Abstract
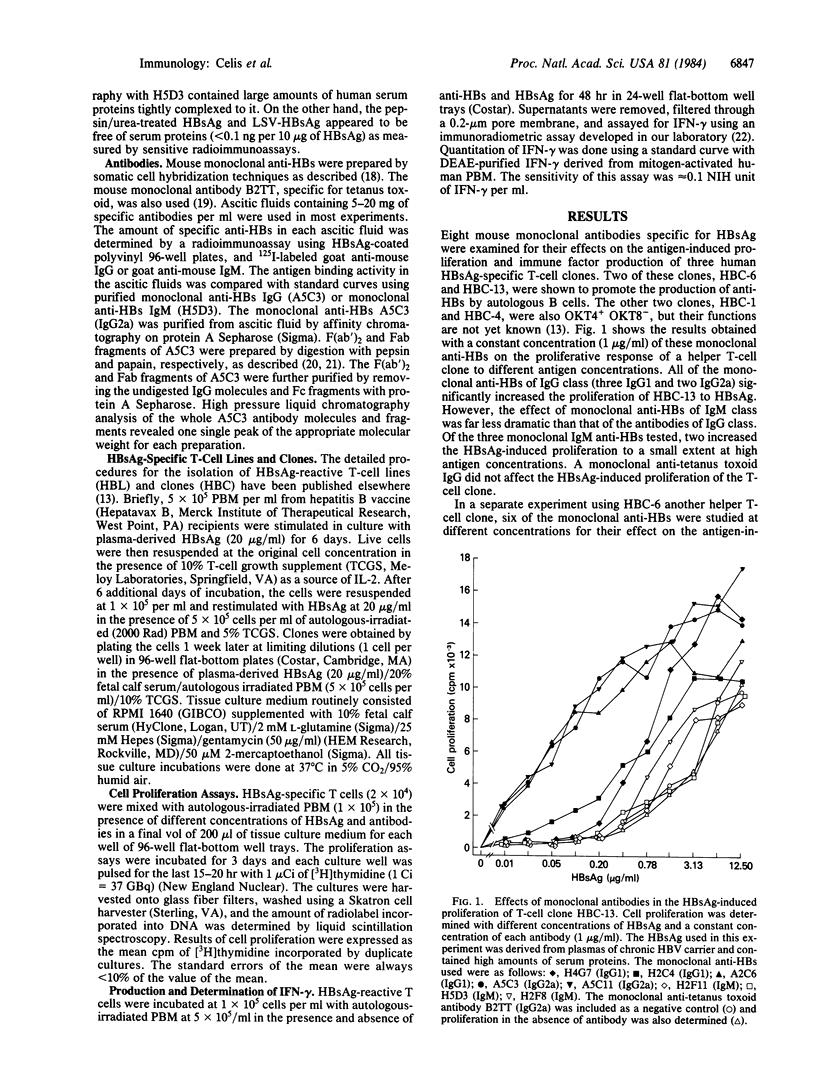
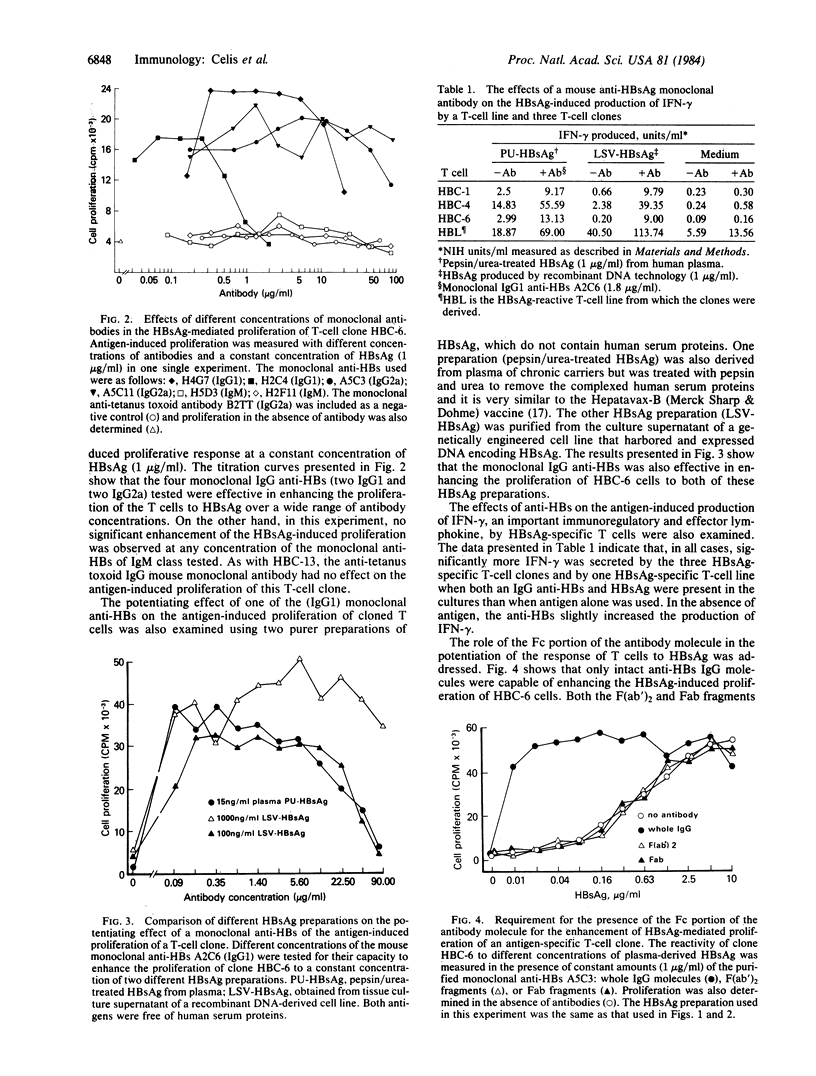
Eight mouse monoclonal antibodies specific for hepatitis B surface antigen (HBsAg) were examined for their effects on the antigen-induced proliferative response and lymphokine production of human HBsAg-specific T-cell clones in vitro. While all specifically enhanced the T-cell proliferative response, antibodies of the IgG class were generally more effective than those of the IgM class. Both the divalent F(ab')2 and the monovalent Fab fragments of an IgG monoclonal antibody had no effects, indicating that the Fc portion of the antibody molecules was required. Since antigen-presenting cells bear surface receptors for the Fc of IgGs and fewer or none for that of IgMs, the above results also suggest that antibodies enhance the capture of antigens by antigen-presenting cells as a result of the binding of antigen-antibody complexes to the Fc receptors on these cells. In addition to potentiating the proliferation of the T-cell clones, antibodies also increased the antigen-induced production of interferon-gamma by these cells. The present in vitro studies suggest that antibodies may regulate immune responses and do so by enhancing antigen presentation and thus augmenting antigen-induced activation and clonal expansion of T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Celis E., Chang T. W. Antibodies to hepatitis B surface antigen potentiate the response of human T lymphocyte clones to the same antigen. Science. 1984 Apr 20;224(4646):297–299. doi: 10.1126/science.6231724. [DOI] [PubMed] [Google Scholar]
- Celis E., Kung P. C., Chang T. W. Hepatitis B virus-reactive human T lymphocyte clones: antigen specificity and helper function for antibody synthesis. J Immunol. 1984 Mar;132(3):1511–1516. [PubMed] [Google Scholar]
- Celis E., Larralde C. Regulation of the binding of antigen to receptors by soluble antibodies: in-vitro competition and synergism for dinitrophenylated human serum albumin and epsilon-DNP-lysine. Immunochemistry. 1978 Aug;15(8):595–601. doi: 10.1016/0161-5890(78)90014-7. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., McKinney S., Liu V., Kung P. C., Vilcek J., Le J. Use of monoclonal antibodies as sensitive and specific probes for biologically active human gamma-interferon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5219–5222. doi: 10.1073/pnas.81.16.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Testa D., Kung P. C., Perry L., Dreskin H. J., Goldstein G. Cellular origin and interactions involved in gamma-interferon production induced by OKt3 monoclonal antibody. J Immunol. 1982 Feb;128(2):585–589. [PubMed] [Google Scholar]
- Cohen B. E., Paul W. E. Macrophage control of time-dependent changes in antigen sensitivity of immune T lymphocyte populations. J Immunol. 1974 Jan;112(1):359–369. [PubMed] [Google Scholar]
- Cohen B. E., Rosenthal A. S., Paul W. E. Antigen-macrophage interaction. II. Relative roles of cytophilic antibody and other membrane sites. J Immunol. 1973 Sep;111(3):820–828. [PubMed] [Google Scholar]
- EISEN H. N., KARUSH F. IMMUNE TOLERANCE AND AN EXTRACELLULAR REGULATORY ROLE FOR BIVALENT ANTIBODY. Nature. 1964 May 16;202:677–682. doi: 10.1038/202677a0. [DOI] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Marrack P. C. Helper T cells recognise antigen and macrophage surface components simultaneously. Nature. 1976 Aug 26;262(5571):797–799. doi: 10.1038/262797a0. [DOI] [PubMed] [Google Scholar]
- Lachman L. B., Hacker M. P., Handschumacher R. E. Partial purification of human lymphocyte-activating factor (LAF) by ultrafiltration and electrophoretic techniques. J Immunol. 1977 Dec;119(6):2019–2023. [PubMed] [Google Scholar]
- Laub O., Rall L. B., Truett M., Shaul Y., Standring D. N., Valenzuela P., Rutter W. J. Synthesis of hepatitis B surface antigen in mammalian cells: expression of the entire gene and the coding region. J Virol. 1983 Oct;48(1):271–280. doi: 10.1128/jvi.48.1.271-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Rosenstreich D. L. Regulation of lymphocyte-activating factor (LAF) production and secretion in P388D1 cells: identification of high molecular weight precursors of LAF. J Immunol. 1979 Jun;122(6):2173–2179. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubin R., Zolla-Pazner S. Markers of macrophage heterogeneity. I. Studies of macrophages from various organs of normal mice. Eur J Immunol. 1979 Dec;9(12):972–978. doi: 10.1002/eji.1830091211. [DOI] [PubMed] [Google Scholar]
- STONER R. D., TERRES G. ENHANCED ANTITOXIN RESPONSES IN IRRADIATED MICE ELICITED BY COMPLEXES OF TETANUS TOXOID AND SPECIFIC ANTIBODY. J Immunol. 1963 Dec;91:761–770. [PubMed] [Google Scholar]
- Schwartz R. H., Paul W. E. T-lymphocyte-enriched murine peritoneal exudate cells. II. Genetic control of antigen-induced T-lymphocyte proliferation. J Exp Med. 1976 Mar 1;143(3):529–540. doi: 10.1084/jem.143.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Sinclair N. R. Modulation of immunity by antibody, antigen-antibody complexes and antigen. Pharmacol Ther. 1979;4(2):355–432. doi: 10.1016/0163-7258(79)90142-6. [DOI] [PubMed] [Google Scholar]
- TERRES G., WOLINS W. Enhanced immunological sensitization of mice by the simultaneous injection of antigen and specific antiserum. I. Effect of varying the amount of antigen used relative to the antiserum. J Immunol. 1961 Apr;86:361–368. [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Voisin G. A. Role of antibody classes in the regulatory facilitation reaction. Immunol Rev. 1980;49:3–59. doi: 10.1111/j.1600-065x.1980.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Wands J. R., Zurawski V. R., Jr High affinity monoclonal antibodies to hepatitis B surface antigen (HBsAg) produced by somatic cell hybrids. Gastroenterology. 1981 Feb;80(2):225–232. [PubMed] [Google Scholar]


