Abstract
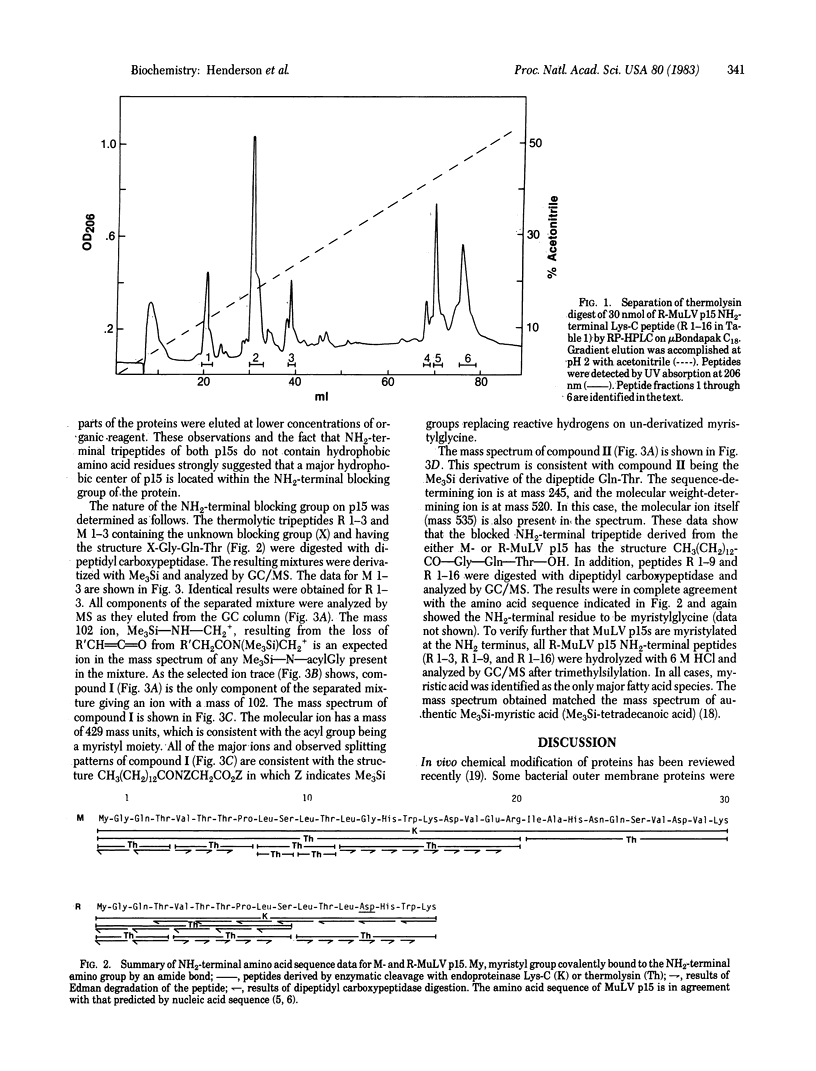
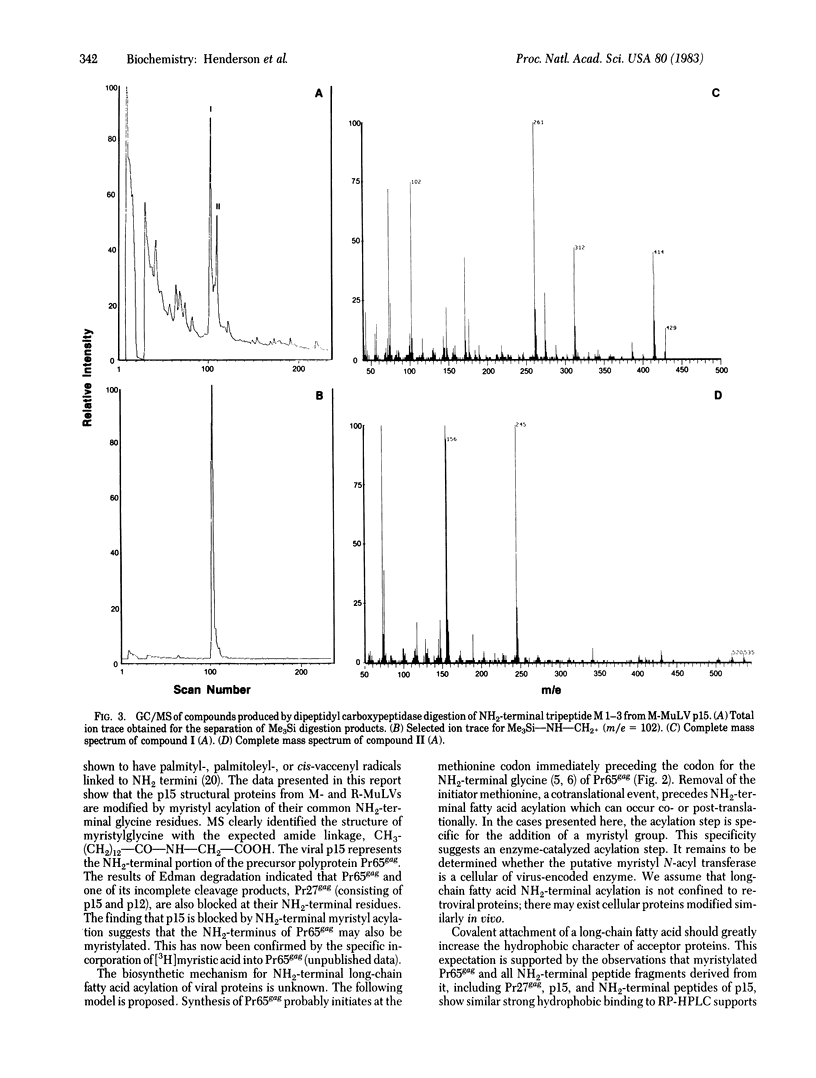
The primary structure of the NH2-terminal region of the gag gene encoded internal membrane-associated protein p15 has been determined for both Rauscher and Moloney murine leukemia viruses. Peptides generated by endopeptidases and purified by HPLC were subjected to semi-automated Edman degradation. Dipeptides obtained with dipeptidyl carboxypeptidase were identified by gas chromatography-mass spectrometry. The amino acid sequence of the first 16-residue segment of Rauscher p15 is identical to the sequence of Moloney p15 except for a single amino acid substitution (Gly→Asp) at position 13. Both proteins were found to have an acylated NH2 terminus. By mass spectroscopy, myristic acid [CH3(CH2)12COOH] was found to be bound through an amide linkage to the NH2-terminal glycyl residue in both p15s. The results of liquid chromatography show that the NH2-terminal myristyl group greatly contributes to the strong binding of these modified proteins and peptides to hydrophobic surfaces. Because p15 is known to be derived from the NH2-terminal region of a precursor polyprotein Pr65gag by proteolytic cleavage in the assembled virus, it is suggested that myristylation in vivo takes place during the biosynthesis of Pr65gag. Preliminary data indicate that such modification of gag precursor polyproteins may be common to mammalian retroviruses. The role of NH2-terminal myristyl acylation of Pr65gag in virus assembly and the possibility of similar NH2-terminal modifications of gag-related fusion proteins of transforming viruses are discussed.
Keywords: gas chromatography-mass spectrometry, membrane proteins, amino acid sequence
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Aaronson S. A. Membrane properties of the gag gene-coded p15 protein of mouse type-C RNA tumor viruses. J Biol Chem. 1978 Mar 10;253(5):1408–1414. [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland T. D., Grandgenett D. P., Oroszlan S. Amino acid sequence analysis of reverse transcriptase subunits from avian myeloblastosis virus. J Virol. 1980 Oct;36(1):115–119. doi: 10.1128/jvi.36.1.115-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Lee J. C., Ihle J. N. Antigenic specificities of the cellular immune response of C57BL/6 mice to the Moloney leukemia/sarcoma virus complex. J Immunol. 1979 Feb;122(2):665–674. [PubMed] [Google Scholar]
- Gallo R. C., Wong-Staal F. Retroviruses as etiologic agents of some animal and human leukemias and lymphomas and as tools for elucidating the molecular mechanism of leukemogenesis. Blood. 1982 Sep;60(3):545–557. [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Oroszlan S. Separation of amino acid phenylthiohydantoins by high-performance liquid chromatography on phenylalkyl support. Anal Biochem. 1980 Feb;102(1):1–7. doi: 10.1016/0003-2697(80)90307-3. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Krutzsch H. C. Determination of polypeptide amino acid sequences from the carboxyl terminus using angiotensin I converting enzyme. Biochemistry. 1980 Nov 11;19(23):5290–5296. doi: 10.1021/bi00564a022. [DOI] [PubMed] [Google Scholar]
- Krutzsch H. C., Pisano J. J. Separation and sequence of dipeptides using gas chromatography and mass spectrometry of their trimethylsilylated derivatives. Biochemistry. 1978 Jul 11;17(14):2791–2797. doi: 10.1021/bi00607a014. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Henderson L. E., Stephenson J. R., Copeland T. D., Long C. W., Ihle J. N., Gilden R. V. Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1404–1408. doi: 10.1073/pnas.75.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Oskarsson M., Maizel J., Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. doi: 10.1128/jvi.34.1.200-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Versteegen R. J., Copeland T. D., Oroszlan S. Complete amino acid sequence of the group-specific antigen gene-encoded phosphorylated proteins of mouse leukemia viruses. J Biol Chem. 1982 Mar 25;257(6):3007–3013. [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold F. In vivo chemical modification of proteins (post-translational modification). Annu Rev Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]
- Yeger H., Kalnins V. I., Stephenson J. R. Type-C retrovirus maturation and assembly: post-translational cleavage of the gag-gene coded precursor polypeptide occurs at the cell membrane. Virology. 1978 Aug;89(1):34–44. doi: 10.1016/0042-6822(78)90037-5. [DOI] [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Rasheed S., Gardner M. B. Different rat-derived transforming retroviruses code for an immunologically related intracellular phosphoprotein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3523–3527. doi: 10.1073/pnas.76.7.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]


