate’ antibodies specific for HIV.50 Regardless of how the antibodies arise, there is evidence that naturally occurring IgM can neutralize HIV, although this does not require Fc-mediated effector function.50 There is also evidence that both neutralizing and non-neutralizing IgG can inhibit infection of macrophages (Mph) and immature monocyte-derived dendritic cells by an Fc-receptor dependent mechanism.51–53 Inhibition of macrophage infection was mediated by FcγR1,51,52 whereas inhibition of immature monocyte-derived dendritic cell infection was mediated by FcγRIIa.53 It is not clear the degree to which this inhibition involves phagocytosis (reviewed in refs 54,55), but phagocytosis has been implicated indirectly in the passive protection of rhesus macaques against a vaginal challenge with SHIV162p3.17 It is possible that it is responsible for the disappearance of virion–antibody complexes from the circulation around day 20 post-T0. If so, it will occur at systemic sites because HIV has spread to secondary lymphoid tissues by this time (Fig. 3). Although the first stage in infection when HIV-specific phagocytosis occurs is unclear, the frequency of FcγR1+ myeloid dendritic cells and the intensity of FcγR1 expression on monocytes is increased in Fiebig Stages IV and V compared with later stages.27 consistent with a role for phagocytosis in the disappearance of virion–IgG complexes in Fiebig Stage IV.27 This hypothesis is supported by the finding that phagocytosis by both monocytes and dendritic cells is increased in acute infection and impaired in chronic infection.27 The impairment in chronic infection was tightly associated with down-regulation of FcγR2a and FcγR3a on monocytes and dendritic cells.27 The expansion of circulating natural killer cells expressing FcγR3 in Fiebig Stages II and III,56 immediately before or at the beginning of seroconversion, suggests that ADCC responses might occur concomitant with emergence of free IgG antibodies to gp41 and gp120. The involvement of Fc-mediated effector function before Fiebig Stage V where ADCC responses are first detectable24,26 is hypothetical and based on indirect indications. This hypothesis can be tested readily with infection models in NHPs where effector cells and antibodies can be quantified at defined times post-infection.
Despite the uncertainty about the role of Fc-mediated effector function in acute infection, a large body of data has accumulated over the years demonstrating correlations between clinical outcome and ADCC titres in HIV-infected individuals. These studies are summarized in Table 1. The earliest report of a correlation between ADCC titres and clinical stage appeared in 198757 and studies with similar conclusions continue to appear up to the time of writing.58 Of the 19 studies listed in Table 1, three failed to detect correlations between ADCC and clinical outcomes whereas the other 16 reported correlations between ADCC and positive clinical outcomes. Further, the negative studies were in the early years of the epidemic when methodology was more challenging. The 15 positive studies, spanning 26 years and involving different cohorts and methods, provide compelling support for the involvement of Fc-mediated effector function, particularly ADCC, and post-infection control of HIV. This conclusion is supported also by similar studies in NHPs, although they are fewer in number. The first NHP study, which appeared in 2002, reported an inverse correlation between ADCC titres and progression to simian AIDS in the simian immunodeficiency virus model of infection.59 A second study appeared in 2011 and reported similar conclusions in the same model.60 A third study reported an inverse correlation between another Fc-mediated effector function, antibody-dependent cellular viral inhibition (ADCVI),24,61 which has elements similar to ADCC, and viral control.62 Collectively, studies in both HIV-infected individuals and simian immunodeficiency virus-infected rhesus macaques strongly support a role for Fc-mediated effector function, and ADCC in particular, in post-infection control of viraemia. If these correlations are causal, they raise the questions of where, when and how do these responses most influence post-infection control?
Table 1.
Studies implicating Fc-mediated effector function and post-infection control of viraemia
| Year | Cohort | Results | References | |
|---|---|---|---|---|
| 1 | 1987 | Healthy seropositives, AIDS with Kaposi's sarcoma and/or opportunistic infections | Higher ADCC titres in healthy seropositives | 57 |
| 2 | 1987 | HIV-infected individuals at all stages of infection | Higher ADCC titres earlier stages of infection | 99 |
| 3 | 1988 | HIV-infected men | Higher ADCC titres in controllers | 100 |
| 4 | 1989 | HIV-infected men, MACS cohort | No correlation between ADCC titres and slow progression. | 101 |
| 5 | 1990 | HIV-infected children with and without AIDS | Higher ADCC titres correlated inversely with progression | 102 |
| 6 | 1990 | HIV-infected men, MACS cohort | Higher ADCC titres earlier stages of infection. | 103 |
| 7 | 1990 | HIV-infected haemophiliacs | No correlation between ADCC titres and progression | 104 |
| 8 | 1993 | HIV-infected children with and without AIDS | Higher ADCC titres correlated inversely with progression | 105 |
| 9 | 1994 | Perinatal transmission cohort | No correlation between ADCC titres and transmission | 106 |
| 10 | 1994 | HIV-infected individuals with high, intermediate, and low CD4+ T-cell counts | Inverse relationship between ADCC titres and CD4+ T-cell counts | 107 |
| 11 | 1999 | Perinatal transmission cohort | Higher ADCC titres were associated with lower progression in infected children | 108 |
| 12 | 2001 | HIV-infected individuals at different stages of disease | Inverse correlation between ADCC titres and progression | 109 |
| 13 | 2001 | HIV-infected individuals at different stages of disease | Inverse correlation between ADCC titres and viral load | 110 |
| 14 | 2001 | Acute infection cohort | Inverse correlation between ADCC titres and with one load | 24 |
| 15 | 2004 | HIV-infected women DATRI-009 Protocol | Higher ADCC titres in cervicovaginal lavage correlated with lower local viral loads | 111 |
| 16 | 2011 | HIV-infected adults | Apparent ADCC-mediated escape at specific epitopes | 26 |
| 17 | 2011 | HIV-infected adults | Inverse correlation with gp140-specific ADCC and progression | 112 |
| 18 | 2013 | HIV-infected adults | Correlation between ADCC epitope breadth and slower progression | 58 |
| 19 | 2013 | HIV-infected controllers | Higher ADCC, lower viral loads | 113 |
If Fc-mediated protection exerts immune pressure, its largest influence is likely to be early in secondary lymphoid tissues at the sites of HIV replication during Fiebig Stage V and early in Fiebig Stage VI. As pointed out above, early transient anti-retroviral5 and immunological interventions16–19 have lasting effects on post-infection control of viraemia, persisting long after the interventions5,16,17,19 are no longer present. At this point, it is important to recognize that Fc-mediated effector function in vivo requires two partners, an appropriate antibody and a functional effector cell. The studies outlined in Table 1 evaluated antibodies for ADCC activity using effector cells from uninfected individuals. Although positive correlations between ADCC titres and favourable clinical pictures were found, these studies do not speak to Fc-mediated effector function in the HIV-infected subjects because they did not examine autologous effector cells. As stated above, there is an early increase in effector cells early in infection accompanied by increased phagocytic activity.27 However, phagocytosis27 and natural killer-mediated ADCC63 are profoundly depressed during progressive HIV infection. Hence, for these effector functions to impact the post-infection control of HIV, it is likely to be early infection where both partners are present. In summary, these studies strongly implicate Fc-mediated effector function in post-infection control of HIV. Further, they indicate that their efficacy is likely to be early infection, in Fiebig Stages V and VI, because both functional effector cells as well as appropriate antibodies must be present and autologous effector cell function wanes during chronic infection. Although the evidence is indirect, the effector mechanisms probably include ADCC, ADCVI and phagocytosis.
Role of Fc-mediated effector function in blocking HIV acquisition
Susceptibility of the acquisition phase to abrogation is established unequivocally by the CAPRISA 009 microbicide trial in at-risk women64 and by the pre-exposure prophylaxis (PREP) trial in men who have sex with men.65 Both studies employed reverse transcriptase inhibitors, which prevent viral replication at a post-entry step. Hence, the protection against acquisition by these drugs must occur very early in the eclipse phase (Fig. 3), most likely either preventing a productive infection of the initial CD4+ CCR5+ T cell or possibly abrogating establishment of a small local founder population. These studies suggest that the ‘window of opportunity’ for blocking acquisition is around 3 days post-exposure (Fig. 3), consistent with similar studies in NHPs.5 The window of opportunity is also framed by passive immunization studies in NHPs where transfer of protective neutralizing antibodies 24 hr after infection fails to prevent infection as mentioned above.38,39
A salient feature of HIV transmission is the low probability of infection per exposure. Approximately 70% of HIV acquisitions worldwide are via heterosexual sex where the frequency of transmission per coital event ranges from 1/200 to 1/3000, which is probably the lower boundary (reviewed in refs 23,66). It is also well established that there is a direct relationship between high viral loads and transmission probability.67 Despite this relationship, as indicated above, 75% of infections are by a single variant.68 Hence, the challenge for blocking of acquisition immunologically becomes one of inhibiting productive infection of a small number of cells by a small number of virions at local mucosal sites within the first 3 days following exposure.
Passive immunization studies in NHPs have established unequivocally that neutralization is a key mechanism of protection against infection with model AIDS viruses such as SHIV162p3.16,69 By contrast, the role of Fc-mediated effector function in blocking acquisition is indirect and more controversial.70,71 Two seminal passive immunization studies in NHPs employing the neutralizing monoclonal antibody (mAb), b12, point toward a role of Fc-mediated effector function in protection against both high-dose70 and low-dose71 vaginal challenges with SHIV162p3. Groups received either wild-type b12 capable of both neutralization and Fc-mediated effector function or b12-LALA, in which Fc-mediated effector function, but not neutralization, was abrogated by L to A mutations at residues 234 and 235 in the CH2 domain of IgG1 (b12-LALA). In both models, protection against SHIV162p3 decreased by approximately 50% for b12-LALA. These are the only passive immunization studies to date unambiguously indicating a role of Fc-mediated effector function in blocking acquisition. The contributing effector function is not known because b12-LALA is incapable of ADCC, ADCVI and phagocytosis. Further, b12 variants with improved Fc receptor binding and biological function did not increase protection in this model, although vaginal mAb levels might not have been optimal to reveal enhanced protection at the times of challenge.72 Hence the precise role of Fc-mediated effector function in blocking acquisition in this model is unknown. There is no evidence that passive immunization with non-neutralizing mAbs can block acquisition by Fc-mediated effector function.
By contrast, a recent study suggested that passive immunization using non-neutralizing antibodies with potent Fc-mediated effector function can increase post-infection control of viraemia.17 That study reported statistically significant post-infection control against a vaginal challenge with SHIV162p3 using a mixture of two non-neutralizing anti-gp41 mAbs specific for its principal immunodominant domain.17 These mAbs were vetted by an algorithm assigning weights based on their abilities to neutralize, mediate ADCC, block infection of monocyte-derived macrophages, bind Fc receptors on cell surfaces and capture free virions. They were compared with a mixture of mAbs that neutralize as well as mediating one or more of these effector functions. The neutralizing mAb mixture prevented acquisition whereas the non-neutralizing mAb mixture did not. On the other hand, this mixture afforded post-infection control of viraemia, suggesting that Fc-mediated effector function contributes to this type of protection. Similar results were reported for another antibody specific for the immunodominant region of gp41 but no functional data other than virus capture was provided in that study.16 Post-infection control is also a common finding for neutralizing mAbs used at doses insufficient to block acquisition (summarized in ref. 19).
Given that the in vivo half-lives of mAbs are short, typically ranging from 3 days to 2 weeks, they must exert their activities early after passive immunization as post-infection control by Fiebig Stage VI.19 The short-term effect probably is to protect components of the immune system early in infection such that they can mature and mediate post-infection control after mAb decay. This possibility is supported by studies in mice showing that NK-mediated lysis of target cells expressing a foreign antigen early in the immune response results in strong CD4+ T cell, CD8+ T cell and antibody responses downstream to release of the foreign antigen.73 It is reasonable to expect that a similar phenomenon would follow ADCC-induced lysis of target cells early in infection. This form of Fc-mediated protection would be most important in limiting the expansion of the local founder population or perhaps decreasing systemic viral spread (Fig. 3). Correlations have been reported repeatedly between ADCC or ADCVI and post-infection control in vaccinated NHPs,74–78 supporting this possibility. Despite the repeated correlations between Fc-mediated effector function and post-infection control in both active and passive immunization studies in NHPs, no study shows that passive immunization with a non-neutralizing mAb can block acquisition. Until a definitive passive immunization study employing a non-neutralizing antibody with Fc-mediated effector function, including an attenuated LALA variant as a negative control, either rules this possibility in or out, the field is left with correlations.
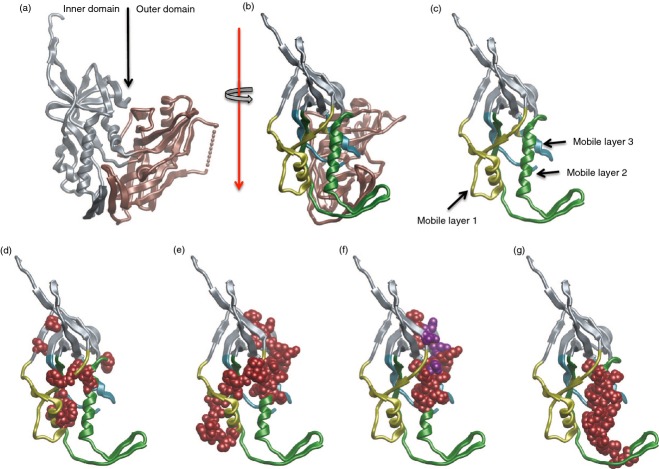
Two recent NHP vaccine studies report an inverse correlation between reduced acquisition and ADCC titres.79,80 In addition to the NHP studies, increasingly solid support indicating a role of Fc-mediated protection in preventing acquisition is developing from studies of infected and vaccinated humans. A recent study in HIV-infected mothers with high viral loads showed an inverse correlation between ADCC titres in breast milk and probability of transmission to their infants.81 No such correlation was found for neutralization.81 The earliest vaccine study reported an inverse correlation between ADCVI titres and risk of infection in a subgroup of vaccines in the VAX004 Phase III efficacy trial, although no overall protection was observed.82 This was followed by the RV144 trial in which modest overall efficacy of 31% was observed83 although subsequent analysis showed that efficacy was as high as 60·5% in the first year of the study,84 which is consistent with the known short half-life of anti-Env antibodies (discussed in ref. 85). ADCC emerged as a correlate of reduced infection risk for vaccinees in the lower two-thirds of titre range for IgA antibodies specific for a C1 peptide,86 raising the possibility that the IgA antibodies competitively inhibited ADCC by IgG in the upper third of the IgA responses. The ability of IgA mAbs isolated from RV144 vaccinees,87 a specific highly conserved ADCC epitope recognized by the A32 mAb,88 to block ADCC mediated by matched IgG1 mAbs specific for the same epitope was confirmed recently.89 This suggests that vaccine-elicited antibodies to this epitope region contribute to decreased infection risk in RV144. This epitope region is not a neutralization target,26,90 although it is a very potent ADCC target.88,90 As shown in Fig. 4(d), mutagenesis studies have mapped the A32 epitope region to the C1 segment of gp120 involving mobile layers one and two,91,92 which we have confirmed and extended by mutagenesis and X-ray crystallography (in preparation). The importance of this region in protective immunity mediated by ADCC is supported by studies in natural infection and the RV144 trial.
Figure 4.
The A32 epitope region in gp120 C1 that is implicated as a dominant antibody-mediated cellular cytotoxicity (ADCC) target of non-neutralizing antibodies implicated in protection against HIV. (a) The inner (grey) and outer domains (bronze) of gp120 from PDB:3JW0.91 (b) gp120 rotated to show inner domain mobile layers 1 (yellow), 2 (green) and 3 (cyan).91 (c) Mobile layers 1, 2 and 3 with the outer domain of gp120 removed for clarity. (d) Point mutations (red) in mobile layers 1 and 2 that alter binding of monoclonal antibody A32 to gp120.92 (e) Peptides (red) in mobile layers 1 and 2 that are ADCC targets in a Thai HIV cohort.93 (f) Peptides (red) in mobile layers 1 and 2 that are ADCC targets in an Australian HIV cohort.26 The purple residues in mobile layer 2 are sites of ADCC escape.26 (g) Peptide (red) recognized by RV144 IgA antibodies that inhibit ADCC mediated by RV144 IgG antibodies to the A32 epitope region.86,87,89
Importance of the A32 epitope region in natural infection is indicated by the ability of A32 Fab fragments to inhibit ADCC in most infected individuals.88 It is also indicated by recognition of C1 peptides by polyclonal antibodies from infected individuals that mediate ADCC88,93 (Fig. 4(e,f) and isolation of A32-like mAbs that mediate potent ADCC from infected individuals.90 Importantly, the A32 epitope region is also a target of ADCC-mediated viral escape early in infection26 (Fig. 4f). With respect to acquisition, the A32 epitope region has been implicated as a target of antibodies that mediate ADCC, which correlate with reduced infection risk in RV144.86,89 The structural details of the A32 epitope region will be described in another report (in preparation) but the key point for this discussion is its identification by four independent groups as a potent ADCC target in infected individuals26,88,90,93 and that it appears to be a target of protective antibodies in the RV144 trial.86,87,89 Collectively, these findings strongly point toward the importance of ADCC responses to the A32 epitope region in both blocking acquisition and in post-infection control of viraemia, raising the questions of where, when and how this happens.
If these responses are important in blocking acquisition, they must occur before the establishment of the latent viral reservoir, which is likely to be in the first 3 days post-exposure when the small, infected founder population is established and expanded locally (Fig. 3). ADCC cannot come in to play until a CD4+ CCR5+ T cell has either bound an entering virion90 or become infected and is budding virions,88 which is at a sub-mucosal site and in the first or second day following exposure. It is paradoxical that the A32 epitope region is a potent ADCC target. This region is typically buried in the native Env trimer,91 becoming exposed as an ADCC target only during cell-to-cell fusion94,95 or viral entry.90 However, there is sound evidence that this epitope can be exposed on Env expressed on infected CD4+ target cells, either by interaction with cell surface CD4 or constitutively for certain viral isolates, including the A/E Env targeted in the RV144 trial (ref 88 and A.L. DeVico, personal communication). These observations inform the questions of when and where but the how is more difficult. This is because a wide variety of cell types mediate ADCC, including natural killer cells, monocytes/macrophages, myeloid dendritic cells, γδ T cells and neutrophils (reviewed in refs 96,97) but little is known about their presence and activity at local sites during mucosal HIV acquisition. Additionally, effector cell phenotype is likely to vary with the mucosal tissue and it is also likely to be affected by ongoing, local innate immune responses as well as by the innate epithelial cell response when HIV crosses mucosal epithelia.98
Conclusion
The large body of data discussed above strongly suggests that Fc-mediated effector function plays a role in blocking HIV acquisition and in post-infection control of viraemia. This picture has emerged over the 27 years since the first report that healthy seropositive individuals had greater ADCC titres than individuals with AIDS.57 Although not all studies support these two conclusions (Table 1), the body of supporting literature is impressive, particularly for post-infection control of viraemia. However, with two exceptions,70,71 the studies implicating a role for Fc-mediated effector function in blocking acquisition are correlative. The same is true for post-infection control of viraemia. Causality will be difficult to evaluate directly in humans but it can be tested by passive immunization studies in NHPs. To date, two independent studies using non-neutralizing mAbs specific for the immunodominant domain of gp41 have failed to demonstrate a role for Fc-mediated effector function in blocking vaginal challenges with high doses of SHIV162p3.16,17 In both of those studies, comparable doses of neutralizing mAbs blocked acquisition. Further, improved Fc-mediated effector function of mAb b12 did not increase its ability to protect against low-dose challenges with SHIV162p3.72 Hence, causality was not established for blocking acquisition in these studies. However, the two earlier studies suggesting that Fc-mediated effector function contributes to blocking of acquisition by the neutralizing mAb b12,70,71 leaves the question open.
By contrast, post-infection control of viraemia was observed in the two passive immunization studies using non-neutralizing anti-gp41 mAbs.16,17 Both anti-gp41 mAbs used in one study17 were active in ADCC and Fc-dependent inhibition of viral replication in macrophages, though they were non-neutralizing in conventional neutralization assays. Taken together, these two studies strongly support a role of Fc-mediated effector function in the post-infection control of viraemia. They also suggest that the protective effect is at a very early step in infection as postulated above. Future studies of a role for Fc-mediated effector function in blocking acquisition and post-infection control would benefit greatly from a better understanding of the effector cells extant at the local site of virus entry, the innate epithelial cell response to virus, and the impact of non-neutralizing mAbs with potent Fc-mediated effector function on early viral dynamics and escape. Characterization of these variables using the approaches reviewed in references 6,36,37 for post-infection control of viraemia mediated by non-neutralizing mAbs, should inform the design of more definitive passive immunization studies to resolve the controversy of whether Fc-mediated effector function plays a role in the blocking of acquisition.
Acknowledgments
The author thanks Drs Yongjun Guan, Mohammed Sajadi, Roberta Kamin-Lewis, Marzena Pazgier, Robert C. Gallo and Tony DeVico for their support and fruitful discussions leading to the ideas discussed in this review. They are not responsible for errors on the part of the author. The exemplary efforts of the laboratory technical staff and postdoctoral fellows are greatly appreciated. This manuscript is supported by Grant #OPP1033109 from The Bill and Melinda Gates Foundation and by R01AI087181 from National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Glossary
- ADCC
antibody-mediated cellular cytotoxicity
- NHPs
non-human primates
- ADCVI
antibody-dependent cellular viral inhibition
- b12-LALA
mAb b12 with L to A mutations at residues 234–235
Disclosures
The author owns stock in Profectus Biosciences, Baltimore, MD.
References
- 1.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 4.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–73. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai CC, Emau P, Follis KE, Beck TW, Benveniste RE, Lifson JD, Morton WR, Bischofberger N. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–73. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–14. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Investig. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estes J, Baker JV, Brenchley JM, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456–64. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, Haase AT. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Investig. 2002;110:1133–9. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–35. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roederer M. T-cell dynamics of immunodeficiency. Nat Med. 1995;1:621–2. doi: 10.1038/nm0795-621. [DOI] [PubMed] [Google Scholar]
- 12.Margolick JB, Munoz A, Donnenberg AD, Park LP, Galai N, Giorgi JV, O'Gorman MR, Ferbas J. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. The Multicenter AIDS Cohort Study. Nat Med. 1995;1:674–80. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 13.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, Schacker TW, Hawse AT. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8:e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schacker TW, Brenchley JM, Beilman GJ, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–60. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton DR, Hessell AJ, Keele BF, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci USA. 2011;108:11181–6. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moog C, Dereuddre-Bosquet N, Teillaud JL, et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2013;7:46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 18.Hansen SG, Ford JC, Lewis MS, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–7. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Ribeiro RM, Mascola JR, Lewis MG, Stiegler G, Katinger H, Perelson AS, Davenport MP. Effects of antibody on viral kinetics in simian/human immunodeficiency virus infection: implications for vaccination. J Virol. 2004;78:5520–2. doi: 10.1128/JVI.78.10.5520-5522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 21.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011;364:1943–54. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw GM, Hunter E. HIV transmission. Cold Spring Harb Perspec Med. 2012;2:1–23. doi: 10.1101/cshperspect.a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–61. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung AW, Navis M, Isitman G, et al. Activation of NK cells by ADCC responses during early HIV infection. Viral Immunol. 2011;24:171–5. doi: 10.1089/vim.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci USA. 2011;108:7505–10. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dugast AS, Tonelli A, Berger CT, et al. Decreased Fc receptor expression on innate immune cells is associated with impaired antibody-mediated cellular phagocytic activity in chronically HIV-1 infected individuals. Virology. 2011;415:160–7. doi: 10.1016/j.virol.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siliciano JD, Siliciano RF. HIV-1 eradication strategies: design and assessment. Curr Opin HIV and AIDS. 2013;8:318–25. doi: 10.1097/COH.0b013e328361eaca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parekh BS, Kennedy MS, Dobbs T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002;18:295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- 31.Kramer HB, Lavender KJ, Qin L, et al. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 2010;6:e1000893. doi: 10.1371/journal.ppat.1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz JE, Johnson RP, McClure HM, et al. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239δ3-vaccinated rhesus macaques. J Virol. 2005;79:8131–41. doi: 10.1128/JVI.79.13.8131-8141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitz JE, Kuroda MJ, Santra S, et al. Effect of humoral immune responses on controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 2003;77:2165–73. doi: 10.1128/JVI.77.3.2165-2173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–39. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 37.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–23. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura Y, Igarashi T, Haigwood NL, et al. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc Natl Acad Sci USA. 2003;100:15131–6. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrantelli F, Buckley KA, Rasmussen RA, et al. Time dependence of protective post-exposure prophylaxis with human monoclonal antibodies against pathogenic SHIV challenge in newborn macaques. Virology. 2007;358:69–78. doi: 10.1016/j.virol.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 40.Hocini H, Belec L, Iscaki S, Garin B, Pillot J, Becquart P, Bomsel M. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res Hum Retroviruses. 1997;13:1179–85. doi: 10.1089/aid.1997.13.1179. [DOI] [PubMed] [Google Scholar]
- 41.Keele BF, Li H, Learn GH, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–34. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–89. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SY, Byrn R, Groopman J, Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989;63:3708–13. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z-Q, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–7. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 45.Miller CJ, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, Lifson JD, Haase AT. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci USA. 2004;101:5640–5. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 48.Ackerman ME, Dugast AS, Alter G. Emerging concepts on the role of innate immunity in the prevention and control of HIV infection. Annu Rev Med. 2012;63:113–30. doi: 10.1146/annurev-med-050310-085221. [DOI] [PubMed] [Google Scholar]
- 49.Forthal D, Hope TJ, Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Curr Opin HIV and AIDS. 2013;8:392–400. doi: 10.1097/COH.0b013e328363d486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lobo PI, Schlegel KH, Yuan W, Townsend GC, White JA. Inhibition of HIV-1 infectivity through an innate mechanism involving naturally occurring IgM anti-leukocyte autoantibodies. J Immunol. 2008;180:1769–79. doi: 10.4049/jimmunol.180.3.1769. [DOI] [PubMed] [Google Scholar]
- 51.Holl V, Hemmerter S, Burrer R, Schmidt S, Bohbot A, Aubertin AM, Moog C. Involvement of FcγRI (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J Immunol. 2004;173:6274–83. doi: 10.4049/jimmunol.173.10.6274. [DOI] [PubMed] [Google Scholar]
- 52.Holl V, Peressin M, Decoville T, Schmidt S, Zolla-Pazner S, Aubertin AM, Moog C. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J Virol. 2006;80:6177–81. doi: 10.1128/JVI.02625-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holl V, Peressin M, Schmidt S, Decoville T, Zolla-Pazner S, Aubertin AM, Moog C. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood. 2006;107:4466–74. doi: 10.1182/blood-2005-08-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holl V, Peressin M, Moog C. Antibody-mediated fcγ receptor-based mechanisms of HIV inhibition: recent findings and new vaccination strategies. Viruses. 2009;1:1265–94. doi: 10.3390/v1031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV and AIDS. 2009;4:388–93. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–9. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 57.Rook AH, Lane HC, Folks T, McCoy S, Alter H, Fauci AS. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol. 1987;138:1064–7. [PubMed] [Google Scholar]
- 58.Wren LH, Chung AW, Isitman G, et al. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology. 2013;138:116–23. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banks ND, Kinsey N, Clements J, Hildreth JE. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses. 2002;18:1197–205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 60.Sun Y, Asmal M, Lane S, Permar SR, Schmidt SD, Mascola JR, Letvin NL. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J Virol. 2011;85:6906–12. doi: 10.1128/JVI.00326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forthal DN, Landucci G, Phan TB, Becerra J. Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1. J Virol. 2005;79:2042–9. doi: 10.1128/JVI.79.4.2042-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asmal M, Sun Y, Lane S, Yeh W, Schmidt SD, Mascola JR, Letvin NL. Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses. J Virol. 2011;85:5465–75. doi: 10.1128/JVI.00313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Q, Sun Y, Rihn S, et al. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol. 2009;83:8705–12. doi: 10.1128/JVI.02666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–57. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 68.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moldt B, Rakasz EG, Schultz N, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012;109:18921–5. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 71.Hessell AJ, Poignard P, Hunter M, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–4. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moldt B, Shibata-Koyama M, Rakasz EG, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86:6189–96. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krebs P, Barnes MJ, Lampe K, Whitley K, Bahjat KS, Beutler B, Janssen E, Hoebe K. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood. 2009;113:6593–602. doi: 10.1182/blood-2009-01-201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demberg T, Brocca-Cofano E, Kuate S, et al. Impact of antibody quality and anamnestic response on viremia control post-challenge in a combined Tat/Env vaccine regimen in rhesus macaques. Virology. 2013;440:210–21. doi: 10.1016/j.virol.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao P, Zhao J, Patterson LJ, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–73. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Florese RH, Demberg T, Xiao P, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with tat/env compared with multigenic vaccines. J Immunol. 2009;182:3718–27. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–9. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 78.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao P, Patterson LJ, Kuate S, et al. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–57. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alpert MD, Harvey JD, Lauer WA, et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog. 2012;8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 2012;8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178:6596–603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 83.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 84.Robb ML, Rerks-Ngarm S, Nitayaphan S, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis. 2012;12:531–7. doi: 10.1016/S1473-3099(12)70088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewis GK. Challenges of antibody-mediated protection against HIV-1. Expert Rev Vaccines. 2010;9:683–7. doi: 10.1586/erv.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonsignori M, Pollara J, Moody MA, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86:11521–32. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferrari G, Pollara J, Kozink D, et al. A HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent ADCC activity and defines a common ADCC epitope in human HIV-1 serum. J Virol. 2011;85:7029–36. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomaras GD, Ferrari G, Shen X, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci USA. 2013;110:9019–24. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guan Y, Pazgier M, Sajadi MM, et al. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci USA. 2013;110:E69–78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pancera M, Majeed S, Ban YE, et al. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proc Natl Acad Sci USA. 2010;107:1166–71. doi: 10.1073/pnas.0911004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Finzi A, Xiang SH, Pacheco B, et al. Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol Cell. 2010;37:656–67. doi: 10.1016/j.molcel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ampol S, Pattanapanyasat K, Sutthent R, Permpikul P, Kantakamalakul W. Comprehensive investigation of common antibody-dependent cell-mediated cytotoxicity antibody epitopes of HIV-1 CRF01_AE gp120. AIDS Res Hum Retroviruses. 2012;28:1250–8. doi: 10.1089/AID.2011.0346. [DOI] [PubMed] [Google Scholar]
- 94.Finnegan CM, Berg W, Lewis GK, DeVico AL. Antigenic properties of the human immunodeficiency virus envelope during cell–cell fusion. J Virol. 2001;75:11096–105. doi: 10.1128/JVI.75.22.11096-11105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DeVico AL. CD4-induced epitopes in the HIV envelope glycoprotein, gp120. Curr HIV Res. 2007;5:561–71. doi: 10.2174/157016207782418560. [DOI] [PubMed] [Google Scholar]
- 96.Houot R, Kohrt HE, Marabelle A, Levy R. Targeting immune effector cells to promote antibody-induced cytotoxicity in cancer immunotherapy. Trends Immunol. 2011;32:510–16. doi: 10.1016/j.it.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, Levy R, Brody J. Combination strategies to enhance antitumor ADCC. Immunotherapy. 2012;4:511–27. doi: 10.2217/imt.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Q, Smith AJ, Schacker TW, Carlis JV, Duan L, Reilly CS, Haase AT. Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J Immunol. 2009;183:1975–82. doi: 10.4049/jimmunol.0803222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ljunggren K, Bottiger B, Biberfeld G, Karlson A, Fenyo EM, Jondal M. Antibody-dependent cellular cytotoxicity-inducing antibodies against human immunodeficiency virus. Presence at different clinical stages. J Immunol. 1987;139:2263–7. [PubMed] [Google Scholar]
- 100.Goudsmit J, Ljunggren K, Smit L, Jondal M, Fenyo EM. Biological significance of the antibody response to HIV antigens expressed on the cell surface. Arch Virol. 1988;103:189–206. doi: 10.1007/BF01311092. [DOI] [PubMed] [Google Scholar]
- 101.Ojo-Amaize E, Nishanian PG, Heitjan DF, et al. Serum and effector-cell antibody-dependent cellular cytotoxicity (ADCC) activity remains high during human immunodeficiency virus (HIV) disease progression. J Clin Immunol. 1989;9:454–61. doi: 10.1007/BF00918014. [DOI] [PubMed] [Google Scholar]
- 102.Ljunggren K, Moschese V, Broliden PA, et al. Antibodies mediating cellular cytotoxicity and neutralization correlate with a better clinical stage in children born to human immunodeficiency virus-infected mothers. J Infect Dis. 1990;161:198–202. doi: 10.1093/infdis/161.2.198. [DOI] [PubMed] [Google Scholar]
- 103.Sawyer LA, Katzenstein DA, Hendry RM, et al. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1990;6:341–56. doi: 10.1089/aid.1990.6.341. [DOI] [PubMed] [Google Scholar]
- 104.Dalgleish A, Sinclair A, Steel M, Beatson D, Ludlam C, Habeshaw J. Failure of ADCC to predict HIV-associated disease progression or outcome in a haemophiliac cohort. Clin Exp Immunol. 1990;81:5–10. doi: 10.1111/j.1365-2249.1990.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Broliden K, Sievers E, Tovo PA, Moschese V, Scarlatti G, Broliden PA, Fundaro C, Rossi P. Antibody-dependent cellular cytotoxicity and neutralizing activity in sera of HIV-1-infected mothers and their children. Clin Exp Immunol. 1993;93:56–64. doi: 10.1111/j.1365-2249.1993.tb06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jenkins M, Landers D, Williams-Herman D, et al. Association between anti-human immunodeficiency virus type 1 (HIV-1) antibody-dependent cellular cytotoxicity antibody titers at birth and vertical transmission of HIV-1. J Infect Dis. 1994;170:308–12. doi: 10.1093/infdis/170.2.308. [DOI] [PubMed] [Google Scholar]
- 107.Ahmad A, Morisset R, Thomas R, Menezes J. Evidence for a defect of antibody-dependent cellular cytotoxic (ADCC) effector function and anti-HIV gp120/41-specific ADCC-mediating antibody titres in HIV-infected individuals. J Acquir Immune Defic Syndr. 1994;7:428–37. [PubMed] [Google Scholar]
- 108.Tranchat C, Perre Van de P, Simonon-Sorel A, et al. Maternal humoral factors associated with perinatal human immunodeficiency virus type-1 transmission in a cohort from Kigali, Rwanda, 1988–1994. J Infect. 1999;39:213–20. doi: 10.1016/s0163-4453(99)90052-x. [DOI] [PubMed] [Google Scholar]
- 109.Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21:227–33. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 110.Forthal DN, Landucci G, Keenan B. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS Res Hum Retroviruses. 2001;17:553–61. doi: 10.1089/08892220151126661. [DOI] [PubMed] [Google Scholar]
- 111.Nag P, Kim J, Sapiega V, et al. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J Infect Dis. 2004;190:1970–8. doi: 10.1086/425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, Kent SJ, Stratov I. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr. 2011;58:127–31. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lambotte O, Pollara J, Boufassa F, et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS ONE. 2013;8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]