Abstract
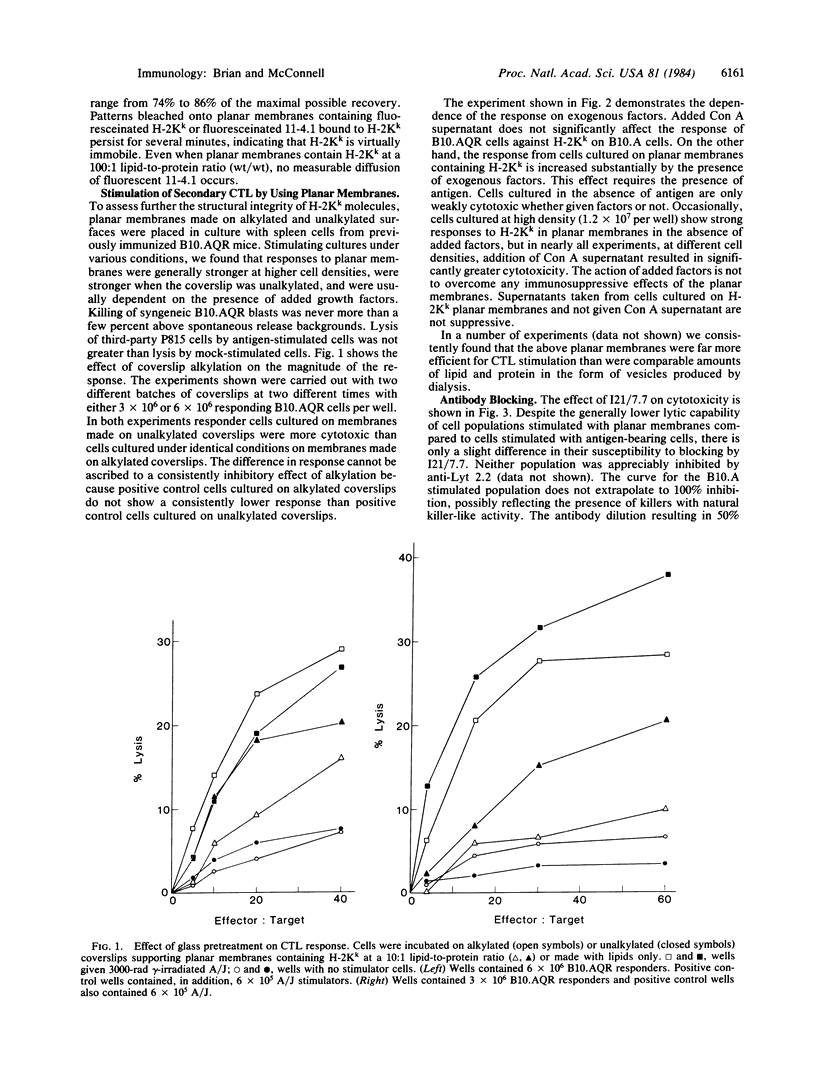
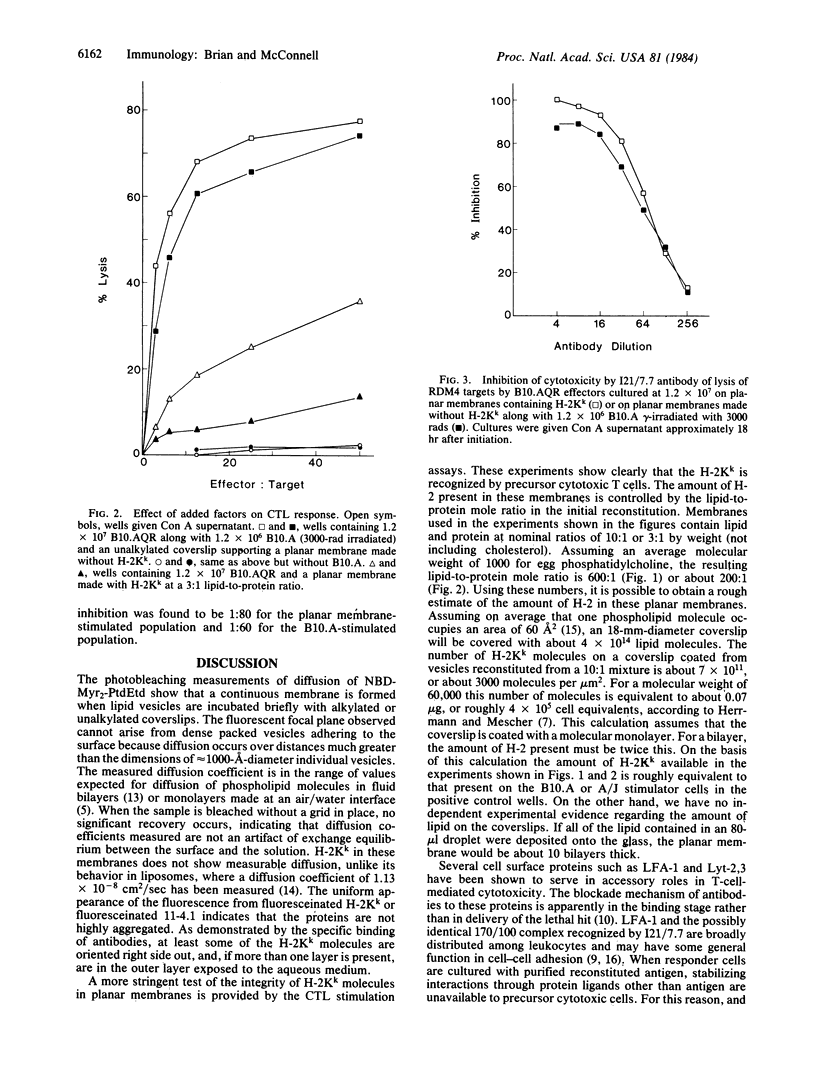
Phospholipid vesicles containing the transmembrane protein H-2Kk spontaneously fuse to form planar membranes when incubated on treated glass surfaces. Pattern photobleaching of fluorescent lipid probes indicates that these planar membranes are continuous and that the lipids are as mobile as they are in conventional fluid bilayers or monolayers. H-2Kk molecules in these planar membranes are immobile. These membranes stimulate cytotoxic T lymphocytes when cultured with immune spleen cells. The response to H-2Kk in planar membranes is greatly enhanced by the addition of supernatant from concanavalin A-stimulated spleen cells, indicating that relatively little antigen processing or presentation by accessory cells occurs. Cytotoxic T cells induced by purified alloantigen are found to be as susceptible to antibody blockade as are effectors from conventional mixed lymphocyte culture, where the antibody is directed against a T-cell surface antigen reputed to strengthen target cell adhesion through an interaction independent of major histocompatibility antigens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert F., Boyer C., Leserman L. D., Schmitt-Verhulst A. M. Immunopurification and insertion into liposomes of native and mutant H-2Kb: quantification by solid phase radioimmunoassay. Mol Immunol. 1983 Jun;20(6):655–667. doi: 10.1016/0161-5890(83)90010-x. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Cartwright G. S., Smith L. M., Heinzelmann E. W., Ruebush M. J., Parce J. W., McConnell H. M. H-2Kk and vesicular stomatitis virus G proteins are not extensively associated in reconstituted membranes recognized by T cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1506–1510. doi: 10.1073/pnas.79.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2382–2388. [PubMed] [Google Scholar]
- Emerson S. G., Cone R. E. I-Kk and H-2Kk antigens are shed as supramolecular particles in association with membrane lipids. J Immunol. 1981 Aug;127(2):482–486. [PubMed] [Google Scholar]
- Emerson S. G., Murphy D. B., Cone R. E. Selective turnover and shedding of H-2K and H-2D antigens is controlled by the major histocompatibility complex. Implications for H-2-restricted recognition. J Exp Med. 1980 Oct 1;152(4):783–795. doi: 10.1084/jem.152.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A., Watson J. Biochemical characterization of lymphocyte regulatory molecules. II. Purification of a class of rat and human lymphokines. J Immunol. 1980 Apr;124(4):1954–1962. [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Purification of the H-2Kk molecule of the murine major histocompatibility complex. J Biol Chem. 1979 Sep 25;254(18):8713–8716. [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Secondary cytolytic T lymphocyte stimulation by purified H-2Kk in liposomes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2488–2492. doi: 10.1073/pnas.78.4.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S. H., Weinberger O., Burakoff S. J., Mescher M. F. Analysis of the two-signal requirement for precursor cytolytic T lymphocyte activation using H-2Kk in liposomes. J Immunol. 1982 May;128(5):1968–1974. [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J Biol Chem. 1979 Jul 10;254(13):6068–6078. [PubMed] [Google Scholar]
- Malissen B., Price M. P., Goverman J. M., McMillan M., White J., Kappler J., Marrack P., Pierres A., Pierres M., Hood L. Gene transfer of H-2 class II genes: antigen presentation by mouse fibroblast and hamster B-cell lines. Cell. 1984 Feb;36(2):319–327. doi: 10.1016/0092-8674(84)90225-3. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Brian A. A., McConnell H. M. Binding of cytotoxic T-lymphocytes to supported lipid monolayers containing trypsinized H-2Kk. Mol Immunol. 1983 Nov;20(11):1227–1231. doi: 10.1016/0161-5890(83)90147-5. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Dialynas D. P., Lancki D. W., Wall K. A., Lorber M. I., Loken M. R., Fitch F. W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Rubenstein J. L., Parce J. W., McConnell H. M. Lateral diffusion of M-13 coat protein in mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1980 Dec 9;19(25):5907–5911. doi: 10.1021/bi00566a037. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Brian A. A., McConnell H. M. Covalent linkage of a synthetic peptide to a fluorescent phospholipid and its incorporation into supported phospholipid monolayers. Biochim Biophys Acta. 1984 Apr 25;772(1):10–19. doi: 10.1016/0005-2736(84)90512-1. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Molecular complexity of leukocyte surface glycoproteins related to the macrophage differentiation antigen Mac-1. J Exp Med. 1981 Nov 1;154(5):1517–1524. doi: 10.1084/jem.154.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger R., Pattus F. Spreading of membranes at the air/water interface. Chem Phys Lipids. 1976 Jul;16(4):285–291. doi: 10.1016/0009-3084(76)90023-2. [DOI] [PubMed] [Google Scholar]
- Weis R. M., Balakrishnan K., Smith B. A., McConnell H. M. Stimulation of fluorescence in a small contact region between rat basophil leukemia cells and planar lipid membrane targets by coherent evanescent radiation. J Biol Chem. 1982 Jun 10;257(11):6440–6445. [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tscharner V., McConnell H. M. Physical properties of lipid monolayers on alkylated planar glass surfaces. Biophys J. 1981 Nov;36(2):421–427. doi: 10.1016/S0006-3495(81)84741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


