Abstract
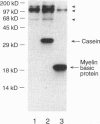
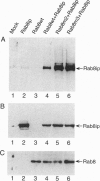
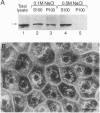
Rab8 is a small GTP-binding protein that plays a role in vesicular transport from the trans-Golgi network to the basolateral plasma membrane in polarized epithelial cells (MDCK), and to the dendritic surface in hippocampal neurons. As is the case for most other rab proteins, the precise molecular interactions by which rab8 carries out its function remain to be elucidated. Here we report the identification and the complete cDNA-derived amino acid sequence of a murine rab8-interacting protein (rab8ip) that specifically interacts with rab8 in a GTP-dependent manner. Rab8ip displays 93% identity with the GC kinase, a serine/threonine protein kinase recently identified in human lymphoid tissue that is activated in the stress response. Like the GC kinase, rab8ip has protein kinase activity manifested by autophosphorylation and phosphorylation of the classical serine/threonine protein kinase substrates, myelin basic protein and casein. When coexpressed in transfected 293T cells, rab8 and the rab8ip/GC kinase formed a complex that could be recovered by immunoprecipitation with antibodies to rab8. Cell fractionation and immunofluorescence analyses indicate that in MDCK cells endogenous rab8ip is present both in the cytosol and as a peripheral membrane protein concentrated in the Golgi region and basolateral plasma membrane domains, sites where rab8 itself is also located. In light of recent evidence that rab proteins may act by promoting the stabilization of SNARE complexes, the specific GTP-dependent association of rab8 with the rab8ip/GC kinase raises the possibility that rab-regulated protein phosphorylation is important for vesicle targeting or fusion. Moreover, the rab8ip/GC kinase may serve to modulate secretion in response to stress stimuli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon R. A., Salminen A., Ruohola H., Novick P., Ferro-Novick S. The GTP-binding protein Ypt1 is required for transport in vitro: the Golgi apparatus is defective in ypt1 mutants. J Cell Biol. 1989 Sep;109(3):1015–1022. doi: 10.1083/jcb.109.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Kearns B., Champion K., Keränen S., Bankaitis V., Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994 Oct 21;79(2):245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. Polarized exocytosis in MDCK cells is regulated by phosphorylation. J Cell Sci. 1995 Feb;108(Pt 2):789–796. doi: 10.1242/jcs.108.2.789. [DOI] [PubMed] [Google Scholar]
- Brondyk W. H., McKiernan C. J., Fortner K. A., Stabila P., Holz R. W., Macara I. G. Interaction cloning of Rabin3, a novel protein that associates with the Ras-like GTPase Rab3A. Mol Cell Biol. 1995 Mar;15(3):1137–1143. doi: 10.1128/mcb.15.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo P. D., Drechsel D., Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995 Dec 8;270(49):29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Cardone M. H., Smith B. L., Song W., Mochly-Rosen D., Mostov K. E. Phorbol myristate acetate-mediated stimulation of transcytosis and apical recycling in MDCK cells. J Cell Biol. 1994 Mar;124(5):717–727. doi: 10.1083/jcb.124.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Vingron M., Sander C., Simons K., Zerial M. Molecular cloning of YPT1/SEC4-related cDNAs from an epithelial cell line. Mol Cell Biol. 1990 Dec;10(12):6578–6585. doi: 10.1128/mcb.10.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Takai Y., Holz R. W. Evidence that the Rab3a-binding protein, rabphilin3a, enhances regulated secretion. Studies in adrenal chromaffin cells. J Biol Chem. 1995 Jul 14;270(28):16714–16718. doi: 10.1074/jbc.270.28.16714. [DOI] [PubMed] [Google Scholar]
- Craighead M. W., Bowden S., Watson R., Armstrong J. Function of the ypt2 gene in the exocytic pathway of Schizosaccharomyces pombe. Mol Biol Cell. 1993 Oct;4(10):1069–1076. doi: 10.1091/mbc.4.10.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasy C. L., Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995 Sep 15;270(37):21695–21700. doi: 10.1074/jbc.270.37.21695. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Balch W. E. Differential inhibition of multiple vesicular transport steps between the endoplasmic reticulum and trans Golgi network. J Biol Chem. 1993 Feb 25;268(6):4216–4226. [PubMed] [Google Scholar]
- Davidson H. W., McGowan C. H., Balch W. E. Evidence for the regulation of exocytic transport by protein phosphorylation. J Cell Biol. 1992 Mar;116(6):1343–1355. doi: 10.1083/jcb.116.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis M. A., Santini G., Kahn R. A., Di Tullio G., Luini A. Receptor and protein kinase C-mediated regulation of ARF binding to the Golgi complex. Nature. 1993 Aug 26;364(6440):818–821. doi: 10.1038/364818a0. [DOI] [PubMed] [Google Scholar]
- Drivas G. T., Shih A., Coutavas E., Rush M. G., D'Eustachio P. Characterization of four novel ras-like genes expressed in a human teratocarcinoma cell line. Mol Cell Biol. 1990 Apr;10(4):1793–1798. doi: 10.1128/mcb.10.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Novick P. The role of GTP-binding proteins in transport along the exocytic pathway. Annu Rev Cell Biol. 1993;9:575–599. doi: 10.1146/annurev.cb.09.110193.003043. [DOI] [PubMed] [Google Scholar]
- Friesen H., Lunz R., Doyle S., Segall J. Mutation of the SPS1-encoded protein kinase of Saccharomyces cerevisiae leads to defects in transcription and morphology during spore formation. Genes Dev. 1994 Sep 15;8(18):2162–2175. doi: 10.1101/gad.8.18.2162. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Gyuris J., Golemis E., Chertkov H., Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993 Nov 19;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Hansen S. H., Casanova J. E. Gs alpha stimulates transcytosis and apical secretion in MDCK cells through cAMP and protein kinase A. J Cell Biol. 1994 Aug;126(3):677–687. doi: 10.1083/jcb.126.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L. A., Pimplikar S., Parton R. G., Virta H., Zerial M., Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993 Oct;123(1):35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L. A., de Hoop M. J., Dupree P., Zerial M., Simons K., Dotti C. Protein transport to the dendritic plasma membrane of cultured neurons is regulated by rab8p. J Cell Biol. 1993 Oct;123(1):47–55. doi: 10.1083/jcb.123.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoueix-Lerosey I., Jollivet F., Camonis J., Marche P. N., Goud B. Two-hybrid system screen with the small GTP-binding protein Rab6. Identification of a novel mouse GDP dissociation inhibitor isoform and two other potential partners of Rab6. J Biol Chem. 1995 Jun 16;270(24):14801–14808. doi: 10.1074/jbc.270.24.14801. [DOI] [PubMed] [Google Scholar]
- Karniguian A., Zahraoui A., Tavitian A. Identification of small GTP-binding rab proteins in human platelets: thrombin-induced phosphorylation of rab3B, rab6, and rab8 proteins. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7647–7651. doi: 10.1073/pnas.90.16.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Whalen G., Kehrl J. H. Differential expression of a novel protein kinase in human B lymphocytes. Preferential localization in the germinal center. J Biol Chem. 1994 Jun 17;269(24):16802–16809. [PubMed] [Google Scholar]
- Leevers S. J., Paterson H. F., Marshall C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994 Jun 2;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Li C., Takei K., Geppert M., Daniell L., Stenius K., Chapman E. R., Jahn R., De Camilli P., Südhof T. C. Synaptic targeting of rabphilin-3A, a synaptic vesicle Ca2+/phospholipid-binding protein, depends on rab3A/3C. Neuron. 1994 Oct;13(4):885–898. doi: 10.1016/0896-6273(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Lian J. P., Stone S., Jiang Y., Lyons P., Ferro-Novick S. Ypt1p implicated in v-SNARE activation. Nature. 1994 Dec 15;372(6507):698–701. doi: 10.1038/372698a0. [DOI] [PubMed] [Google Scholar]
- Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994 Jan 6;367(6458):40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Nuoffer C., Balch W. E. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Ohashi M., Huttner W. B. An elevation of cytosolic protein phosphorylation modulates trimeric G-protein regulation of secretory vesicle formation from the trans-Golgi network. J Biol Chem. 1994 Oct 7;269(40):24897–24905. [PubMed] [Google Scholar]
- Pimplikar S. W., Simons K. Activators of protein kinase A stimulate apical but not basolateral transport in epithelial Madin-Darby canine kidney cells. J Biol Chem. 1994 Jul 22;269(29):19054–19059. [PubMed] [Google Scholar]
- Polakis P., McCormick F. Structural requirements for the interaction of p21ras with GAP, exchange factors, and its biological effector target. J Biol Chem. 1993 May 5;268(13):9157–9160. [PubMed] [Google Scholar]
- Pombo C. M., Kehrl J. H., Sánchez I., Katz P., Avruch J., Zon L. I., Woodgett J. R., Force T., Kyriakis J. M. Activation of the SAPK pathway by the human STE20 homologue germinal centre kinase. Nature. 1995 Oct 26;377(6551):750–754. doi: 10.1038/377750a0. [DOI] [PubMed] [Google Scholar]
- Ramer S. W., Davis R. W. A dominant truncation allele identifies a gene, STE20, that encodes a putative protein kinase necessary for mating in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):452–456. doi: 10.1073/pnas.90.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirataki H., Kaibuchi K., Sakoda T., Kishida S., Yamaguchi T., Wada K., Miyazaki M., Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993 Apr;13(4):2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. N., De Virgilio C., Souza B., Pringle J. R., Abo A., Reed S. I. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature. 1995 Aug 24;376(6542):702–705. doi: 10.1038/376702a0. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Vitale G., Ullrich O., Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995 Nov 3;83(3):423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994 Jun 3;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Søgaard M., Tani K., Ye R. R., Geromanos S., Tempst P., Kirchhausen T., Rothman J. E., Söllner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994 Sep 23;78(6):937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Xu H., Greengard P., Gandy S. Regulated formation of Golgi secretory vesicles containing Alzheimer beta-amyloid precursor protein. J Biol Chem. 1995 Oct 6;270(40):23243–23245. doi: 10.1074/jbc.270.40.23243. [DOI] [PubMed] [Google Scholar]