This editorial refers to ‘Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis’†, by S. Kaptoge et al., on page 578
In the 15 years that have passed since C-reactive protein was linked to future cardiovascular events in otherwise healthy individuals,1 no alternative biomarker has proved to be a superior surrogate for vascular inflammation in the prediction of global cardiovascular risk. This observation is re-affirmed in population-based prospective cohort data from the Danish Research Center of Prevention and Health published by Kaptoge and colleagues in this issue of the European Heart Journal.2 In brief, in age- and gender-adjusted analyses, as well as in full multivariate models, basal levels of the ‘upstream’ pro-inflammatory cytokines interleukin-6 (IL-6), interleukin-18, and tumour necrosis factor-α (TNF-α) were all associated with future vascular events in an approximately log-linear manner, as were levels of matrix metalloproteinase-9. However, and also consistent with prior work in this arena, the magnitudes of these effects were smaller than that associated with the ‘downstream’ inflammatory biomarker, C-reactive protein. Furthermore, among those study participants in whom direct comparison could be performed, only two biomarkers—downstream C-reactive protein (hazard ratio 1.69 per 1 SD higher baseline level) and upstream TNF-α (hazard ratio 1.32 per 1 SD higher baseline level)—remained statistically significant predictors of future vascular risk when simultaneously adjusting for the full panel of cytokines and chemokines.
The core question raised in the present analysis, however, does not relate to which inflammatory biomarker is best suited for clinical practice. That issue was addressed in a prior comprehensive meta-analysis from the Emerging Risk Factors Collaboration, in which the incremental utility of C-reactive protein for predicting vascular risk was found to be fully comparable in magnitude to that of total and high-density lipoprotein cholesterol.3 Rather, what the present data address is how epidemiological evidence can help us to understand potential causal pathways of inflammation better, and how those insights can be used in clinical trials to test the inflammatory hypothesis of atherothrombosis directly.
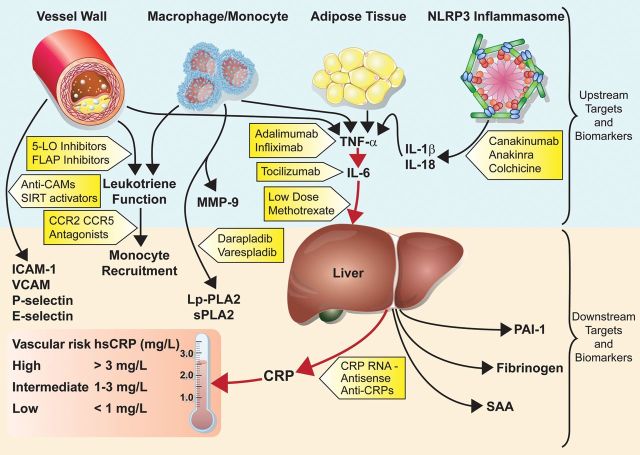
The recognition that both ‘upstream’ and ‘downstream’ biomarkers of inflammation all associate with vascular risk supports the broader concept that targeting causal pathways of inflammation rather than any single biomarker is likely to be the most promising direction for drug development. Most prominent among these is the interleukin-1 (IL-1), TNF-α, IL-6 pathway, which, when activated, subsequently results in elevated levels of hepatic acute phase proteins, including C-reactive protein, fibrinogen, and plasminogen activator inhibitor type-1 (Figure 1). As summarized in the current European Heart Journal report,2 a wealth of epidemiological data consistently show that basal IL-6 levels predict future vascular events. Moreover, two recent Mendelian randomization studies demonstrate that genetic polymorphism in the IL-6 receptor signalling pathway concordantly associate with lifelong lower levels of C-reactive protein and fibrinogen, as well as proportional reductions in cardiovascular event rates, data that elegantly support a causal relationship between classical inflammatory activation and future plaque rupture.4,5 These upstream data for the IL-6 signalling pathway also provide a counterpoint to earlier Mendelian randomization studies of single downstream biomarkers, such as C-reactive protein and fibrinogen, that may be less likely to ‘cause’ atherosclerosis, even if they are superior biomarkers for daily clinical practice.
Figure 1.
Targeting inflammatory pathways for the treatment of cardiovascular disease. Abbreviations: CCR2/CCR5, chemokine receptor types 2 and 5; CRP, C-reactive protein; FLAP, 5-liopoxygenase-activating protein; hsCRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule type 1; IL-1β, interleukin-1β; IL-18, interleukin-18; IL-6, interleukin-6; 5-LO, 5-lipoxygenase; Lp-PLA2, lipoprotein-associated phospholipase A2; MMP-9, matirx metalloproteinase-9; PAI-1, plasminogen activator inhibitor type-1; SAA, serum amyloid A; SIRT1, sirtuin-1; sPLA2, secretory phospholipse A2; TNF-α, tumour necrosis factor-α; and VCAM, vascular cellular adhesion molecule.
Our group has been fortunate to launch two multinational clinical trials designed to test the inflammatory hypothesis of atherothrombosis directly using pathway approaches that, by design, significantly reduce the upstream pro-inflammatory cytokines IL-1, TNF-α, and IL-6, as well as downstream biomarkers, such as C-reactive protein. One on-going trial, the Canakinumab Anti-inflammatory Thrombosis Outcomes Trial (CANTOS), is designed to address whether a human monoclonal antibody that specifically inhibits IL-1β can reduce recurrent vascular events.6 Canakinumab is an approved drug for use in several rare heritable paediatric conditions associated with IL-1β over-expression. It is also an effective agent for systemic juvenile idiopathic arthritis,7 and recent phase II trial data among diabetics show dose-dependent reductions in IL-6 and C-reactive protein of >50%.8 A major mechanism of this effect is inhibition of the nod-like receptor pyrin domain 3 (NLRP-3) inflammasome, which is responsible for activation and local secretion of IL-1β following recognition of crystalline structures, most importantly cholesterol crystals that deposit within a growing atheroma.9 What CANTOS fundamentally asks, therefore, is whether a systematic anti-inflammatory shift in IL-1β activity (and the downstream pathways that follow) will safely result in fewer heart attacks, strokes, and cardiovascular deaths.
In our second on-going multinational trial, the National Heart, Lung and Blood Institute-sponsored Cardiovascular Inflammation Reduction Trial (CIRT), we are randomly assigning post-myocardial infarction patients to methotrexate at a low dose currently used worldwide as a safe first-line treatment for rheumatoid arthritis (15–20 mg weekly).10 Like canakinumab, low-dose methotrexate inhibits upstream cytokines (including TNF-α and IL-6) and concordant downstream production of hepatic acute-phase proteins. Methotrexate, a proven systemic anti-inflammatory with over 40 years of clinical use, has recently been shown in the cholesterol-fed rabbit model to slow atherosclerotic lesion progression and reduce intimal–medial thickening.11 These pathway effects parallel non-randomized observations that low-dose methotrexate may reduce vascular events by 20% among patients being treated for rheumatoid arthritis or psoriatic arthritis (two inflammatory conditions known to be associated with excess vascular risk). Thus, in parallel to CANTOS, what CIRT fundamentally asks is whether a generic broad-spectrum anti-inflammatory agent might also reduce recurrent event rates. Together, CIRT and CANTOS provide two swings of the bat to test the inflammation reduction hypothesis.
Methotrexate is not the only ‘old’ agent being repurposed as a potential anti-inflammatory drug with vascular implications (Figure 1). Colchicine, an agent long used to treat gout and reduce disease invoked by uric acid crystals, is now understood also to suppress the crystal-recognizing NLRP-3 inflammasome and thus reduce levels of IL-1β. Provocative trial data provided by the Australian LoDoCo investigators in 2012 raise the possibility that this anti-inflammatory agent might also have clinical efficacy in reducing cardiovascular event rates.12 Likewise, the TINSAL-2D investigators have reported that the anti-inflammatory salsalate improves glycaemic control in diabetics and lowers circulating leucocyte, neutrophil, and lymphocyte counts.13 These latter trial data are of interest given the common ground shared by insulin resistance and atherosclerosis, as well as evidence that diabetes is also an inflammatory disorder of the innate immune system.
Canakinumab is not the only ‘new’ anti-inflammatory under investigation as a potential vasculoprotective drug (Figure 1). Anakinra, an IL-1β receptor antagonist, has shown efficacy in diabetes and undergone one short-term trial of C-reactive protein reduction in patients with acute coronary ischaemia.14 The IL-6 inhibitor tocilizumab and the TNF-α inhibitors adalimumab and infliximab, in wide use for rheumatoid arthritis, could also prove to have vascular benefits, although these monoclonal antibodies may adversely increase low-density lipoprotein cholesterol.15 Major phaseIII trials of the lipoprotein-associated phospholipase A2 inhibitor darapladib are due to report in the next year (though a smaller trial of the secretory phospholipase A2 inhibitor varespladib was stopped due to futility). Other approaches to inhibition of vascular inflammation include agents such as 5-lipoxygenase and 5-lipoxygenase-activating protein inhibitors that reduce leukotriene function, and chemokine antagonists to chemokine receptor type 2 and chemokine receptor type 5 that inhibit monocyte recruitment (although it is uncertain whether these will enter phase III study). Sirtuin-1, a metabolic regulator that impacts upon longevity and cell death, has anti-inflammatory effects on endothelial cell activation. As such, sirtuin-1 activators could be protective in atherosclerosis. Finally, downstream C-reactive protein antagonists have been developed, which, if pursued, could provide a direct method to resolve controversy about whether C-reactive protein is more than a clinical biomarker indicative of higher risk patients.
While anti-inflammatory therapies hold promise in cardiovascular disease, it is crucial to acknowledge that atherosclerosis is a chronic process, which could require long-term immunosupression, an approach that carries risk for infection and possibly oncogenesis. On-going pathway trials will need to demonstrate not only efficacy for reducing vascular events, but also acceptable safety profiles. For this reason, post-trial registries will be needed in the phase III development process to determine long-term safety with particular respect to opportunistic infections, the potential for reactivation of tuberculosis, and cancer. Work is also needed to determine whether narrow targeting of vascular inflammation is of greater relevance for atherothrombotic protection. As examples, whereas methotrexate is a broad anti-proliferative, anti-inflammatory agent, canakinumab has a narrower spectrum of effect, because it specifically targets IL-1β and leaves IL-α signalling intact (an effect that may reduce adverse events).In order to test the inflammatory hypothesis of atherothrombosis successfully, researchers will need carefully to target individuals who are at high risk for vascular events due to a persistent pro-inflammatory response (who thus have the greatest likelihood of benefiting from an anti-inflammatory approach), while avoiding enrolment of those without inflammation (to reduce drug exposure and unnecessary toxicity). It is for this reason that CIRT is enrolling only post-myocardial infarction patients who have diabetes or metabolic syndrome (two conditions associated with a systemic pro-inflammatory response) and why CANTOS is enrolling only post-myocardial infarction patients with persistent elevation of C-reactive protein despite statin therapy. Another promising approach for patient selection and event rate enrichment is the use of molecular imaging to define inflammatory lesions. In this regard, advances in dynamic contrast-enhanced magnetic resonance imaging as well as positron emission tomography technologies capable of addressing glucose uptake (FDG-PET) or coronary flow reserve have been promising.16 Finally, researchers, regulators, and the clinical community will need to evaluate inflammation inhibition trials with an open mind, cognizant of the balance between benefits and risks; post-myocardial infarction patients with a persistent pro-inflammatory response represent a very high-risk group for life-threatening events, in whom reasonable levels of drug-related toxicity can be accepted.
The next decade will inform cardiologists as to whether treatments to prevent vascular disease should continue to focus solely on evermore aggressive low-density lipoprotein reduction, or whether there is additional room for therapies targeting the innate immune process that, along with hyperlipidaemia, drives atherogenesis.17 Atherosclerosis is a systemic disease in need of systemic therapies. Moving forward, the cardiology community can no longer simply address the mechanical reversal of coronary stenosis.
Conflict of interest: P.M.R. is listed as a co-inventor on patents held by the Brigham and Women's Hospital (BWH) that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to Siemens and AstraZeneca. P.M.R. is the Principal Investigator of the Cardiovascular Inflammation Reduction Trial (CIRT; funded by the National Heart Lung and Blood Institute) and the Principal Investigator of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS; funded by Novartis). Neither P.M.R. nor the BWH receives any royalties related to the use of the CRP test in either of these trials.
References
- 1.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 2.Kaptoge S, Seshasai SRK, Gao P, Freitag DF, Butterworth AS, Borglykke A, Angelantoino AD, Gudnason V, Rumley A, Lowe GDO, Jorgenesn T, Danesh J. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35:578–589. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Björkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D'Agostino RB, Sr, Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engstrom G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jørgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjærg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundström J, Wassertheil-Smoller S, Mellström D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O'Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten Å, Ljunggren Ö, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Hólm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hingorani AD, Casas JP Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, Brik R, McCann L, Kasapcopur O, Rutkowska-Sak L, Schneider R, Berkun Y, Calvo I, Erguven M, Goffin L, Hofer M, Kallinich T, Oliveira SK, Uziel Y, Viola S, Nistala K, Wouters C, Cimaz R, Ferrandiz MA, Flato B, Gamir ML, Kone-Paut I, Grom A, Magnusson B, Ozen S, Sztajnbok F, Lheritier K, Abrams K, Kim D, Martini A, Lovell DJ PRINTO; PRCSG. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2396–2406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T. CANTOS Pilot Investigative Group. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 9.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett BM, Pradhan A, Solomon DH, Paynter N, MacFadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AAK, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulgarelli A, Dias AAM, Caramelli B, Maranhão RC. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 2012;59:308–314. doi: 10.1097/FJC.0b013e318241c385. [DOI] [PubMed] [Google Scholar]
- 12.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Goldfine AB, Fonseca V, Jablonski KA, Chen YDI, Tipton L, Staten MA. Shoelson SE for the Targeting Inflammation Using Salsalate in Type 2 Diabetes Study Team. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 15.Foltz IN, Karow M, Wasserman SM. Evolution and emergence of therapeutic monoclonal antibodies: what cardiologists need to know. Circulation. 2013;127:2222–2230. doi: 10.1161/CIRCULATIONAHA.113.002033. [DOI] [PubMed] [Google Scholar]
- 16.Camici PG, Rimoldi OE, Gaemperli O, Libby P. Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J. 2012;33:1309–1317. doi: 10.1093/eurheartj/ehs067. [DOI] [PubMed] [Google Scholar]
- 17.Libby P, Ridker PM, Hannson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]