Abstract
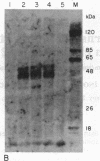
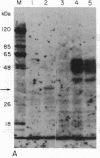
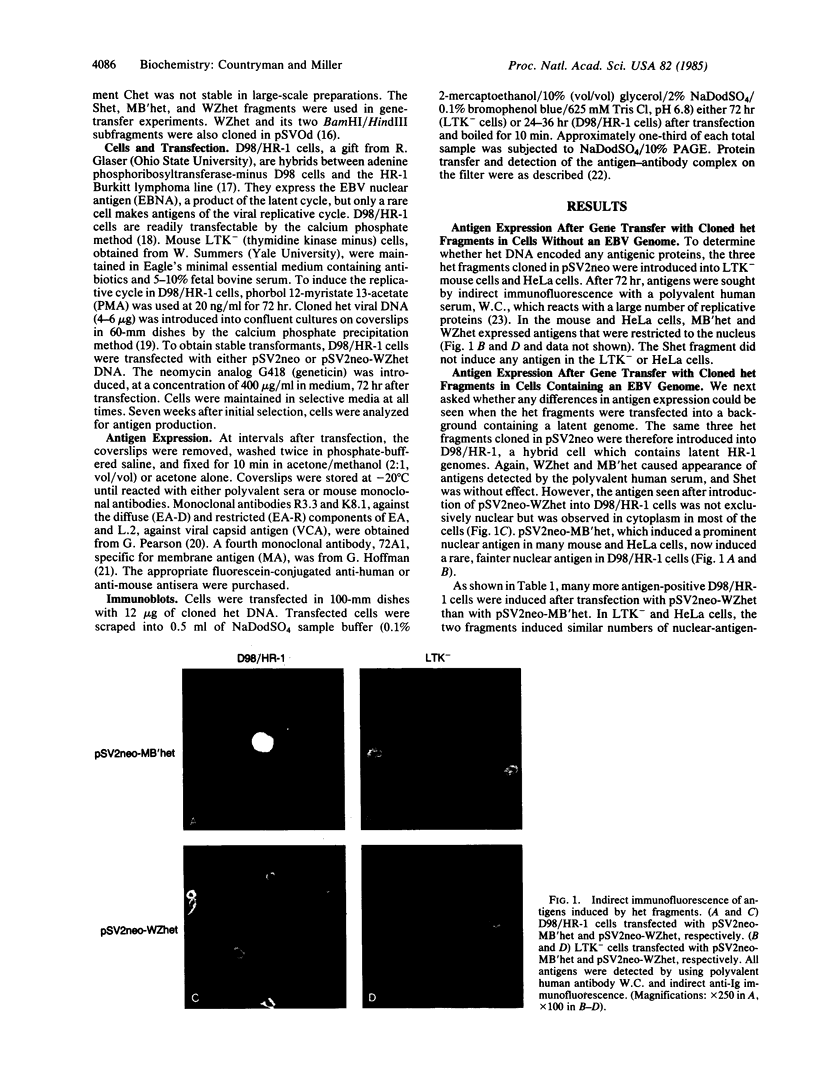
We previously found that a form of Epstein-Barr virus with rearranged DNA induces replication of latent Epstein-Barr virus. We now have found that one of three fragments of this rearranged DNA, when cloned in recombinant plasmids and used to transfect cells, can activate expression of several polypeptides from a latent viral genome. The 33-kDa protein that is the product of the active fragment is likely to be responsible for disruption of latency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer G., Höfler P., Zur Hausen H. Epstein-Barr virus induction by a serum factor. I. Induction and cooperation with additional inducers. Virology. 1982 Aug;121(1):184–194. doi: 10.1016/0042-6822(82)90128-3. [DOI] [PubMed] [Google Scholar]
- Cho M. S., Bornkamm G. W., zur Hausen H. Structure of defective DNA molecules in Epstein-Barr virus preparations from P3HR-1 cells. J Virol. 1984 Jul;51(1):199–207. doi: 10.1128/jvi.51.1.199-207.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Isom H. C., Rapp F. Involvement of an early human cytomegalovirus function in reactivation of quiescent herpes simplex virus type 2. J Virol. 1981 Mar;37(3):1051–1059. doi: 10.1128/jvi.37.3.1051-1059.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson C. M., Cohen L. K., Henle W., Strominger J. L. An unusually high-titer human anti-Epstein Barr virus (EBV) serum and its use in the study of EBV-specific proteins synthesized in vitro and in vivo. J Immunol. 1983 Feb;130(2):919–924. [PubMed] [Google Scholar]
- Glaser R., Rapp F. Rescue of Epstein-Barr virus from somatic cell hybrids of Burkitt lymphoblastoid cells. J Virol. 1972 Aug;10(2):288–296. doi: 10.1128/jvi.10.2.288-296.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- Hoffman G. J., Lazarowitz S. G., Hayward S. D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci U S A. 1980 May;77(5):2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Thorley-Lawson D., Kieff E. An Epstein-Barr virus DNA fragment encodes messages for the two major envelope glycoproteins (gp350/300 and gp220/200). J Virol. 1984 Feb;49(2):413–417. doi: 10.1128/jvi.49.2.413-417.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Miller G., Heston L., Countryman J. P3HR-1 Epstein-Barr virus with heterogeneous DNA is an independent replicon maintained by cell-to-cell spread. J Virol. 1985 Apr;54(1):45–52. doi: 10.1128/jvi.54.1.45-52.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Rabson M., Heston L. Epstein-Barr virus with heterogeneous DNA disrupts latency. J Virol. 1984 Apr;50(1):174–182. doi: 10.1128/jvi.50.1.174-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana T., Kieff E. Epstein-barr virus-specific RNA. II. Analysis of polyadenylated viral RNA in restringent, abortive, and prooductive infections. J Virol. 1977 May;22(2):321–330. doi: 10.1128/jvi.22.2.321-330.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R., Vroman B., Chase B., Sculley T., Hummel M., Kieff E. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J Virol. 1983 Jul;47(1):193–201. doi: 10.1128/jvi.47.1.193-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Gradoville L., Heston L., Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982 Dec;44(3):834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Heston L., Miller G. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2762–2766. doi: 10.1073/pnas.80.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M. F., Shedd D., Weigel R. J., Fischer D. K., Miller G. Expression in COS-1 cells of Epstein-Barr virus nuclear antigen from a complete gene and a deleted gene. J Virol. 1984 Jun;50(3):822–831. doi: 10.1128/jvi.50.3.822-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stoerker J., Glaser R. Rescue of transforming Epstein-Barr virus (EBV) from EBV-genome-positive epithelial hybrid cells transfected with subgenomic fragments of EBV DNA. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1726–1729. doi: 10.1073/pnas.80.6.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M. G., Lenoir G., Begon-Lours J. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature. 1978 Nov 16;276(5685):270–272. doi: 10.1038/276270a0. [DOI] [PubMed] [Google Scholar]
- Weigel R., Miller G. Major EB virus-specific cytoplasmic transcripts in a cellular clone of the HR-1 Burkitt lymphoma line during latency and after induction of viral replicative cycle by phorbol esters. Virology. 1983 Mar;125(2):287–298. doi: 10.1016/0042-6822(83)90202-7. [DOI] [PubMed] [Google Scholar]
- Wigdahl B., Smith C. A., Traglia H. M., Rapp F. Herpes simplex virus latency in isolated human neurons. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6217–6221. doi: 10.1073/pnas.81.19.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]