Abstract
The Nlrp3 inflammasome has been proposed to play an important role in antifungal host defense. However, studies exploring the role of the inflammasome in antifungal host defense have been limited to the direct effects on IL-1β processing. Although IL-1β has important direct effects on the innate immune response, important effects of the caspase-1-dependent cytokines IL-1β and IL-18 are exerted on the initiation of adaptive cellular responses Th1 and Th17. No studies have been employed to assess the impact of the inflammasome on the Th1/Th17 defense mechanisms in-vivo during candidiasis. In the present study we demonstrate an essential role for caspase-1 and ASC in disseminated candidiasis through regulating antifungal Th1 and Th17 responses. Caspase-1−/− and ASC−/− mice display diminished Th1/Th17 responses, followed by increased fungal outgrowth and lower survival. These observations identify a critical role for the inflammasome in controlling protective adaptive immune responses during invasive fungal infection.
INTRODUCTION
The availability of novel treatment options during the last decade did not reduce the mortality and morbidity associated with invasive Candida infections. A better understanding of the host defense against Candida is crucial to develop new strategies that will improve clinical outcome in patients with disseminated candidiasis. IL-1β and IL-18 play an important role in anti-Candida host defense and these cytokines are cleaved into active proteins by the cysteine protease caspase-1. In turn, activation of caspase-1 is controlled by the inflammasome, a multimeric protein complex composed of ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), caspase-1 and one or more nucleotide-binding domain leucine-rich repeat receptors (NLRs) [1].
Recently, it has been reported that Nlrp3 is the crucial NLR family member that connects Candida recognition to caspase-1 activation. Mice deficient in Nlrp3 were highly susceptible to disseminated candidiasis [2, 3] and mucosal candidiasis [4]. It has therefore been suggested that the inflammasome is crucial for antifungal host defense. The underlying protective host defense mechanism induced by the inflammasome has been attributed to the activation of the innate immune system by caspase-1-dependent IL-1β. However, the inflammasome/caspase-1 also processes the proinflammatory cytokine IL-18. Noteworthy, both IL-1β and IL-18 play a major role in the development of specific T helper responses. IL-1β is important for the induction of Th17 responses and IL-18 drives Th1 responses [5–8]. Nlrp3 has been shown to play an important role in directing Th1/Th17 responses in experimental autoimmune encephalomyelitis [9]. In a mouse model with a mutation in Nlrp3, it was demonstrated that inflammasome hyperactivation potentiated predominantly Th17 immune responses [10]. Furthermore, the inflammasome can modulate Th1 and Th17 responses during bacterial infection [11, 12]. Th17 responses are crucial for anti-Candida host defense by recruiting neutrophils [13–15], and the importance of the Th17 response in anti-Candida host defense is underlined by the observation that patients with IL-17 deficiency suffer specifically from Candida infections [16, 17]. The Th1 response is predominantly responsible for activation of neutrophils and macrophages during disseminated candidiasis [18]. We hypothesized that the inflammasome would exert protective antifungal effects by providing the substrates that are needed for optimal protective antifungal Th1 and Th17 responses. By studying mice deficient in ASC and caspase-1 we observed that caspase-1 and ASC have strong protective antifungal capacities by controlling Th1 and Th17 responses during disseminated candidiasis.
RESULTS
Caspase-1−/− and ASC−/− are more susceptible to disseminated candidiasis
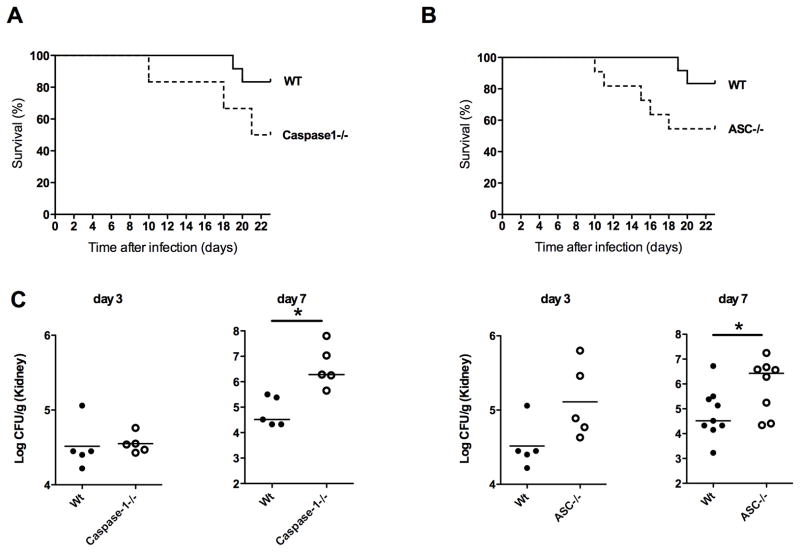
Recent studies suggest that the production of IL-1β in response to C. albicans is critically dependent on the Nlrp3 inflammasome [2]. However, the essential role of Nlrp3 and caspase-1 in anti-Candida host defense is controversial, since it was demonstrated that Candida albicans can bypass the need for the induction of Nlrp3 to activate the inflammasome in monocytes and an earlier study of disseminated candidiasis in caspase-1−/− mice did not show an essential role for caspase-1 [19, 20]. Because of these inconsistencies in the literature concerning this potentially important anti-Candida host defense mechanism, we aimed to assess the role of the inflammasome during disseminated candidiasis using knock-out animals deficient in the crucial components of the inflammasome; caspase-1 and ASC. Twenty two days after intravenous injection of 2×105 colony forming units (CFU) of C. albicans, 83% of the WT mice survived the infection (Figure 1A). When caspase-1−/− mice were infected, they showed a higher susceptibility to disseminated candidiasis, with 50% overall survival after 22 days of infection (Figure 1A). ASC−/− mice also showed a higher mortality than WT mice with 54% survival of ASC−/− mice 22 days after infection (Figure 1B).
Figure 1.
Caspase-1−/− and ASC−/− are more susceptible to disseminated candidiasis. (A) Kaplan-Meier survival plots of WT (n=10), caspase-1−/− (n=6) (B) Kaplan-Meier survival plots of WT, ASC−/−, n=10 mice per group. (C) Fungal burden of kidneys of WT, Caspase-1−/−, and ASC−/− mice at day 3 and 7 after infection. n ≥ 5 mice per group.
Increased fungal burden in the kidneys of caspase-1−/− and ASC−/− mice
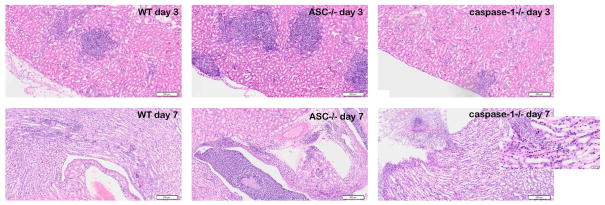
Mice with hematogenously disseminated candidiasis die because of progressive sepsis [21]. Notably, kidney fungal burden is correlated with severity of renal failure and systemic acidosis, which are hallmarks of severe sepsis [21]. We therefore investigated the fungal loads in the kidneys of the WT mice and knockout mice. In line with the higher susceptibility to disseminated candidiasis demonstrated by the survival experiments, caspase-1−/− mice had a 62-fold increase, and ASC−/− mice had a 13-fold increase in fungal load on day 7 compared to wild-type mice (Figure 1C). Histology showed that large amounts of C. albicans had accumulated on day 7 of infection in the collecting ducts of caspase-1−/− mice that directly invaded the tissue, which was not observed in the WT mice and ASC −/− mice (Figure 2).
Figure 2.
Histopathologic assessment of the kidneys of WT, caspase-1 −/−and ASC−/− mice 3 and 7 days after intravenous injection with 2×10e5 CFU C. albicans. Kidneys from caspase-1−/− mice show C. albicans hyphae invading the tissue in the presence of little inflammation at day 7 of infection. Scale 200μm hematoxylin and eosin stainings.
Caspase-1 and ASC deficiency results in impaired Th1 and Th17 responses
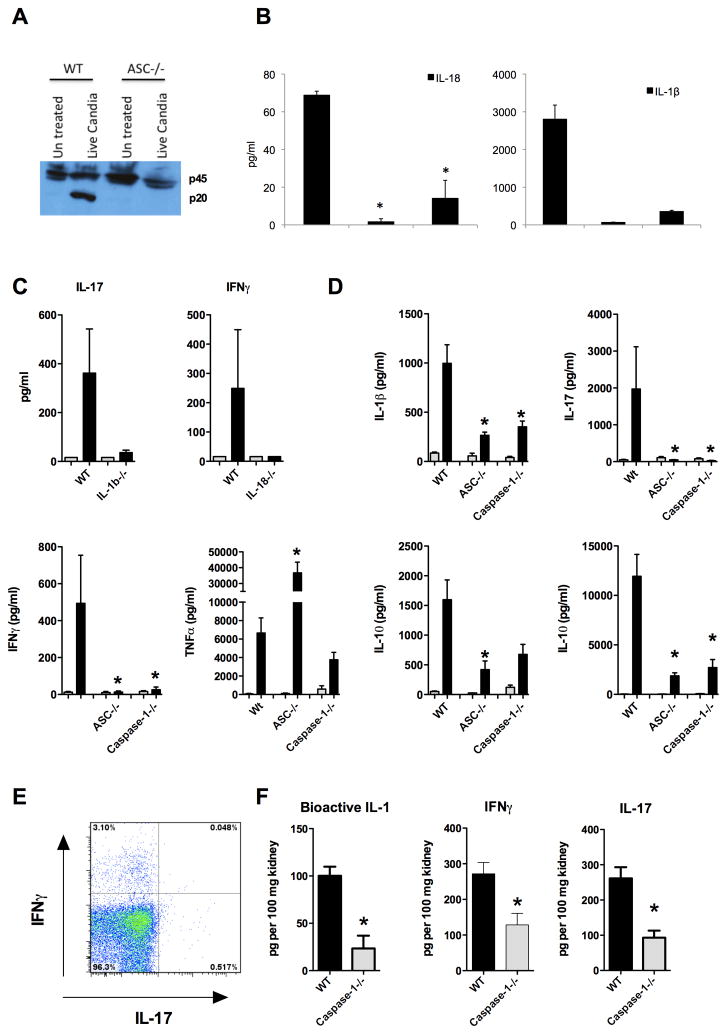
Caspase-1, the essential enzyme of the inflammasome, is able to cleave the precursors pro-IL-1β an pro-IL-18 into their mature active forms [22, 23]. IL-1β plays an important role in the induction of the Th17 response, which is characterized by the production of IL-17, a cytokine that is important for recruiting neutrophils and maintaining optimal neutrophil responses at the site of infection [6]. Th17 responses are crucial for protective anti-Candida host defense, since mice that are deficient in the IL-17 signaling pathway are highly susceptible to disseminated candidiasis [13], and patients that are deficient in Th17 responses are highly susceptible to mucosal candidiasis [16]. IL-18 production is controlled by the inflammasome and is necessary for an optimal Th1 response. Th1 responses are characterized by IFNγ production, and IFNγ plays an important role in disseminated candidiasis [24, 25]. We therefore tested the hypothesis that the inflammasome could play an important role in the induction of protective antifungal Th17 and Th1 responses. ASC is important for caspase-1 activation in BMDCs stimulated with C. albicans (Figure 3A). BMDCs deficient in ASC and caspase-1 showed an impaired production of IL-1β and IL-18 in response to C. albicans (Figure 3B). To test whether these cytokines were directly involved in the production of the production of the characteristic T cell cytokines IL-17 and IFNγ, splenocytes deficient in IL-1β and IL-18 were stimulated with C. albicans and IL-17 and IFNγ were measured respectively (Figure 3C). We observed that splenocytes deficient in IL-1β had an impaired IL-17 production and IL-18−/− splenocytes had an impaired IFNγ production (Figure 3C). Splenocytes from mice deficient in caspase-1 or ASC that were infected intravenously with Candida, had an impaired IL-1β response and were unable to mount normal production of IL-17 after restimulation with Candida (Figure 3D). We observed that during systemic infection with C. albicans, CD4 positive splenocytes showed intracellular staining of IL-17 and IFNγ (Fig 3E). Histology supported these findings, since we observed remarkable little influx of neutrophils in the tissues of caspase-1 deficient mice (Figure 2). Levels of bioactive IL-1, IL-17, and IFNγ were also significantly lower in vivo at the site of infection (Figure 3F).
Figure 3.
(A) Lysates from bone marrow-derived dendritic cells (BMDCs) from WT and ASC−/− mice were collected 4 hours after exposure to 1×104 CFU C. albicans/ml, and immunoblotted with anti-caspase-1 antibody. p45 indicates procaspase-1 and p20 processed caspase-1. (B) BMDCs are stimulated overnight live C. albicans 1×10e5/ml, IL18 and IL-1β were measured by ELISA. (C) Splenocytes from WT, IL-1β−/− and IL-18−/− mice were stimulated with RPMI (grey bars) or heat-killed 1×106 C. albicans hyphae cells/ml. Cytokines were measured 5 days after stimulation. n=4 mice per group. (D) Splenocytes from WT, ASC−/−, and caspase-1−/− mice were re-stimulated with RPMI (grey bars) or heat-killed 1×106 C. albicans hyphae cells/ml (black bars) 7 days after intravenous infection with C. albicans. Cytokines were measured 48 hours after stimulation with ELISA. *p<0.05. n=5 mice per group. (E) FACS analysis of splenocytes isolated 7 days after infection with intravenous infection with C. albicans gated for CD4, and stained for intracellular IL-17 and IFNγ. The data shown is representative for data observed in 2 separate experiments with a total of n=5 mice. (F) IFNγ, IL-17 and bioactive IL-1β were measured in the kidney homogenates from WT and caspase-1−/− mice 7 days after intravenous infection with C. albicans. n=5 mice per group.
ASC−/− mice have an increased inflammatory response during early infection
Three days after the intravenous injection of 2×10e5 colony forming units (CFU) of C. albicans, the ASC−/− mice showed a strong granulomatous inflammatory response in the kidneys, which was not observed in the caspase-1 deficient mice or WT mice (Figure 4A). Granulomatous responses in disseminated candidiasis are largely dependent on TNFα production [26]. Therefore, we investigated the production of TNFα at three days of infection in WT, ASC−/− and caspase-1−/−mice. Indeed, splenocytes isolated from infected ASC−/− mice after three days of infection showed an increased TNFα production compared to WT and caspase-1−/− mice (Figure 4B). These data indicate that in addition to its role in inflammasome activation, ASC has a caspase-1- independent biological activity, controlling TNFα production during early infection.
Figure 4.
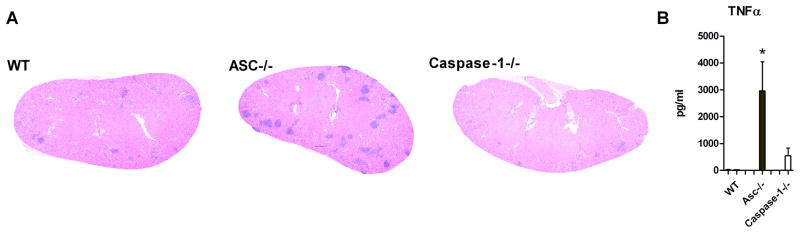
ASC−/− mice have an increased inflammatory response during early infection. (A) Histopathologic assessment of the kidneys of WT, ASC−/− and caspase-1 −/− mice three days after intravenous injection with 2×105 CFU C. albicans. Kidneys from ASC−/− mice show markedly more inflammatory granulomatous reaction. (B) Splenocytes from WT, ASC−/−, and caspase-1−/−mice were re-stimulated with RPMI (grey bars) or heat-killed 1×106 C. albicans hyphae cells/ml (black bars) three days after intravenous infection with C. albicans. TNFα was measured 48 hours after stimulation with ELISA. *p<0.05. n=5 mice per group.
DISCUSSION
An increasing interest has been elicited in the role of the inflammasome and IL-1β for the host defense against Candida infections. The importance of the inflammasome for antifungal host defense has been ascribed to the direct effects of caspase-1 on IL-1β processing and subsequently the activation of the innate immune response. However, the role of the caspase-1-dependent cytokines IL-1β and IL-18 is not restricted to innate immunity, since both are also important for the initiation of the adaptive cellular Th17 and Th1 responses respectively. No studies to date have addressed the importance of the inflammasome on Th1/Th17 responses in fungal infection. In the present study we have dissected the contribution of the inflammasome components caspase-1 and ASC to the host defense against disseminated candidiasis, and demonstrate that caspase-1 and ASC are crucial for anti-Candida host defense during disseminated candidiasis by promoting optimal antifungal protective Th1 and Th17 responses.
Our findings are different from a previous study which reported that caspase-1 deficiency had no critical effect on primary disseminated candidiasis [20]. In the latter study however, infection with a virulent C. albicans strain resulted in 100% mortality in both the WT group and caspase-1 deficient mice, while infection with an avirulent C. albicans strain resulted in almost 100% survival in both groups. This makes it difficult to draw conclusions about the susceptibility of the caspase-1 deficient mice during these infections, as no intermediary (LD50) mortality rates were performed in any of the experiments. Overall our data clearly demonstrate an important role for the inflammasome components ASC and caspase-1 in antifungal host defense against disseminated candidiasis. Furthermore, they are in line with previous data, which show that IL-1β deficient mice and Nlrp3 deficient mice are more susceptible to disseminated candidiasis [2, 3, 27].
The inflammasome-processed IL-1β and IL-18 play a prominent role in polarizing T helper responses. IL-18 is important for the induction of the Th1 response, which is characterized by the production of IFN-γ [5], while IL-1β and its receptor IL-1R1 are important for the early differentiation of Th17 responses, and IL-1β in the presence of IL-23 can amplify Th17 responses [6–8]. Mice deficient in Th1 or Th17 responses have both been reported to be highly susceptible to disseminated candidiasis [13, 15, 25, 28]. These data suggest that the inflammasome might be an important component of the innate immune system that regulates T helper responses during disseminated Candida infection, yet this concept has not been studied in-vivo. Here we report that caspase-1 and ASC play a role in Candida-induced IL-1β and IL-18 by bone marrow derived dendritic cells and are important for optimal Th1 and Th17 responses during disseminated candidiasis. This seems to be at odds with a recent study showing that the Nlrp3 inflammasome is dispensible for mounting T cell responses [29]. An important difference is that this study only investigated the impact of Nlrp3 deficiency on T cell responses, but not the impact of caspase-1 and ASC deficiency. Furthermore, the T cell responses of splenocytes were analysed in mice immunized with OVA and curdlan, which is a different experimental setting compared to restimulation of splenocytes from Candida infected mice. Since we did not investigate the dynamics of T helper subsets in lymphoid tissue during infection in Wt and knockout mice, it remains to be elucidated which T helper subsets are locally altered and to what extend. However, our data strongly suggest that Th1 and Th17 subsets are deficient in ASC and caspase-1 deficiency, because of the measured differences in the production of the characteristic T helper cytokines IL-17 and IFNγ. Our observations are supported by several other studies that have shown that Nlrp3 can modulate Th17 responses in experimental models of autoimmune, autoinflammatory and infectious diseases [9–12].
Interestingly, we and others have observed that during early infection (day 3), the caspase-1 deficient splenocytes did not have an impaired IL-1β or IFNγ production when re-stimulated with C. albicans (data not shown, [20]). These observations suggest that during the early stages of infection proIL-1β and pro-IL-18 can be processed without the need of inflammasome induction and subsequently caspase-1 activation. Most likely the activity of neutrophil-derived serine proteases, which can also cleave process pro-IL-1β and pro-IL-18, is responsible for these effects [30, 31]. The relative importance of caspase-1 and serine proteases changes during the later stages of infection, a time point where Th1 and Th17 responses are critical for protective immunity. Since caspase-1 activity and IL-1β production are deficient in ASC−/− and caspase-1−/− mice, and subsequently the Th1 and Th17 responses are impaired, this would indicate that the inflammasome is a critical link between the innate and adaptive immune response during the subacute phase of disseminated candidiasis.
A recent study defined the protective mechanisms of immunity during Candida albicans bloodstream infections in mice immunized with the recombinant N-terminus of Als3p (rAls3p-N) vaccine plus aluminum hydroxide (alum) [32]. Interestingly, vaccination resulted in protective Th1 and Th17 responses that enhanced phagocytic killing of C. albicans, increased neutrophil influx, and decreased fungal burden in tissues. Since alum activates caspase-1 [33], it is tempting to speculate that the adjuvant alum modulated the immune response towards protective Th1 and Th17 responses by activating the inflammasome, which would be in line with our findings.
An unexpected finding was the increased inflammatory response observed in ASC deficient mice during early infection (day 3). The higher TNFα production at day 3 in ASC deficient mice might well be responsible for the increased inflammation in the beginning stage of the infection, since TNFα is important for early neutrophil influx in disseminated candidiasis [26]. The difference in TNF production between ASC−/− and caspase-1−/− might also explain the differences in histological findings in these knockout mice during infection. The presence of high levels of TNF might be responsible for the absence of aggressive fungal invasion in the kidney in ASC deficient mice. More studies are needed to elucidate these findings, and the role of ASC in inflammation and TNF responses is currently under investigation. Notably, these data demonstrate that ASC−/− and caspase-1−/− do not share an identical phenotype during early infection, and highlight the possibility that ASC can also have caspase-1-independent effects during infection. These findings are in line with a previous study reporting that ASC enhances induction of TNF via a caspase-1 independent pathway [34]. Modulatory effects of ASC on NF-κB activation and gene transcription have been described and suggest that ASC can directly affect transcription [35]. However, other mechanisms remain to be elucidated. Effects of ASC on MAPK or other signaling pathways are a possible explanation of how ASC regulates TNF production. Other studies that investigated the role of ASC and caspase-1 in experimental autoimmune models [34, 36, 37] have reported an important role for ASC in inflammatory conditions that are independent of caspase-1. These observations might have important implications for understanding the mechanisms leading to acute inflammation and the design of novel therapeutic strategies, and need further evaluation.
In summary, we present data that demonstrate a crucial role of the inflammasome components caspase-1 and ASC in disseminated candidiasis, which is in line with the earlier studies reporting increased susceptibility to candidiasis of Nlrp3 and IL-1β-deficient mice. In addition, Th1 and Th17 responses during the later stages of disseminated candidiasis are dependent on caspase-1 and ASC, a finding that provides new insight into the mechanisms of inflammasome-mediated antifungal host defense and supporting the concept that the inflammasome has an important regulatory function in the development of protective proinflammatory T helper responses during infection.
MATERIALS AND METHODS
Ethics statement
Animal studies were conducted under protocols approved by St. Jude Children’s Research Hospital. Mice were housed in a pathogen-free facility.
Mice and bone marrow derived dendritic cells (BMDCs)
ASC−/− and caspase-1−/− backcrossed to C57BL/6 background for at least 10 generations have been described before [38, 39]. IL-1β gene-deficient mice were kindly provided by J. Mudgett, Merck (Rahway, NJ, USA). The generation of IL-18 knockout mice was previously described [40]. Bone marrow was prepared from the leg bones of 8–20-week-old mice. The legs were dissected, and the bone marrow flushed out. DCs were differentiated from bone marrow cells cultured with RPMI-1640 supplemented with 20ng/ml GM-CSF along with 10% heat inactivated fetal bovine serum (Invitrogen), 100 U/ml penicillin and 100 mg/ml streptomycin at 37 °C in 5% CO2 for 7 days.
C. albicans growth conditions
C. albicans ATCC MYA-3573 (UC 820), a strain well described elsewhere [41], was used in all experiments. Candida was grown overnight in Sabouraud broth at 37°C, cells were harvested by centrifugation, washed twice, and resuspended in culture medium in culture medium (RPMI-1640 Dutch modification, ICN Biomedicals, Aurora, OH) [42]. For in-vitro experiments, C. albicans was heat-killed for 1h at 100°C. To generate pseudohyphae, C. albicans blastoconidia were grown at 37°C in culture medium, adjusted to pH 6.4 by using hydrochloric acid. Pseudohyphae were killed for 1h at 100°C and resuspended in culture medium to a hyphal inoculom size that originated from 106/ml blastoconidia (referred to as 106/ml pseudohyphae) [42].
C. albicans infection model
Knock-out mice and WT mice were injected intravenously with C. albicans blastoconidia (2 × 105 CFU/mouse) in a 100 μl volume of sterile pyrogen-free phosphate-buffered saline (PBS). Survival was assessed daily for 22 days. Subgroups of 5 animals were killed on days 3 or 7 of infection. To assess the tissue outgrowth of the microorganisms, the kidneys of the sacrificed animals were removed aseptically, weighed, and homogenized in sterile saline in a tissue grinder. The number of viable Candida cells in the tissues was determined by plating serial dilutions on Sabouraud dextrose agar plates as previously described [43]. The CFU were counted after 24h of incubation at 37°C, and expressed as log CFU/g tissue. For histologic analysis, kidneys of subgroups of mice (5 mice/group) were fixed in buffered formaldehyde (4%). Paraffin-embedded sections were stained with hematoxylin-eosin.
In vitro cytokine production by primed splenocytes and BMDCs
To assess cytokine production, primed spleen cells from mice on day 7 of infection with 2×105 CFU of C. albicans per mouse were stimulated in vitro with heat-killed Candida conidia or pseudohyphae (1×106 microorganisms/ml). Spleen cells were obtained by gently squeezing spleens in a sterile 200 mm filter chamber. The cells were washed and resuspended in RPMI1640, counted in a Bürker counting chamber and the number was adjusted to 5×106/ml. 500 μL of the cell suspension was stimulated with 1×106 heat killed C. albicans/ml. Measurement of IFNγ, IL-1β, IL-17, and TNFα concentrations was performed in supernatants collected after 48 h of incubation at 37 1C in 5% CO2 in 48-well plate. 2×106 BMDCs were stimulated overnight with 105/ml of live candida-blastoconidia in RP-10 complete medium.
Cytokine quantification
A Bioplex mouse X-plex assay (BioRad) was used according to the manufacturer’s instructions in order to evaluate the quantity of the following cytokines: IL-1β, IFN-γ, IL-17. Bioactive IL-1 secretion was determined in a bioassay using the murine thymoma cell lineEL4/NOB1 as an IL-1-specificcell-producing IL-2 response. IL-2 was subsequently determined by ELISA (R&D). Mouse IL-18−/−IL-1F4 in the supernatants of BMDCs was measured with an ELISA Kit from R&D Systems.
Immunoblotting for caspase-1
BMDMs were first stimulated for 4 hours with medium, live C. albicans in a concentration of 1×104 CFU/ml, or LPS + ATP as a control (data not shown) washed twice with phosphate-buffered saline and scraped in lysis buffer solution (150 mm NaCl, 10 mm Tris, pH 7.4, 5 mm EDTA, 1 mm EGTA, 0.1% Nonidet P-40) supplemented with a protease inhibitor mixture tablet (Roche Applied Science). Samples were clarified, denatured with SDS buffer, and boiled for 5 min. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were immunoblotted with primary antibodies and proteins detected with appropriate secondary anti-rabbit antibody conjugated to horseradish peroxidase followed by enhanced chemiluminescence. Rabbit anti-mouse caspase-1 was a generous gift from Dr. P. Vandenabeele (Gent University, Belgium).
Flow cytometry
Total spleen cells were isolated on day-7 from candida infected (105/mice i.v) mice and passed through 70 μm filters. Cells were stimulated for two days with heat-killed candida-blastoconidia (107/ml) in RP-10 complete medium (RPMI 1640, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine, 10% FBS, 50 μM 2-ME) in 96 well plates, with the nal 2 hr of culture in the presence of PMA(50ng/ml), Ionomycin (500ng/ml) and the monensin(eBioscience). Cells were harvested, extracellularly stained with anti-CD4 mAb(CD4 (L3T4)), and then intracellularly stained for IFN-γ (XMG1.2) and IL-17A (eBio17B7), from eBioscience. Samples were acquired on a FACSCalibur LSR II (BD Biosciences) and analyzed with Flowjo software (TreeStar).
Statistical analysis
Data were analyzed using GraphPad software. The differences between groups were analyzed by the Mann-Whitney U test or Student’s t-test where appropriate. Comparison of two survival curves was done using the Logrank test. The level of significance between groups was set at p<0.05.
Acknowledgments
This work was supported by Grant number AR05629 from NIH/NIAMS and the American Lebanese Syrian Associated Charities (ALSAC) to T-D.K. M.G.N. was supported by a Vici Grant of the Netherlands Organization for Scientific Research. We thank Tim Koenen and Jeroen van der Laak for their help with the histology.
References
- 1.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 3.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello CA. IL-18: A Th1-inducing, proinflammatory cytokine and a new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 6.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, Huang M, Schneider M, Miller SD, Ting JP. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE, Iwakura Y, Tschopp J, Sebo P, Mills KH. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185:1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 12.Oosting M, van de Veerdonk FL, Kanneganti TD, Sturm P, Verschueren I, Berende A, van der Meer JW, Kullberg BJ, Netea MG, Joosten LA. Borrelia species induce inflammasome activation and IL-17 production through a caspase-1-dependent mechanism. Eur J Immunol. 2011;41:172–181. doi: 10.1002/eji.201040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 14.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper- IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H, Ring J, Traidl-Hoffmann C. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol. 2008;128:2640–2645. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 18.Gozalbo D, Gil ML. IFN-gamma in Candida albicans infections. Front Biosci. 2009;14:1970–1978. doi: 10.2741/3356. [DOI] [PubMed] [Google Scholar]
- 19.van de Veerdonk FL, Joosten LA, Devesa I, Mora-Montes HM, Kanneganti TD, Dinarello CA, van der Meer JW, Gow NA, Kullberg BJ, Netea MG. Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1beta production by the fungal pathogen Candida albicans. J Infect Dis. 2009;199:1087–1096. doi: 10.1086/597274. [DOI] [PubMed] [Google Scholar]
- 20.Mencacci A, Bacci A, Cenci E, Montagnoli C, Fiorucci S, Casagrande A, Flavell RA, Bistoni F, Romani L. Interleukin 18 restores defective Th1 immunity to Candida albicans in Caspase 1-deficient mice. Infect Immun. 2000;68:5126–5131. doi: 10.1128/iai.68.9.5126-5131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis. 2005;192:336–343. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 23.Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Muhl H, Yoon DY, Reznikov LL, Kim SH, Rubinstein M. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leuk Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- 24.Stuyt RJ, Netea MG, Verschueren I, Fantuzzi G, Dinarello CA, Van der Meer JWM, Kullberg BJ. Role of interleukin-18 in host defense against disseminated Candida albicans infection. Infect Immun. 2002;70:3284–3286. doi: 10.1128/IAI.70.6.3284-3286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balish E, Wagner RD, Vasquez-Torres A, Pierson C, Warner T. Candidiasis in interferon-gamma knock-out (IFN-gamma−/−) mice. J Infect Dis. 1998;178:478–487. doi: 10.1086/515645. [DOI] [PubMed] [Google Scholar]
- 26.Netea MG, Van Tits LJH, Curfs JHAJ, Amiot F, Meis JFGM, Van der Meer JWM, Kullberg BJ. The increased susceptibility of TNFaLTa double knock-out mice to systemic candidiasis is due to defective recruitment and phagocytosis by neutrophils. J Immunol. 1999;163:1498–1505. [PubMed] [Google Scholar]
- 27.Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, Kullberg BJ. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 28.Lavigne LM, Schopf LR, Chung CL, Maylor R, Sypek JP. The role of recombinant IL-12 and IFN-gamma in the pathogenesis of a murine Candida albicans infection. J Immunol. 1998;160:284–292. [PubMed] [Google Scholar]
- 29.Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol. 2009;183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 30.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, Pham CT, van der Meer JW, Dinarello CA, van den Berg WB. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spellberg BJ, Ibrahim AS, Avenissian V, Filler SG, Myers CL, Fu Y, Edwards JE., Jr The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect Immun. 2005;73:6191–6193. doi: 10.1128/IAI.73.9.6191-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taxman DJ, Zhang J, Champagne C, Bergstralh DT, Iocca HA, Lich JD, Ting JP. Cutting edge: ASC mediates the induction of multiple cytokines by Porphyromonas gingivalis via caspase-1-dependent and -independent pathways. J Immunol. 2006;177:4252–4256. doi: 10.4049/jimmunol.177.7.4252. [DOI] [PubMed] [Google Scholar]
- 35.Stehlik C, Fiorentino L, Dorfleutner A, Bruey JM, Ariza EM, Sagara J, Reed JC. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. J Exp Med. 2002;196:1605–1615. doi: 10.1084/jem.20021552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ippagunta SK, Brand DD, Luo J, Boyd KL, Calabrese C, Stienstra R, Van de Veerdonk FL, Netea MG, Joosten LA, Lamkanfi M, Kanneganti TD. Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. J Biol Chem. 285:12454–12462. doi: 10.1074/jbc.M109.093252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw PJ, Lukens JR, Burns S, Chi H, McGargill MA, Kanneganti TD. Cutting Edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol. 184:4610–4614. doi: 10.4049/jimmunol.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 41.Lehrer RI, Cline MJ. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969;98:996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Graaf CA, Netea MG, Verschueren I, van der Meer JW, Kullberg BJ. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458–7464. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kullberg BJ, Van 't Wout JW, Van Furth R. Role of granulocytes in enhanced host resistance to Candida albicans induced by recombinant interleukin-1. Infect Immun. 1990;58:3319–3324. doi: 10.1128/iai.58.10.3319-3324.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]