Abstract
Clinical outcomes in ovarian cancer are heterogeneous even when considering common features such as stage, response to therapy, and grade. This disparity in outcomes warrants further exploration into tumor and host characteristics. One compelling host characteristic is the immune response to ovarian cancer. While several studies have confirmed a prominent role for the immune system in modifying the clinical course of the disease, recent genetic and protein analyses also suggest a role in disease incidence. Recent studies also show that anti-tumor immunity is often negated by immune suppressive cells present in the tumor microenvironment. These suppressive immune cells also directly enhance the pathogenesis through the release of various cytokines and chemokines, which together form an integrated pathologic network. Thus, future research into immunotherapy targeting ovarian cancer will likely become increasingly focused on combination approaches that simultaneously augment immunity while preventing local immune suppression or by disrupting critical cytokine networks.
Keywords: Cytokines, T cells, macrophages, single nucleotide polymorphisms, dendritic cells
II. INTRODUCTION
Ovarian cancer has the highest mortality rate of the cancers unique to women. According to Siegel and colleagues, in 2012 there were an estimated 22,280 new cases of ovarian cancer and estimated 15,500 deaths.1 Moreover, the majority (65%–75%) of women with ovarian cancer are diagnosed with advanced stage disease (III and IV), and only about 15%–20% of these women are free of disease recurrence at ten years.2–4 Major subtypes of ovarian cancer include serous, endometrioid, clear cell and mucinous histologies, with heterogeneous characteristics.5 There are nevertheless unique features about ovarian cancer that may shed light on its basic biology, hopefully leading to improvements in therapeutics and outcome. One of those unique features is the complex interaction of the immune system in both ovarian cancer initiation and progression which has been well documented over the last two decades and is the central topic of this review. We will cover a broad range of topics related to inflammation and ovarian cancer, beginning with epidemiologic and genetic evidence in ovarian cancer risk. We will then discuss how immune mediators might serve as biomarkers for detection and diagnosis of ovarian cancer. Finally, we will focus on tumor microenvironment and consider how different subsets of immune cells and cytokines can either promote tumor growth and progression or target tumor cells for destruction and improve patient survival.
III. THE IMMUNE SYSTEM IN DISEASE ONSET AND INITIATION
A. Models of inflammation in ovarian cancer initiation
The immune system is thought to be an important mediator of ovarian carcinogenesis.6 There are two models for the role of the immune system in ovarian cancer, incessant ovulation and chronic inflammation. The incessant ovulation hypothesis proposes that ovulation damage, due to rupturing of the ovulating follicle, traumatizes the ovarian surface causing an immediate inflammatory response and wound repair. The process of continuous damage and epithelial proliferation to repair the wound places strain on the epithelium and increases the risk of malignant transformation. Several epidemiologic studies beginning around five decades ago have implicated increased number of ovulations as a risk factor for ovarian cancer.7 Protection from ovarian cancer has been observed in association with increased parity,8, 9 oral contraceptive use,10, 11 breast feeding,12, 13 older age at first menses (for premenopausal women),14 which all impact number of lifetime ovulations. One of these early studies, which has been further substantiated by more recent investigations, found protective effects of “anovulatory time” by combining information on both increased oral contraceptive use and parity as well as age at first and last menses,15 supporting the theory of incessant ovulation as an underlying mechanism of carcinogenesis. Although the proliferative effect of hormones on ovarian epithelium is one postulated biological mechanism explaining these associations, emerging studies support a role of macrophages in inducing DNA damage. For example, a recent study found not only that superovulated mice had elevated macrophage counts in their oviducts, but that oxidative stress induced by hydrogen peroxide or macrophage secreted mediators initiated DNA damage in immortalized baboon tubal epithelial cell lines.16 In vivo, it has been clearly established that macrophages are abundant within epithelial inclusion cysts.17
As with the incessant ovulation hypothesis, the chronic inflammation model of carcinogenesis proposes that chronic exposures to external or endogenous triggers of immunity and persistent immune cells that cause injury to surrounding epithelium, damage DNA through release of reactive oxygen species, or produce cytokines that promote proliferation. One proposed environmental exposure shown to induce inflammation in animal models and human lungs is talcum powder.18 Composed primarily of magnesium silicate, this moisture absorbing substance has been linked to ovarian cancer risk in a number of studies,6, 19–21 although selection bias and confounding were strong concerns for the studies that found these associations.22, 23 The strong correlation between endometriosis and ovarian cancer also supports the chronic inflammation hypothesis. Endometriosis involves endometrial cells growing outside of the uterus and has been investigated extensively as a risk factor for ovarian cancer. Several epidemiologic studies have found an association between history of endometriosis and invasive epithelial ovarian cancer and most recently a pooled analysis of 13 case-control studies demonstrated that history of endometriosis increased risk of clear cell, low grade serous, and endometrioid invasive ovarian cancers, but not mucinous or high grade serous ovarian cancers.24 Evidence strongly suggests that endometriosis is a pelvic inflammatory condition.25 Although the causes are unknown, the results of some studies suggest that endometriosis may be caused by toxic inflammation-inducing substances, especially poly-halogenated aromatic hydrocarbons and xeno-estrogens.26
Exposure to pathogens might also cause chronic inflammation that would aid in tumor promotion and progression. Retrograde menstruation provides a potential mechanism by which pathogens could reach fallopian tube epithelial cells to establish infection.27 While there is little evidence that specific pathogens contribute to most ovarian cancer cases, some studies have found a relationship between pelvic inflammatory disease and ovarian cancer risk.6, 28, 29 Lastly, consistent with the idea that chronic inflammation - whether mediated by ovulation induced damage, foreign agents, or both - is an underlying cause of ovarian cancer, non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with a decreased risk of ovarian cancer, but results have been inconsistent.6, 21, 30
B. Inherited variation and risk: Germline polymorphisms in immune-related genes and ovarian cancer risk
Inherited single nucleotide changes in genes can affect expression and function of their protein products.31 Apart from typical epidemiologic approaches, the ability to examine the impact of genetic variation in disease processes over the past decade represents a significant advance in understanding the role of the immune system in disease risk. While genome wide association studies to date have found significant associations with ovarian cancer and single nucleotide polymorphisms (SNPs) in genes that are not directly immune-related,32–34 several groups have carried out candidate gene studies that examined polymorphisms in immune-related genes in risk of ovarian cancer. NF-κB is a pro-inflammatory transcription factor complex that can be activated by multiple pathways. In addition to hematopoietic cells, epithelial cells also use NF-κB to produce signals that alert the immune system to stress and infection. The NFKB1 gene encodes the p50 subunit of the NF-κB protein and the deletion allele of the NFKB1 variant (−94 ins/del ATTG) was previously reported to have reduced promoter activity by luciferase assay in HeLa or HT-29 cell lines.35 This variant was also recently evaluated in a Chinese case-control study, where those homozygous for the insertion had increased risk of advanced ovarian cancer (OR=2.7, 95% CI: 1.4–5.1) compared with those homozygous for the deletion.36
One mechanism through which NF-κB is activated is by NOD2 in response to muramyl dipeptide from intracellular bacterial lipopolysaccharide. The NOD2 3020insC polymorphism results in a frameshift and premature stop codon, truncating the NOD2 protein.37 A Polish case-control study found an increased risk for all ovarian cancer (OR=1.6, 95% CI: 1.1– 2.3) with the NOD2 3020insC allele.38 SNPs in two NF-κB inhibitor genes, NFKBIA and NFKBIB, were assessed in an ovarian case-control study from two sites (Mayo Clinic and Duke University). A marginally statistically significant association was found between the NFKBIA synonymous coding SNP rs1957106 and reduced risk of all epithelial ovarian cancer (EOC) (ORABvsAA=0.8, 95% CI: 0.6–0.9, ORBBvsAA=0.9, 95% CI: 0.6–1.3; p=0.03), and in the Mayo Clinic study only an increased risk for all EOC with 5' upstream SNP rs3138050 in the recessive model (OR=2.2, 95% CI: 1.1–4.6; p=0.03).39 No known functional studies to date have assessed the direct effect these SNPs have on protein levels or function. No associations were found with EOC and the NFKBIB SNPs genotyped in this study.39
Caspase-8 (CASP8) is activated following ligation of several important immune receptors to their ligands, including T cell receptors, tumor necrosis factor receptor, and Fas. This activation ultimately leads to apoptosis. Ma and colleagues examined 8 tag SNPs in the CASP8 gene for association with risk of epithelial ovarian cancer of combined histologies in a Chinese population case-control study.40 They found that several SNPs in this gene were associated with reduced risk of developing EOC, including promoter indel SNP rs3834129 (ORallelic=0.6, 95% CI: 0.5–0.8; p=0.002), intronic rs3769827 (ORallelic=0.6, 95% CI: 0.5–0.8; p=0.0008), and intronic rs6704688 (ORallelic=0.7, 95% CI: 0.5–0.9; p=0.004).40 Participants carrying at least one copy of the minor allele for rs3834129 and rs3769827 were also more likely to have an older age of disease onset.40 The rs3834129 result is not surprising, as the deletion removes the stimulatory protein 1 transcription factor binding site, resulting in reduced caspase-8 activity and reduced activation induced cell death in T-cells after exposure to cancer cells.41 In a cohort of BRCA1/2 mutation carriers of European descent from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA), the CASP8 D302H variant was significantly associated with decreased risk of ovarian cancer in BRCA1 mutation carriers (HRallelic=0.7, 95% CI: 0.5–0.9).42 This group also assessed a functional polymorphism in the gene for a CASP8 cooperating protein, CASP10, and found no association with ovarian cancer risk in either BRCA1 or BRCA2 carriers.42 When this SNP, also known as rs1045485, was assessed in a population that was not restricted on BRCA1 carriers, it was not found to be associated with invasive EOC (ORminor allele homozygous=0.81, 95% CI: 0.59–1.12) in participants of European descent in a large consortium of 14 case-control studies, the Ovarian Cancer Association Consortium.43
Mannose-binding lectin is a component of the complement system, recognizing microbial and tumor associated mannose and initiating activation of the complement cascade, another important pathway in both the innate and adaptive immune responses.44, 45 A functional polymorphism in exon 1 of mannose-binding lectin was recently assessed in relation to vaginal concentrations of the protein in study participants. This polymorphism, also known as rs1800450 was also analyzed for frequency among healthy controls, patients with benign conditions, and patients with gynecologic malignancies, including ovarian cancer.46 They found that the variant allele was elevated in ovarian cancer patients (17.1%) compared to healthy women (8.7%, p=0.02) and women with benign conditions (8.4%, p=0.03) and that this allele was associated with lower concentrations of vaginal mannose-binding lectin, suggesting a role of this pathway in disease pathogenesis.46
Cyclooxygenase 2, or prostaglandin endoperoxide synthase 2 (PTGS2), is an enzyme involved in synthesis of prostaglandin, an important mediator of inflammation. A pooled analysis of two population-based case-control studies (Hawaii Ovarian Cancer Case-Control Study and the New England Case-Control Study) of combined non-serous ovarian cancer found the C allele of PTGS2 SNP rs5275, found in one study to be associated with a modest reduction of PTGS2 mRNA levels in lymphoblastoid cell lines,47 was also associated with reduced risk of combined non-serous subtypes of ovarian cancer (ORrecessive=0.7, 95% CI: 0.4–0.98).48 When all invasive ovarian cancers were assessed together information on rs5275 genotype and NSAID use were combined, non-aspirin NSAID users with the CC genotype had the lowest risk of developing ovarian cancer compared to NSAID nonusers with TT genotype.48 Pinheiro et al also assessed associations with NSAID use in a case-control study including participants from the New England Case-Control study and the Nurses' Health Studies. They however, did not find an association with ovarian cancer risk, nor did they find significant interactions between NSAID use and the rs5275 or the rs20417 PTGS2 polymorphism in relation to ovarian cancer risk.49 In a recent report by White et al, a panel of inflammation related genes, eight PTGS2 tagSNPs were included and no association with EOC in a case-control study combining two US study sites (Mayo Clinic and North Carolina Ovarian Cancer Study).50 However, the same study revealed an association between ALOX5 SNP, rs1864414, and EOC, an association which remained even after combining these studies with additional Ovarian Cancer Association Consortium studies (OR=0.9, 95% CI:0.8–0.95; p=0.002).50 Arachidonate 5-lipoxigenase (ALOX5) converts arachidonic acid into leukotrienes.
Several associations between cytokines and cytokine receptors also suggest a relationship between inflammation and immunity and ovarian cancer initiation. The IL1A gene encodes the IL-1α cytokine that binds to IL-1R1 and is an important inflammatory mediator. The case-control study reported above by White et al, also assessed several cytokine gene polymorphisms. The strongest association was seen between the minor allele of the IL1A SNP rs17561, which results in a missense mutation, and an elevated risk of clear cell (OR=0.8, 95% CI: 0.6–0.9), mucinous (OR=0.8, 95% CI: 0.7–0.96), and endometrioid (OR=0.8, 95% CI: 0.7–0.96), but not serous ovarian cancers.50 The minor (T) allele of rs17561 leads to an amino acid change to serine at position 114 in pro-IL-1α, which has been shown to be more sensitive to calpain cleavage than the major (G) allele encoded alanine at this position.51 The cleaved form has recently been demonstrated to have higher binding affinity to IL-1R1 and leading to increased cytokine expression at lower concentrations than the uncleaved form.52 When examining polymorphisms in miRNA binding sites of immune-related genes in a US population case-control study, Liang and colleagues found that a SNP (rs3917328) in a putative miR-335 binding site in the IL1R1 gene was related to increased risk of developing EOC (ORdominant=1.6, 95% CI: 1.0, 2.6).53
IL-18 promotes IFN-γ production in NK and TH1 cells. Assessment of 11 IL18 tagSNPs in the North Carolina Ovarian Cancer Study found one SNP, rs1834481 was related to risk of advanced ovarian cancer (ORper-allele=1.2, 95% CI: 1.1–1.4). However, when they assessed this SNP in a larger ovarian cancer consortium, the Ovarian Cancer Association Consortium, the significance was attenuated (ORper-allele=0.99, 95% CI: 0.9–1.05).54 Interestingly, in a genome-wide association study that assessed 704,409 SNPs for association with IL-18 levels in a cohort of 1523 women (with and without type 2 diabetes) from the Women’s Health Study and 435 women (with no history of chronic disease) from the Women’s Genome Health Study, rs1834481 was significantly associated with plasma IL-18 levels.55 It should be noted that a number of SNPs in other immune-related genes assessed for association with ovarian cancer risk were not found to be significantly associated.50 While this is not an all-inclusive list of studies that have assessed immune related SNPs in relation to ovarian cancer risk, it does start to provide insight into contribution of immune genes in this disease.
C. Protein-based inflammatory markers: Association with risk of developing ovarian cancer
In addition to the genetic approaches there has been interest in developing protein-based biomarker studies that can aid in diagnosis. The suggestion that initiation of ovarian cancer is mediated by inflammation is somewhat supported by studies evaluating systemic markers of inflammation such as C-reactive protein (CRP). CRP levels in the blood rise rapidly in response to IL-6 released during local inflammatory processes, and thus it is not a disease-specific marker.56 Its physiological role is to bind to phosphocholine on the surface of dead or dying cells in order to activate the complement system. CRP protein levels in the blood are associated with a wide range of diseases, such as diabetes and atherosclerosis.57 Toriola and colleagues conducted a prospective population based case-control study of serum CRP nested within the Finnish Maternity Cohort to determine if there is an association between elevated pre-diagnostic CRP levels in the blood and ovarian cancer.58 In the Finnish Maternity Cohort, 170 women developed ovarian cancer. and CRP levels were found to be associated with ovarian cancer in samples collected and average of 6.4 years prior to diagnosis (OR=2.0, 95% CI: 1.1–3.4 but not those collected an average of 8.9 years prior to diagnosis (OR=1.6, 95% CI: 0.9–2.8). They also reported an increase over time with CRP serum levels and ovarian cancer risk (OR=1.9, 95% CI: 1.1–3.2).58 In another analysis, which included participants from three pooled prospective nested cohort studies, Lundin and colleagues reported a similar finding in which high levels of CRP (>10mg/ml) were associated with a 4.4 fold increased risk of developing ovarian cancer.59
The increased availability of assays to determine cytokine levels has recently led to studies evaluating other circulating markers of inflammation. Using a nested approach with three prospective cohort studies, Clenenden and colleagues found that IL-6, as well as IL-2, IL-4, IL-12, and IL-13 levels were significantly associated with increased risk of developing epithelial ovarian cancer of combined histologies.60 The majority of cases were serous and the results looked similar when they restricted on serous subtype.
Overall, the studies demonstrating that elevated CRP and IL-6 levels are associated with an elevated risk of ovarian cancer support a role for inflammation, likely subclinical, in initiating the disease. The recent findings of genetic associations with risk further support this contention. Furthermore, recent data showing elevated IL-2, IL-4 and IL-13 years prior to diagnosis, although not definitive, suggest that the chronic inflammation may have an adaptive T and B cell component that remains to be discovered.
In summary, there is some support of the notion that chronic inflammation in the reproductive tract is involved in ovarian cancer development. Unfortunately, to the best of our knowledge no studies have been reported which directly compare in either a prospective cohort or case-control setting that smoldering subclinical inflammation drives the development of neoplasia of the ovary. The two models of ovarian cancer initiation involving the immune system that have been proposed, the incessant ovulation and chronic inflammation models, also likely are not exclusive and could act together to increase incidental ovarian cancer. With high-throughput genetic approaches having been developed in recent years, such as genome-wide SNP analysis and multiplexed cytokine analysis, it has become possible to interrogate mechanisms at the population level. Although difficult and with weaknesses, these population-based approaches have provided substantial additional evidence suggesting that the immune system is a mediator of epithelial transformation. The potential mediators span both innate and adaptive immunity. The studies being largely association-based, remain difficult to interpret at present, particularly the genetic studies; however, the identified targets provide a future pipeline of possible biochemical approaches evaluating the impact of their gene products.
IV. IMMUNE SIGNATURES IN THE DIAGNOSIS OF OVARIAN CANCE
There have been several reports over the decades that ovarian cancer patients have elevated levels of serum cytokines leading to speculation that the immune response against ovarian cancer may have diagnostic, in addition to prognostic, value.61 Despite that, there is a realization that that individual cytokines are of limited use as diagnostics because of specificity and sensitivity concerns. However, with the recent introduction of methods to measure a variety of cytokines, particularly in a multiplexed manner, studies are now being done to assess panels of markers as diagnostics with several significant advances.
CA-125 is the most widely used biomarker for ovarian cancer. Despite the widespread use of CA-125, this biomarker has poor positive and negative predictive value.62 Besides ovarian cancer, CA-125 is also elevated in a variety of other malignant and non-malignant conditions,63, 64 limiting its ability to distinguish benign and malignant adnexal masses. Given the growing body of evidence of a role for the immune system in the clinical course of ovarian cancer, there have been a number of studies evaluating circulating cytokine profiles as signatures of ovarian cancer. In a retrospective case control study, Egdell and colleagues found that combining plasma IL-6, IL-8, and two markers of inflammation, CRP and serum amyloid A with CA-125 levels increased diagnostic efficiency compared to CA-125 alone.65 Despite the impressive area under the curve values of 0.988 and 0.985 for the test and validation cohorts, respectively, this phase II biomarker study did not examine the utility of this panel in distinguishing ovarian malignancies from benign masses. Lambeck and colleagues compared several cytokines (IL-7, IL-6, IL-8 IL-10, MCP-1, and IP-10) with CA-125 for the ability to correctly predict malignancy versus benign masses 66. While none of the cytokines alone were useful in predicting malignancy, low levels of IL-7 in combination with high CA-125 resulted in a high specificity of 100% but only 56% sensitivity.
In another study, Gorelik and colleagues used multiplexed cytokine analysis to establish a signature that would distinguish early stage ovarian cancer from healthy controls and patients with benign masses.67 Although not validated in an independent cohort, their preliminary study found that the combination of CA-125, IL-6, epidermal growth factor, vascular endothelial growth factor, and IL-8 was superior in distinguishing early stage ovarian cancer from controls with 100% sensitivity and 86% specificity. In contrast, the use of CA-125 alone in this cohort resulted in sensitivities between 70% and 80%. Further analysis showed that a five marker set (CA-125, G-CSF, IL-6, vascular endothelial growth factor and epidermal growth factor) correctly distinguished benign masses from cancer with 84% sensitivity and 76% specificity. Collectively these results, while preliminary, demonstrate that immune factors when considered in the context of complex biomarker sets may be useful for early detection and pre-surgical diagnosis.
V. IMMUNITY IN DISEASE PROGRESSION
A. Microenvironment: Role of tumor infiltrating immune cells in disease progression
A completely different kind of inflammation follows tumor development.68 Chronic inflammation can foster and support various cellular events resulting in induction and/or selection of aggressive cancer cells. Observations that date back several decades have established that there are natural immune responses to ovarian tumors and these immune responses can have a profound impact on the clinical course of the disease. Although infiltration of immune effectors into ovarian cancers was observed as early as 1982 by Haskill and colleagues,69 it would not be until nearly two decades later that the prognostic significance of these cells was appreciated. In their seminal publication in 2003, Zhang, Conejo-Garcia and colleagues showed that T cell infiltration into ovarian tumors was associated with improved survival.70 Among 74 patients with complete clinical responses after debulking and platinum-based therapy, the five-year survival rate was an extraordinary 73.9% among those patients with CD3+ T cells within their tumor compared to 11.9% among patients without infiltrating T cells.70 This study also revealed that in tumors with high numbers of tumor-infiltrating T cells, the expression of monokines induced by IFN-γ, macrophage-derived chemokines, and secondary lymphoid-tissue chemokines were significantly increased as compared with tumors lacking T cells, suggesting that these chemokines may be involved in modulating the local anti-tumor response and therefore represent novel targets for improving anti-tumor immunity.70
CD8 cytotoxic T lymphocytes (CTL) are generally thought to be the primary mediators of anti-tumor immune responses. These cells recognize antigens displayed in the context of major histocompatibility complex (HLA) class I molecules expressed on ovarian cancer cells. Upon recognition of their cognate antigen, CD8 T cells release, among several mediators, perforin and granzyme which induce apoptosis in target cells.71 To examine a role for CTL in ovarian cancer, Sato and colleagues studied 117 ovarian cancer cases and observed improved survival in patients who had higher numbers of intraepithelial CD8+ T cells compared to patients without intraepithelial CD8 T cells (median survival 55 vs. 26 months).72 These findings were largely confirmed by Leffers and colleagues in an independent cohort.73 Although the recent identification of regulatory subsets of CD8 T cells has clouded interpretations of tumor-infiltrating CD8 cells, the idea that the vast majority of CD8 T cells are cytotoxic is reasonable given recent findings of a strong positive correlation between levels of CD8 T cells and granzyme B within tumors.74, 75 Gene expression profiling of serous ovarian tumors with high and low CD8 T cell infiltration found two genes differentially expressed in cancers with high versus low CD8 T cell infiltration: interferon regulatory factor 1 and C-X-C chemokine receptor 6.76, 77 Upregulation of interferon regulatory factor 1 results in both major histocompatibility complex class I and II expression thereby fostering a tumor immune microenvironment favoring immune recognition. Paradoxically, while C-X-C chemokine receptor 6 expression enhances T cell proliferation to foster immune eradication of tumor, upregulation of C-X-C chemokine receptor 6 has been associated with tumor promotion and metastasis of several cancers.78 These findings highlight the double edged nature of the immune system in cancer. A recent meta-analysis combined results of 10 prior studies that had examined intraepithelial tumor infiltrating lymphocyte numbers in relation to ovarian cancer survival. Utilizing studies that assessed either CD3 (a pan-T cell marker) or CD8 (a cytotoxic T cell marker) positive cells, they found that the presence of tumor infiltrating lymphocytes, as indicated by either marker, favored overall survival for patient populations that included mixed histologies and grades of ovarian cancer, although most were serous and high grade.79
The role of CD4 helper T cell infiltration is less clear due to shared marker expression with regulatory T cells (Tregs). Both Sato and Milne observed similar outcomes among patients with or without CD4+ T cell staining within tumors,72, 74 while Le Page observed that increased CD4+ cells in 199 high grade serous cancers were associated with improved survival.80 Kryczek and colleagues found that high levels of IL-17 were associated with greatly improved outcome suggesting that Th17 cells, a subset of CD4 helper T cells that produce IL-17, may have a direct role eradicating tumor.81 Given the abundant expression of IL-17 among innate immune effectors, it is difficult to draw a conclusion as to whether intratumoral Th17 cells might impact the clinical course of the disease;82 however, the fact that levels of Th17 cells have a strong inverse correlation with Tregs, suggests that they might be associated with tumor eradication.81
Other specific subsets of antitumor immune effectors have also been studied but with less than clear results. One example is the subset of natural killer (NK) cells, a group of cytotoxic lymphocytes that have two different mature phenotypes: CD16+ CD56dim NK cells found in the periphery, which have high cytolytic function, and CD16− CD56bright NK cells found in the secondary lymphoid tissue with inefficient cytotoxicity.83 NK cells have two types of surface receptors: activating (i.e., NKG2) receptors and inhibitory (i.e., KIR) receptors, and can lyse cells without having to recognize specific antigens.84 The balance between inhibitory and activating signals through the different NK receptors is important in the NK cell activation process. NK cells can also be activated in an antigen-dependent manner to mediate antigen-dependent cellular cytotoxicity, which involves binding of cell bound antibodies to Fc receptors on the NK cells. The activating NKG2 receptors bind to stress ligands such as MICA, MICB, and ULBP1–3, while KIR receptors bind to major histocompatibility complex class I and class I associated ligands.84, 85 Like other cancers, nearly all ovarian cancers express MICA, MICB and ULBP2.86 Furthermore, higher NK cell activity in the peripheral blood of ovarian cancer patients at the time of surgery is predictive of improved progression-free survival.87 However, increased numbers of NK cells in peritoneal and pleural effusions of metastatic ovarian carcinoma have been associated with poorer prognosis.88
The generation of antibody responses to ovarian cancer is a common observation suggesting a role for B cells in disease prognosis.89–91 Although antibody-secreting B cells do not need to be at the tumor site to exert antitumor activity, studies evaluating whether B cell infiltration is associated with improved survival show mixed results.74, 88 Another recent evaluation of infiltrating cells constructed from tumors of women with high grade serous ovarian cancer from 3 patient cohorts, found that infiltration of tumors by both CD8+ and CD20+ (B cell marker) lymphocytes (the two subsets were correlated and often found near each other within the tumor) was associated with increased survival.92 The CD20+ B cells did not express CD27, a memory B cell marker, but IgG sequencing suggested they had been exposed to antigen. The authors hypothesized that these cells are likely involved in modulating CTL recruitment or activity.92
Although studies are somewhat limited in regard to immune cell profiles by histologic subtype, recently, Milne and colleagues assessed leukocyte infiltration and immune marker expression in a large group of ovarian cancer specimens, including 199 high grade serous, 31 mucinous, 125 endometrioid and 132 clear cell EOC cases. They found that high-grade serous had the highest number of tissue cores that stained with the pan leukocyte marker, CD45, and also more frequently contained other immune cell marker positive infiltrates, such as FoxP3, CD25 and CD20, compared to the other subtypes. Tumors with endometrioid histology had the second highest and clear cell and mucinous subtypes had lower percentages with infiltrates overall.74 This study underscores the possibility that the subtypes of ovarian cancer are uniquely recognized by the immune system and, thus, may require different immunotherapeutic approaches.
Evidence suggests that ovarian cancer cells, like other malignancies, manipulate cytotoxic and other effector cells in the microenvironment to suppress cytotoxic responses.93 While CTL are known to be involved in immune surveillance and tumor clearance in ovarian cancer,94, 95 it is well accepted that Tregs and tumor-associated macrophages (M2) inhibitor these cells in the tumor microenvironment.96–98 The term Treg, as previously alluded to, most commonly refers to CD4+CD25+ T cells that were either selected early in development in the thymus with the function of protecting against autoimmune reactions (natural Tregs) or developed in the periphery to regulate immune response to other antigens (adaptive Tregs).99 Several studies have assessed the presence of Tregs in ovarian tumors and found them to be positively associated with poor prognosis/outcome72, 100, 101 or unfavorable clinical characteristics, such as high grade, advanced stage and suboptimal tumor debulking.102 Tregs have multiple tumor promoting effects. In addition to their direct immune suppressive effects, Treg-derived factors such as TGF-β act directly on tumor cells to induce increased aggressiveness.103
Under normal healthy conditions, macrophages are localized to tissue where they carry out various functions including surveying for infectious agents and phagocytizing dead cells. When faced with an infectious challenge, they typically activate and acquire an immune activating phenotype often referred to as an M1 phenotype. Macrophages that infiltrate tumors adopt an alternative phenotype referred to as an M2 phenotype characterized by enhanced tissue regenerative responses and local immune suppression. Tumor associated macrophages (M2) express different markers than classically activated macrophages (M1). Hagemann and colleagues demonstrated that ovarian cancer cells were capable of inducing macrophages to become M2.104 A few studies assessed the presence of infiltrating macrophages using CD68, but did not find an association between these cells and ovarian cancer survival.74, 105 However, one study that assessed M2 maker, CD206, found that patients with tumors that had a higher proportion of CD206+/CD68+ cells also had an increased risk of disease progression.80
In addition to macrophages, ovarian tumors have a pronounced infiltration by dendritic cells (DCs). DCs are classified into two subtypes according to their lineage: plasmacytoid DCs and myeloid DCs.106 Under quiescent conditions, DCs are present in the body in an immature form and are responsible for detecting danger and sampling of antigens. Upon detection of danger through their pathogen-associated molecular pattern receptors or danger-associated molecular pattern receptors, DCs mature and migrate to the lymph nodes to activate T helper cells and CTL.106 Although tumors are able to produce danger signals, they are ineffective in inducing DC maturation and trafficking to lymph nodes which is thought to be due to tumor-induced alterations in DC differentiation thus reducing the number of functional cells available for effective T cell activation.107 For example, ovarian cancers secrete large amounts of IL-10 which promotes differentiation of DC to CD14+CD1a− macrophage-like cells with reduced T cell activation properties.107
Human ovarian cancers contain plasmacytoid and myeloid DCs as well as their precursors. CXCR4+ plasmacytoid precursor cells are attracted into the tumor microenvironment by tumor derived stroma-derived factor 1. Plasmacytoid precursor cells recruited into the ovarian cancer microenvironment induce T cells to release large amounts of IL-10 preventing local T cell activation.108 Myeloid-derived DCs (MDCs) are the dominant DCs in the blood under normal health conditions. Some early studies appear to suggest that there is little accumulation of MDCs in ovarian cancer,108 but more recent studies demonstrate their infiltration. Their role in local immune suppression remains unclear, and may differ in the solid tumor mass compared to actions in ascites fluid.109 Immature MDCs promote angiogenesis and vasculogenesis during tumor growth.110–112 One study suggests that MDCs mediate local immune suppression in ovarian cancer by suppressing the effector function of T cells through the engagement of MDC expressed B7-H1.113 DCs expressing B7-H1 have been shown to inhibit T cell proliferation directly and also indirectly by promoting the induction of CD25highFoxP3+ Tregs, thus suggesting that B7-H1 is a key player in the tumor microenvironment immune suppression.114 B7-H1 is expressed on the surface of several gynecological cancers including ovarian and is associated with poor overall survival in ovarian cancer.115–117 PD-1 was also shown to be expressed on various adaptive immune effectors in the ovarian tumor microenvironment, notably CD4 and CD8 T cells, where it negatively regulates cell activation.118–121 The molecular interactions, particularly those associated with PD-1 remain elusive.113 MDCs mediate local immune suppression not only by expressing PD-1 and B7-H1 but also by generating immune suppressive mediators including arginase, indoleamine 2,3-dioxygenase, nitric oxide and reactive oxygen species.122–124 Overall, the DC component of ovarian cancers remains an intriguing and underexplored area of ovarian cancer research.
One mechanism by which several different types of immune cells are suppressed in the tumor microenvironment is through the production of indoleamine 2,3-dioxygenase (IDO).125, 126 IDO mediates immune tolerance in T cells and natural killer cells by consuming tryptophan and forming kynurenine resulting in inhibition of proliferation and functional impairment.127–131 It also promotes a regulatory T-cell phenotype.132–134 IDO is expressed by many tumor types, including ovarian cancer.135–137 Takao and colleagues reported protein expression of IDO in serous (57.5%), clear cell (49.2%), and endometrioid (73.3%) ovarian adenocarcinomas.137 They additionally found an association between IDO staining in ovarian cancer tumors and lower overall survival for serous ovarian cancer patients with advanced stage disease who had optimal surgery and were given first-line paclitaxel-carboplatin chemotherapy.137 Inaba and colleagues reported a similar association between high compared to low expression of IDO in ovarian cancer tumors of mixed histology, stage and grade and lower overall and progression-free survival. In addition, they found reduced numbers of CD8+ TILS in the tumors expressing high IDO.135 Finally, they reported that SCID mice given IDO-transfected SKOV3 human ovarian cancer xenografts had higher peritoneal dissemination of the tumor compared to mice given the cell line xenograft with a control vector.135 IDO plays an important role in immune suppression and tumor immune evasion and may have potential as a therapeutic target
Several studies have investigated the efficacy of blocking IDO in tumor models.135, 138–142Several matters complicate this, however. First, two IDO genes (IDO1 and IDO2) contribute to tryptophan metabolism, and the contribution of each is not entirely clear (reviewed in Soliman et al..143 Common polymorphisms in IDO2 have been identified that render it inactive in as many as 50% of Caucasians, making it less likely to contribute to any immunosuppressive effects.144, 145 Second, two isomers of the most commonly studied inhibitor, 1-methyl-tryptophan, exist.146, 147 Many early studies used a racemic mixture of the two, however a study by Metz and colleagues found that the L isomer of 1-methyl-tryptophan preferentially inhibits IDO1 while the D isomer preferentially inhibits IDO2.144 In vitro studies demonstrated that the L isoform was superior in restoring T cell and antigen presenting functions in vitro. However, the D isomer, in conjunction with conventional chemotherapy drugs, was more potent than the L isomer in in vivo studies, which led to using this form in early clinical trials.144 Currently, which isoform to use is still contentious. Third, in addition to IDO1 and IDO2, increased expression of TDO (tryptophan 2,3-dioxygenase), normally expressed at high levels in the liver, has recently been reported in several different tumor types, including ovarian cancer.148 Kynurenin, one of the metabolites of tryptophan metabolism, was found to bind to the aryl hydrocarbon receptor, causing this transcription factor to translocate to the nucleus and activate several genes, including those supporting the promotion of regulatory T cells. 148, 149 The structure of TDO differs greatly from IDO1 and IDO2, and neither isomer of 1MT is able to inhibit it. Pilotte and colleagues found that a derivative (LM10) of a known TDO inhibitor (680C91) was bioavailable in mice after oral administration and able to prevent the growth TDO positive tumors.150 Several phase 1 and 2 clinical trials involving IDO inhibitors are currently underway, including NCT01685255, comparing the IDO inhibitor INCB024360 to tamoxifen in biochemical recurrent ovarian cancer (http://www.clinicaltrials.gov/ct2/search/advanced, accessed 2/25/2013). As of yet, no results have been published on the trials.
B. Inherited variation and ovarian cancer survival
Further understanding the role of the immune system in established disease may also be attainable through evaluating associations between genetic variation and recurrence or progression in ovarian cancer. Previously, germline variation in 26 inflammation-related genes was assessed in association with survival in a population of 325 patients with invasive EOC.151 Of those 26 genes, SNPs in CCR3, CCL2, IL1B, IL18, and ALOX5 were related to survival in patients with EOC. As described in the section on inherited variation and ovarian cancer risk, IL18 and ALOX5 encode genes with important immune functions and were found in association with ovarian cancer risk as well. CCR3 encodes the pleiotropic chemokine receptor CCR3 which binds and responds to a variety of chemokines, including CCL11, CCL26, CCL7, CCL13, and CCL5. The receptor is involved in chemotaxis in a wide variety of leukocytes including eosinophils, basophils, and CD4 T cells. CCR2, also known as MCP1 or monocyte chemotactic protein 1, is important for chemotaxis of monocytes and basophils to sites of inflammation. Given the role of macrophages in tumorigenesis, it seems quite plausible that a SNP in this gene could alter macrophage recruitment and therefore survival. IL1B encodes IL1beta, a pro-inflammatory cytokine that also has pleiotropic effects on both the immune system and directly on ovarian cancer cells.152 Still another prospective study of 147 advanced ovarian cancer patients found an association between two IL10 promoter polymorphisms and disease-free and overall survival, possibly due to its relationship with optimal tumor debulking.153 Lastly, in addition to cytokines, genes encoding other modulators of the immune system have been implicated in disease recurrence and progression. TLR4, normally expressed at high levels on immune cells, detects lipopolysaccharide from Gram-negative bacteria and is important in the activation of the innate immune system through NF-κB. In a clinical outcomes study of ovarian cancer patients, a putative microRNA binding site polymorphism in Toll-like receptor 4 (TLR4) (rs7869402) was associated with inferior survival and response to treatment.53 Another putative microRNA binding site SNP in NFKBIB (rs3136642) examined in this study was also marginally associated with superior survival.53
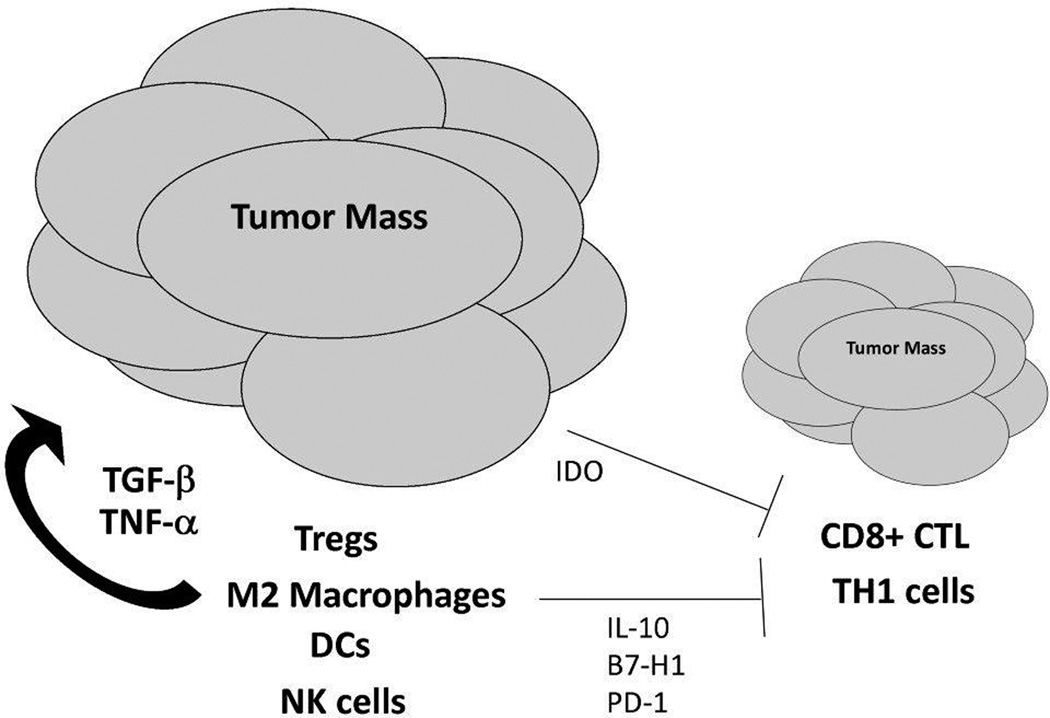
In conclusion, it is clear from the multitude of reports that the immune system is a central component of the ovarian cancer microenvironment and contributes to the variable outcomes seen in patients. Figure 1 shows that there are many immune effectors that favor tumor progression either by releasing factors that directly promote growth or through direct blockade of immune effectors that elicit immune destruction. Based on this paradigm, there has been significant interest in combination approaches that induce T cell immunity in conjunction with other agents that reduce suppressive cells. Much work has focused on blocking Tregs along with CTL inducing vaccines. Agents like cyclophosphamide which augment immune-based therapies in both human and mouse studies are thought to have pro-immune functions due to dose-dependent Treg depletion.154 Other strategies that are being explored to inhibit the function of Tregs include anti-CTLA-4 monoclonal antibodies and CD25-targeted agents such as Denileukin Diftitox.155
Figure 1.
The immune system favors tumor progression in ovarian cancer
VI. MOLECULAR INTERACTIONS IN THE OVARIAN CANCER IMMUNE MICROENVIRONMENT: THE TNFα, CXCL12, IL-6 AXIS
In contrast to agents that directly increase or decrease specific immune cell subsets, another concept that has recently been introduced is the disruption of integrated cytokine networks. One integrated cytokine network for which there is considerable published support, termed the ‘TNF network’ – consisting of TNF, CXCL12, and IL-6 – was recently identified by Kulbe and colleagues in human ovarian cancer specimens.156 High level expression of IL-6 has been found in multiple cancers, including ovarian,157 breast,158, 159 prostate,160 colon,161, 162 lung,163 and multiple myeloma.164–166 In addition to direct synthesis by cancer cells, infiltrating T cells and macrophages are also major sources of IL-6. IL-6 directly promotes cancer growth and progression through several mechanisms, including proliferation, angiogenesis, and decreased sensitivity to apoptotic signals.167, 168 High levels of IL-6 in the serum of ovarian cancer patients have been associated with shorter survival.169, 170 Additionally, high levels of IL-6 are often detected in the malignant ascites of ovarian cancer patients.171 IL-6 mediates its pathologic effects on cancers via several mechanisms. First, IL-6 signaling results in the activation of several pathways, including the JAK/STAT, PI3K/Akt, and Ras/MEK/IRK pathways.169, 170, 172, 173 Second, phosphorylated Stat3, resulting from the JAK/STAT pathway activation, can bind to and inhibit p53 responses to pro-apoptotic signals.167
Because of the multiple pro-tumor effects of IL-6, it has emerged as a logical candidate for targeted therapy [reviewed in 167, 174]. Several antagonists have been developed to inhibit IL-6 signaling. BE-8, a murine anti-IL-6 monoclonal antibody was initially used in clinical trials; however, the short half-life and inability to block higher levels of IL-6 often seen in patients make it a less than ideal choice for advanced studies.175 Siltuximab (CNTO328), a human-mouse chimeric IL-6 antibody, has been used in multiple clinical trials for a variety of cancers, including ovarian, renal, and prostate.156, 176–179 CNTO328 is generally well tolerated, however, responses are mixed. In a phase II trial of 18 ovarian cancer patients, one individual had a partial response and 7 had stable disease for a period of time.168 The effects of IL-6 blockade appear to be cancer specific. For example, a phase I study using CNT0328 in castration-resistant prostate cancer patients resulted in decreased expression of genes downstream of the IL-6 signaling pathway but no improvement in outcome.178 Additional strategies, such as targeting the IL-6R (Tocilizumab) or inhibition of Jak/Stat signaling through a Jak inhibitor, have been tried in various cancers and diseases; at this point it is not clear if these will offer a benefit over targeting IL-6 directly.180–184
CXCR4 has been previously reported to be constitutively expressed in ovarian cancer; the corresponding ligand, CXCL12 is secreted by supporting stromal and endothelial cells in addition to ovarian cancer cells [reviewed in 185]. High levels of CXCL12 are also found in malignant ascites.186–188 Chemokine signaling through CXCR4 has multiple effects, including stimulation of proliferation, vascular endothelial growth factor-mediated angiogenesis, and altered recruitment of immune cells toward tumors.189–192 Righi and colleagues studied the effects of CXCL12 blockade in an immunocompetent mouse model.192 Syngeneic tumor cells expressing CXCL12 and CXCR4 were injected intraperitoneally into mice and chemokine signaling was interrupted by either knocking down expression of CXCL12 or via administration of AMD3100, a CXCR4 antagonist. Not only did blockade of the CXCL12/CXCR4 signaling increase survival and tumor cell apoptosis, it also resulted in a specific shift of the types of T cells recruited to the tumors. Tregs express higher levels of CXCR4 than CD8 T cells do, making them more sensitive to changes in availability of the ligand. Thus, blockade of the CXCL12/CXCR4 signaling cascade resulted in decreased recruitment of Tregs to the tumor and a subsequent significant increase in the CD8/Treg cell ratio and an increase in the antitumor immune response.192
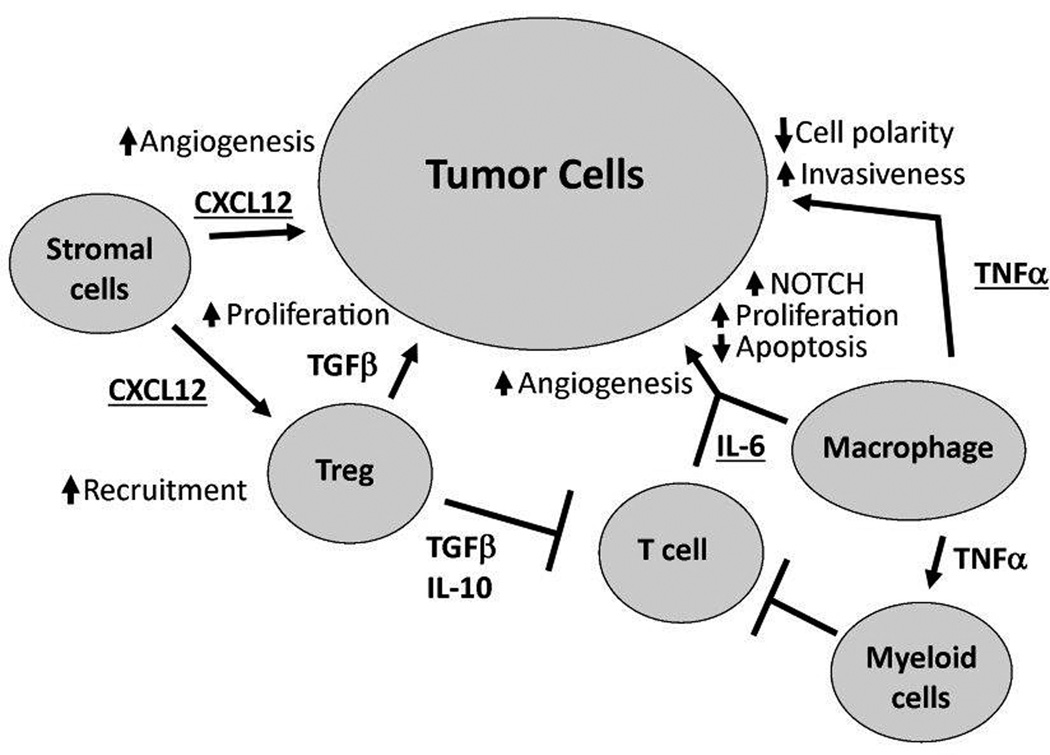
TNFα expression in various cancers including ovarian cancer has long been studied. Its expression is highly correlated with increased expression CXCL12 and IL-6,193 and this results in the ability of TNFα to mediate a variety of effects. The resultant effect of TNFα on a tumor can depend on both the timing and dosage; a single, high-dose exposure may promote tumor regression while chronic, low-dose expression of TNFα may be part of a tumor promoting network in ovarian cancer.194, 195 In vitro, exposure of ovarian surface epithelial cells grown as organoids to TNFα resulted in a precancerous phenotype marked by increased proliferation, decreased cellular organization, disruption of epithelial cell polarity, multilayer stratification, and loss of a functional basement membrane.196 At the same time, the cells increased expression of CA125, MUC1, Claudin 4, and CXCR4.196 In vitro studies of ovarian cancer cell lines using RNAi to decrease expression of TNFα did not result in any changes in proliferation or apoptosis; however, when the same cells were injected in vivo, a decrease in tumor burden, restricted distribution, and increased apoptosis was evident compared to control cells.193 Kulbe and colleagues suggest that these differential effects of TNFα blockade in vivo are due to disruption of a multi-cytokine network constituted by TNFα, CXCL12 and IL-6 that results in reduced angiogenesis, infiltration of myeloid cells and NOTCH signaling (Figure 2).156
Figure 2.
The TNF network contributes to ovarian cancer pathogenesis
VII. IMMUNE INTERACTIONS IN THE DEVELOPMENT OF CHEMORESISTANCE
Expression of IL-6 has also been linked to increased expression of the multidrug resistance gene 1, leading to resistance to paclitaxel.169, 197 Paclitaxel is useful in treating ovarian cancer as it binds to microtubules, preventing their depolymerization, resulting in an interruption of mitosis and pro-apoptotic signaling. Paclitaxel exposure can also increase the expression of IL-6, resulting in a positive feedback loop promoting increased drug resistance and additional IL-6 expression.173 Development of chemoresistance is a major hurdle in the treatment of ovarian cancer.198 Several factors related to inflammation have been shown to modulate the response of ovarian cancer cells to the common therapeutic agents, paclitaxel and cisplatin. However, it is notable that paclitaxel has also been shown to be able to bind and activate TLR4.199 As mentioned above, TLR4, is normally expressed at high levels on immune cells, detects bacterial lipopolysaccharide and is important in the activation of the innate immune system. TLR4 is expressed on many ovarian cancer cell lines and in ovarian tumors; expression of the TLR adaptor protein MyD88 is cell line specific, however.199 Several studies have shown that MyD88 positive ovarian cancer cells are resistant to paclitaxel while the MyD88 negative counterparts remain sensitive.199–203 The mechanisms for resistance include increased expression of anti-apoptotic proteins BCL2 and XIAP, activation of Akt survival signaling, and increased production of pro-inflammatory cytokines such as IL-6, IL-8, and RANTES through activation of NFkB.199, 202, 203 The sum of these pro-inflammatory pro-survival signals is the ability of paclitaxel to prevent the induction of apoptosis. Thus, paclitaxel can paradoxically cause increased apoptosis or survival, depending on the MyD88 and TLR4 status of the cells. In addition to binding lipopolysaccharide and paclitaxel, in recent years it has become clear that TLR4 is also able to bind host derived products. Heparan sulfate is one such product of relevance to ovarian cancer, as its degradation fragments are known to be potent ligands for TLR4.204 Furthermore, overexpression of the enzyme that generates these fragments, heparanase, correlates with poor survival in ovarian cancer.205
VIII. CONCLUSIONS
From initiating events to long-term survival, the immune system plays a multifaceted role in ovarian cancer pathogenesis. Although most studies to this point have been limited in numbers to determine subtype-specific immune profiles, there is some evidence to suggest different immune gene products (e.g. cytokines and chemokines) and cells may play an integrated role in development and progression of ovarian cancer. Thus, it appears that ovarian cancer, because of its complex interactions with the immune system must be thought of in a systems biology model in order to achieve a better understanding of how to employ novel immune therapies to determine risk, diagnose, treat and understand the prognosis of those afflicted by this detrimental disease.
ACKNOWLEDGEMENTS
The authors are supported by the Mayo Clinic Ovarian Cancer SPORE (P50-CA136393, Awardee: Dr. Lynn Hartmann of the Mayo Clinic) and National Cancer Institute Research Project Grant (R01-CA-122443, Awardee: Dr. Ellen Goode of the Mayo Clinic). BC and MSD are supported by a National Cancer Institute Training Program Grant (R25 CA92049, Awardee: Dr. Gloria Petersen of the Mayo Clinic).
Abbreviations
- ALOX5
arachidonate 5-lipoxigenase
- CASP8
caspase-8
- CRP
C-reactive protein
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- EOC
epithelial ovarian cancer
- IDO
indoleamine 2,3-dioxygenase
- MDC
myeloid-derived dendritic cell
- NK
natural killer (cell)
- NSAIDs
non-steroidal anti-inflammatory drugs
- OR
odds ratio
- PTGS2
prostaglandin endoperoxide synthase 2
- SNP
single nucleotide polymorphism
- TLR4
toll-like receptor 4
- Treg
regulatory T cell
Contributor Information
Bridget Charbonneau, Email: charbonneau.bridget@mayo.edu, Department of Health Sciences Research, Mayo Clinic, Rochester, MN 55906.
Ellen L. Goode, Email: egoode@mayo.edu, Department of Health Sciences Research, Mayo Clinic, Rochester, MN 55906.
Kimberly R. Kalli, Email: kalli.kimberly@mayo.edu, Division of Oncology, Mayo Clinic, Rochester, MN 55906.
Keith L. Knutson, Email: knutson.keith@mayo.edu, Department of Immunology, Mayo Clinic, Rochester, MN 55906.
Melissa S. DeRycke, Email: derycke.melissa@mayo.edu, Department of Health Sciences Research, Mayo Clinic, Rochester, MN 55906.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007;82:751–770. doi: 10.4065/82.6.751. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK, Stronach EA, Walczak H, Bowtell DD, Balkwill FR. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122:170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 7.Mahdavi A, Pejovic T, Nezhat F. Induction of ovulation and ovarian cancer: a critical review of the literature. Fertil Steril. 2006;85:819–826. doi: 10.1016/j.fertnstert.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 8.Modan B, Hartge P, Hirsh-Yechezkel G, Chetrit A, Lubin F, Beller U, Ben-Baruch G, Fishman A, Menczer J, Struewing JP, Tucker MA, Wacholder S. Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235–240. doi: 10.1056/NEJM200107263450401. [DOI] [PubMed] [Google Scholar]
- 9.Adami HO, Hsieh CC, Lambe M, Trichopoulos D, Leon D, Persson I, Ekbom A, Janson PO. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. 1994;344:1250–1254. doi: 10.1016/s0140-6736(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 10.Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 11.Narod SA, Risch H, Moslehi R, Dorum A, Neuhausen S, Olsson H, Provencher D, Radice P, Evans G, Bishop S, Brunet JS, Ponder BA. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med. 1998;339:424–428. doi: 10.1056/NEJM199808133390702. [DOI] [PubMed] [Google Scholar]
- 12.Jordan SJ, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Breast-feeding and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23:919–927. doi: 10.1007/s10552-012-9963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan SJ, Siskind V, A CG, Whiteman DC, Webb PM. Breastfeeding and risk of epithelial ovarian cancer. Cancer Causes Control. 2010;21:109–116. doi: 10.1007/s10552-009-9440-x. [DOI] [PubMed] [Google Scholar]
- 14.Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer. 2001;84:714–721. doi: 10.1054/bjoc.2000.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casagrande JT, Louie EW, Pike MC, Roy S, Ross RK, Henderson BE. "Incessant ovulation" and ovarian cancer. Lancet. 1979;2:170–173. doi: 10.1016/s0140-6736(79)91435-1. [DOI] [PubMed] [Google Scholar]
- 16.King SM, Hilliard TS, Wu LY, Jaffe RC, Fazleabas AT, Burdette JE. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627–642. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaytan M, Morales C, Bellido C, Sanchez-Criado JE, Gaytan F. Macrophages in human fallopian tube and ovarian epithelial inclusion cysts. J Reprod Immunol. 2007;73:66–73. doi: 10.1016/j.jri.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Wehner AP. Biological effects of cosmetic talc. Food Chem Toxicol. 1994;32:1173–1184. doi: 10.1016/0278-6915(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 19.Mills PK, Riordan DG, Cress RD, Young HA. Perineal talc exposure and epithelial ovarian cancer risk in the Central Valley of California. Int J Cancer. 2004;112:458–464. doi: 10.1002/ijc.20434. [DOI] [PubMed] [Google Scholar]
- 20.Rosenblatt KA, Weiss NS, Cushing-Haugen KL, Wicklund KG, Rossing MA. Genital powder exposure and the risk of epithelial ovarian cancer. Cancer Causes Control. 2011;22:737–742. doi: 10.1007/s10552-011-9746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124:1409–1415. doi: 10.1002/ijc.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huncharek M, Geschwind JF, Kupelnick B. Perineal application of cosmetic talc and risk of invasive epithelial ovarian cancer: a meta-analysis of 11,933 subjects from sixteen observational studies. Anticancer Res. 2003;23:1955–1960. [PubMed] [Google Scholar]
- 23.Huncharek M, Muscat J. Perineal talc use and ovarian cancer risk: a case study of scientific standards in environmental epidemiology. Eur J Cancer Prev. 2011;20:501–507. doi: 10.1097/CEJ.0b013e3283476242. [DOI] [PubMed] [Google Scholar]
- 24.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139–147. doi: 10.1159/000093121. [DOI] [PubMed] [Google Scholar]
- 26.Rier SE. The potential role of exposure to environmental toxicants in the pathophysiology of endometriosis. Ann N Y Acad Sci. 2002;955:201–212. doi: 10.1111/j.1749-6632.2002.tb02781.x. discussion 230-202, 396–406. [DOI] [PubMed] [Google Scholar]
- 27.D'Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update. 2002;8:84–88. doi: 10.1093/humupd/8.1.84. [DOI] [PubMed] [Google Scholar]
- 28.Risch HA, Howe GR. Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:447–451. [PubMed] [Google Scholar]
- 29.Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin WZ, Wu SC, Lai YL. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol. 2011;12:900–904. doi: 10.1016/S1470-2045(11)70165-6. [DOI] [PubMed] [Google Scholar]
- 30.Bonovas S, Filioussi K, Sitaras NM. Do nonsteroidal anti-inflammatory drugs affect the risk of developing ovarian cancer? A meta-analysis. Br J Clin Pharmacol. 2005;60:194–203. doi: 10.1111/j.1365-2125.2005.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Moult J. SNPs, protein structure, and disease. Hum Mutat. 2001;17:263–270. doi: 10.1002/humu.22. [DOI] [PubMed] [Google Scholar]
- 32.Bolton KL, Tyrer J, Song H, Ramus SJ, Notaridou M, Jones C, Sher T, Gentry-Maharaj A, Wozniak E, Tsai YY, Weidhaas J, Paik D, Van Den Berg DJ, Stram DO, Pearce CL, Wu AH, Brewster W, Anton-Culver H, Ziogas A, Narod SA, Levine DA, Kaye SB, Brown R, Paul J, Flanagan J, Sieh W, McGuire V, Whittemore AS, Campbell I, Gore ME, Lissowska J, Yang HP, Medrek K, Gronwald J, Lubinski J, Jakubowska A, Le ND, Cook LS, Kelemen LE, Brook-Wilson A, Massuger LF, Kiemeney LA, Aben KK, van Altena AM, Houlston R, Tomlinson I, Palmieri RT, Moorman PG, Schildkraut J, Iversen ES, Phelan C, Vierkant RA, Cunningham JM, Goode EL, Fridley BL, Kruger-Kjaer S, Blaeker J, Hogdall E, Hogdall C, Gross J, Karlan BY, Ness RB, Edwards RP, Odunsi K, Moyisch KB, Baker JA, Modugno F, Heikkinenen T, Butzow R, Nevanlinna H, Leminen A, Bogdanova N, Antonenkova N, Doerk T, Hillemanns P, Durst M, Runnebaum I, Thompson PJ, Carney ME, Goodman MT, Lurie G, Wang-Gohrke S, Hein R, Chang-Claude J, Rossing MA, Cushing-Haugen KL, Doherty J, Chen C, Rafnar T, Besenbacher S, Sulem P, Stefansson K, Birrer MJ, Terry KL, Hernandez D, Cramer DW, Vergote I, Amant F, Lambrechts D, Despierre E, Fasching PA, Beckmann MW, Thiel FC, Ekici AB, Chen X, Johnatty SE, Webb PM, Beesley J, Chanock S, Garcia-Closas M, Sellers T, Easton DF, Berchuck A, Chenevix-Trench G, Pharoah PD, Gayther SA. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet. 2010;42:880–884. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goode EL, Chenevix-Trench G, Song H, Ramus SJ, Notaridou M, Lawrenson K, Widschwendter M, Vierkant RA, Larson MC, Kjaer SK, Birrer MJ, Berchuck A, Schildkraut J, Tomlinson I, Kiemeney LA, Cook LS, Gronwald J, Garcia-Closas M, Gore ME, Campbell I, Whittemore AS, Sutphen R, Phelan C, Anton-Culver H, Pearce CL, Lambrechts D, Rossing MA, Chang-Claude J, Moysich KB, Goodman MT, Dork T, Nevanlinna H, Ness RB, Rafnar T, Hogdall C, Hogdall E, Fridley BL, Cunningham JM, Sieh W, McGuire V, Godwin AK, Cramer DW, Hernandez D, Levine D, Lu K, Iversen ES, Palmieri RT, Houlston R, van Altena AM, Aben KKH, Massuger LFAG, Brooks-Wilson A, Kelemen LE, Le ND, Jakubowska A, Lubinski J, Medrek K, Stafford A, Easton DF, Tyrer J, Bolton KL, Harrington P, Eccles D, Chen A, Molina AN, Davila BN, Arango H, Tsai Y-Y, Chen Z, Risch HA, McLaughlin J, Narod SA, Ziogas A, Brewster W, Gentry-Maharaj A, Menon U, Wu AH, Stram DO, Pike MC, Beesley J, Webb PM. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet. 2010;42:874–879. doi: 10.1038/ng.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song H, Ramus SJ, Tyrer J, Bolton KL, Gentry-Maharaj A, Wozniak E, Anton-Culver H, Chang-Claude J, Cramer DW, DiCioccio R, Dork T, Goode EL, Goodman MT, Schildkraut JM, Sellers T, Baglietto L, Beckmann MW, Beesley J, Blaakaer J, Carney ME, Chanock S, Chen Z, Cunningham JM, Dicks E, Doherty JA, Durst M, Ekici AB, Fenstermacher D, Fridley BL, Giles G, Gore ME, De Vivo I, Hillemanns P, Hogdall C, Hogdall E, Iversen ES, Jacobs IJ, Jakubowska A, Li D, Lissowska J, Lubinski J, Lurie G, McGuire V, McLaughlin J, Medrek K, Moorman PG, Moysich K, Narod S, Phelan C, Pye C, Risch H, Runnebaum IB, Severi G, Southey M, Stram DO, Thiel FC, Terry KL, Tsai YY, Tworoger SS, Van Den Berg DJ, Vierkant RA, Wang-Gohrke S, Webb PM, Wilkens LR, Wu AH, Yang H, Brewster W, Ziogas A, Houlston R, Tomlinson I, Whittemore AS, Rossing MA, Ponder BA, Pearce CL, Ness RB, Menon U, Kjaer SK, Gronwald J, Garcia-Closas M, Fasching PA, Easton DF, Chenevix-Trench G, Berchuck A, Pharoah PD, Gayther SA. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet. 2009;41:996–1000. doi: 10.1038/ng.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karban AS, Okazaki T, Panhuysen CI, Gallegos T, Potter JJ, Bailey-Wilson JE, Silverberg MS, Duerr RH, Cho JH, Gregersen PK, Wu Y, Achkar JP, Dassopoulos T, Mezey E, Bayless TM, Nouvet FJ, Brant SR. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet. 2004;13:35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- 36.Fan Y, Yu W, Ye P, Wang H, Wang Z, Meng Q, Duan Y, Liang X, An W. NFKB1 insertion/deletion promoter polymorphism increases the risk of advanced ovarian cancer in a Chinese population. DNA Cell Biol. 2011;30:241–245. doi: 10.1089/dna.2010.1107. [DOI] [PubMed] [Google Scholar]
- 37.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 38.Lubinski J, Huzarski T, Kurzawski G, Suchy J, Masojc B, Mierzejewski M, Lener M, Domagala W, Chosia M, Teodorczyk U, Medrek K, Debniak T, Zlowocka E, Gronwald J, Byrski T, Grabowska E, Nej K, Szymanska A, Szymanska J, Matyjasik J, Cybulski C, Jakubowska A, Gorski B, Narod SA. The 3020insC Allele of NOD2 Predisposes to Cancers of Multiple Organs. Hered Cancer Clin Pract. 2005;3:59–63. doi: 10.1186/1897-4287-3-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White KL, Vierkant RA, Phelan CM, Fridley BL, Anderson S, Knutson KL, Schildkraut JM, Cunningham JM, Kelemen LE, Pankratz VS, Rider DN, Liebow M, Hartmann LC, Sellers TA, Goode EL. Polymorphisms in NF-kappaB inhibitors and risk of epithelial ovarian cancer. BMC Cancer. 2009;9:170. doi: 10.1186/1471-2407-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X, Zhang J, Liu S, Huang Y, Chen B, Wang D. Polymorphisms in the CASP8 gene and the risk of epithelial ovarian cancer. Gynecol Oncol. 2011;122:554–559. doi: 10.1016/j.ygyno.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Sun T, Gao Y, Tan W, Ma S, Shi Y, Yao J, Guo Y, Yang M, Zhang X, Zhang Q, Zeng C, Lin D. A six-nucleotide insertion-deletion polymorphism in the CASP8 promoter is associated with susceptibility to multiple cancers. Nat Genet. 2007;39:605–613. doi: 10.1038/ng2030. [DOI] [PubMed] [Google Scholar]
- 42.Engel C, Versmold B, Wappenschmidt B, Simard J, Easton DF, Peock S, Cook M, Oliver C, Frost D, Mayes R, Evans DG, Eeles R, Paterson J, Brewer C, McGuffog L, Antoniou AC, Stoppa-Lyonnet D, Sinilnikova OM, Barjhoux L, Frenay M, Michel C, Leroux D, Dreyfus H, Toulas C, Gladieff L, Uhrhammer N, Bignon YJ, Meindl A, Arnold N, Varon-Mateeva R, Niederacher D, Preisler-Adams S, Kast K, Deissler H, Sutter C, Gadzicki D, Chenevix-Trench G, Spurdle AB, Chen X, Beesley J, Olsson H, Kristoffersson U, Ehrencrona H, Liljegren A, van der Luijt RB, van Os TA, van Leeuwen FE, Domchek SM, Rebbeck TR, Nathanson KL, Osorio A, Ramon y Cajal T, Konstantopoulou I, Benitez J, Friedman E, Kaufman B, Laitman Y, Mai PL, Greene MH, Nevanlinna H, Aittomaki K, Szabo CI, Caldes T, Couch FJ, Andrulis IL, Godwin AK, Hamann U, Schmutzler RK. Association of the variants CASP8 D302H and CASP10 V410I with breast and ovarian cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2010;19:2859–2868. doi: 10.1158/1055-9965.EPI-10-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramus SJ, Vierkant RA, Johnatty SE, Pike MC, Van Den Berg DJ, Wu AH, Pearce CL, Menon U, Gentry-Maharaj A, Gayther SA, Dicioccio RA, McGuire V, Whittemore AS, Song H, Easton DF, Pharoah PD, Garcia-Closas M, Chanock S, Lissowska J, Brinton L, Terry KL, Cramer DW, Tworoger SS, Hankinson SE, Berchuck A, Moorman PG, Schildkraut JM, Cunningham JM, Liebow M, Kjaer SK, Hogdall E, Hogdall C, Blaakaer J, Ness RB, Moysich KB, Edwards RP, Carney ME, Lurie G, Goodman MT, Wang-Gohrke S, Kropp S, Chang-Claude J, Webb PM, Chen X, Beesley J, Chenevix-Trench G, Goode EL. Consortium analysis of 7 candidate SNPs for ovarian cancer. Int J Cancer. 2008;123:380–388. doi: 10.1002/ijc.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita T, Taira S, Kodama N, Matsushita M. Mannose-binding protein recognizes glioma cells: in vitro analysis of complement activation on glioma cells via the lectin pathway. Jpn J Cancer Res. 1995;86:187–192. doi: 10.1111/j.1349-7006.1995.tb03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Y, Uemura K, Oka S, Kozutsumi Y, Kawasaki N, Kawasaki T. Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding proteindependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1999;96:371–375. doi: 10.1073/pnas.96.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nevadunsky NS, Korneeva I, Caputo T, Witkin SS. Mannose-binding lectin codon 54 genetic polymorphism and vaginal protein levels in women with gynecologic malignancies. Eur J Obstet Gynecol Reprod Biol. 2012;163:216–218. doi: 10.1016/j.ejogrb.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang H, Gu J, Lin X, Grossman HB, Ye Y, Dinney CP, Wu X. Profiling of genetic variations in inflammation pathway genes in relation to bladder cancer predisposition. Clin Cancer Res. 2008;14:2236–2244. doi: 10.1158/1078-0432.CCR-07-1670. [DOI] [PubMed] [Google Scholar]
- 48.Lurie G, Terry KL, Wilkens LR, Thompson PJ, McDuffie KE, Carney ME, Palmieri RT, Cramer DW, Goodman MT. Pooled analysis of the association of PTGS2 rs5275 polymorphism and NSAID use with invasive ovarian carcinoma risk. Cancer Causes Control. 2010;21:1731–1741. doi: 10.1007/s10552-010-9602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinheiro SP, Gates MA, DeVivo I, Rosner BA, Tworoger SS, Titus-Ernstoff L, Hankinson SE, Cramer DW. Interaction between use of non-steroidal anti-inflammatory drugs and selected genetic polymorphisms in ovarian cancer risk. Int J Mol Epidemiol Genet. 2010;1:320–331. [PMC free article] [PubMed] [Google Scholar]
- 50.White KL, Schildkraut JM, Palmieri RT, Iversen ES, Jr, Berchuck A, Vierkant RA, Rider DN, Charbonneau B, Cicek MS, Sutphen R, Birrer MJ, Pharoah PP, Song H, Tyrer J, Gayther SA, Ramus SJ, Wentzensen N, Yang HP, Garcia-Closas M, Phelan CM, Cunningham JM, Fridley BL, Sellers TA, Goode EL. Ovarian Cancer Risk Associated with Inherited Inflammation-Related Variants. Cancer Res. 2012;72:1064–1069. doi: 10.1158/0008-5472.CAN-11-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawaguchi Y, Tochimoto A, Hara M, Kawamoto M, Sugiura T, Saito S, Kamatani N. Contribution of single nucleotide polymorphisms of the IL1A gene to the cleavage of precursor IL-1alpha and its transcription activity. Immunogenetics. 2007;59:441–448. doi: 10.1007/s00251-007-0213-y. [DOI] [PubMed] [Google Scholar]
- 52.Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang D, Meyer L, Chang DW, Lin J, Pu X, Ye Y, Gu J, Wu X, Lu K. Genetic variants in MicroRNA biosynthesis pathways and binding sites modify ovarian cancer risk, survival, and treatment response. Cancer Res. 2010;70:9765–9776. doi: 10.1158/0008-5472.CAN-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmieri RT, Wilson MA, Iversen ES, Clyde MA, Calingaert B, Moorman PG, Poole C, Anderson AR, Anderson S, Anton-Culver H, Beesley J, Hogdall E, Brewster W, Carney ME, Chen X, Chenevix-Trench G, Chang-Claude J, Cunningham JM, Dicioccio RA, Doherty JA, Easton DF, Edlund CK, Gayther SA, Gentry-Maharaj A, Goode EL, Goodman MT, Kjaer SK, Hogdall CK, Hopkins MP, Jenison EL, Blaakaer J, Lurie G, McGuire V, Menon U, Moysich KB, Ness RB, Pearce CL, Pharoah PD, Pike MC, Ramus SJ, Rossing MA, Song H, Terada KY, Vandenberg D, Vierkant RA, Wang-Gohrke S, Webb PM, Whittemore AS, Wu AH, Ziogas A, Berchuck A, Schildkraut JM. Polymorphism in the IL18 gene and epithelial ovarian cancer in non-Hispanic white women. Cancer Epidemiol Biomarkers Prev. 2008;17:3567–3572. doi: 10.1158/1055-9965.EPI-08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He M, Cornelis MC, Kraft P, van Dam RM, Sun Q, Laurie CC, Mirel DB, Chasman DI, Ridker PM, Hunter DJ, Hu FB, Qi L. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol. 2010;30:885–890. doi: 10.1161/ATVBAHA.109.199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 57.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 2007;65:S253–S259. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 58.Toriola AT, Grankvist K, Agborsangaya CB, Lukanova A, Lehtinen M, Surcel HM. Changes in pre-diagnostic serum C-reactive protein concentrations and ovarian cancer risk: a longitudinal study. Ann Oncol. 2011;22:1916–1921. doi: 10.1093/annonc/mdq694. [DOI] [PubMed] [Google Scholar]
- 59.Lundin E, Dossus L, Clendenen T, Krogh V, Grankvist K, Wulff M, Sieri S, Arslan AA, Lenner P, Berrino F, Hallmans G, Zeleniuch-Jacquotte A, Toniolo P, Lukanova A. C-reactive protein and ovarian cancer: a prospective study nested in three cohorts (Sweden, USA, Italy) Cancer Causes Control. 2009;20:1151–1159. doi: 10.1007/s10552-009-9330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, Koenig KL, Berrino F, Lukanova A, Lokshin AE, Idahl A, Ohlson N, Hallmans G, Krogh V, Sieri S, Muti P, Marrangoni A, Nolen BM, Liu M, Shore RE, Arslan AA. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:799–810. doi: 10.1158/1055-9965.EPI-10-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol. 1997;66:27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- 62.Jacobs IJ, Skates SJ, MacDonald N, Menon U, Rosenthal AN, Davies AP, Woolas R, Jeyarajah AR, Sibley K, Lowe DG, Oram DH. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353:1207–1210. doi: 10.1016/S0140-6736(98)10261-1. [DOI] [PubMed] [Google Scholar]
- 63.Jacobs I, Bast RC. The CA 125 tumour-associated antigen: a review of the literature. Human Reproduction. 1989;4:1–12. doi: 10.1093/oxfordjournals.humrep.a136832. [DOI] [PubMed] [Google Scholar]
- 64.Ruibal A, Encabo G, Martinezmiralles E, Murcia C, Capdevila JA, Salgado A, Martinezvasquez JM. CA125 SERIC LEVELS IN NON MALIGNANT PATHOLOGIES. Bulletin Du Cancer. 1984;71:145–148. [PubMed] [Google Scholar]
- 65.Edgell T, Martin-Roussety G, Barker G, Autelitano DJ, Allen D, Grant P, Rice GE. Phase II biomarker trial of a multimarker diagnostic for ovarian cancer. J Cancer Res Clin Oncol. 2010;136:1079–1088. doi: 10.1007/s00432-009-0755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lambeck AJ, Crijns AP, Leffers N, Sluiter WJ, ten Hoor KA, Braid M, van der Zee AG, Daemen T, Nijman HW, Kast WM. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res. 2007;13:2385–2391. doi: 10.1158/1078-0432.CCR-06-1828. [DOI] [PubMed] [Google Scholar]
- 67.Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB, Lokshin AE. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:981–987. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- 68.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haskill S, Becker S, Fowler W, Walton L. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br J Cancer. 1982;45:728–736. doi: 10.1038/bjc.1982.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 71.Ashton-Rickardt PG. The granule pathway of programmed cell death. Crit Rev Immunol. 2005;25:161–182. doi: 10.1615/critrevimmunol.v25.i3.10. [DOI] [PubMed] [Google Scholar]
- 72.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, Ferrera F, Balestra P, Ghio M, Contini P, Setti M, Olive D, Azzarone B, Carmignani G, Ravetti JL, Torre G, Indiveri F. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 76.Callahan MJ, Nagymanyoki Z, Bonome T, Johnson ME, Litkouhi B, Sullivan EH, Hirsch MS, Matulonis UA, Liu J, Birrer MJ, Berkowitz RS, Mok SC. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin Cancer Res. 2008;14:7667–7673. doi: 10.1158/1078-0432.CCR-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leffers N, Fehrmann RS, Gooden MJ, Schulze UR, Ten Hoor KA, Hollema H, Boezen HM, Daemen T, de Jong S, Nijman HW, van der Zee AG. Identification of genes and pathways associated with cytotoxic T lymphocyte infiltration of serous ovarian cancer. Br J Cancer. 2010;103:685–692. doi: 10.1038/sj.bjc.6605820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darash-Yahana M, Gillespie JW, Hewitt SM, Chen YY, Maeda S, Stein I, Singh SP, Bedolla RB, Peled A, Troyer DA, Pikarsky E, Karin M, Farber JM. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS One. 2009;4:e6695. doi: 10.1371/journal.pone.0006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Page C, Marineau A, Bonza PK, Rahimi K, Cyr L, Labouba I, Madore J, Delvoye N, Mes-Masson AM, Provencher DM, Cailhier JF. BTN3A2 Expression in Epithelial Ovarian Cancer Is Associated with Higher Tumor Infiltrating T Cells and a Better Prognosis. PLoS ONE. 2012;7:e38541. doi: 10.1371/journal.pone.0038541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 83.Perussia B, Chen Y, Loza MJ. Peripheral NK cell phenotypes: multiple changing of faces of an adapting, developing cell. Mol Immunol. 2005;42:385–395. doi: 10.1016/j.molimm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 84.Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Lopez-Larrea C, Suarez-Alvarez B, Lopez-Soto A, Lopez-Vazquez A, Gonzalez S. The NKG2D receptor: sensing stressed cells. Trends Mol Med. 2008;14:179–189. doi: 10.1016/j.molmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, Yagi H, Yamaguchi K, Baba T, Fujii S, Konishi I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother. 2009;58:641–652. doi: 10.1007/s00262-008-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garzetti GG, Cignitti M, Ciavattini A, Fabris N, Romanini C. Natural killer cell activity and progression-free survival in ovarian cancer. Gynecol Obstet Invest. 1993;35:118–120. doi: 10.1159/000292678. [DOI] [PubMed] [Google Scholar]
- 88.Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, Davidson B, Risberg B. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125:451–458. [PubMed] [Google Scholar]
- 89.Goodell V, Salazar LG, Urban N, Drescher CW, Gray H, Swensen RE, McIntosh MW, Disis ML. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J Clin Oncol. 2006;24:762–768. doi: 10.1200/JCO.2005.03.2813. [DOI] [PubMed] [Google Scholar]
- 90.Knutson KL, Krco CJ, Erskine CL, Goodman K, Kelemen LE, Wettstein PJ, Low PS, Hartmann LC, Kalli KR. T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J Clin Oncol. 2006;24:4254–4261. doi: 10.1200/JCO.2006.05.9311. [DOI] [PubMed] [Google Scholar]
- 91.Tchabo NE, Mhawech-Fauceglia P, Caballero OL, Villella J, Beck AF, Miliotto AJ, Liao J, Andrews C, Lele S, Old LJ, Odunsi K. Expression and serum immunoreactivity of developmentally restricted differentiation antigens in epithelial ovarian cancer. Cancer Immun. 2009;9:6. [PMC free article] [PubMed] [Google Scholar]
- 92.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ Tumor-Infiltrating Lymphocytes Have an Atypical CD27− Memory Phenotype and Together with CD8+ T Cells Promote Favorable Prognosis in Ovarian Cancer. Clin Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 93.Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–372. doi: 10.1016/j.ygyno.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 94.Pittet MJ. Behavior of immune players in the tumor microenvironment. Curr Opin Oncol. 2009;21:53–59. doi: 10.1097/CCO.0b013e32831bc38a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stojanovic A, Cerwenka A. Natural killer cells and solid tumors. J Innate Immun. 2011;3:355–364. doi: 10.1159/000325465. [DOI] [PubMed] [Google Scholar]
- 96.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167:195–205. doi: 10.1111/j.1365-2249.2011.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Facciabene A, Santoro S, Coukos G. Know thy enemy: Why are tumor-infiltrating regulatory T cells so deleterious? Oncoimmunology. 2012;1:575–577. doi: 10.4161/onci.19401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peng DJ, Liu R, Zou W. Regulatory T cells in human ovarian cancer. J Oncol. 2012;2012:345164. doi: 10.1155/2012/345164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 101.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 102.Barnett JC, Bean SM, Whitaker RS, Kondoh E, Baba T, Fujii S, Marks JR, Dressman HK, Murphy SK, Berchuck A. Ovarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol Oncol. 2010;116:556–562. doi: 10.1016/j.ygyno.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 103.Rodriguez GC, Haisley C, Hurteau J, Moser TL, Whitaker R, Bast RC, Jr, Stack MS. Regulation of invasion of epithelial ovarian cancer by transforming growth factor-beta. Gynecol Oncol. 2001;80:245–253. doi: 10.1006/gyno.2000.6042. [DOI] [PubMed] [Google Scholar]
- 104.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 105.Shah CA, Allison KH, Garcia RL, Gray HJ, Goff BA, Swisher EM. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol. 2008;109:215–219. doi: 10.1016/j.ygyno.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 106.Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest. 2006;35:459–483. doi: 10.1080/08820130600803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen F, Hou M, Ye F, Lv W, Xie X. Ovarian cancer cells induce peripheral mature dendritic cells to differentiate into macrophagelike cells in vitro. Int J Gynecol Cancer. 2009;19:1487–1493. doi: 10.1111/IGC.0b013e3181bb70c6. [DOI] [PubMed] [Google Scholar]
- 108.Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, Capron F, Emilie D, Curiel TJ. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 109.Wertel I, Polak G, Bednarek W, Barczynski B, Rolinski J, Kotarski J. Dendritic cell subsets in the peritoneal fluid and peripheral blood of women suffering from ovarian cancer. Cytometry B Clin Cytom. 2008;74:251–258. doi: 10.1002/cyto.b.20410. [DOI] [PubMed] [Google Scholar]
- 110.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol. 2010;2010:201026. doi: 10.1155/2010/201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 114.Wilcox RA, Feldman AL, Wada DA, Yang ZZ, Comfere NI, Dong H, Kwon ED, Novak AJ, Markovic SN, Pittelkow MR, Witzig TE, Ansell SM. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–2158. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 118.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 120.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, Ahonen CL, Conejo-Garcia JR. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69:7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cubillos-Ruiz JR, Rutkowski M, Conejo-Garcia JR. Blocking ovarian cancer progression by targeting tumor microenvironmental leukocytes. Cell Cycle. 2010;9:260–268. doi: 10.4161/cc.9.2.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sperner-Unterweger B, Neurauter G, Klieber M, Kurz K, Meraner V, Zeimet A, Fuchs D. Enhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markers. Immunobiology. 2011;216:296–301. doi: 10.1016/j.imbio.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 125.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 126.Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Current Opin Immunol. 2006;18:220–225. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 127.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 129.Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R, Moretta L, Moretta A, Vitale M. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood. 2006;108:4118–4125. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 130.Chiesa S, Mingueneau M, Fuseri N, Malissen B, Raulet DH, Malissen M, Vivier E, Tomasello E. Multiplicity and plasticity of natural killer cell signaling pathways. Blood. 2006;107:2364–2372. doi: 10.1182/blood-2005-08-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. The Journal of experimental medicine. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 134.Hill M, Tanguy-Royer S, Royer P, Chauveau C, Asghar K, Tesson L, Lavainne F, Remy S, Brion R, Hubert FX, Heslan M, Rimbert M, Berthelot L, Moffett JR, Josien R, Gregoire M, Anegon I. IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur J Immunol. 2007;37:3054–3062. doi: 10.1002/eji.200636704. [DOI] [PubMed] [Google Scholar]
- 135.Inaba T, Ino K, Kajiyama H, Yamamoto E, Shibata K, Nawa A, Nagasaka T, Akimoto H, Takikawa O, Kikkawa F. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol. 2009;115:185–192. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 136.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, Kawaguchi R, Isonishi S, Tanaka T, Urashima M. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 137.Takao M, Okamoto A, Nikaido T, Urashima M, Takakura S, Saito M, Okamoto S, Takikawa O, Sasaki H, Yasuda M, Ochiai K, Tanaka T. Increased synthesis of indoleamine-2,3-dioxygenase protein is positively associated with impaired survival in patients with serous-type, but not with other types of, ovarian cancer. Oncol Rep. 2007;17:1333–1339. [PubMed] [Google Scholar]
- 138.Zheng X, Koropatnick J, Li M, Zhang X, Ling F, Ren X, Hao X, Sun H, Vladau C, Franek JA, Feng B, Urquhart BL, Zhong R, Freeman DJ, Garcia B, Min W-P. Reinstalling Antitumor Immunity by Inhibiting Tumor-Derived Immunosuppressive Molecule IDO through RNA Interference. J Immunol. 2006;177:5639–5646. doi: 10.4049/jimmunol.177.8.5639. [DOI] [PubMed] [Google Scholar]
- 139.Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Let. 2007;111:69–75. doi: 10.1016/j.imlet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 140.Löb S, Königsrainer A, Zieker D, Brücher BDM, Rammensee H-G, Opelz G, Terness P. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 142.Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, Combs AP, Scherle PA, Vaddi K, Fridman JS. Hydroxyamidine Inhibitors of Indoleamine-2,3-dioxygenase Potently Suppress Systemic Tryptophan Catabolism and the Growth of IDO-Expressing Tumors. Mol Cancer Ther. 2010;9:489–498. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 143.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16:354–359. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Metz R, DuHadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel Tryptophan Catabolic Enzyme IDO2 Is the Preferred Biochemical Target of the Antitumor Indoleamine 2,3-Dioxygenase Inhibitory Compound d-1-Methyl-Tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 145.Lob S, Konigsrainer A, Schafer R, Rammensee H-G, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–2154. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 146.Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 147.Hou D-Y, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of Indoleamine 2,3-Dioxygenase in Dendritic Cells by Stereoisomers of 1-Methyl-Tryptophan Correlates with Antitumor Responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 148.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 149.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 150.Pilotte L, Larrieu P, Stroobant V, Colau D, Dolušić E, Frédérick R, De Plaen E, Uyttenhove C, Wouters J, Masereel B, Van den Eynde BJ. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci. 2012;109:2497–2502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, Vierkant RA, Armasu SM, White KL, Keeney GL, Cliby WA, Rider DN, Kelemen LE, Jones MB, Peethambaram PP, Lancaster JM, Olson JE, Schildkraut JM, Cunningham JM, Hartmann LC. Inherited determinants of ovarian cancer survival. Clin Cancer Res. 2010;16:995–1007. doi: 10.1158/1078-0432.CCR-09-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frede S, Freitag P, Otto T, Heilmaier C, Fandrey J. The proinflammatory cytokine interleukin 1beta and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 2005;65:4690–4697. doi: 10.1158/0008-5472.CAN-04-3877. [DOI] [PubMed] [Google Scholar]
- 153.Ioana Braicu E, Mustea A, Toliat MR, Pirvulescu C, Konsgen D, Sun P, Nurnberg P, Lichtenegger W, Sehouli J. Polymorphism of IL-1alpha, IL-1beta and IL-10 in patients with advanced ovarian cancer: results of a prospective study with 147 patients. Gynecol Oncol. 2007;104:680–685. doi: 10.1016/j.ygyno.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 154.Ercolini AM, Machiels JP, Chen YC, Slansky JE, Giedlen M, Reilly RT, Jaffee EM. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol. 2003;170:4273–4280. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 155.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 156.Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, Coward JI, Schioppa T, Robinson SC, Gallagher WM, Galletta L, Salako MA, Smyth JF, Hagemann T, Brennan DJ, Bowtell DD, Balkwill FR. A Dynamic Inflammatory Cytokine Network in the Human Ovarian Cancer Microenvironment. Cancer Research. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE, Duska LR, Fuller AF, Goodman AK, Nikrui N, MacNeill KM, Matulonis UA, Preffer FI, Seiden MV. Cytokines IL-1β, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFα in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 158.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 159.Knüpfer H, Preiß R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 160.Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95:497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- 161.Belluco C, Nitti D, Frantz M, Toppan P, Basso D, Plebani M, Lise M, Jessup J. Interleukin-6 Blood Level Is Associated With Circulating Carcinoembryonic Antigen and Prognosis in Patients With Colorectal Cancer. Ann of Surg Oncol. 2000;7:133–138. doi: 10.1007/s10434-000-0133-7. [DOI] [PubMed] [Google Scholar]
- 162.Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. Int J Colorectal Dis. 2010;25:135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 163.Songur N, Kuru B, Kalkan F, Ozdilekcan C, Cakmak H, Hizel N. Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori. 2004;90:196–200. doi: 10.1177/030089160409000207. [DOI] [PubMed] [Google Scholar]
- 164.Hata H, Xiao H, Petrucci M, Woodliff J, Chang R, Epstein J. Interleukin-6 gene expression in multiple myeloma: a characteristic of immature tumor cells. Blood. 1993;81:3357–3364. [PubMed] [Google Scholar]
- 165.Bommert K, Bargou RC, Stühmer T. Signalling and survival pathways in multiple myeloma. Eur J Cancer. 2006;42:1574–1580. doi: 10.1016/j.ejca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 166.Shi Y, Frost PJ, Hoang BQ, Benavides A, Sharma S, Gera JF, Lichtenstein AK. IL-6–Induced Stimulation of c-Myc Translation in Multiple Myeloma Cells Is Mediated by Myc Internal Ribosome Entry Site Function and the RNA-Binding Protein, hnRNP A1. Cancer Res. 2008;68:10215–10222. doi: 10.1158/0008-5472.CAN-08-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 168.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J, Vermeulen J, Singh N, Avril N, Cummings J, Rexhepaj E, Jirström K, Gallagher WM, Brennan DJ, McNeish IA, Balkwill FR. Interleukin-6 as a Therapeutic Target in Human Ovarian Cancer. Clin Cancer Res. 2011;17:6083–6096. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, Hampel C, Lee H, Seiden MV. Signal Transducers and Activators of Transcription 3 Pathway Activation in Drug-Resistant Ovarian Cancer. Clin Cancer Res. 2006;12:5055–5063. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 170.Lo C-W, Chen M-W, Hsiao M, Wang S, Chen C-A, Hsiao S-M, Chang J-S, Lai T-C, Rose-John S, Kuo M-L, Wei L-H. IL-6 Trans-Signaling in Formation and Progression of Malignant Ascites in Ovarian Cancer. Cancer Res. 2011;71:424–434. doi: 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- 171.Plante M, Rubin SC, Wong GY, Federici MG, Finstad CL, Gastl GA. Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73:1882–1888. doi: 10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 172.Yang L, Wang L, Lin H-K, Kan P-Y, Xie S, Tsai M-Y, Wang P-H, Chen Y-T, Chang C. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem Biophys Res Comm. 2003;305:462–469. doi: 10.1016/s0006-291x(03)00792-7. [DOI] [PubMed] [Google Scholar]
- 173.Wang TH, Chan YH, Chen CW, Kung WH, Lee YS, Wang ST, Chang TC, Wang HS. Paclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathways. Oncogene. 2006;25:4857–4866. doi: 10.1038/sj.onc.1209498. [DOI] [PubMed] [Google Scholar]
- 174.Trikha M, Corringham R, Klein B, Rossi J-F. Targeted Anti-Interleukin-6 Monoclonal Antibody Therapy for Cancer. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 175.Lu Z, Brailly H, Wijdenes J, Bataille R, Rossi J, Klein B. Measurement of whole body interleukin-6 (IL-6) production: prediction of the efficacy of anti-IL-6 treatments. Blood. 1995;86:3123–3131. [PubMed] [Google Scholar]
- 176.Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Karkera J, Steiner H, Li W, Skradski V, Moser PL, Riethdorf S, Reddy M, Puchalski T, Safer K, Prabhakar U, Pantel K, Qi M, Culig Z. The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. The Prostate. 2011;71:1455–1465. doi: 10.1002/pros.21362. [DOI] [PubMed] [Google Scholar]
- 178.Fizazi K, De Bono JS, Flechon A, Heidenreich A, Voog E, Davis NB, Qi M, Bandekar R, Vermeulen JT, Cornfeld M, Hudes GR. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48:85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 179.Dorff TB, Goldman B, Pinski JK, Mack PC, Lara PN, Van Veldhuizen PJ, Quinn DI, Vogelzang NJ, Thompson IM, Hussain MHA. Clinical and Correlative Results of SWOG S0354: A Phase II Trial of CNTO328 (Siltuximab), a Monoclonal Antibody against Interleukin-6, in Chemotherapy-Pretreated Patients with Castration-Resistant Prostate Cancer. Clin Cancer Res. 2010;16:3028–3034. doi: 10.1158/1078-0432.CCR-09-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shinriki S, Jono H, Ota K, Ueda M, Kudo M, Ota T, Oike Y, Endo M, Ibusuki M, Hiraki A, Nakayama H, Yoshitake Y, Shinohara M, Ando Y. Humanized Anti-Interleukin-6 Receptor Antibody Suppresses Tumor Angiogenesis and In vivo Growth of Human Oral Squamous Cell Carcinoma. Clin Cancer Res. 2009;15:5426–5434. doi: 10.1158/1078-0432.CCR-09-0287. [DOI] [PubMed] [Google Scholar]
- 181.Mihara M, Nishimoto N, Ohsugi Y. The therapy of autoimmune diseases by anti-interleukin-6 receptor antibody. Expert Opin Biol Ther. 2005;5:683–690. doi: 10.1517/14712598.5.5.683. [DOI] [PubMed] [Google Scholar]
- 182.Dawson MA, Curry JE, Barber K, Beer PA, Graham B, Lyons JF, Richardson CJ, Scott MA, Smyth T, Squires MS, Thompson NT, Green AR, Wallis NG. AT9283, a potent inhibitor of the Aurora kinases and Jak2, has therapeutic potential in myeloproliferative disorders. Br J Haematol. 2010;150:46–57. doi: 10.1111/j.1365-2141.2010.08175.x. [DOI] [PubMed] [Google Scholar]
- 183.Pardanani A, Vannucchi AM, Passamonti F, Cervantes F, Barbui T, Tefferi A. JAK inhibitor therapy for myelofibrosis: critical assessment of value and limitations. Leukemia. 2011;25:218–225. doi: 10.1038/leu.2010.269. [DOI] [PubMed] [Google Scholar]
- 184.William AD, Lee ACH, Blanchard Sp, Poulsen A, Teo EL, Nagaraj H, Tan E, Chen D, Williams M, Sun ET, Goh KC, Ong WC, Goh SK, Hart S, Jayaraman R, Pasha MK, Ethirajulu K, Wood JM, Dymock BW. Discovery of the Macrocycle 11-(2-Pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a Potent Janus Kinase 2/Fms-Like Tyrosine Kinase-3 (JAK2/FLT3) Inhibitor for the Treatment of Myelofibrosis and Lymphoma. J Med Chem. 2011;54:4638–4658. doi: 10.1021/jm200326p. [DOI] [PubMed] [Google Scholar]
- 185.Barbieri F, Bajetto A, Florio T. Role of chemokine network in the development and progression of ovarian cancer: a potential novel pharmacological target. J Oncol. 2010;2010:15. doi: 10.1155/2010/426956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial Cancer Cell Migration. Cancer Res. 2001;61:4961–4965. [PubMed] [Google Scholar]
- 187.Milliken D, Scotton C, Raju S, Balkwill F, Wilson J. Analysis of Chemokines and Chemokine Receptor Expression in Ovarian Cancer Ascites. Clin Cancer Res. 2002;8:1108–1114. [PubMed] [Google Scholar]
- 188.Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR. Multiple Actions of the Chemokine CXCL12 on Epithelial Tumor Cells in Human Ovarian Cancer. Cancer Res. 2002;62:5930–5938. [PubMed] [Google Scholar]
- 189.Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, Emilie D, Terrassa M, Lackner A, Curiel TJ, Carmeliet P, Zou W. CXCL12 and Vascular Endothelial Growth Factor Synergistically Induce Neoangiogenesis in Human Ovarian Cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 190.Porcile C, Bajetto A, Barbieri F, Barbero S, Bonavia R, Biglieri M, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1α (SDF-1α/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308:241–253. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 191.Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 192.Righi E, Kashiwagi S, Yuan J, Santosuosso M, Leblanc P, Ingraham R, Forbes B, Edelblute B, Collette B, Xing D, Kowalski M, Mingari MC, Vianello F, Birrer M, Orsulic S, Dranoff G, Poznansky MC. CXCL12/CXCR4 Blockade Induces Multimodal Antitumor Effects That Prolong Survival in an Immunocompetent Mouse Model of Ovarian Cancer. Cancer Res. 2011;71:5522–5534. doi: 10.1158/0008-5472.CAN-10-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, Gould D, Ayhan A, Balkwill F. The Inflammatory Cytokine Tumor Necrosis Factor-α Generates an Autocrine Tumor-Promoting Network in Epithelial Ovarian Cancer Cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–141. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]
- 195.Anderson GM, Nakada MT, DeWitte M. Tumor necrosis factor-α in the pathogenesis and treatment of cancer. Curr Opin Pharm. 2004;4:314–320. doi: 10.1016/j.coph.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 196.Kwong J, Chan FL, Wong KK, Birrer MJ, Archibald KM, Balkwill FR, Berkowitz RS, Mok SC. Inflammatory cytokine tumor necrosis factor alpha confers precancerous phenotype in an organoid model of normal human ovarian surface epithelial cells. Neoplasia. 2009;11:529–541. doi: 10.1593/neo.09112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Duan Z, Feller AJ, Penson RT, Chabner BA, Seiden MV. Discovery of Differentially Expressed Genes Associated with Paclitaxel Resistance Using cDNA Array Technology: Analysis of Interleukin (IL) 6, IL-8, and Monocyte Chemotactic Protein 1 in the Paclitaxel-resistant Phenotype. Clin Cancer Res. 1999;5:3445–3453. [PubMed] [Google Scholar]
- 198.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 199.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 200.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 201.Silasi DA, Alvero AB, Illuzzi J, Kelly M, Chen R, Fu HH, Schwartz P, Rutherford T, Azodi M, Mor G. MyD88 predicts chemoresistance to paclitaxel in epithelial ovarian cancer. Yale J Biol Med. 2006;79:153–163. [PMC free article] [PubMed] [Google Scholar]
- 202.Szajnik M, Szczepanski MJ, Czystowska M, Elishaev E, Mandapathil M, Nowak-Markwitz E, Spaczynski M, Whiteside TL. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–4363. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang AC, Su QB, Wu FX, Zhang XL, Liu PS. Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells. Eur J Clin Invest. 2009;39:157–164. doi: 10.1111/j.1365-2362.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 204.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 205.Davidson B, Shafat I, Risberg B, Ilan N, Trope CG, Vlodavsky I, Reich R. Heparanase expression correlates with poor survival in metastatic ovarian carcinoma. Gynecol Oncol. 2007;104:311–319. doi: 10.1016/j.ygyno.2006.08.045. [DOI] [PubMed] [Google Scholar]